The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression
Abstract
1. Introduction
2. Methodology
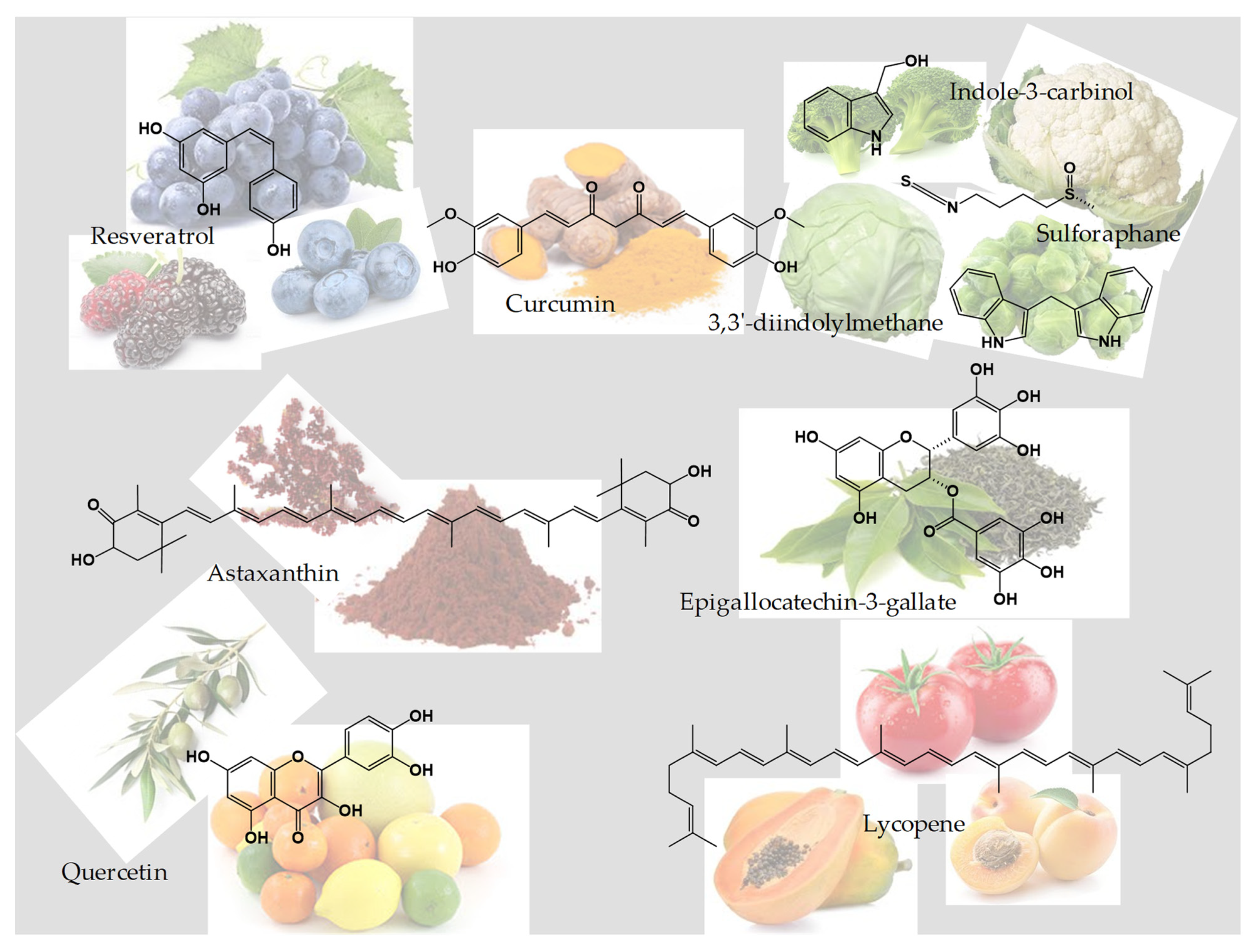
3. Nutraceuticals
3.1. Curcumin
3.1.1. Anticancer Activity and the Suppression of Carcinogenesis
3.1.2. Inhibition of Angiogenesis
3.1.3. Anti-Inflammatory Properties
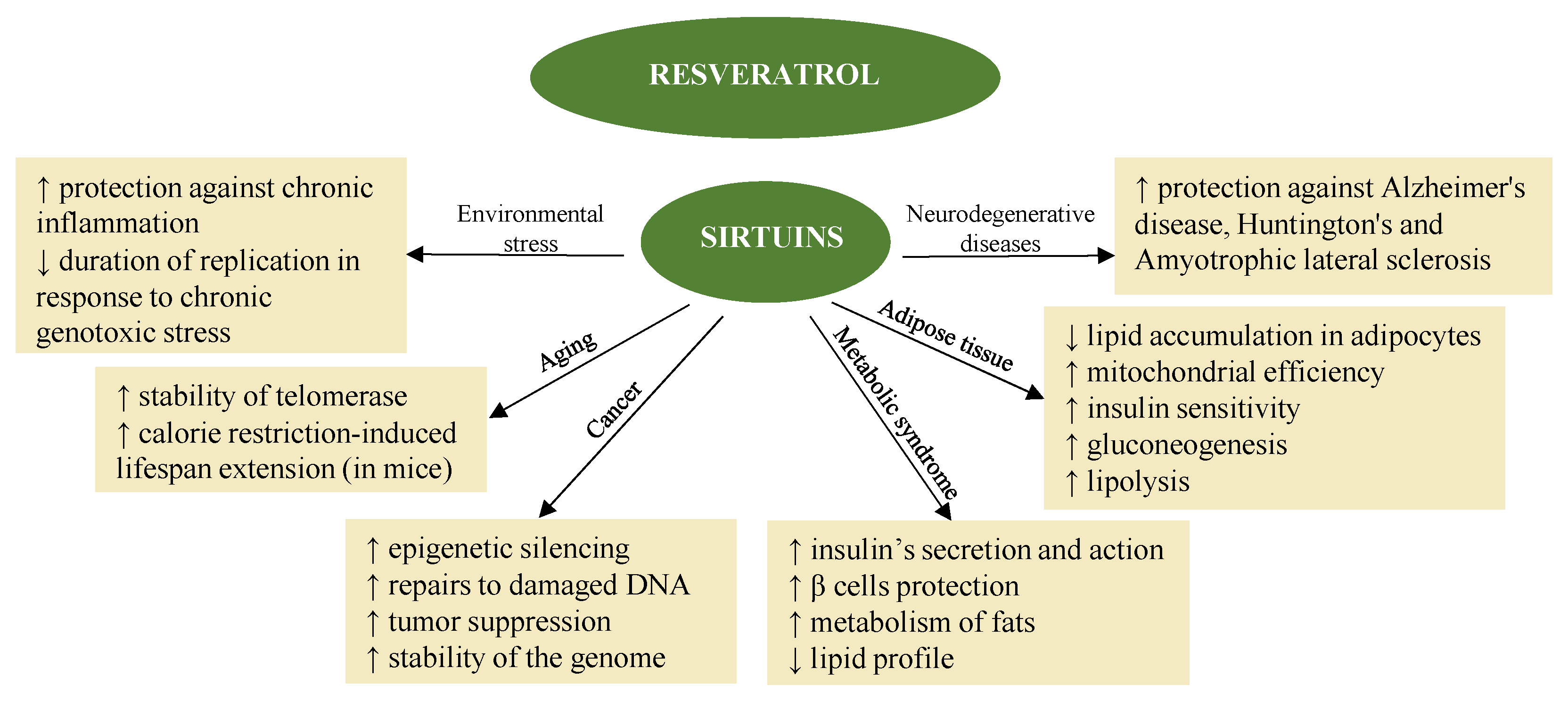
3.2. Resveratrol
3.2.1. Antioxidant and Anti-Inflammatory Activity
3.2.2. Resveratrol and Cells Apoptosis
3.3. Sulforaphane, Indole-3-Carbinol, and 3,3′-Diindolylmethane
3.3.1. Chemopreventive Activity and Epigenetic Role
3.3.2. Effect of Estrogen Analog and Anticarcinogenic in Mammary Tumor Cells
3.3.3. Anticancer Activity
3.3.4. Anti-Inflammatory Activity
3.4. Astaxanthin
3.5. Quercetin
3.6. Epigallocatechin-3-Gallate
3.7. Lycopene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/ (accessed on 5 July 2022).
- Waheed, R.; Aleksandra, N.; Matthias, R. Scientific Evaluation of Dietary Factors in Cancer. J. Nutr. Med. Diet Care 2018, 4, 1–13. [Google Scholar] [CrossRef]
- Saldanha, S.N.; Tollefsbol, T.O. The role of nutraceuticals in chemoprevention and chemotherapy and their clinical outcomes. J. Oncol. 2012, 2012, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Boba, A.; Czemplik, M.; Kulma, A.; Wojtasik, W. Influence of the Bioactive Diet Components on the Gene Expression Regulation. Nutrients 2021, 13, 3673. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D. Nutrigenomica ed Epigenetica: Dalla Biologia alla Clinica, 1st ed.; Edra: Milano, Italy, 2017; ISBN 8821442357. [Google Scholar]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef]
- Darwiche, N. Epigenetic mechanisms and the hallmarks of cancer: An intimate affair. Am. J. Cancer Res. 2020, 10, 1954–1978. [Google Scholar]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef]
- Ahuja, N.; Sharma, A.R.; Baylin, S.B. Epigenetic Therapeutics: A New Weapon in the War Against Cancer. Annu. Rev. Med. 2016, 67, 73–89. [Google Scholar] [CrossRef]
- Sut, S.; Baldan, V.; Faggian, M.; Peron, G.; Dall’Acqua, S. Nutraceuticals, A New Challenge for Medicinal Chemistry. Curr. Med. Chem. 2016, 23, 3198–3223. [Google Scholar] [CrossRef]
- Calvani, M.; Pasha, A.; Favre, C. Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 1936. [Google Scholar] [CrossRef]
- Bergamin, A.; Mantzioris, E.; Cross, G.; Deo, P.; Garg, S.; Hill, A.M. Nutraceuticals: Reviewing their Role in Chronic Disease Prevention and Management. Pharm. Med. 2019, 33, 291–309. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Baradaran, A.; Rafieian, M. Plants antioxidants: From laboratory to clinic. J. Nephropathol. 2013, 2, 152–153. [Google Scholar] [CrossRef]
- Parsaei, P.; Karimi, M.; Asadi, S.Y.; Rafieian-kopaei, M. Bioactive components and preventive effect of green tea (Camellia sinensis) extract on post-laparotomy intra-abdominal adhesion in rats. Int. J. Surg. 2013, 11, 811–815. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Lachance, P.A.; Das, Y.T. Nutraceuticals. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Oxford, UK, 2007; pp. 449–461. ISBN 9780080450445. [Google Scholar]
- Teiten, M.H.; Dicato, M.; Diederich, M. Curcumin as a regulator of epigenetic events. Mol. Nutr. Food Res. 2013, 57, 1619–1629. [Google Scholar] [CrossRef]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The Curcumin Analog EF24 Targets NF-κB and miRNA-21, and Has Potent Anticancer Activity In Vitro and In Vivo. PLoS ONE 2013, 8, e71130. [Google Scholar] [CrossRef]
