Cork Development: What Lies Within
Abstract
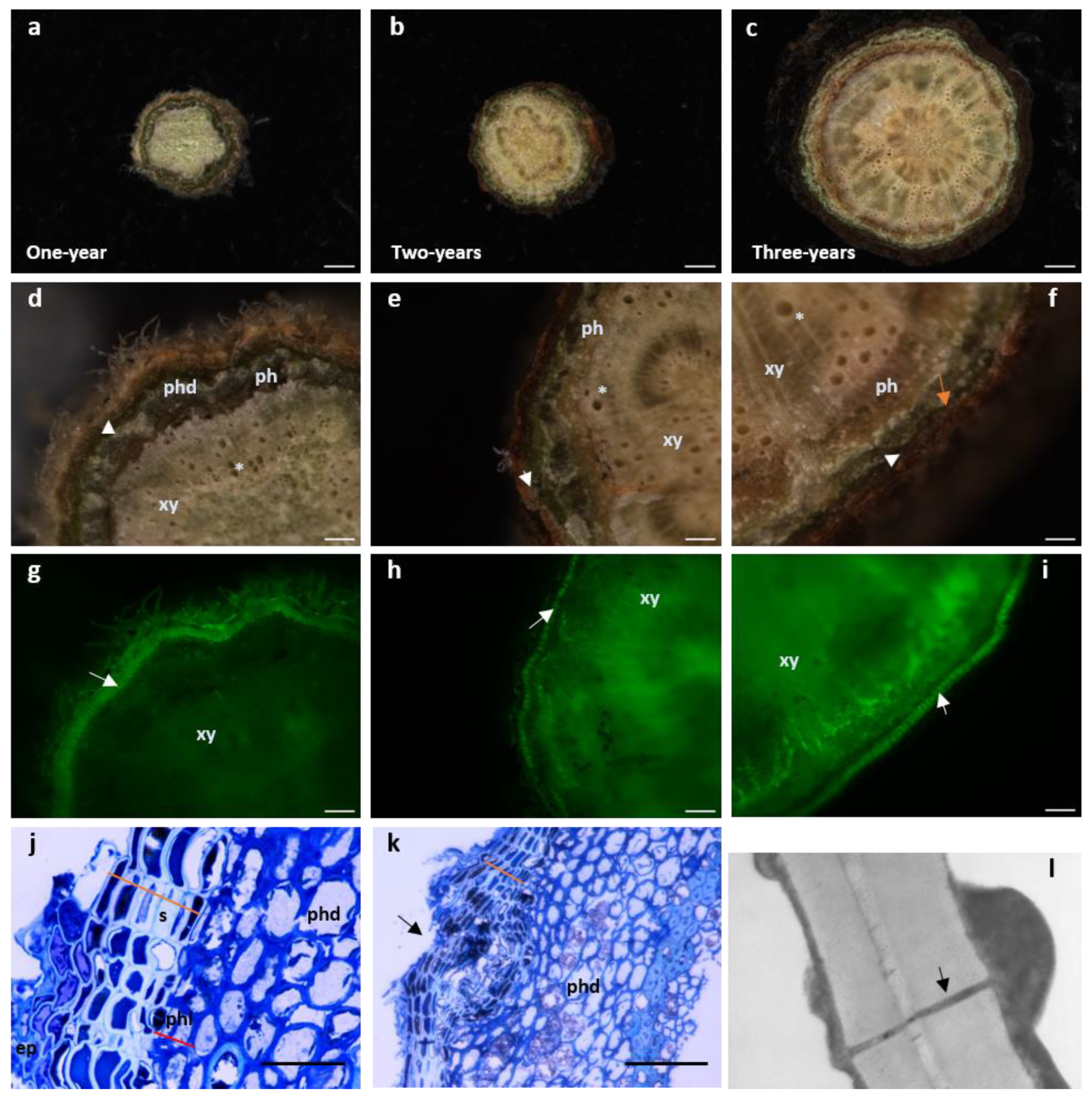
1. Growth and Uses
2. Biology and Adaptation
3. Chemical Composition
4. Molecular Basis of Cork Development
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, H. Cork Biology, Production and Uses; Elsevier Publications: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Aroso, I.M.; Araújo, A.R.; Pires, R.A.; Reis, R.L. Cork: Current Technological Developments and Future Perspectives for This Natural, Renewable, and Sustainable Saterial. ACS Sustain. Chem. Eng. 2017, 5, 11130–11146. [Google Scholar] [CrossRef]
- Camilo-Alves, C.; Dinis, C.; Vaz, M.; Barroso, J.M.; Ribeiro, N.A. Irrigation of Young Cork Oaks under Field Conditions—Testing the Best Water Volume. Forests 2020, 11, 88. [Google Scholar] [CrossRef]
- Gil, L.; Suib, S.L.; Ferreira, J.L.; Coelho, A.; Irmaos, S.A. Cork: A Strategic Material. Front. Chem. 2014, 2, 1–2. [Google Scholar] [CrossRef]
- Duarte, A.P.; Bordado, J.C.; Mariz, G.; Oliveira Barra, D.; Park, C.H.; Nationale, E. Cork—A Renewable Raw Material: Forecast of Industrial Potential and Development Priorities. Front. Mater. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Sen, A.; Zhianski, M.; Glushkova, M.; Petkova, K.; Ferreira, J.; Pereira, H. Chemical Composition and Cellular Structure of Corks from Quercus Suber Trees Planted in Bulgaria and Turkey. Wood Sci. Technol. 2016, 50, 1261–1276. [Google Scholar] [CrossRef]
- Chubar, N.; Carvalho, J.R.; Correia, M.J.N. Cork Biomass as Biosorbent for Cu(II), Zn(II) and Ni(II). Colloids Surf. A Physicochem. Eng. Asp. 2003, 230, 57–65. [Google Scholar] [CrossRef]
- Sen, A.U.; Olivella, A.; Fiol, N.; Miranda, I.; Villaescusa, I.; Pereira, H. Removal of Chromium (VI) in Aqueous Environments Using Cork and Heat-Treated Cork Samples from Quercus Cerris and Quercus Suber. BioResources 2012, 7, 4843–4857. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Silvestre-Albero, A.M.; Ferreira, C.I.A.; Pereira, J.P.C.; Vilar, V.J.P.; Botelho, C.M.S.; Rodríguez-Reinoso, F.; Boaventura, R.A.R. Textural and Surface Characterization of Cork-Based Sorbents for the Removal of Oil from Water. Ind. Eng. Chem. Res. 2013, 52, 16427–16435. [Google Scholar] [CrossRef]
- Olivella, M.; Patrícia, J.; Şen, A.; Pereira, H.; Villaescusa, I.; Fiol, N. Quercus Cerris Cork Sorbent. BioResources 2011, 6, 3363–3375. [Google Scholar]
- Oliveira, V.; Pereira, H. Cork and Cork Stoppers: Quality and Performance. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; Cosme, F., Nunes, F.M., Filipe-Ribeiro, L., Eds.; IntechOpen: London, UK, 2020; ISBN 9781839625763. [Google Scholar]
- Costa, A.; Oliveira, G. Cork Oak (Quercus Suber L.): A Case of Sustainable Bark Harvesting in Southern Europe. In Ecological Sustainability for Non-Timber Forest Products: Dynamics and Case Studies of Harvesting; Shackleton, C.M., Pandey, A.K., Ticktin, T., Eds.; Taylor and Francis Inc.: Abingdon, UK, 2015; pp. 179–198. ISBN 9781317916123. [Google Scholar]
