Germination Behavior and Early Seedling Growth in Abies pinsapo Boiss. Seeds
Abstract
1. Introduction
2. Results
2.1. Seed Viability
2.2. Seed Germination
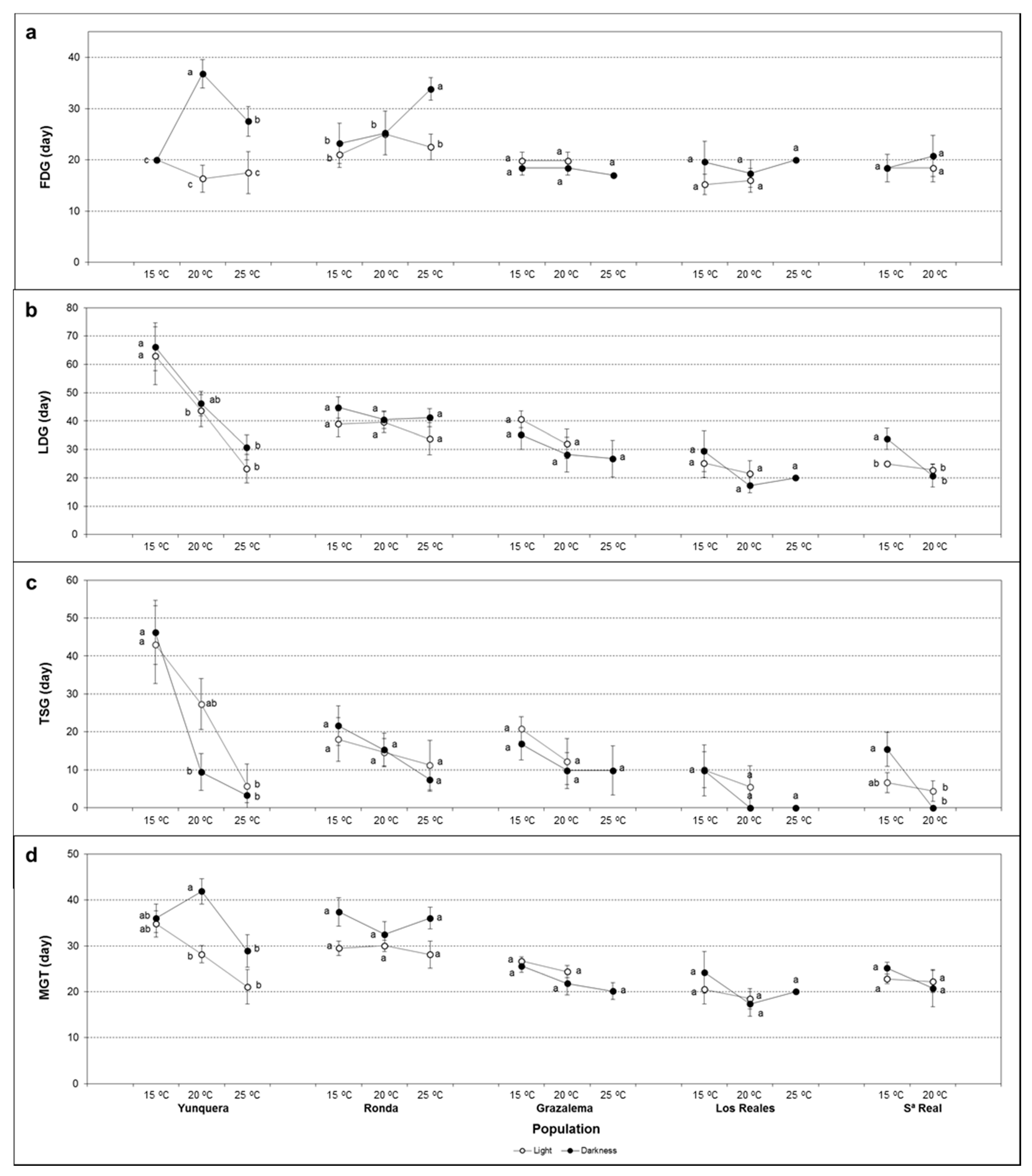
2.3. Germination Kinetics
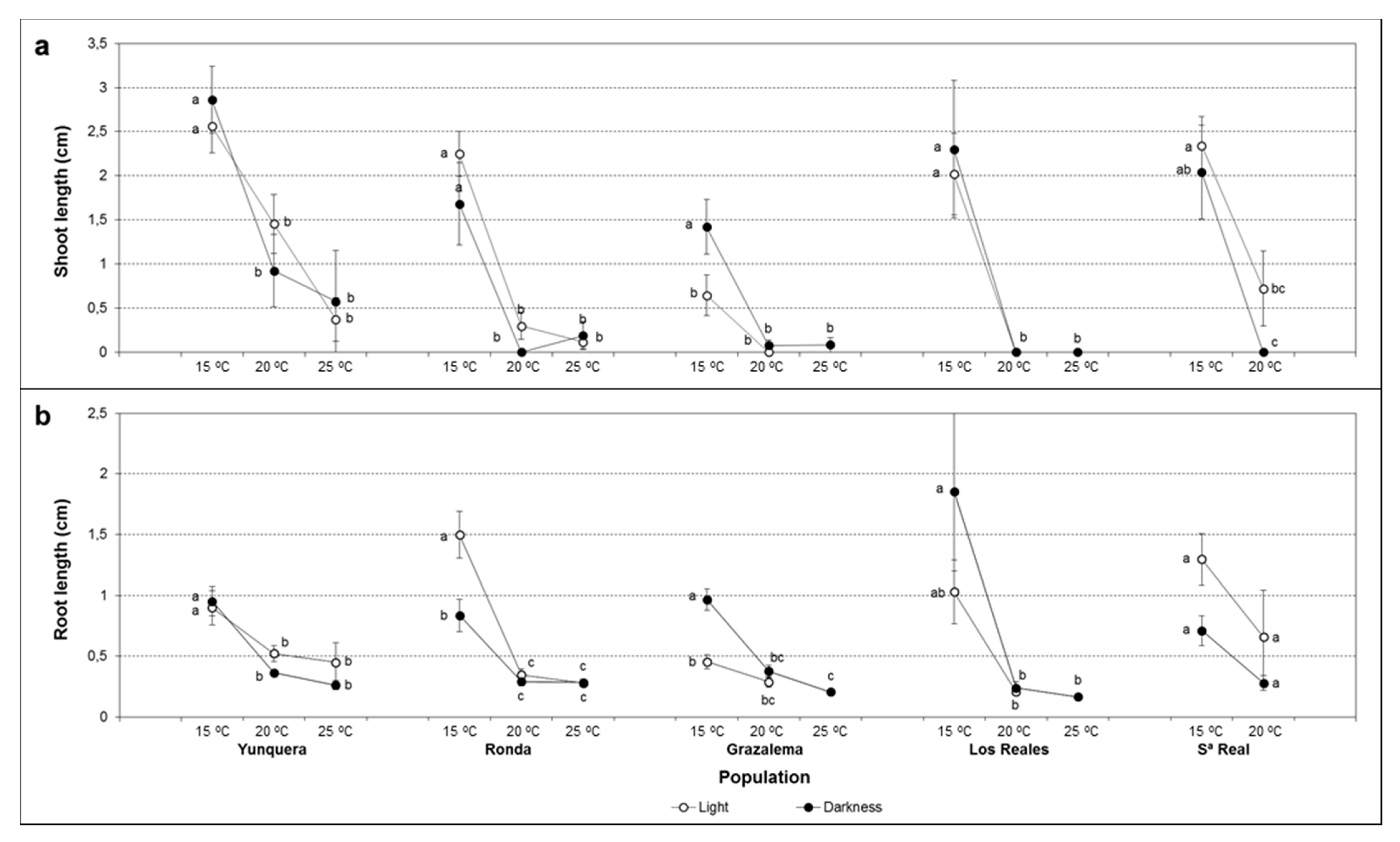
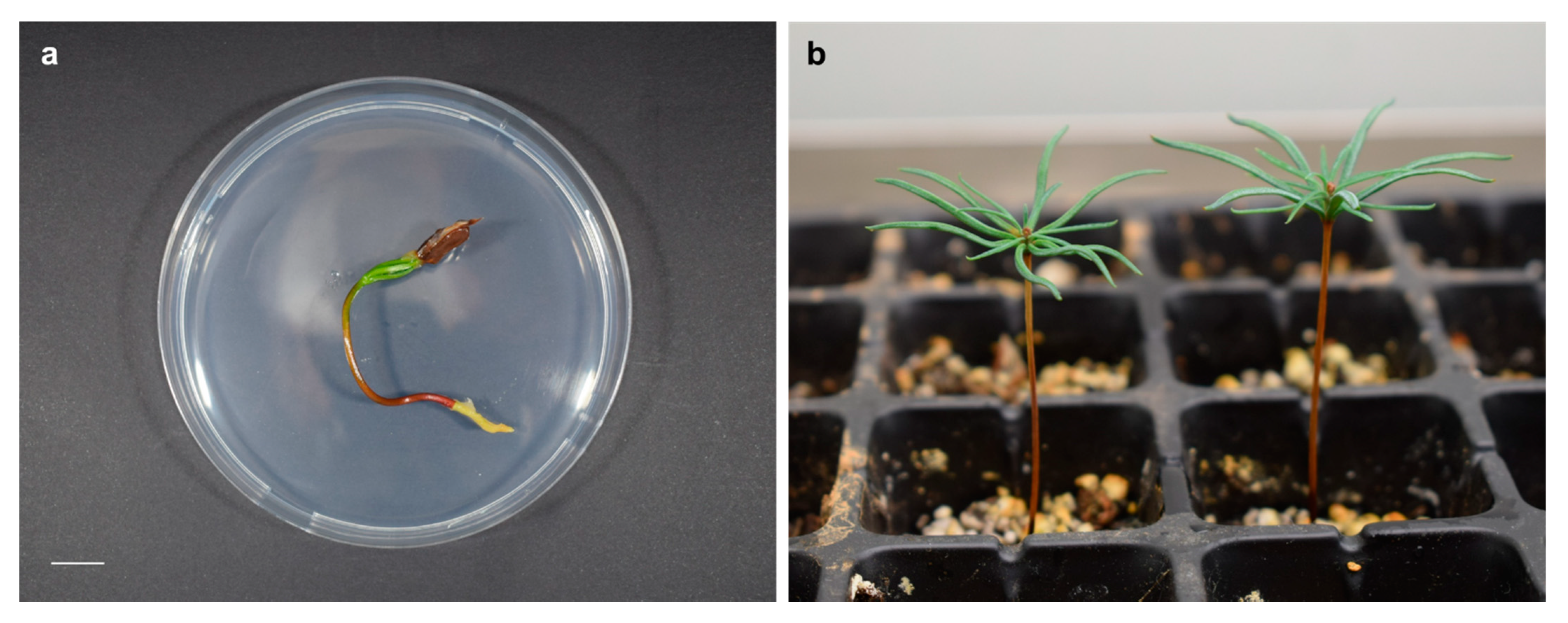
2.4. Early Seedling Growth
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Seed Viability
4.3. Germination Experiments
4.4. Data Taken and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrión, J.S.; Riquelme, J.A.; Navarro, C.; Munuera, M. Pollen in hyaena coprolites reflects late glacial landscape in southern Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 176, 193–205. [Google Scholar] [CrossRef]
- Martín, M.A.; Alvarez, J.B.; Martín, L.M. Genetic diversity of Spanish fir (Abies pinsapo Boiss.) populations by means of megagametophyte storage proteins. Ann. For. Sci. 2010, 67, 603. [Google Scholar] [CrossRef]
