Potential Use of Colored LED Lights to Increase the Production of Bioactive Metabolites Hedyotis corymbosa (L.) Lam
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
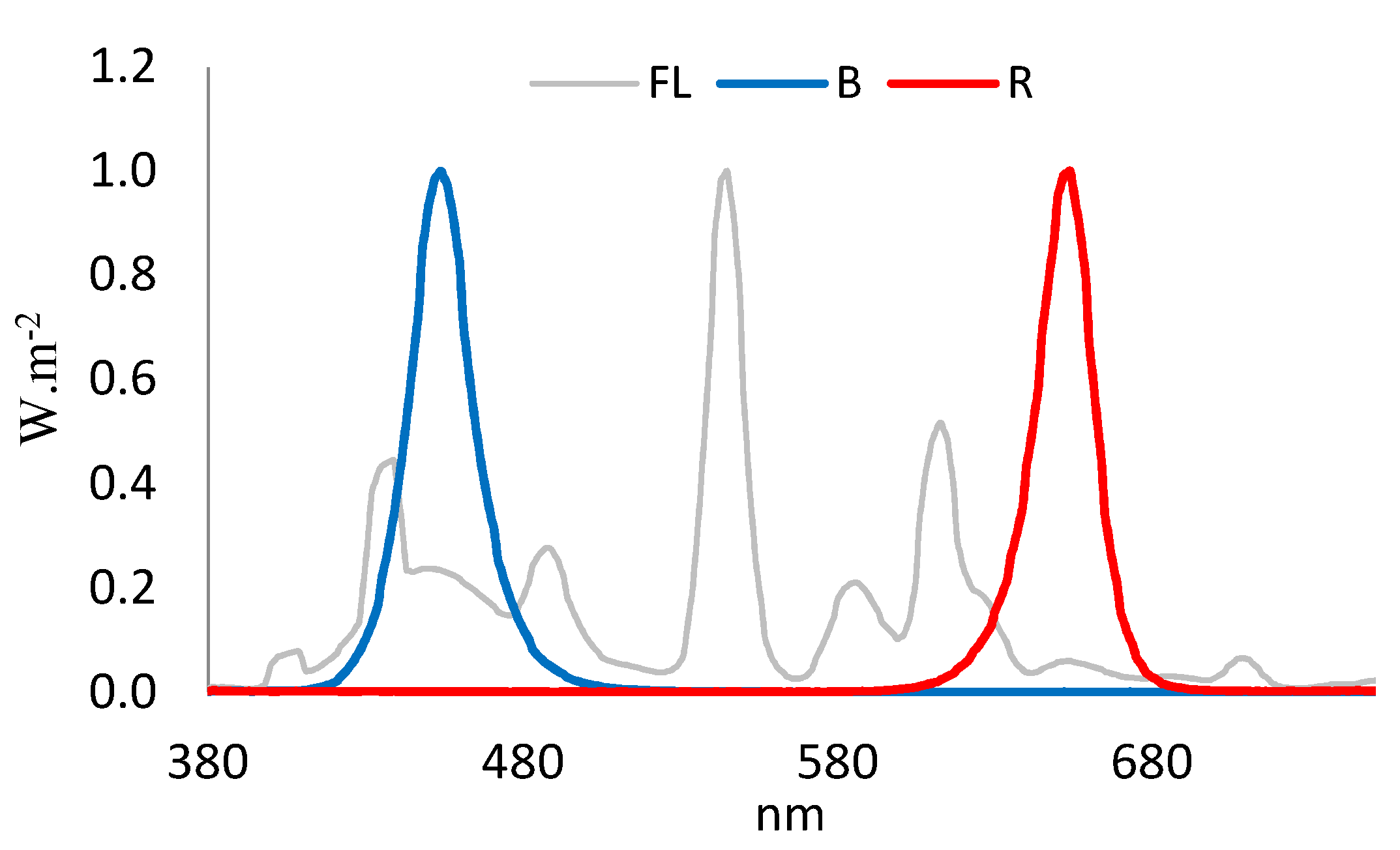
4.2. Light Treatment
4.3. Measurements
4.4. Compound Extraction
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, W.H. Oldenlandia corymbosa (rubiaceae). Grana Palynol. 1964, 5, 330–341. [Google Scholar] [CrossRef]
- Sasikumar, J.M.; Maheshu, V.; Aseervatham, G.S.B.; Darsini, D.T.P. In vitro antioxidant activity of Hedyotis corymbosa (L.) Lam. aerial parts. Indian J. Biochem. Biophys. 2010, 47, 49–52. [Google Scholar] [PubMed]
- Yadav, R.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011, 3, 10–14. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh, R.K.; Swain, S.R.; Hussain, T.; Rao, C.V. Anti-hepatotoxic potential of Hedyotis corymbosa against D-galactosamine-induced hepatopathy in experimental rodents. Asian Pac. J. Trop. Biomed. 2012, 2, 1542–1547. [Google Scholar] [CrossRef]
- Sultana, T.; Rashid, M.A.; Ali, M.A.; Mahmood, S.F. Hepatoprotective and antibacterial activity of ursolic acid extracted from Hedyotis corymbos (L.). Bangladesh J. Sci. Ind. Res. 2010, 45, 27–34. [Google Scholar] [CrossRef]
- Andriyani, R.; Risdian, C.; Udin, Z. Cytotoxicity assay from fractions of hedyotis corymbosa extract against breast cancer cell line T47D. Indones. J. Cancer Chemoprev. 2011, 2, 182. [Google Scholar] [CrossRef][Green Version]
- Endrini, S. Antioxidant activity and anticarcinogenic properties of “rumput mutiara” {Hedyotis corymbosa (L.) Lam.} and “pohpohan” {Pilea trinervia (Roxb.) Wight}. J. Med. Plants Res. 2011, 5, 3715–3718. [Google Scholar]
- Sharma, S.; Sharma, S.; Kukreja, S.; Jadon, V.S.; Sharma, V. Plant tissue culture methods in secondary metabolite production-A mini review. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 144–153. [Google Scholar]
- Chia, T.F.; Hew, C.S.; Lee, Y.K. Cell suspension culture of Hedyotis spp. Bot. Gaz. 1988, 149, 376–381. [Google Scholar] [CrossRef]
- Norrizah, J.S.; Suhaimi, M.Y.; Rohaya, A.; Roslan, N.N. Ursolic acid and oleanolic acid productions in elicited cell suspension culltures of Hedyotis corymbosa. Biotechnology 2012, 11, 238–242. [Google Scholar] [CrossRef]
- Le, A.T.; Hoang, T.T.T.; Phan, N.H. Development of shoots of Hedyotis corymbosa (L.) Lam. In Vitro Culture 2015, 5, 75–84. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Zeinali, M.; Naghavi, M.R. CRISPR-based metabolic editing: Next-generation metabolic engineering in plants. Gene 2020, 759, 144993. [Google Scholar] [CrossRef]