- Oliveira, V.; Lauw, A.; Pereira, H. Sensitivity of Cork Growth to Drought Events: Insights from a 24-Year Chronology. Clim. Chang. 2016, 137, 261–274. [Google Scholar] [CrossRef]
- Leite, C.; Oliveira, V.; Miranda, I.; Pereira, H. Cork Oak and Climate Change: Disentangling Drought Effects on Cork Chemical Composition. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Fernández-Piñán, S.; Boher, P.; Soler, M.; Figueras, M.; Serra, O. Transcriptomic Analysis of Cork during Seasonal Growth Highlights Regulatory and Developmental Processes from Phellogen to Phellem Formation. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Evert, R.F. (Ed.) Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body, 3rd ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 9780471738435. [Google Scholar]
- Caritat, A.; Molinas, M.; Gutierrez, E. Annual Cork-Ring Width Variability of Quercus Suber L in Relation to Temperature and Precipitation (Extremadura, Southwestern Spain). For. Ecol. Manage. 1996, 86, 113–120. [Google Scholar] [CrossRef]
- Graça, J.; Pereira, H. The Periderm Development in Quercus Suber. Iawa J. 2004, 25, 325–335. [Google Scholar] [CrossRef]
- Poeiras, A.P.; Oliveira, T.; Reis, J.; Surový, P.; Silva, M.E.; De, N.; Ribeiro, A. Influence of Water Supply on Cork Increment and Quality in Quercus Suber L. Cent. Eur. For. J. 2022, 68, 3–14. [Google Scholar] [CrossRef]
- Caritat, A.; Gutiérrez, E.; Molinas, M. Influence of Weather on Cork-Ring Width. Tree Physiol. 2000, 20, 893–900. [Google Scholar] [CrossRef]
- Costa, A.; Barbosa, I.; Roussado, C.; Graça, J.; Spiecker, H. Climate Response of Cork Growth in the Mediterranean Oak (Quercus suber L.) Woodlands of Southwestern Portugal. Dendrochronologia 2016, 38, 72–81. [Google Scholar] [CrossRef]
- Costa, A.; Graça, J.; Barbosa, I.; Spiecker, H. Effect of Climate on Cork-Ring Width and Density of Quercus Suber L. in Southern Portugal. Trees 2022, 1–10. [Google Scholar] [CrossRef]
- Campilho, A.; Nieminen, K.; Ragni, L. The Development of the Periderm: The Final Frontier between a Plant and Its Environment. Curr. Opin. Plant Biol. 2020, 53, 10–14. [Google Scholar] [CrossRef]
- Lendzian, K.J. Survival Strategies of Plants during Secondary Growth: Barrier Properties of Phellems and Lenticels towards Water, Oxygen, and Carbon Dioxide. J. Exp. Bot. 2006, 57, 2535–2546. [Google Scholar] [CrossRef]
- Pereira, H. Variability of the Chemical Composition of Cork. BioResources 2013, 8, 2246–2256. [Google Scholar] [CrossRef]
- Oliveira, V.; Rosa, M.E.; Pereira, H. Variability of the Compression Properties of Cork. Wood Sci. Technol. 2014, 48, 937–948. [Google Scholar] [CrossRef]
- Costa, R.; Lourenço, A.; Oliveira, V.; Pereira, H. Chemical Characterization of Cork, Phloem and Wood from Different Quercus Suber Provenances and Trees. Heliyon 2019, 5, e02910. [Google Scholar] [CrossRef] [PubMed]
