Far-Red Light Effects on Lettuce Growth and Morphology in Indoor Production Are Cultivar Specific
Abstract
1. Introduction
2. Results
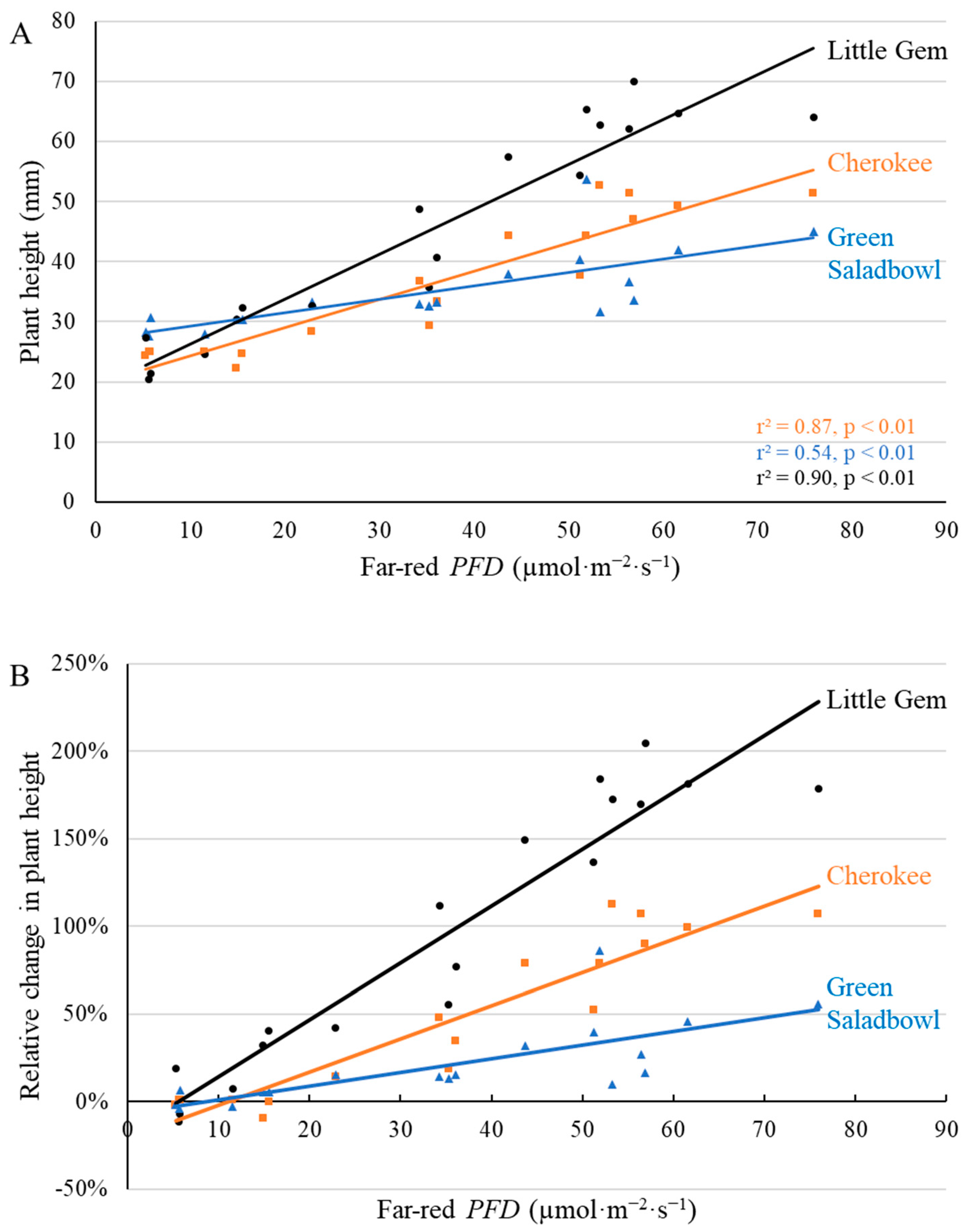
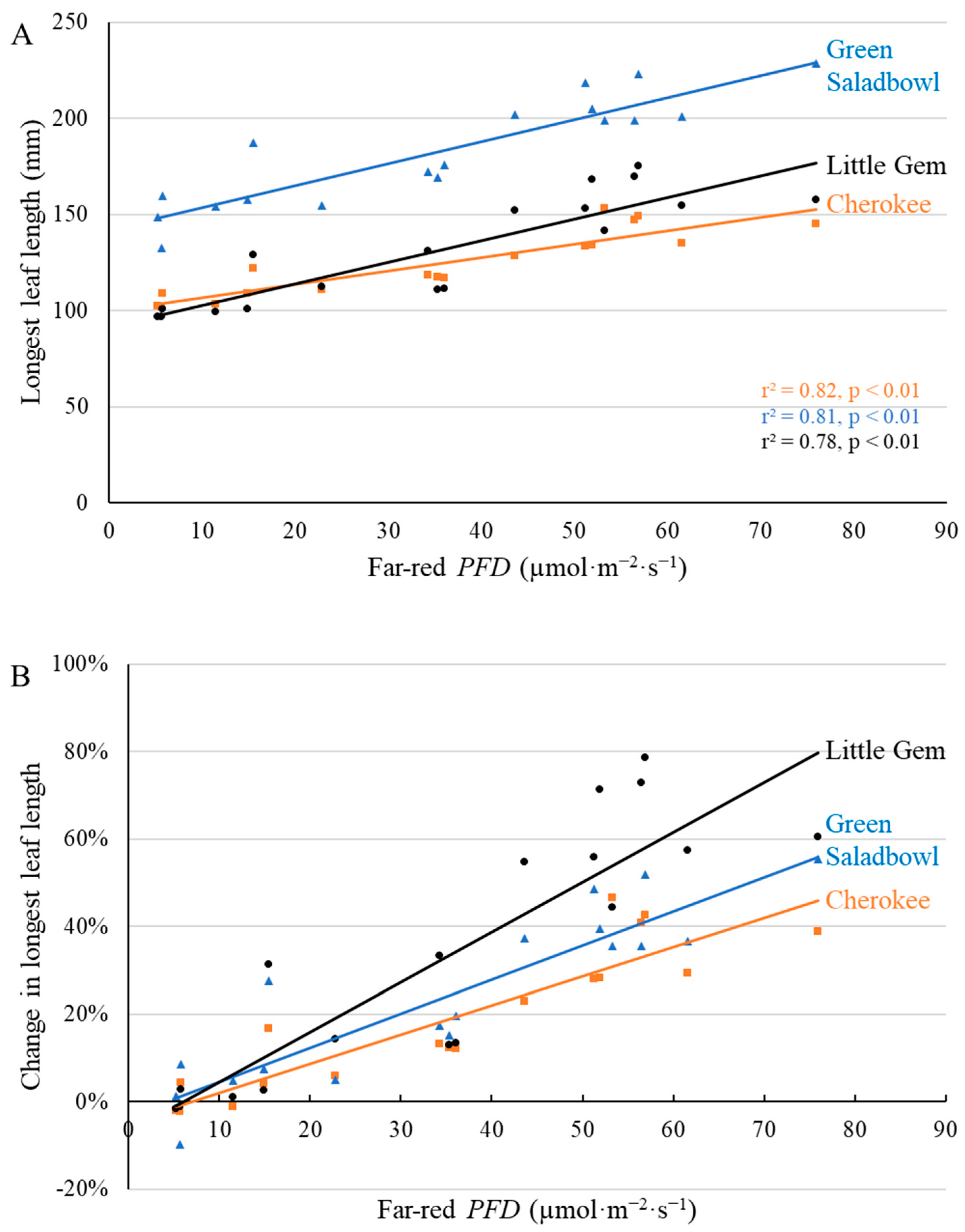
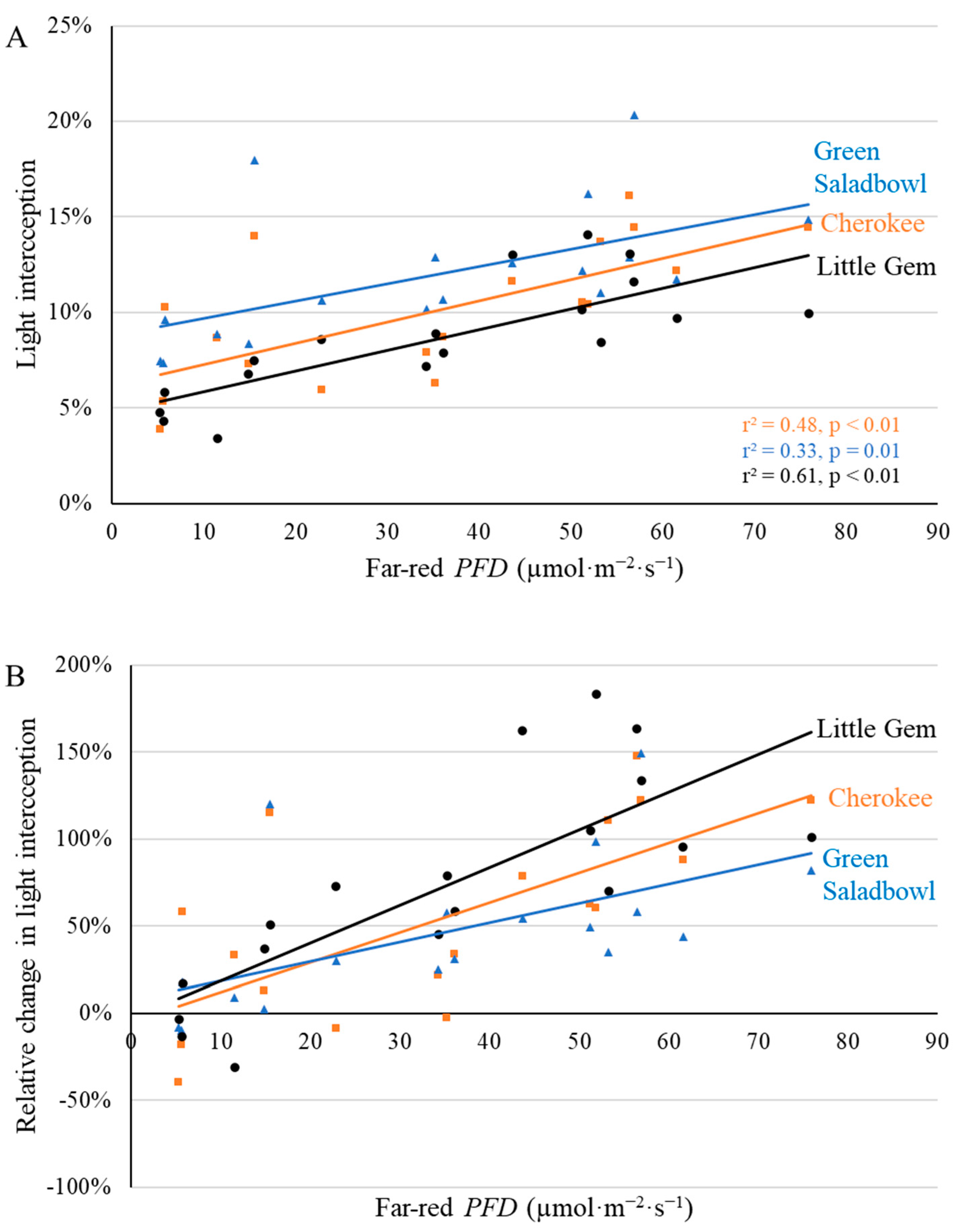
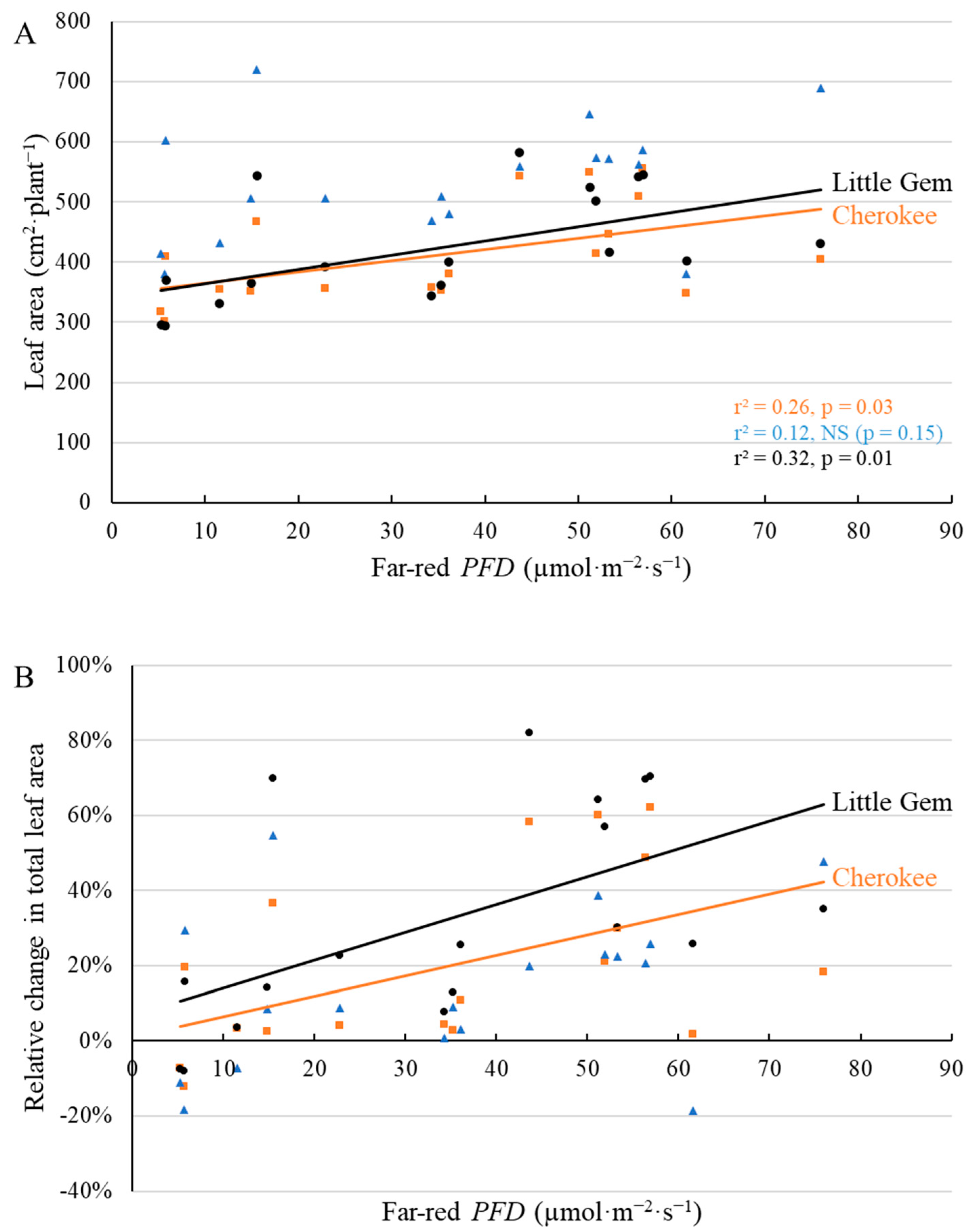
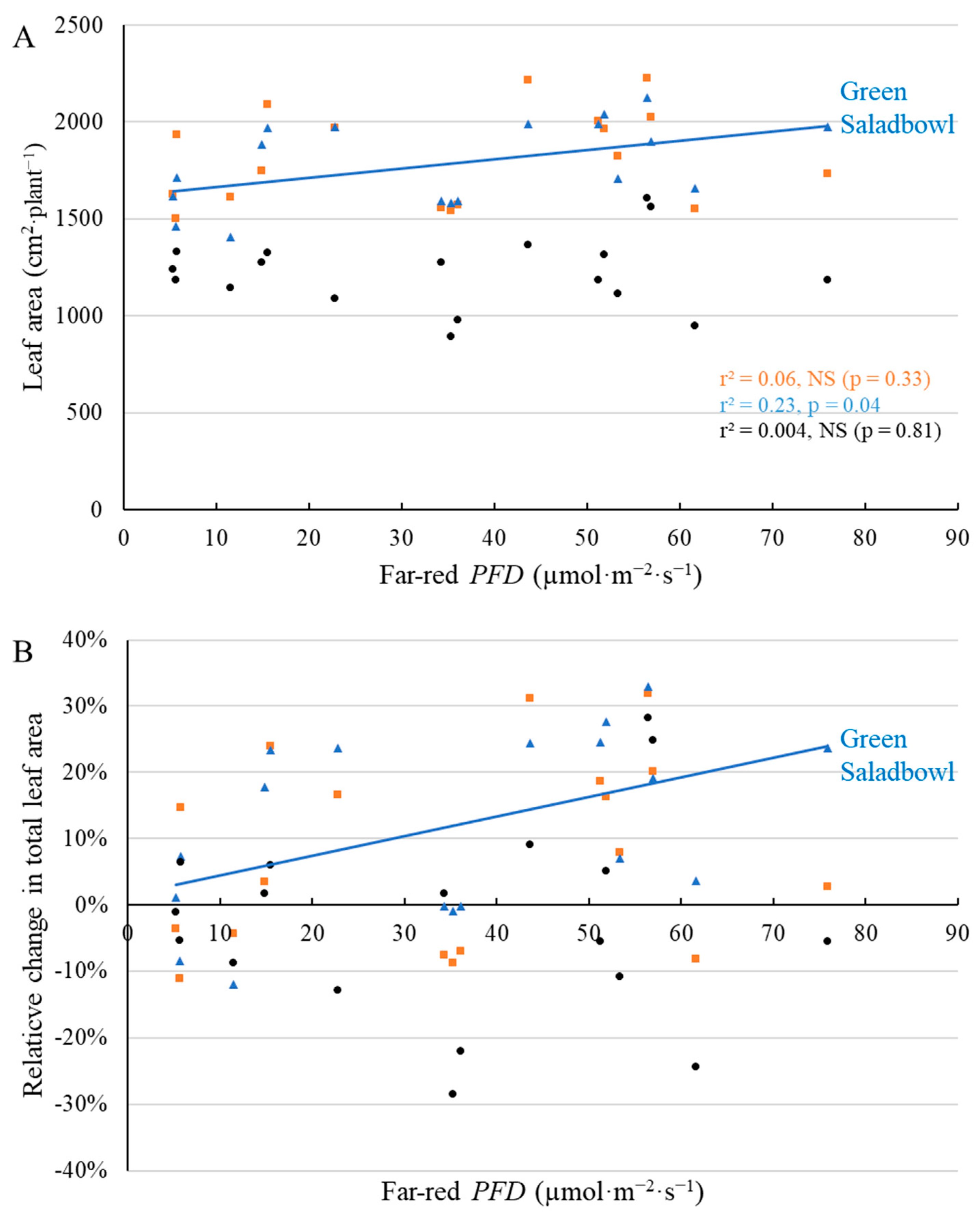
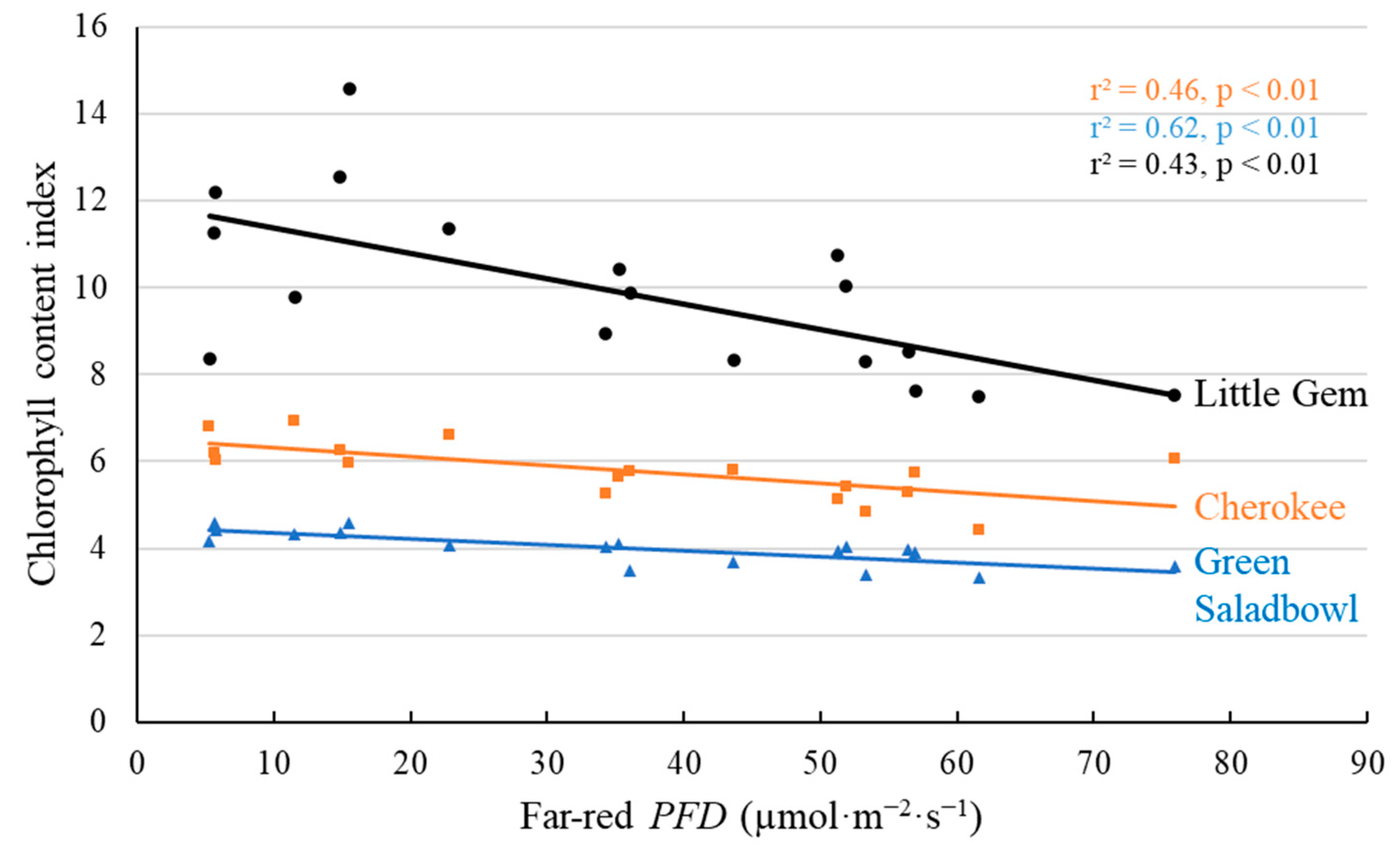
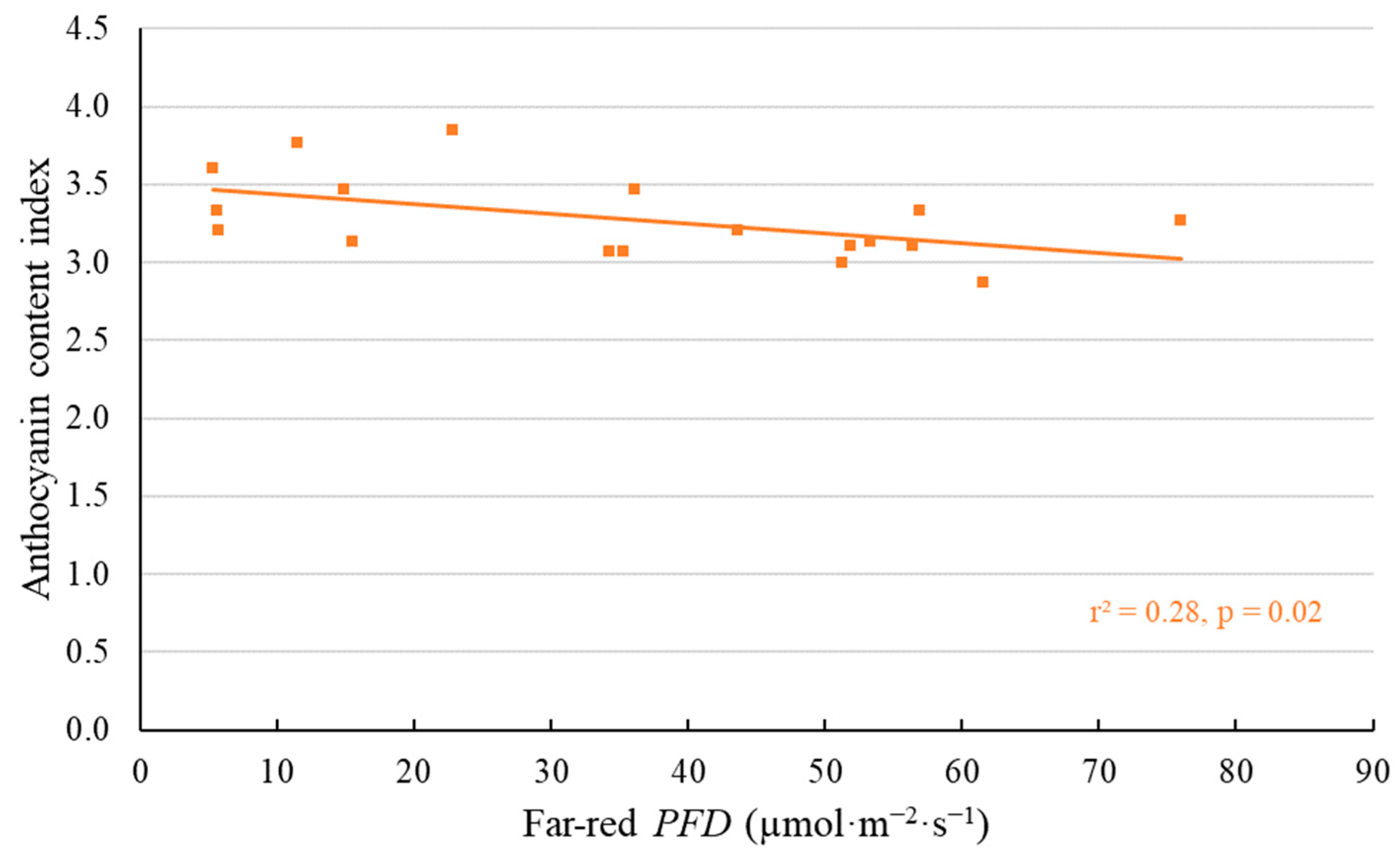
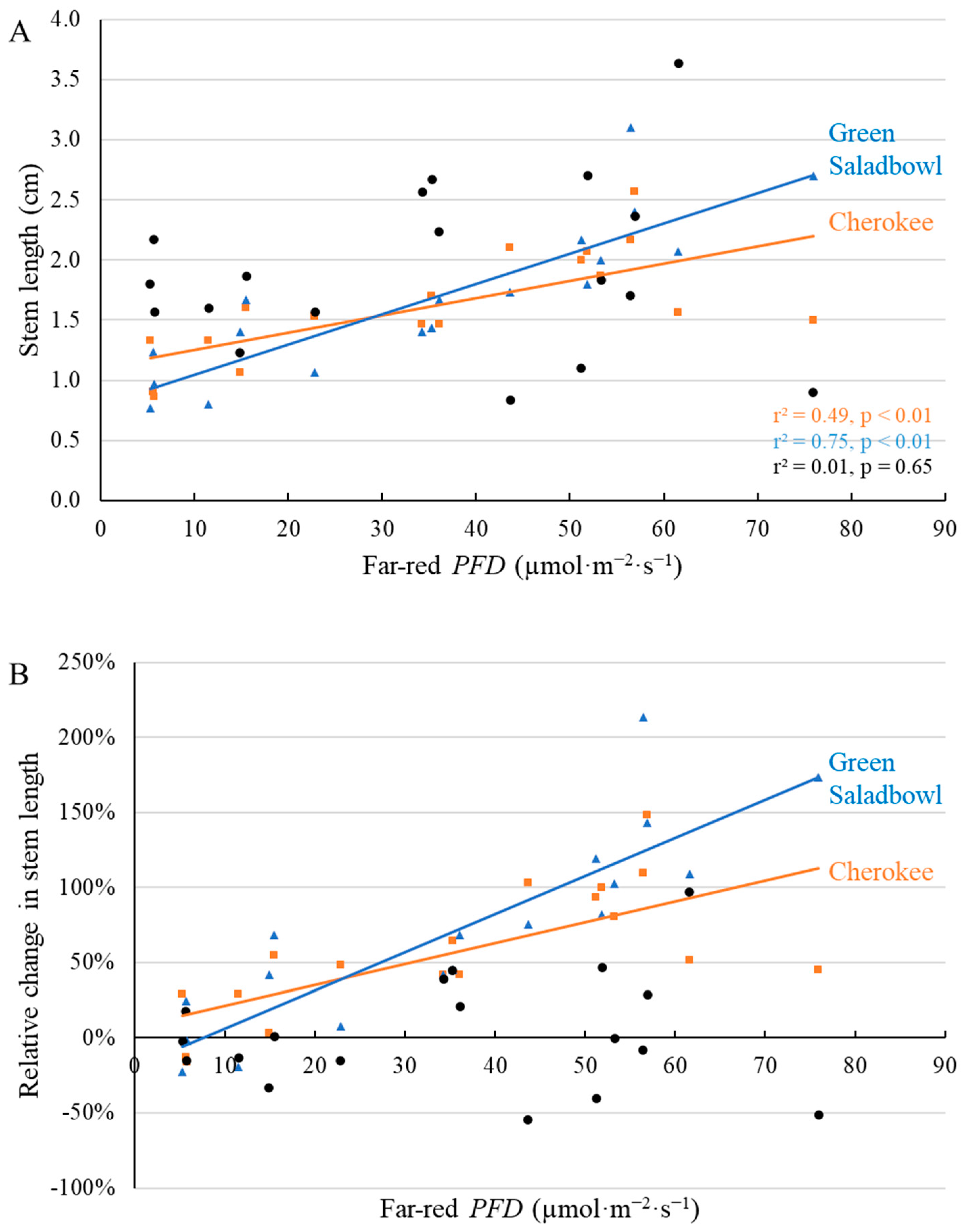
2.1. Morphological Responses to Far-Red Light
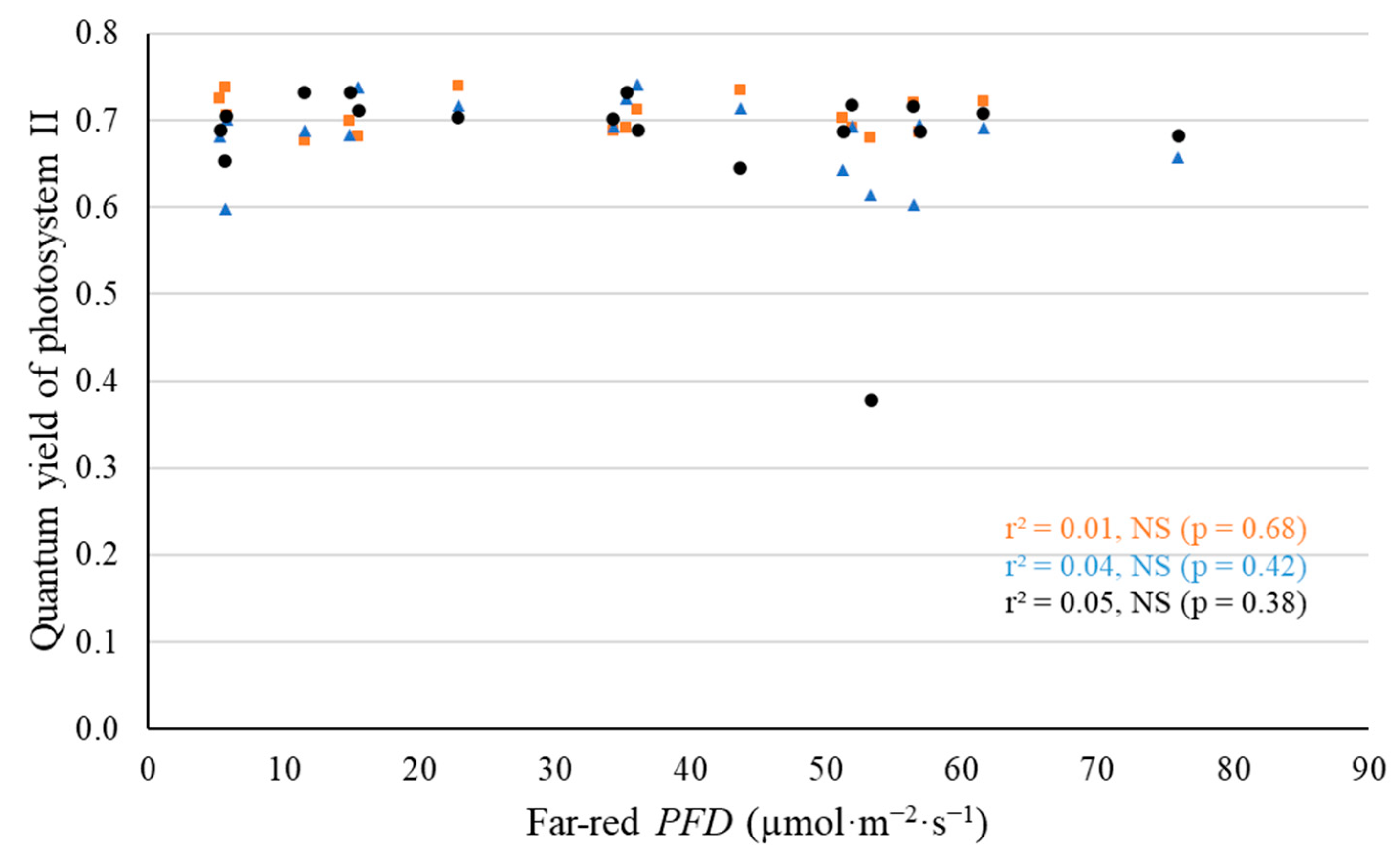
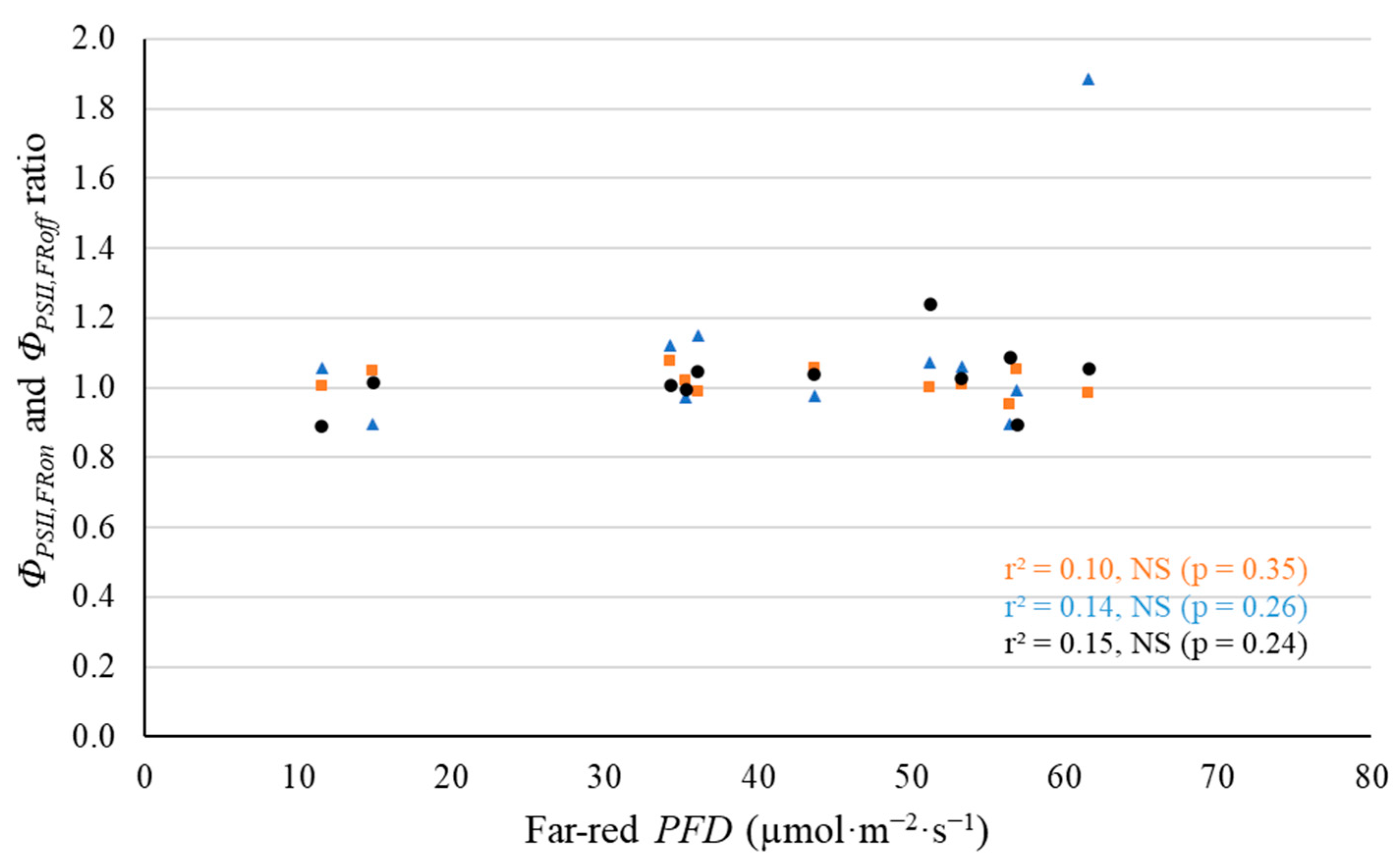
2.2. Leaf-Level Photosynthetic Response to Supplemental Far-Red Light
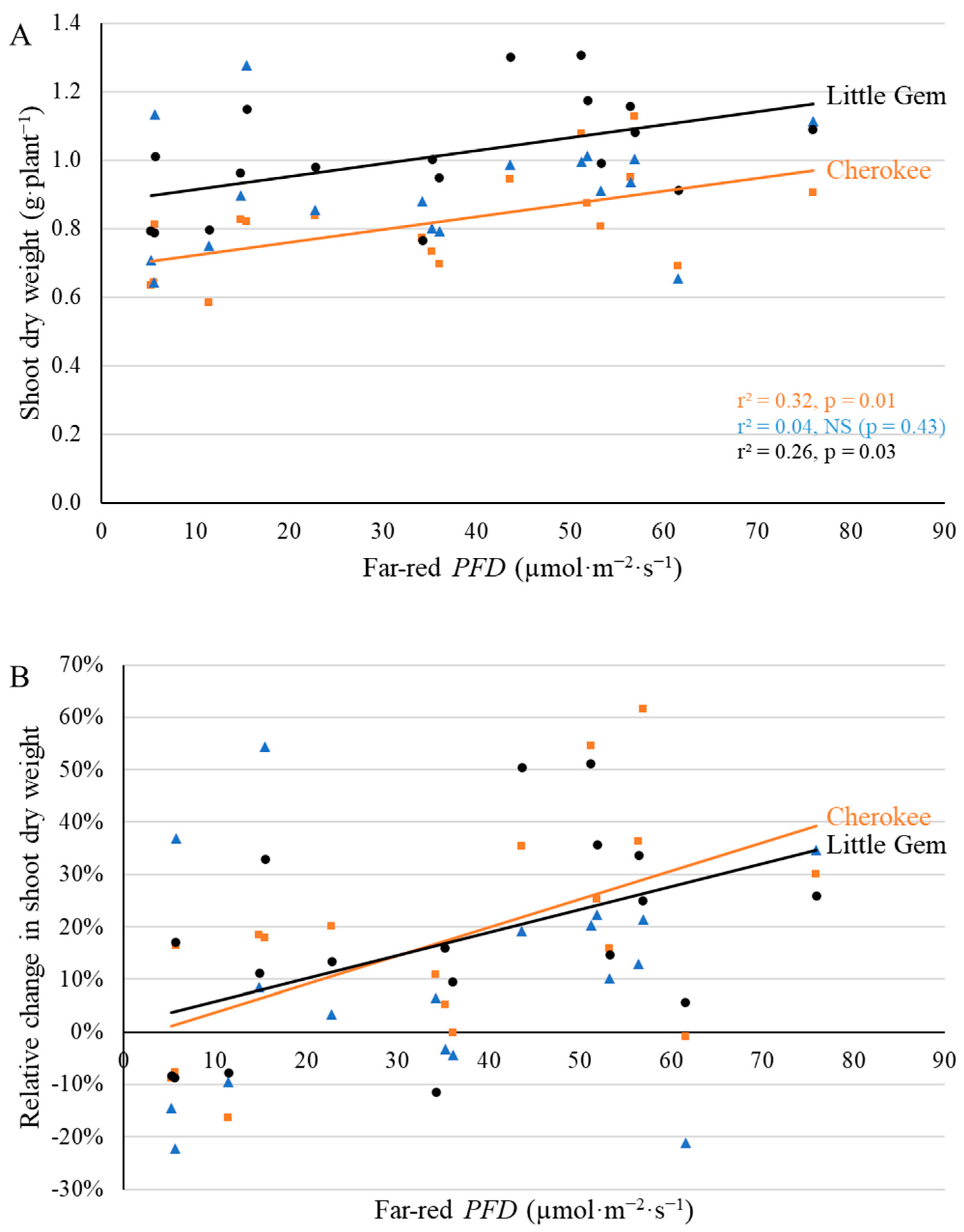
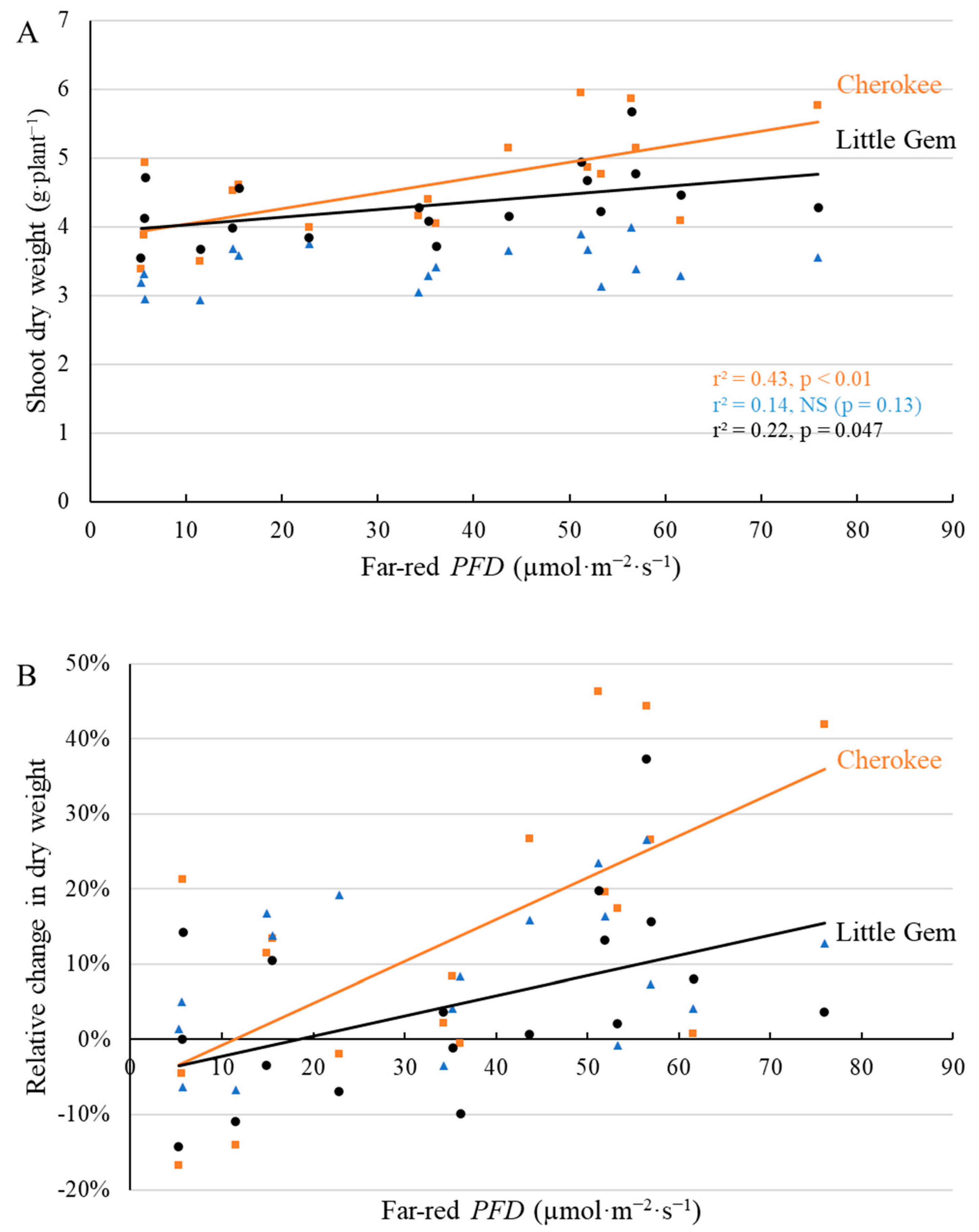
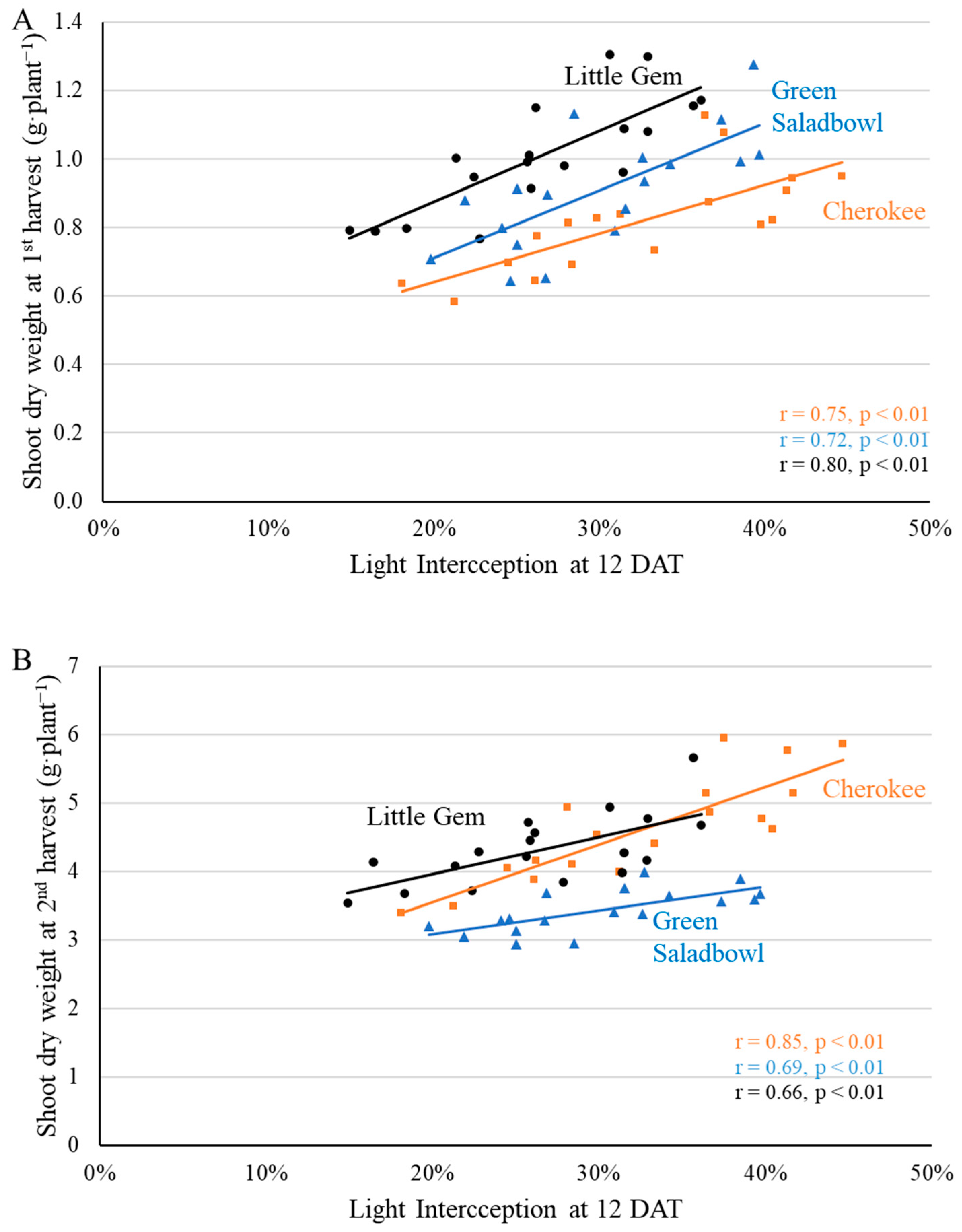
2.3. Final Yield
3. Discussion