- Qadir, M.; Naqvi, S.; Muhammad, S. Curcumin: A Polyphenol with Molecular Targets for Cancer Control. Asian Pac. J. Cancer Prev. 2016, 17, 2735–2739. [Google Scholar]
- Vadukoot, A.K.; Mottemmal, S.; Vekaria, P.H. Curcumin as a Potential Therapeutic Agent in Certain Cancer Types. Cureus 2022, 14, e22825. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Ti, X.; Shi, J.; Wu, C.; Ren, X.; Yin, H. Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through a miRNA signaling pathway. Biochem. Biophys. Res. Commun. 2010, 399, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Rehman, M.S.U.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an alternative epigenetic modulator: Mechanism of action and potential effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef]
- Yu, J.; Peng, Y.; Wu, L.C.; Xie, Z.; Deng, Y.; Hughes, T.; He, S.; Mo, X.K.; Chiu, M.; Wang, Q.E.; et al. Curcumin Down-Regulates DNA Methyltransferase 1 and Plays an Anti-Leukemic Role in Acute Myeloid Leukemia. PLoS ONE 2013, 8, e55934. [Google Scholar] [CrossRef] [PubMed]
- Boyanapalli, S.S.S.; Kong, A.N.T. “Curcumin, the King of Spices”: Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr. Pharmacol. Rep. 2015, 1, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Nagoor, N.H.; Aggarwal, B. Cancer-linked targets modulated by curcumin. Int. J. Biochem. Mol. Biol. 2012, 3, 328–351. [Google Scholar]
- Farghadani, R.; Naidu, R. Curcumin: Modulator of Key Molecular Signaling Pathways in Hormone-Independent Breast Cancer. Cancers 2021, 13, 3427. [Google Scholar] [CrossRef]
- Zhou, H.; Beevers, C.S.; Huang, S. Targets of curcumin. Curr. Drug Targets 2011, 12, 332. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef]
- Issinger, O.G.; Guerra, B. Phytochemicals in cancer and their effect on the PI3K/AKT-mediated cellular signalling. Biomed. Pharmacother. 2021, 139, 111650. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Silva, G.D.B.; Pavan, A.R.; Chiba, D.E.; Chin, C.M.; Dos Santos, J.L. Epigenetic Regulatory Mechanisms Induced by Resveratrol. Nutrients 2017, 9, 1201. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Alder, H.; Volinia, S.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem. Pharmacol. 2010, 80, 2057–2065. [Google Scholar] [CrossRef]
- Kumazaki, M.; Noguchi, S.; Yasui, Y.; Iwasaki, J.; Shinohara, H.; Yamada, N.; Akao, Y. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J. Nutr. Biochem. 2013, 24, 1849–1858. [Google Scholar] [CrossRef]
- Hagiwara, K.; Kosaka, N.; Yoshioka, Y.; Takahashi, R.U.; Takeshita, F.; Ochiya, T. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci. Rep. 2012, 2, 314. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef]
- Williams, D.E. Indoles Derived from Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3′-Diindolylmethane. Front. Nutr. 2021, 8, 4334. [Google Scholar] [CrossRef]
- Beaver, L.M.; Yu, T.W.; Sokolowski, E.I.; Williams, D.E.; Dashwood, R.H.; Ho, E. 3,3′-Diindolylmethane, but not indole-3-carbinol, inhibits histone deacetylase activity in prostate cancer cells. Toxicol. Appl. Pharmacol. 2012, 263, 345–351. [Google Scholar] [CrossRef]