- Alba-Sánchez, F.; López-Sáez, J.A.; Benito de Pando, B.; Linares, J.C.; Nieto-Lugilde, D.; López-Merino, L. Past and present potential distribution of the Iberian Abies species: A phytogeographic approach using fossil pollen data and species distribution models. Divers. Distrib. 2010, 16, 214–228. [Google Scholar] [CrossRef]
- Alba-Sánchez, F.; López-Sáez, J.A.; Abel-Schaad, D.; Sabariego Ruiz, S.; Pérez-Díaz, S.; González-Hernández, A.; Linares, J.C. The impact of climate and land-use changes on the most southerly fir forests (Abies pinsapo) in Europe. Holocene 2019, 29, 1176–1188. [Google Scholar] [CrossRef]
- Sánchez-Robles, J.M.; Balao, F.; García-Castaño, J.L.; Terrab, A.; Navarro-Sampedro, L.; Talavera, S. Nuclear microsatellite primers for the endangered relict fir, Abies pinsapo (Pinaceae) and cross-amplification in related Mediterranean species. Int. J. Mol. Sci. 2012, 13, 14243–14250. [Google Scholar] [CrossRef]
- Ruíz de la Torre, J.; Ceballos, L. Árboles y Arbustos de la España PENINSULAR; Escuela Técnica Superior de Ingenieros de Montes: Madrid, Spain, 1979. [Google Scholar]
- Valladares, A. Abetales de Abies pinsapo Boiss. In Bases Ecológicas Preliminares para la Conservación de los Tipos de Hábitat de Interés Comunitario en España; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2009; ISBN 9788449109119. [Google Scholar]
- Esteban, L.G.; De Palacios, P.; Aguado, L.R.L. Abies pinsapo forests in Spain and Morocco: Threats and conservation. Oryx 2010, 44, 276–284. [Google Scholar] [CrossRef][Green Version]
- IUCN. Available online: https://www.iucnredlist.org/fr/species/42295/10679577 (accessed on 5 September 2022).