3.1. Morphological Changes Induced by Adding Far-Red Light
3.2. No Evidence That Far-Red Photons Affected Leaf-Level Photosynthesis
3.3. Supplemental Far-Red Light Increased Final Yield in a Cultivar-Specific Manner
3.4. Conclusions
4. Materials and Methods
4.1. Walk-In Cooler Setup
4.2. Plant Material
4.3. Far-Red Light Treatments
4.4. Physiological and Morphological Measurements
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Holmes, M.; Smith, H. The function of phytochrome in the natural environment—II. The influence of vegetation canopies on the spectral energy distribution of natural daylight. Photochem. Photobiol. 1977, 25, 539–545. [Google Scholar] [CrossRef]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant adaptation to dynamically changing environment: The shade avoidance response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Gommers, C.M.M.; Visser, E.J.W.; Onge, K.R.S.; Voesenek, L.A.C.J.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Craver, J.K.; Boldt, J.K.; Lopez, R.G. Radiation Intensity and Quality from Sole-source Light-emitting Diodes Affect Seedling Quality and Subsequent Flowering of Long-day Bedding Plant Species. HortScience 2018, 53, 1407–1415. [Google Scholar] [CrossRef]
- Kubota, C.; Chia, P.; Yang, Z.; Li, Q. Applications of far-red light emitting diodes in plant production under controlled environments. In Proceedings of the International Symposium on Advanced Technologies and Management Towards Sustainable Greenhouse Ecosystems: Greensys, Neos Marmaras-Sithonia, Chalkidiki, Greece, 5–10 June 2011; pp. 59–66. [Google Scholar]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Spalholz, H. Characterization of Solar Radiation Spectral