- Mitsunobu, H.; Teramoto, J.; Nishida, K.; Kondo, A. Beyond native Cas9: Manipulating genomic information and function. Trends Biotechnol. 2017, 35, 983–996. [Google Scholar] [CrossRef]
- Soyars, C.L.; Peterson, B.A.; Burr, C.A.; Nimchuk, Z.L. Cutting edge genetics: Crispr/cas9 editing of plant genomes. Plant Cell Physiol. 2018, 59, 1608–1620. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of light-emitting diodes for improving the nutritional quality and bioactive compound levels of some crops and medicinal plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Viršile, A.; Brazaityte, A.; Sirtautas, R.; Duchovskis, P. Light spectral effects on phenolic compounds in Perilla frutescens leaves as related to the leaf age, color and duration of exposure. Acta Hortic. 2017, 1170, 981–988. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Manukyan, A. Effects of PAR and UV-B radiation on herbal yield, bioactive compounds and their antioxidant capacity of some medicinal plants under controlled environmental conditions. Photochem. Photobiol. 2013, 89, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Kong, Y.; Nemali, K. Blue and far-red light affect area and number of individual leaves to influence vegetative growth and pigment synthesis in lettuce. Front. Plant Sci. 2021, 12, 667407. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.X.; Wang, X.Z.; Gao, L.H.; Chen, Q.Y.; Mei, Q.U. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Nam, M.W.; Choi, D.H.; Lee, H.I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, K.H.; Oh, M.M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Maluta, F.A.; Bordignon, S.R.; Rossi, M.L.; Ambrosano, G.M.B.; Rodrigues, P.H.V. In vitro culture of sugarcane exposed to different light sources. Pesqui. Agropecu. Bras. 2013, 48, 1303–1307. [Google Scholar] [CrossRef][Green Version]
- Patrick, J.W.; Botha, F.C.; Birch, R.G. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, A.; Padilla-Chacon, D. Subcellular compartmentation of sugar signaling: Links among carbon cellular status, route of sucrolysis, sink-source allocation, and metabolic partitioning. Front. Plant Sci. 2013, 3, 306. [Google Scholar] [CrossRef] [PubMed]
- Fünfgeld, M.M.F.F.; Wang, W.; Ishihara, H.; Arrivault, S.; Feil, R.; Smith, A.M.; Stitt, M.; Lunn, J.E.; Niittylä, T.; Lunn, J.; et al. The pathway of starch synthesis in Arabidopsis thaliana leaves. bioRxiv 2021, 1–31. [Google Scholar] [CrossRef]