- Spanish Fir Recovery Plan. Available online: https://www.juntadeandalucia.es/medioambiente/portal/landing-page-planificacion/-/asset_publisher/Jw7AHImcvbx0/content/plan-de-recuperaci-c3-b3n-del-pinsapo-plan-/20151 (accessed on 5 September 2022).
- Roeder, M.; Yang, W.; Tomlinson, K.W. Influence of smoke, heat and fire on germination of woody species occurring in the dry valleys of southwest China. J. Plant Ecol. 2019, 12, 931–940. [Google Scholar] [CrossRef]
- Meyer, S.; Allen, P.; Beckstead, J. Seed germination regulation in Bromus tectorum (Poaceae) and its ecological significance. OIKOS 1997, 78, 475–485. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Arroyo, M.T.K. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae): Altitudinal variation in the Mediterranean Andes of central Chile. Plant Ecol. 2000, 149, 1–8. [Google Scholar] [CrossRef]
- Angevine, M.W.; Chabot, B.F. Seed germination syndromes in higher plants. In Topics in Plant Population Biology; Solbrig, O., Jain, S., Johnson, G.B., Raven, P., Eds.; Columbia University Press: New York, NY, USA, 1979; pp. 188–206. [Google Scholar]
- Seiwa, K.; Ando, M.; Imaji, A.; Tomita, M.; Kanou, K. Spatio-temporal variation of environmental signals inducing seed germination in temperate conifer plantations and natural hardwood forests in northern Japan. For. Ecol. Manag. 2009, 257, 361–369. [Google Scholar] [CrossRef]
- Kurt, Y.; Frampton, J.; Isik, F.; Landgren, C.; Chastagner, G. Variation in needle and cone characteristics and seed germination ability of Abies bornmuelleriana and Abies equi-trojani populations from Turkey. Turk. J. Agric. For. 2016, 40, 169–176. [Google Scholar] [CrossRef]
- Arista, M.; Talavera, S.; Herrera, J. Viabilidad y germinación de las semillas de Abies pinsapo Boiss. Acta Bot. Malacit. 1992, 17, 223–228. [Google Scholar] [CrossRef]
- Arista, M. Germinación de las semillas y supervivencia de las plántulas de Abies pinsapo Boiss. Acta Bot. Malacit. 1993, 18, 173–177. [Google Scholar] [CrossRef]
- Escudero, A.; Pérez-García, F.; Luzuriaga, A.L. Effects of light, temperature and population variability on the germination of seven Spanish pines. Seed Sci. Res. 2002, 12, 261–271. [Google Scholar] [CrossRef]
- Skordilis, A.; Thanos, C.A. Seed stratification and germination strategy in the Mediterranean pines Pinus brutia and P. halepensis. Seed Sci. Res. 1995, 5, 151–160. [Google Scholar] [CrossRef]
- Guo, C.; Shen, Y.; Shi, F. Effect of temperature, light, and storage time on the seed germination of Pinus bungeana Zucc. ex Endl.: The role of seed-covering layers and abscisic acid changes. Forests 2020, 11, 300. [Google Scholar] [CrossRef]
- Caliskan, S.; Makineci, E. Carbon and nitrogen of seed and some germination parameters at different test temperatures in Anatolian black pine populations. J. Sustain. For. 2020, 39, 23–34. [Google Scholar] [CrossRef]
- Thanos, C.A.; Skordilis, A. The effects of light, temperature and osmotic stress on the germination of Pinus halepensis and P. brutia seeds. Seed Sci. Technol. 1987, 15, 163–174. [Google Scholar]
- Skordilis, A.; Thanos, C.A. Comparative ecophysiology of seed germination strategies in the seven pine species naturally growing in Greece. In Proceedings of the Basic and Applied Aspects of Seed Biology: Proceedings of the Fifth International Workshop on Seeds; Ellis, R., Black, M., Murdoch, A., Hong, T., Eds.; Kluwer Academic Publishers: Reading, UK, 1997; pp. 623–632. [Google Scholar]