- Graça, J.; Pereira, H. Cork Suberin: A Glyceryl Based Polyester. Holzforschung 1997, 51, 225–234. [Google Scholar] [CrossRef]
- Marques, A.V.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Pereira, H. Ferulates and Lignin Structural Composition in Cork. Holzforschung 2016, 70, 275–289. [Google Scholar] [CrossRef]
- Pereira, H. The Rationale behind Cork Properties: A Review of Structure and Chemistry. BioResources 2015, 10, 6207–6229. [Google Scholar] [CrossRef]
- Bernards, M.A. Demystifying Suberin. Can. J. Bot. 2002, 80, 227–240. [Google Scholar] [CrossRef]
- Graça, J.; Pereira, H. Suberin Structure in Potato Periderm: Glycerol, Long-Chain Monomers, and Glyceryl and Feruloyl Dimers. J. Agric. Food Chem. 2000, 48, 5476–5483. [Google Scholar] [CrossRef]
- Graça, J.; Santos, S. Suberin: A Biopolyester OfpPlants’ Skin. Macromol. Biosci. 2007, 7, 128–135. [Google Scholar] [CrossRef]
- Sitte, P. Zum Feinbau Der Suberinschichten Im Flaschenkork. Protoplasma 1962, 54, 555–559. [Google Scholar] [CrossRef]
- Teixeira, R.T.; Pereira, H. Suberized Cell Walls of Cork from Cork Oak Differ from Other Species. Microsc. Microanal. 2010, 16, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, Capabilities and Applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Miranda, I.; Gominho, J.; Pereira, H. Chemical Characterization of Cork and Phloem from Douglas Fir Outer Bark. Holzforschung 2016, 70, 475–483. [Google Scholar] [CrossRef]
- Mota, G.S.; Sartori, C.J.; Ferreira, J.; Miranda, I.; Quilhó, T.; Mori, F.A.; Pereira, H. Cellular Structure and Chemical Composition of Cork from Plathymenia Reticulata Occurring in the Brazilian Cerrado. Ind. Crops Prod. 2016, 90, 65–75. [Google Scholar] [CrossRef]
- Pereira, H. Chemical Composition and Variability of Cork from Quercus suber L. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building Lipid Barriers: Biosynthesis of Cutin and Suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef]
- Schreiber, L.; Franke, R.; Hartmann, K. Wax and Suberin Development of Native and Wound Periderm of Potato (Solanum tuberosum L.) and Its Relation to Peridermal Transpiration. Planta 2005, 220, 520–530. [Google Scholar] [CrossRef]
- Teixeira, R.T.; Pereira, H. Ultrastructural Observations Reveal the Presence of Channels between Cork Cells. Microsc. Microanal. 2009, 15, 539–544. [Google Scholar] [CrossRef]
- Machado, A.; Pereira, H.; Teixeira, R.T. Anatomy and Development of the Endodermis and Phellem of Quercus suber L. Roots. Microsc. Microanal. 2013, 19, 525–534. [Google Scholar] [CrossRef]
- Pinheiro, C.; Wienkoop, S.; de Almeida, J.F.; Brunetti, C.; Zarrouk, O.; Planchon, S.; Gori, A.; Tattini, M.; Ricardo, C.P.; Renaut, J.; et al. Phellem Cell-Wall Components Are Discriminants of Cork Quality in Quercus suber. Front. Plant Sci. 2019, 10, 944. [Google Scholar] [CrossRef]