Contribution in Lettuce Bolting and Flowering Using LEDs in an Indoor Setting. In Proceedings of the 2019 ASHS Annual Conference, Online, 21–25 July 2019. [Google Scholar]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Emerson, R.; Lewis, C.M. The dependence of the quantum yield of Chlorella photosynthesis on wave length of light. Am. J. Bot. 1943, 30, 165–178. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Evans, J. The dependence of quantum yield on wavelength and growth irradiance. Funct. Plant Biol. 1987, 14, 69–79. [Google Scholar] [CrossRef]
- Emerson, R. Dependence of yield of photosynthesis in long-wave red on wavelength and intensity of supplementary light. Science 1957, 125, 746. [Google Scholar] [CrossRef]
- Myers, J. Enhancement studies in photosynthesis. Annu. Rev. Plant Physiol. 1971, 22, 289–312. [Google Scholar] [CrossRef]
- McCree, K.J. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric. Meteorol. 1972, 10, 443–453. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Substituting Far-Red for Traditionally Defined Photosynthetic Photons Results in Equal Canopy Quantum Yield for CO2 Fixation and Increased Photon Capture During Long-Term Studies: Implications for Re-Defining PAR. Front. Plant Sci. 2020, 11, 581156. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 56. [Google Scholar] [CrossRef]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding Far-Red to Red-Blue Light-Emitting Diode Light Promotes Yield of Lettuce at Different Planting Densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef]