- Knapp, K.; Smith, W. Factors influencing understory seedling establishment of Engelmann spruce (Picea engelmannii) and subalpine fir (Abies lasiocarpa) in southeast Wyoming. Can. J. Bot. 1982, 60, 2753–2761. [Google Scholar] [CrossRef]
- Kueppers, L.M.; Faist, A.; Ferrenberg, S.; Castanha, C.; Conlisk, E.; Wolf, J. Lab and field warming similarly advance germination date and limit germination rate for high and low elevation provenances of two widespread subalpine conifers. Forests 2017, 8, 433. [Google Scholar] [CrossRef]
- Cristaudo, A.; Catara, S.; Mingo, A.; Restuccia, A.; Onofri, A. Temperature and storage time strongly affect the germination success of perennial Euphorbia species in Mediterranean regions. Ecol. Evol. 2019, 9, 10984–10999. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 20–26. ISBN 9780124166776. [Google Scholar]
- Nyman, B. Effect of red and far-red irradiation on the germination process in seeds of Pinus sylvestris L. Nature 1961, 191, 1219–1220. [Google Scholar] [CrossRef]
- Orlandini, M.; Malcoste, R. Etude du phytochrome des graines de Pinus nigra Arn par spectrophotométrie bichromatique in vivo. Planta 1972, 105, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chanyenga, T.F.; Geldenhuys, C.J.; Sileshi, G.W. Germination response and viability of an endangered tropical conifer Widdringtonia whytei seeds to temperature and light. S. Afr. J. Bot. 2012, 81, 25–28. [Google Scholar] [CrossRef]
- Blazich, F.; Hinesley, L. Low temperature germination of Fraser fir seed. Can. J. For. Res. 1984, 14, 948–949. [Google Scholar] [CrossRef]
- Ahola, V.; Leinonen, K. Responses of Betula pendula, Picea abies, and Pinus sylvestris seeds to red/far-red ratios as affected by moist chilling and germination temperature. Can. J. For. Res. 1999, 29, 1709–1717. [Google Scholar] [CrossRef]
- Daskalakou, E.N.; Koutsovoulou, K.; Mavroeidi, L.; Tsiamitas, C.; Kafali, E.; Radaiou, P.E.; Ganatsas, P.; Thanos, C.A. Interannual variability of germination and cone/seed morphometric characteristics in the endemic Grecian fir (Abies cephalonica) over an 8-year-long study. Seed Sci. Res. 2017, 28, 24–33. [Google Scholar] [CrossRef]
- Jull, L.G.; Blazich, F.A. Seed germination of selected provenances of Atlantic white-cedar as influenced by stratification, temperature, and light. HortScience 2000, 35, 132–135. [Google Scholar] [CrossRef]
- Strandby Andersen, U.; Prado Córdova, J.P.; Bräuner Nielsen, U.; Kollmann, J. Provenance variation in germination and seedling growth of Abies guatemalensis Rehder. For. Ecol. Manag. 2008, 255, 1831–1840. [Google Scholar] [CrossRef]
- Song, J.; Jang, K.; Hur, S. Variation of seed and germination characteristics of natural populations of Abies koreana Wilson, a Korean endemic species. J. Korean For. Soc. 2013, 99, 849–854. [Google Scholar]
- Wang, Z.M.; MacDonald, S.E. Peatland and upland black spruce populations in Alberta: Morphology and ecophysiology. Can. J. For. Res. 1993, 23, 33–40. [Google Scholar] [CrossRef]
- Gutterman, Y. Maternal effects on seeds during development. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 59–84. [Google Scholar]
- Liu, Y.; El-Kassaby, Y.A. Timing of seed germination correlated with temperature-based environmental conditions during seed development in conifers. Seed Sci. Res. 2015, 25, 29–45. [Google Scholar] [CrossRef]
- Ferreira, A.; Handro, W. Aspects of seed germination in Araucaria angustifolia (Bert.) O. Ktre. Rev. Bras. Bot. 1979, 2, 7–14. [Google Scholar]
- Anderson, L.J.; Winterton, A.J. Germination as a determinant of seedling distributions among natural substrates in Picea engelmannii (Pinaceae) and Abies lasiocarpa (Pinaceae). Am. J. Bot. 1996, 83, 112–117. [Google Scholar] [CrossRef]