- Teixeira, R.T.; Fortes, A.M.; Pinheiro, C.; Pereira, H. Comparison of Good- and Bad-Quality Cork: Application of High-Throughput Sequencing of Phellogenic Tissue. J. Exp. Bot. 2014, 65, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Şen, A.; Miranda, I.; Santos, S.; Graça, J.; Pereira, H. The Chemical Composition of Cork and Phloem in the Rhytidome of Quercus Cerris Bark. Ind. Crops Prod. 2010, 31, 417–422. [Google Scholar] [CrossRef]
- Boher, P.; Soler, M.; Sánchez, A.; Hoede, C.; Noirot, C.; Almiro, J.; Paiva, P.; Serra, O.; Figueras, M. A Comparative Transcriptomic Approach to Understanding the Formation of Cork. Plant Mol. Biol. 2018, 96, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.T.; Fortes, A.M.; Bai, H.; Pinheiro, C.; Pereira, H. Transcriptional Profiling of Cork Oak Phellogenic Cells Isolated by Laser Microdissection. Planta 2018, 247, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Inácio, V.; Barros, P.M.; Costa, A.; Roussado, C.; Gonçalves, E.; Costa, R.; Graça, J.; Oliveira, M.M.; Morais-Cecílio, L. Differential DNA Methylation Patterns Are Related to Phellogen Origin and Quality of Quercus suber Cork. PLoS ONE 2017, 12, e0169018. [Google Scholar] [CrossRef]
- Silva, H.G.; Sobral, R.S.; Magalhães, A.P.; Morais-Cecílio, L.; Costa, M.M.R. Genome-Wide Identification of Epigenetic Regulators in Quercus suber L. Int. J. Mol. Sci. 2020, 21, 3783. [Google Scholar] [CrossRef]
- Ramos, M.; Rocheta, M.; Carvalho, L.; Inácio, V.; Graça, J.; Morais-Cecilio, L. Expression of DNA Methyltransferases Is Involved in Quercus suber Cork Quality. Tree Genet. Genomes 2013, 9, 1481–1492. [Google Scholar] [CrossRef][Green Version]
- Cao, X.; Springer, N.M.; Muszynski, M.G.; Phillips, R.L.; Kaeppler, S.; Jacobsen, S.E. Conserved Plant Genes with Similarity to Mammalian de Novo DNA Methyltransferases. Proc. Natl. Acad. Sci. USA 2000, 97, 4979–4984. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Xie, X.; Zhang, D.; Liu, Y.; Guo, G. Roles of DNA Methyltransferases in Arabidopsis Development. African J. Biotechnol. 2015, 9, 8506–8514. [Google Scholar] [CrossRef]
- Negishi, M.; Chiba, T.; Saraya, A.; Miyagi, S.; Iwama, A. Dmap1 Plays an Essential Role in the Maintenance of Genome Integrity through the DNA Repair Process. Genes Cells 2009, 14, 1347–1357. [Google Scholar] [CrossRef]
- Correia, B.; Valledor, L.; nica Meijó, M.; Luis Rodriguez, J.; Celeste Dias, M.; Santos, C.; Jesus Cañ Al, M.; Rodriguez, R.; ria Pinto, G. Is the Interplay between Epigenetic Markers Related to the Acclimation of Cork Oak Plants to High Temperatures? PLoS ONE 2013, 8, e53543. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.T.; Sheng, X.; Brunner, A.M. Activity of the Shoot Apical and Cambial Meristems: Coordination and Responses to Environmental Signals; Academic Press: Cambridge, MA, USA, 2019; Volume 89, ISBN 9780128154656. [Google Scholar]