- Sarkar, D.; Shetty, K. Metabolic stimulation of plant phenolics for food preservation and health. Annu. Rev. Food Sci. Technol. 2014, 5, 395–413. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B Biol. 2018, 183, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of light-emitting diodes on the accumulation of glucosinolates and phenolic compounds in sprouting canola (Brassica napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef]
- Heo, J.-W.; Kang, D.-H.; Bang, H.-S.; Hong, S.-G.; Chun, C.-H.; Kang, K.-K. Early growth, pigmentation, protein content, and phenylalanine ammonia-lyase activity of red curled lettuces grown under different lighting conditions. Korean J. Hortic. Sci. Technol. 2012, 30, 6–12. [Google Scholar] [CrossRef]
- Park, W.T.; Yeo, S.K.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Influence of light-emitting diodes on phenylpropanoid biosynthetic gene expression and phenylpropanoid accumulation in Agastache rugosa. Appl. Biol. Chem. 2020, 63, 25. [Google Scholar] [CrossRef]
- Son, K.H.; Park, J.H.; Kim, D.; Oh, M.M. Leaf shape index, growth, and phytochemicals in two leaf lettuce cultivars grown under monochromatic light-emitting diodes. Korean J. Hortic. Sci. Technol. 2012, 31, 664–672. [Google Scholar] [CrossRef]
- Beadle, C.L.; Ludlow, M.M.; Honeysett, J.L. Water relations. In Techniques in Bioproductivity and Photosynthesis; Coombs, J., Hall, D.O., Long, S.P., Eds.; Pergamon Press Ltd.: Oxford, UK, 1985; pp. 50–61. [Google Scholar]
- Henselová, M.; Regecová, M.; Sováková, A. Isolation of chloroplasts in the Karwinskia species and determination of their photochemical activity under in vitro conditions. Plant Soil Environ. 2004, 50, 149–156. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.; Hind, G.; Leegood, R.C.; Tieszen, L.L.; Vonshak, A. Analytical techniques. In Techniques in Bioproductivity and Photosynthesis; Coombs, J., Hall, D.O., Long, S.P., Eds.; Pergamon Press Ltd.: Oxford, UK, 1985; pp. 219–228. [Google Scholar]
- Victório, C.P.; Lage, C.L.S.; Kuster, R.M. Flavonoid extraction from Alpinia zerumbet (Pers.) Burtt et Smith leaves using different techniques and solvents. Eclet. Quim. 2009, 34, 19–24. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef]
| Light Source | Leaf Area (cm2) | Stomatal Aperture (µm) | Hill Reaction Activity (nmol DCIP.Million of Chloroplast−1·min−1) | Respiratory Rate (µmol O2·cm−2·min−1) |
|---|---|---|---|---|