- Davidson, R.H.; Edwards, D.G.W.; Sziklai, O.; El-Kassaby, Y. Genetic variation in germination parameters among Pacific silver fir populations. Silvae Genet. 1996, 45, 165–171. [Google Scholar]
- Adkins, C.R.; Hinesley, L.E.; Blazich, F.A. Role of stratification, temperature, and light in Fraser fir germination. Can. J. For. Res. 1984, 14, 88–93. [Google Scholar] [CrossRef]
- Liesebach, M.; Konig, A.O.; Ujvári-Jármay, E. Provenance-environment interactions of Norway spruce (Picea abies Karst.) on German and Hungarian test sites. In Genetic Response of Forest Systems to Changing Environmental Conditions; Müller-Starck, G., Schubert, R., Eds.; Kluwer Academic Publishers: London, UK, 2001; pp. 353–363. [Google Scholar]
- Chazarra Bernabé, A.; Flórez García, E.; Peraza Sánchez, B.; Tohá Rebull, T.; Lorenzo Mariño, B.; Criado Pinto, E.; Moreno García, J.V.; Romero Fresneda, R.; Botey Fullat, R. Mapas climáticos de España (1981–2010) Y ETo (1996–2016); Ministerio para la Transición Ecológica Agencia Estatal de Meteorología: Madrid, Spain, 2018. [Google Scholar]
- Atlas Climático de la Península y Baleares. Available online: https://www.aemet.es/es/serviciosclimaticos/datosclimatologicos/atlas_climatico/visor_atlas_climatico (accessed on 23 September 2022).
- Hartmann, H.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann & Kester’ s Plant Propagation Principles and Practices Hartmann Kester Davies Geneve, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2014; pp. 188–189. ISBN 9781292020884. [Google Scholar]
- Witty, M. Topographischer nachweis der keimfähigkeit der getreidefrüchte durch tetrazoliumsalze (Topographic detection of germination in cereal crops by tetrazolium salts)—A Translation of Lakon’s 1942 paper on tetrazolium seed testing. Seed Technol. 2012, 34, 275–282. [Google Scholar]
- Kader, M.A. A Comparison of seed germination calculation formulae and the associated interpretation of resulting data. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar]
- Ellis, R.; Roberts, E. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Sokal, R.; Rohlf, F. Biometry: The Principles and Practice of Statistics in Biological Research; W.H. Freeman and Company: New York, NY, USA, 2013; pp. 319–378. [Google Scholar]

| Location | Latitude | Longitude | Altitude (m.a.s.l.) |
|---|---|---|---|
| Pinsapar de Ronda | 36°41′37′′ N | 5°07′20′′ W | 1300–1700 |
| Sierra de Yunquera | 36°43′51′′ N | 4°57′11′′ W | 1000–1500 |
| Sierra Real | 36°37′13′′ N | 4°58′41′′ W | 1000–1449 |
| Sierra de Grazalema | 36°46′11′′ N | 5°24′03′′ W | 1000–1400 |
| Los Reales de Sierra Bermeja | 36°29′29′′ N | 5°12′02′′ W | 1100–1449 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Navas, M.V.; Sánchez-Romero, C. Germination Behavior and Early Seedling Growth in Abies pinsapo Boiss. Seeds. Plants 2022, 11, 2715. https://doi.org/10.3390/plants11202715
Bravo-Navas MV, Sánchez-Romero C. Germination Behavior and Early Seedling Growth in Abies pinsapo Boiss. Seeds. Plants. 2022; 11(20):2715. https://doi.org/10.3390/plants11202715
Chicago/Turabian StyleBravo-Navas, María Victoria, and Carolina Sánchez-Romero. 2022. "Germination Behavior and Early Seedling Growth in Abies pinsapo Boiss. Seeds" Plants 11, no. 20: 2715. https://doi.org/10.3390/plants11202715
APA StyleBravo-Navas, M. V., & Sánchez-Romero, C. (2022). Germination Behavior and Early Seedling Growth in Abies pinsapo Boiss. Seeds. Plants, 11(20), 2715. https://doi.org/10.3390/plants11202715