- Lubecka-Pietruszewska, K.; Kaufman-Szymczyk, A.; Stefanska, B.; Cebula-Obrzut, B.; Smolewski, P.; Fabianowska-Majewska, K. Sulforaphane Alone and in Combination with Clofarabine Epigenetically Regulates the Expression of DNA Methylation-Silenced Tumour Suppressor Genes in Human Breast Cancer Cells. J. Nutrigenet. Nutr. 2015, 8, 91–101. [Google Scholar] [CrossRef]
- Hsu, A.; Wong, C.P.; Yu, Z.; Williams, D.E.; Dashwood, R.H.; Ho, E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin. Epigenetics 2011, 3, 3. [Google Scholar] [CrossRef]
- Phuah, N.H.; Nagoor, N.H. Regulation of microRNAs by natural agents: New strategies in cancer therapies. Biomed. Res. Int. 2014, 2014, 804510. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, S.M.; Gamal-Eldeen, A.M.; Gouhar, S.A.; Abo-elfadl, M.T.; El-Saeed, G. Modulatory Effect of Indoles on the Expression of miRNAs Regulating G1/S Cell Cycle Phase in Breast Cancer Cells. Appl. Biochem. Biotechnol. 2020, 192, 1208–1223. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yu, Z.Y.; Chuang, Y.S.; Huang, R.M.; Wang, T.C.V. Sulforaphane attenuates EGFR signaling in NSCLC cells. J. Biomed. Sci. 2015, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ganai, S.A. Histone deacetylase inhibitor sulforaphane: The phytochemical with vibrant activity against prostate cancer. Biomed. Pharmacother. 2016, 81, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Patel, S.N.; Tollefsbol, T.O. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS ONE 2010, 5, e11457. [Google Scholar] [CrossRef]
- Yan, T.; Li, H.Y.; Wu, J.S.; Niu, Q.; Duan, W.H.; Han, Q.Z.; Ji, W.M.; Zhang, T.; Lv, W. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol. Lett. 2017, 14, 5400–5408. [Google Scholar] [CrossRef]
- Yang, Y.; Fuentes, F.; Shu, L.; Wang, C.; Pung, D.; Li, W.; Zhang, C.; Guo, Y.; Kong, A.N. Epigenetic CpG Methylation of the Promoter and Reactivation of the Expression of GSTP1 by Astaxanthin in Human Prostate LNCaP Cells. AAPS J. 2017, 19, 421–430. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, I.; Cao, M.; Su, Z.; Wu, R.; Guo, Y.; Fang, M.; Kong, A.N. Fucoxanthin Elicits Epigenetic Modifications, Nrf2 Activation and Blocking Transformation in Mouse Skin JB6 P+ Cells. AAPS J. 2018, 20, 32. [Google Scholar] [CrossRef]
- Ni, X.; Yu, H.; Wang, S.; Zhang, C.; Shen, S. Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice. Mar. Drugs 2017, 15, 66. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, Y.M.; Hong, S. Astaxanthin suppresses the metastasis of colon cancer by inhibiting the MYC-mediated downregulation of microRNA-29a-3p and microRNA-200a. Sci. Rep. 2019, 9, 9457. [Google Scholar] [CrossRef]
- Tomasini, P.; Barlesi, F.; Mascaux, C.; Greillier, L. Pemetrexed for advanced stage nonsquamous non-small cell lung cancer: Latest evidence about its extended use and outcomes. Ther. Adv. Med. Oncol. 2016, 8, 198–208. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, X.; Gu, J.; Li, X.; Cao, Y.; Xu, J.; Xue, C. Influence of molecular structure of astaxanthin esters on their stability and bioavailability. Food Chem. 2021, 343, 128497. [Google Scholar] [CrossRef]
- Mirazimi, S.M.A.; Dashti, F.; Tobeiha, M.; Shahini, A.; Jafari, R.; Khoddami, M.; Sheida, A.H.; Esna Ashari, P.; Aflatoonian, A.H.; Elikaii, F.; et al. Application of Quercetin in the Treatment of Gastrointestinal Cancers. Front. Pharmacol. 2022, 13, 921. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.H.; Wang, Z.J. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol. In Vitro 2009, 23, 797–807. [Google Scholar] [CrossRef]