- Brunner, A.M.; Varkonyi-Gasic, E.; Jones, R.C. Phase Change and Phenology in Trees; Springer: Cham, Switzerland, 2017; pp. 227–274. [Google Scholar] [CrossRef]
- Horvath, D.P.; Sung, S.; Kim, D.; Chao, W.; Anderson, J. Characterization, Expression and Function of Dormancy Associated Mads-Box Genes from Leafy Spurge. Plant Mol. Biol. 2010, 73, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Leida, C.; Conesa, A.; Llácer, G.; Badenes, M.L.; Ríos, G. Histone Modifications and Expression of DAM6 Gene in Peach Are Modulated during Bud Dormancy Release in a Cultivar-Dependent Manner. New Phytol. 2012, 193, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Rains, M.K.; De Silva, N.D.G.; Molina, I. Reconstructing the Suberin Pathway in Poplar by Chemical and Transcriptomic Analysis of Bark Tissues. Tree Physiol. 2018, 38, 340–361. [Google Scholar] [CrossRef]
- Vulavala, V.K.R.; Fogelman, E.; Faigenboim, A.; Shoseyov, O.; Ginzberg, I. The Transcriptome of Potato Tuber Phellogen Reveals Cellular Functions of Cork Cambium and Genes Involved in Periderm Formation and Maturation. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Aloni, R. Vascular Differentiation in Branch Junctions of Trees: Circular Patterns and Functional Significance. Trees 1990, 4, 49–54. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. The Ethylene Signaling Pathway: New Insights. Curr. Opin. Plant Biol. 2004, 7, 40–49. [Google Scholar] [CrossRef]
- Lopes, S.T.; Sobral, D.; Costa, B.; Perdiguero, P.; Chaves, I.; Costa, A.; Miguel, C.M. Phellem versus Xylem: Genome-Wide Transcriptomic Analysis Reveals Novel Regulators of Cork Formation in Cork Oak. Tree Physiol. 2019, 40, 129–141. [Google Scholar] [CrossRef]
- Zhu, C.; Gan, L.; Shen, Z.; Xia, K. Interactions between Jasmonates and Ethylene in the Regulation of Root Hair Development in Arabidopsis. J. Exp. Bot. 2006, 57, 1299–1308. [Google Scholar] [CrossRef]
- Lorenzo, O.; Piqueras, R.; Sánchez-Serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 Integrates Signals from Ethylene and Jasmonate Pathways in Plant Defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.; Pinto, G.; Correia, B.; Santos, C.; Gonçalves, S. QsMYB1 Expression Is Modulated in Response to Heat and Drought Stresses and during Plant Recovery in Quercus suber. Plant Physiol. Biochem. 2013, 73, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.; Menéndez, E.; Capote, T.; Ribeiro, T.; Santos, C.; Gonçalves, S. Molecular Characterization of Quercus suber MYB1, a Transcription Factor up-Regulated in Cork Tissues. J. Plant Physiol. 2013, 170, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Boher, P.; Serra, O.; Soler, M.; Molinas, M.; Figueras, M. The Potato Suberin Feruloyl Transferase FHT Which Accumulates in the Phellogen Is Induced by Wounding and Regulated by Abscisic and Salicylic Acids. J. Exp. Bot. 2013, 64, 3225. [Google Scholar] [CrossRef]