| Fluorescent lamp | 1.64 ± 0.08 a | 3.30 ± 0.08 b | 0.087 ± 0.001 b | 0.145 ± 0.02 b |
| Blue LED | 1.13 ± 0.05 c | 4.22 ± 0.17 a | 0.108 ± 0.002 a | 0.219 ± 0.02 a |
| Red LED | 1.39 ± 0.05 b | 3.30 ± 0.06 b | 0.083 ± 0.001 b | 0.166 ± 0.01 b |
| Light Source | Fv/Fm | qP | qN | ETR (µmol Electron·m−2·s−1) |
|---|---|---|---|---|
| Blue LED | 0.70 ± 0.01 | 0.87 ± 0.02 | 0.20 ± 0.02 | 24.48 ± 0.53 |
| Red LED | 0.58 ± 0.02 * | 0.45 ± 0.04 * | 0.38 ± 0.02 * | 9.15 ± 0.43 * |
| Light Source | Pigment Content (mg/g FW) | a/b Ratio | (a+b)/c Ratio | ||
|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Carotenoid | |||
| Fluorescent lamp | 2.05 ± 0.09 a | 0.54 ± 0.03 a | 0.74 ± 0.03 a | 3.77 ± 0.03 b | 3.56 ± 0.03 b |
| Blue LED | 2.04 ± 0.02 a | 0.48 ± 0.01 b | 0.74 ± 0.02 a | 4.25 ± 0.02 a | 3.47 ± 0.08 b |
| Red LED | 2.09 ± 0.05 a | 0.57 ± 0.02 a | 0.68 ± 0.01 b | 3.70 ± 0.03 b | 3.93 ± 0.11 a |
| Light Source | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|
| Fluorescent lamp | 21.13 ± 0.33 b | 1.67 ± 0.08 b |
| Blue LED | 24.89 ± 1.51 a | 2.07 ± 0.13 a |
| Red LED | 18.36 ± 0.15 c | 1.69 ± 0.05 b |
| Light Source | Content (mg/g FW) | |||
|---|---|---|---|---|
| Sucrose | Starch | Total Phenolic | Total Flavonoid | |
| Fluorescent lamp | 25.85 ± 3.64 a | 80.01 ± 5.45 b | 1.56 ± 0.07 c | 0.68 ± 0.06 b |
| Blue LED | 18.38 ± 1.08 b | 101.31 ± 6.56 a | 1.90 ± 0.08 b | 1.48 ± 0.30 a |
| Red LED | 17.69 ± 1.45 b | 78.04 ± 8.20 b | 3.50 ± 0.16 a | 0.58 ± 0.08 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, A.T.; Yu, J.-K.; Han, G.-D.; Do, T.K.; Chung, Y.-S. Potential Use of Colored LED Lights to Increase the Production of Bioactive Metabolites Hedyotis corymbosa (L.) Lam. Plants 2022, 11, 225. https://doi.org/10.3390/plants11020225
Le AT, Yu J-K, Han G-D, Do TK, Chung Y-S. Potential Use of Colored LED Lights to Increase the Production of Bioactive Metabolites Hedyotis corymbosa (L.) Lam. Plants. 2022; 11(2):225. https://doi.org/10.3390/plants11020225
Chicago/Turabian StyleLe, Anh Tuan, Ju-Kyung Yu, Gyung-Deok Han, Thuong Kiet Do, and Yong-Suk Chung. 2022. "Potential Use of Colored LED Lights to Increase the Production of Bioactive Metabolites Hedyotis corymbosa (L.) Lam" Plants 11, no. 2: 225. https://doi.org/10.3390/plants11020225
APA StyleLe, A. T., Yu, J.-K., Han, G.-D., Do, T. K., & Chung, Y.-S. (2022). Potential Use of Colored LED Lights to Increase the Production of Bioactive Metabolites Hedyotis corymbosa (L.) Lam. Plants, 11(2), 225. https://doi.org/10.3390/plants11020225