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.S.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol. Res. 2019, 147, 104346. [Google Scholar] [CrossRef]
- Tan, J.; Wang, B.; Zhu, L. Regulation of survivin and Bcl-2 in HepG2 cell apoptosis induced by quercetin. Chem. Biodivers. 2009, 6, 1101–1110. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci. Biotechnol. Biochem. 2008, 72, 797–804. [Google Scholar] [CrossRef]
- Xi, L.; Zhang, Y.; Kong, S.; Liang, W. miR-34 inhibits growth and promotes apoptosis of osteosarcoma in nude mice through targetly regulating TGIF2 expression. Biosci. Rep. 2018, 38, 20180078. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Chen, D.; Ma, Q.; Zheng, Y.; Liao, S.; Wang, Y.; Zhang, J. Quercetin preferentially induces apoptosis in KRAS-mutant colorectal cancer cells via JNK signaling pathways. Cell Biol. Int. 2019, 43, 117–124. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar] [CrossRef]
- Vijayababu, M.R.; Arunkumar, A.; Kanagaraj, P.; Arunakaran, J. Effects of quercetin on insulin-like growth factors (IGFs) and their binding protein-3 (IGFBP-3) secretion and induction of apoptosis in human prostate cancer cells. J. Carcinog. 2006, 5, 10. [Google Scholar] [CrossRef]
- Luo, C.L.; Liu, Y.Q.; Wang, P.; Song, C.H.; Wang, K.J.; Dai, L.P.; Zhang, J.Y.; Ye, H. The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Biomed. Pharmacother. 2016, 82, 595–605. [Google Scholar] [CrossRef]
- Alvarez, M.C.; Maso, V.; Torello, C.O.; Ferro, K.P.; Saad, S.T.O. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin. Epigenetics 2018, 10, 139. [Google Scholar] [CrossRef]
- Yiannakopoulou, E.C. Targeting DNA methylation with green tea catechins. Pharmacology 2015, 95, 111–116. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729. [Google Scholar] [CrossRef]
- Deb, G.; Thakur, V.S.; Limaye, A.M.; Gupta, S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol. Carcinog. 2015, 54, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Cadieux, Z.; Lewis, H.; Esquela-Kerscher, A. Role of Nutrition, the Epigenome, and MicroRNAs in Cancer Pathogenesis. In MicroRNAs in Diseases and Disorders: Emerging Therapeutic Targets; Royal Society of Chemistry: London, UK, 2019; pp. 1–35. ISBN 9781782621454. [Google Scholar]
- Qi, W.J.; Sheng, W.S.; Peng, C.; Xiaodong, M.; Yao, T.Z. Investigating into anti-cancer potential of lycopene: Molecular targets. Biomed. Pharmacother. 2021, 138, 111546. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-J.; Ding, Y.-B.; Wu, L.-X.; Wen, C.-J.; Qu, Q.; Zhang, X.; Zhou, H.-H. The Effects of Lycopene on the Methylation of the GSTP1 Promoter and Global Methylation in Prostatic Cancer Cell Lines PC3 and LNCaP. Int. J. Endocrinol. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Edwards, A.; Chen, Z.; Tseng, T.S.; Li, M.; Gonzalez, G.V.; Zhang, K. Insufficient Lycopene Intake Is Associated With High Risk of Prostate Cancer: A Cross-Sectional Study From the National Health and Nutrition Examination Survey (2003–2010). Front. Public Health 2021, 9, 2041. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Zhao, W.; Hao, J.; An, R. MicroRNA-let-7f-1 is induced by lycopene and inhibits cell proliferation and triggers apoptosis in prostate cancer. Mol. Med. Rep. 2016, 13, 2708–2714. [Google Scholar] [CrossRef]
- Home—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 6 September 2022).