- Oelze-Karow, H.; Mohr, H. Phytochrome Action on Chlorophyll Synthesis—A Study of the Escape from Photoreversibility. Plant Physiol. 1982, 70, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Yanovsky, M.J.; Alconada-Magliano, T.M.; Mazzella, M.A.; Gatz, C.; Thomas, B.; Casal, J.J. Phytochrome A affects stem growth, anthocyanin synthesis, sucrose-phosphate-synthase activity and neighbour detection in sunlight-grown potato. Planta 1998, 205, 235–241. [Google Scholar] [CrossRef]
- Lange, H.; Shropshire, W., Jr.; Mohr, H. An Analysis of Phytochrome-mediated Anthocyanin Synthesis. Plant Physiol. 1971, 47, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.L. Light-dependent anthocyanin synthesis: A model system for the study of plant photomorphogenesis. Bot. Rev. 1985, 51, 107–157. [Google Scholar] [CrossRef]
- Parry, C.; Blonquist, J.M., Jr.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, W.L.; Weston, D.J.; Bowden, J.D.; Dudley, J.B.; Toler, J.E. Leaf absorptance of photosynthetically active radiation in relation to chlorophyll meter estimates among woody plant species. Sci. Hortic. 2004, 101, 169–178. [Google Scholar] [CrossRef]
- Zhen, S.; Kusuma, P. Accuracy of the Generic Equation to Convert CCI to Chlorophyll Concentration in the Apogee Model MC-100 Chlorophyll Concentration Meter; Techniques and Instruments. Paper 17; Utah State University: Logan, UT, USA, 2020; Available online: https://digitalcommons.usu.edu/cpl_techniquesinstruments/17 (accessed on 16 September 2022).
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. Green light drives CO2 fixation deep within leaves. Plant Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Zelitch, I. The close relationship between net photosynthesis and crop yield. Bioscience 1982, 32, 796–802. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; van Iersel, M.W. Far-Red Light Effects on Lettuce Growth and Morphology in Indoor Production Are Cultivar Specific. Plants 2022, 11, 2714. https://doi.org/10.3390/plants11202714
Liu J, van Iersel MW. Far-Red Light Effects on Lettuce Growth and Morphology in Indoor Production Are Cultivar Specific. Plants. 2022; 11(20):2714. https://doi.org/10.3390/plants11202714
Chicago/Turabian StyleLiu, Jun, and Marc W. van Iersel. 2022. "Far-Red Light Effects on Lettuce Growth and Morphology in Indoor Production Are Cultivar Specific" Plants 11, no. 20: 2714. https://doi.org/10.3390/plants11202714
APA StyleLiu, J., & van Iersel, M. W. (2022). Far-Red Light Effects on Lettuce Growth and Morphology in Indoor Production Are Cultivar Specific. Plants, 11(20), 2714. https://doi.org/10.3390/plants11202714