- Company-Arumí, D.; Figueras, M.; Salvadó, V.; Molinas, M.; Serra, O.; Anticó, E. The Identification and Quantification of Suberin Monomers of Root and Tuber Periderm from Potato (Solanum tuberosum) as Fatty Acyl Tert-Butyldimethylsilyl Derivatives. Phytochem. Anal. 2016, 27, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Pinot, F.; Beisson, F. Cytochrome P450 Metabolizing Fatty Acids in Plants: Characterization and Physiological Roles. FEBS J. 2011, 278, 195–205. [Google Scholar] [CrossRef]
- Soler, M.; Serra, O.; Molinas, M.; Huguet, G.; Fluch, S.; Figueras, M. A Genomic Approach to Suberin Biosynthesis and Cork Differentiation. Plant Physiol. 2007, 144, 419–431. [Google Scholar] [CrossRef]
- Hofer, R.; Briesen, I.; Beck, M.; Pinot, F.; Schreiber, L.; Franke, R. The Arabidopsis Cytochrome P450 CYP86A1 Encodes a Fatty Acid-Hydroxylase Involved in Suberin Monomer Biosynthesis. J. Exp. Bot. 2008, 59, 2347–2360. [Google Scholar] [CrossRef]
- Leal, A.R.; Barros, P.M.; Parizot, B.; Sapeta, H.; Vangheluwe, N.; Andersen, T.G.; Beeckman, T.; Oliveira, M.M. Translational Profile of Developing Phellem Cells in Arabidopsis Thaliana Roots. Plant J. 2022, 110, 899–915. [Google Scholar] [CrossRef]
- Joos, H.-J.; Hahlbrock, K. Phenylalanine Ammonia-Lyase in Potato (Solanum tuberosum L.). Eur. J. Biochem. 1992, 204, 621–629. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Lulai, E.C.; Suttle, J.C.; Knowles, N.R. Age-Induced Loss of Wound-Healing Ability in Potato Tubers Is Partly Regulated by ABA. Planta 2010, 232, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Reddy, M.S.S.; Temple, S.; Jackson, L.; Shadle, G.; Dixon, R.A. Multi-Site Genetic Modulation of Monolignol Biosynthesis Suggests New Routes for Formation of Syringyl Lignin and Wall-Bound Ferulic Acid in Alfalfa (Medicago sativa L.). Plant J. 2006, 48, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A Final Frontier in Flavonoid Research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Inácio, V.; Lobato, C.; Graça, J.; Morais-Cecílio, L. Cork Cells in Cork Oak Periderms Undergo Programmed Cell Death and Proanthocyanidin Deposition. Tree Physiol. 2021, 41, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.; MaheriSis, N.; Eshratkhah, B.; Baghbani, F. Plants and Secondary Metabolites (Tannins): A Review. Int. J. For. Soil Erosionr. 2011, 1, 47–53. [Google Scholar]
- Conde, E.; Cadahía, E.; García-Vallejo, M.C.; de Simón, B.F. Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances. J. Agric. Food Chem 1998, 46, 3166–3171. [Google Scholar] [CrossRef]
- Tanner, G.J.; Francki, K.T.; Abrahams, S.; Watson, J.M.; Larkin, P.J.; Ashton, A.R. Proanthocyanidin Biosynthesis in Plants. Purification of Legume Leucoanthocyanidin Reductase and Molecular Cloning of Its CDNA. J. Biol. Chem. 2003, 278, 31647–31656. [Google Scholar] [CrossRef]