- Sharifi-Rad, J.; Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Hwang, K.W.; Son, D.; Jo, H.W.; Kim, C.H.; Seong, K.C.; Moon, J.K. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. Appl. Biol. Chem. 2016, 59, 209–215. [Google Scholar] [CrossRef]
- Dosoky, N.; Setzer, W. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumour Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, X.; Zhang, K. New Discovery of Curcumin Combination Therapy and Action Mechanism. Evid. Based Complement. Altern. Med. 2020, 2020, 4793058. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The Multifaceted Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Ruan, D.; Zhu, Y.W.; Fouad, A.M.; Yan, S.J.; Chen, W.; Zhang, Y.N.; Xia, W.G.; Wang, S.; Jiang, S.Q.; Yang, L.; et al. Dietary curcumin enhances intestinal antioxidant capacity in ducklings via altering gene expression of antioxidant and key detoxification enzymes. Poult. Sci. 2019, 98, 3705–3714. [Google Scholar] [CrossRef]
- Shaikh, S.; Shaikh, J.; Naba, Y.S.; Doke, K.; Ahmed, K.; Yusufi, M. Curcumin: Reclaiming the lost ground against cancer resistance. Cancer Drug Resist. 2021, 4, 298–320. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent advances to improve curcumin oral bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266. [Google Scholar] [CrossRef]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2013, 66, 222–307. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Cas, M.D.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Utilizing food matrix effects to enhance nutraceutical bioavailability: Increase of curcumin bioaccessibility using excipient emulsions. J. Agric. Food Chem. 2015, 63, 2052–2062. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Paolino, D.; Vero, A.; Cosco, D.; Pecora, T.M.G.; Cianciolo, S.; Fresta, M.; Pignatello, R. Improvement of Oral Bioavailability of Curcumin upon Microencapsulation with Methacrylic Copolymers. Front. Pharmacol. 2016, 7, 485. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Nedaeinia, R.; Shamloo, A.S.; Nikdoust, S.; Oskuee, R.K. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J. Phytomed. 2016, 6, 383. [Google Scholar] [PubMed]
- Porat, D.; Dahan, A. Active intestinal drug absorption and the solubility-permeability interplay. Int. J. Pharm. 2018, 537, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study—Delivery by Design of curcumin. J. Colloid Interface Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B. Biointerfaces 2018, 168, 29–34. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M.; Romero-Pérez, A.I.; Waterhouse, A.L.; de la Torre-Boronat, M.C. Direct HPLC Analysis of cis- and trans-Resveratrol and Piceid Isomers in Spanish Red Vitis vinifera Wines. J. Agric. Food Chem. 1995, 43, 281–283. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Anisimova, N.Y.U.; Kiselevsky, M.V.; Sosnov, A.V.; Sadovnikov, S.V.; Stankov, I.N.; Gakh, A.A. Trans-, cis-, and dihydro-resveratrol: A comparative study. Chem. Cent. J. 2011, 5, 88. [Google Scholar] [CrossRef]
- Ducimetiere, P.; Cambien, F.; Richard, J.L.; Rakotovao, R.; Claude, J.R. Coronary heart disease in middle-aged Frenchmen. Comparisons between Paris Prospective Study, Seven Countries Study, and Pooling Project. Lancet 1980, 1, 1346–1350. [Google Scholar] [CrossRef]
- Ferrières, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107. [Google Scholar] [CrossRef]
- Miura, T.; Muraoka, S.; Ikeda, N.; Watanabe, M.; Fujimoto, Y. Antioxidative and prooxidative action of stilbene derivatives. Pharmacol. Toxicol. 2000, 86, 203–208. [Google Scholar] [CrossRef]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (NOX4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Lubecka, K.; Kurzava, L.; Flower, K.; Buvala, H.; Zhang, H.; Teegarden, D.; Camarillo, I.; Suderman, M.; Kuang, S.; Andrisani, O.; et al. Stilbenoids remodel the DNA methylation patterns in breast cancer cells and inhibit oncogenic NOTCH signaling through epigenetic regulation of MAML2 transcriptional activity. Carcinogenesis 2016, 37, 656–668. [Google Scholar] [CrossRef]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef]
- Kai, L.; Samuel, S.K.; Levenson, A.S. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int. J. Cancer 2010, 126, 1538–1548. [Google Scholar] [CrossRef]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Caloric restriction, resveratrol and melatonin: Role of SIRT1 and implications for aging and related-diseases. Mech. Ageing Dev. 2015, 146–148, 28–41. [Google Scholar] [CrossRef]
- Komorowska, J.; Wątroba, M.; Szukiewicz, D. Review of beneficial effects of resveratrol in neurodegenerative diseases such as Alzheimer’s disease. Adv. Med. Sci. 2020, 65, 415–423. [Google Scholar] [CrossRef]
- Ahmed, T.; Javed, S.; Javed, S.; Tariq, A.; Šamec, D.; Tejada, S.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Resveratrol and Alzheimer’s Disease: Mechanistic Insights. Mol. Neurobiol. 2017, 54, 2622–2635. [Google Scholar] [CrossRef]
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.G.; Park, H.; Ryu, S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016, 6, 21772. [Google Scholar] [CrossRef]
- Taniguchi, T.; Iizumi, Y.; Watanabe, M.; Masuda, M.; Morita, M.; Aono, Y.; Toriyama, S.; Oishi, M.; Goi, W.; Sakai, T. Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer. Cell Death Dis. 2016, 7, e2211. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Gaballo, A.; Pradhan, B.; Patra, S.; Jena, M.; Ragusa, A. Beneficial Oxidative Stress-Related trans-Resveratrol Effects in the Treatment and Prevention of Breast Cancer. Appl. Sci. 2021, 11, 11041. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 3095. [Google Scholar] [CrossRef]
- de Vries, K.; Strydom, M.; Steenkamp, V. A Brief Updated Review of Advances to Enhance Resveratrol’s Bioavailability. Molecules 2021, 26, 4367. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B. Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef] [PubMed]
- De Vries, K.; Strydom, M.; Steenkamp, V. Bioavailability of resveratrol: Possibilities for enhancement. J. Herb. Med. 2018, 11, 71–77. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Banc, R.; Loghin, F.; Miere, D.; Ranga, F.; Socaciu, C. Phenolic composition and antioxidant activity of red, rosé and white wines originating from Romanian grape cultivars. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 716–734. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Brotons-Canto, A.; Gonzalez-Navarro, C.J.; Gurrea, J.; González-Ferrero, C.; Irache, J.M. Zein nanoparticles improve the oral bioavailability of resveratrol in humans. J. Drug Deliv. Sci. Technol. 2020, 57, 101704. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, W.H.; Ku, C.F.; Zhang, H.J.; Tsang, S.W. Design, synthesis and evaluation of novel dihydrostilbene derivatives as potential anti-melanogenic skin-protecting agents. Eur. J. Med. Chem. 2018, 143, 1254–1260. [Google Scholar] [CrossRef]
- Tang, Y.L.; Chan, S.W. A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phytother. Res. 2014, 28, 1581–1588. [Google Scholar] [CrossRef]
- Neves, A.R.; Martins, S.; Segundo, M.A.; Reis, S. Nanoscale Delivery of Resveratrol towards Enhancement of Supplements and Nutraceuticals. Nutrients 2016, 8, 131. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates from Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 2964. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Iahtisham-Ul-Haq; Khan, S.; Awan, K.A.; Iqbal, M.J. Sulforaphane as a potential remedy against cancer: Comprehensive mechanistic review. J. Food Biochem. 2022, 46, e13886. [Google Scholar] [CrossRef]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed]
- Shekarri, Q.; Dekker, M. A physiological-based model for simulating the bioavailability and kinetics of sulforaphane from broccoli products. Foods 2021, 10, 2761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kwan, M.L.; Pratt, R.; Roh, J.M.; Kushi, L.H.; Danforth, K.N.; Zhang, Y.; Ambrosone, C.B.; Tang, L. Effects of cooking methods on total isothiocyanate yield from cruciferous vegetables. Food Sci. Nutr. 2020, 8, 5673–5682. [Google Scholar] [CrossRef]
- Orlando, P.; Nartea, A.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Dludla, P.V.; Fiorini, R.; Pacetti, D.; Loizzo, M.R.; Lucci, P.; et al. Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. var. Italica Boiled or Steamed: Functional Food or Dietary Supplement? Antioxidants 2022, 11, 209. [Google Scholar] [CrossRef]
- Okunade, O.; Niranjan, K.; Ghawi, S.K.; Kuhnle, G.; Methven, L. Supplementation of the Diet by Exogenous Myrosinase via Mustard Seeds to Increase the Bioavailability of Sulforaphane in Healthy Human Subjects after the Consumption of Cooked Broccoli. Mol. Nutr. Food Res. 2018, 62, e1700980. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef]
- Bouranis, J.A.; Beaver, L.M.; Ho, E. Metabolic Fate of Dietary Glucosinolates and Their Metabolites: A Role for the Microbiome. Front. Nutr. 2021, 8, 690. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.A.J.; Beresford, S.A.A.; Lampe, J.W. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.W.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef]
- Aronica, L.; Ordovas, J.M.; Volkov, A.; Lamb, J.J.; Stone, P.M.; Minich, D.; Leary, M.; Class, M.; Metti, D.; Larson, I.A.; et al. Genetic Biomarkers of Metabolic Detoxification for Personalized Lifestyle Medicine. Nutrients 2022, 14, 768. [Google Scholar] [CrossRef]
- Hauder, J.; Winkler, S.; Bub, A.; Rüfer, C.E.; Pignitter, M.; Somoza, V. LC-MS/MS quantification of sulforaphane and indole-3-carbinol metabolites in human plasma and urine after dietary intake of selenium-fortified broccoli. J. Agric. Food Chem. 2011, 59, 8047–8057. [Google Scholar] [CrossRef]
- Sun, J.; Charron, C.S.; Novotny, J.A.; Peng, B.; Yu, L.; Chen, P. Profiling glucosinolate metabolites in human urine and plasma after broccoli consumption using non-targeted and targeted metabolomic analyses. Food Chem. 2020, 309, 125660. [Google Scholar] [CrossRef]