- Xie, D.Y.; Sharma, S.B.; Paiva, N.L.; Ferreira, D.; Dixon, R.A. Role of Anthocyanidin Reductase, Encoded by BANYULS in Plant Flavonoid Biosynthesis. Science 2003, 299, 396–399. [Google Scholar] [CrossRef]
- Daneva, A.; Gao, Z.; Van Durme, M.; Nowack, M.K. Functions and Regulation of Programmed Cell Death in Plant Development. Annu. Rev. Cell Dev. Biol. 2016, 32, 441–468. [Google Scholar] [CrossRef]
- Rantong, G.; Gunawardena, A.H.L.A.N. Programmed Cell Death: Genes Involved in Signaling, Regulation, and Execution in Plants and Animals. Botany 2015, 93, 193–210. [Google Scholar] [CrossRef]
- Olvera-Carrillo, Y.; Van Bel, M.; Hautegem, T.V.; Fendrych, M.; Huysmans, M.; Simaskova, M.; Van Durme, M.; Buscaill, P.; Rivas, S.; Coll, N.S.; et al. A Conserved Core of Programmed Cell Death Indicator Genes Discriminates Developmentally and Environmentally Induced Programmed Cell Death in Plants. Plant Physiol. 2015, 169, 2684–2699. [Google Scholar] [CrossRef] [PubMed]
- Wunderling, A.; Ripper, D.; Barra-Jimenez, A.; Mahn, S.; Sajak, K.; Targem, M.B.; Ragni, L. A Molecular Framework to Study Periderm Formation in Arabidopsis. New Phytol. 2018, 219, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Inácio, V.; Martins, M.T.; Graça, J.; Morais-Cecílio, L. Cork Oak Young and Traumatic Periderms Show PCD Typical Chromatin Patterns but Different Chromatin-Modifying Genes Expression. Front. Plant Sci. 2018, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.; Dolan, L. Origin and Diversification of Basic-Helix-Loop-Helix Proteins in Plants. Mol. Biol. Evol. 2010, 27, 862–874. [Google Scholar] [CrossRef]
- Macalister, C.A.; Bergmann, D.C. Sequence and Function of BHLHs Required for Stomatal Development in Arabidopsis Are Deeply Conserved in Land Plants. Evol. Dev. 2011, 13, 182. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Torii, K.U. Breaking the Silence: Three BHLH Proteins Direct Cell-Fate Decisions during Stomatal Development. BioEssays 2007, 29, 861–870. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Bogenschutz, N.L.; Torii, K.U. The BHLH Protein, MUTE, Controls Differentiation of Stomata and the Hydathode Pore in Arabidopsis. Plant Cell Physiol. 2008, 49, 934–943. [Google Scholar] [CrossRef]
- Ricardo, C.P.P.; Martins, I.; Francisco, R.; Sergeant, K.; Pinheiro, C.; Campos, A.; Fevereiro, P. Proteins Associated with Cork Formation in Quercus suber L. Stem Tissues. J. Proteom. 2011, 74, 1266–1278. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, R.T. Cork Development: What Lies Within. Plants 2022, 11, 2671. https://doi.org/10.3390/plants11202671
Teixeira RT. Cork Development: What Lies Within. Plants. 2022; 11(20):2671. https://doi.org/10.3390/plants11202671
Chicago/Turabian StyleTeixeira, Rita Teresa. 2022. "Cork Development: What Lies Within" Plants 11, no. 20: 2671. https://doi.org/10.3390/plants11202671
APA StyleTeixeira, R. T. (2022). Cork Development: What Lies Within. Plants, 11(20), 2671. https://doi.org/10.3390/plants11202671