- Bradlow, H.; Zeligs, M. Diindolylmethane (DIM) spontaneously forms from indole-3-carbinol (I3C) during cell culture experiments. In Vivo 2010, 24, 387–391. [Google Scholar]
- Anderton, M.J.; Manson, M.M.; Verschoyle, R.; Gescher, A.; Steward, W.P.; Williams, M.L.; Mager, D.E. Physiological modeling of formulated and crystalline 3,3’-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab. Dispos. 2004, 32, 632–638. [Google Scholar] [CrossRef]
- Anderton, M.J.; Manson, M.M.; Verschoyle, R.D.; Gescher, A.; Lamb, J.H.; Farmer, P.B.; Steward, W.P.; Williams, M.L. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res. 2004, 10, 5233–5241. [Google Scholar] [CrossRef]
- Acharya, A.; Das, I.; Singh, S.; Saha, T. Chemopreventive properties of indole-3-carbinol, diindolylmethane and other constituents of cardamom against carcinogenesis. Recent Pat. Food. Nutr. Agric. 2010, 2, 166–177. [Google Scholar] [CrossRef]
- Nian, H.; Delage, B.; Ho, E.; Dashwood, R.H. Modulation of Histone Deacetylase Activity by Dietary Isothiocyanates and Allyl Sulfides: Studies with Sulforaphane and Garlic Organosulfur Compounds. Environ. Mol. Mutagen. 2009, 50, 213. [Google Scholar] [CrossRef]
- Jump, S.M.; Kung, J.; Staub, R.; Kinseth, M.A.; Cram, E.J.; Yudina, L.N.; Preobrazhenskaya, M.N.; Bjeldanes, L.F.; Firestone, G.L. N-Alkoxy derivatization of indole-3-carbinol increases the efficacy of the G1 cell cycle arrest and of I3C-specific regulation of cell cycle gene transcription and activity in human breast cancer cells. Biochem. Pharmacol. 2008, 75, 713–724. [Google Scholar] [CrossRef]
- Szaefer, H.; Licznerska, B.; Krajka-Kuniak, V.; Bartoszek, A.; Baer-Dubowska, W. Modulation of CYP1A1, CYP1A2 and CYP1B1 expression by cabbage juices and indoles in human breast cell lines. Nutr. Cancer 2012, 64, 879–888. [Google Scholar] [CrossRef]
- Reed, G.A.; Arneson, D.W.; Putnam, W.C.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. Single-dose and multiple-dose administration of indole-3-carbinol to women: Pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2477–2481. [Google Scholar] [CrossRef]
- Arslan, A.A.; Koenig, K.L.; Lenner, P.; Afanasyeva, Y.; Shore, R.E.; Chen, Y.; Lundin, E.; Toniolo, P.; Hallmans, G.; Zeleniuch-Jacquotte, A. Circulating Estrogen Metabolites and Risk of Breast Cancer in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1290. [Google Scholar] [CrossRef]
- Zeleniuch-Jacquotte, A.; Shore, R.E.; Afanasyeva, Y.; Lukanova, A.; Sieri, S.; Koenig, K.L.; Idahl, A.; Krogh, V.; Liu, M.; Ohlson, N.; et al. Postmenopausal circulating levels of 2- and 16α-hydroxyestrone and risk of endometrial cancer. Br. J. Cancer 2011, 105, 1458–1464. [Google Scholar] [CrossRef]
- McCann, S.E.; Wactawski-Wende, J.; Kufel, K.; Olson, J.; Ovando, B.; Kadlubar, S.N.; Davis, W.; Carter, L.; Muti, P.; Shields, P.G.; et al. Changes in 2-hydroxyestrone and 16alpha-hydroxyestrone metabolism with flaxseed consumption: Modification by COMT and CYP1B1 genotype. Cancer Epidemiol. Biomark. Prev. 2007, 16, 256–262. [Google Scholar] [CrossRef]
- Lee, S.U.; Rhee, M.; Min, Y.K.; Kim, S.H. Involvement of peroxiredoxin IV in the 16alpha-hydroxyestrone-induced proliferation of human MCF-7 breast cancer cells. Cell Biol. Int. 2008, 32, 401–405. [Google Scholar] [CrossRef]
- Firestone, G.L.; Sundar, S.N. Minireview: Modulation of hormone receptor signaling by dietary anticancer indoles. Mol. Endocrinol. 2009, 23, 1940–1947. [Google Scholar] [CrossRef]
- Le, H.T.; Schaldach, C.M.; Firestone, G.L.; Bjeldanes, L.F. Plant-derived 3,3’-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem. 2003, 278, 21136–21145. [Google Scholar] [CrossRef]
- Bradlow, H.L.; Davis, D.L.; Lin, G.; Sepkovic, D.; Tiwari, R. Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: A biologic marker of breast cancer risk. Environ. Health Perspect. 1995, 103, 147. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Hall, J.A.; Patterson, W.L.; Ho, E.; Hsu, A.; Al-Mulla, F.; Georgel, P.T. Sulforaphane modulates telomerase activity via epigenetic regulation in prostate cancer cell lines. Biochem. Cell Biol. 2016, 94, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, H.; Kim, J.; Park, J.H.Y. 3,3’-diindolylmethane suppresses 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and tumor promotion in mouse skin via the downregulation of inflammatory mediators. Mol. Carcinog. 2010, 49, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179. [Google Scholar] [CrossRef]
- Royston, K.J.; Tollefsbol, T.O. The Epigenetic Impact of Cruciferous Vegetables on Cancer Prevention. Curr. Pharmacol. Rep. 2015, 1, 46–51. [Google Scholar] [CrossRef]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid. Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef]
- Thomson, C.A.; Ho, E.; Strom, M.B. Chemopreventive properties of 3,30-diindolylmethane in breast cancer: Evidence from experimental and human studies. Nutr. Rev. 2016, 74, 432–443. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Zhang, S.; Akbari, M.; Salmena, L.; Llacuachaqui, M.; Zeligs, M.; Sun, P.; Narod, S.A. BRCA1 mRNA levels following a 4–6-week intervention with oral 3,3′-diindolylmethane. Br. J. Cancer 2014, 111, 1269. [Google Scholar] [CrossRef]
- Fujioka, N.; Fritz, V.; Upadhyaya, P.; Kassie, F.; Hecht, S.S. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee, W. Wattenberg. Mol. Nutr. Food Res. 2016, 60, 1228–1238. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, Y. Nanodelivery of natural isothiocyanates as a cancer therapeutic. Free Radic. Biol. Med. 2021, 167, 125–140. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, T.T.Y.; Teng, Z.; Chen, P.; Sun, J.; Wang, Q. Encapsulation of indole-3-carbinol and 3,3′-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. 2013, 139, 224–230. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Karimian, A.; Hadi Bahadori, M.; Moghaddam, A.H.; Mir Mohammadrezaei, F.; Mohammadrezaei, F.M. Effect of Astaxanthin on cell viability in T-47D and MDA-MB-231 Breast Cancer Cell Lines. Multidiscip. Cancer Investig. 2017, 124, 151832. [Google Scholar] [CrossRef]
- McCall, B.; McPartland, C.K.; Moore, R.; Frank-Kamenetskii, A.; Booth, B.W. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro. Antioxidants 2018, 7, 135. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Cheng, K.W.; Chen, F. Inhibition of autophagy modulates astaxanthin and total fatty acid biosynthesis in Chlorella zofingiensis under nitrogen starvation. Bioresour. Technol. 2018, 247, 610–615. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA. Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Liao, K.S.; Wei, C.L.; Chen, J.C.; Zheng, H.Y.; Chen, W.C.; Wu, C.H.; Wang, T.J.; Peng, Y.S.; Chang, P.Y.; Lin, Y.W. Astaxanthin enhances pemetrexed-induced cytotoxicity by downregulation of thymidylate synthase expression in human lung cancer cells. Regul. Toxicol. Pharmacol. 2016, 81, 353–361. [Google Scholar] [CrossRef]
- Chen, J.C.; Wu, C.H.; Peng, Y.S.; Zheng, H.Y.; Lin, Y.C.; Ma, P.F.; Yen, T.C.; Chen, T.Y.; Lin, Y.W. Astaxanthin enhances erlotinib-induced cytotoxicity by p38 MAPK mediated xeroderma pigmentosum complementation group C (XPC) down-regulation in human lung cancer cells. Toxicol. Res. 2018, 7, 1247. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lim, J.W.; Kim, H. Astaxanthin induces NADPH oxidase activation and receptor-interacting protein kinase 1-mediated necroptosis in gastric cancer AGS cells. Mol. Med. Rep. 2021, 24, 1–12. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Osawa, Y.; Hirasawa, K.; Kuroda, I. Evaluation and improvement of storage stability of astaxanthin 3 isomers in oils and fats. Food Chem. 2021, 352, 129371. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef]
- Madhavi, D.; Kagan, D.; Seshadri, S. A Study on the Bioavailability of a Proprietary, Sustained-release Formulation of Astaxanthin. Integr. Med. A Clin. J. 2018, 17, 38. [Google Scholar]
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato protein- based carriers for enhancing bioavailability of astaxanthin. Food Hydrocoll. 2019, 96, 72–80. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.S.; Lee, J.H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits-A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Genç, Y.; Bardakci, H.; Yücel, Ç.; Karatoprak, G.Ş.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E. Oxidative Stress and Marine Carotenoids: Application by Using Nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis-Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Snell, T.W.; Carberry, J. Astaxanthin Bioactivity Is Determined by Stereoisomer Composition and Extraction Method. Nutrients 2022, 14, 1522. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hua, S.; Huang, Z.; Gu, Z.; Cheng, L.; Hong, Y. Comparison of bioaccessibility of astaxanthin encapsulated in starch-based double emulsion with different structures. Carbohydr. Polym. 2021, 272, 118475. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Marin, D.P.; Bolin, A.P.; De Cássia Santos Macedo, R.; Campoio, T.R.; Fineto, C.; Guerra, B.A.; Polotow, T.G.; Vardaris, C.; Mattei, R.; et al. Combined astaxanthin and fish oil supplementation improves glutathione-based redox balance in rat plasma and neutrophils. Chem. Biol. Interact. 2012, 197, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Ferro-Furtado, R.; Favaro-Trindade, C.S. Encapsulation of an astaxanthin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin–cashew gum complex. Food Hydrocoll. 2016, 61, 155–162. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Toma, C.-C.; Simu, G.M.; Hanganu, D.; Olah, N.; Vata, F.M.G.; Hammami, C.; Hammami, M. Chemical composition of the Tunisian Nigella sativa. Note I. Profile on essential oil. Farmacia 2010, 58, 458–464. [Google Scholar]
- Fabre, G.; Bayach, I.; Berka, K.; Paloncýová, M.; Starok, M.; Rossi, C.; Duroux, J.L.; Otyepka, M.; Trouillas, P. Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes. Chem. Commun. 2015, 51, 7713–7716. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.J.; Choi, J.; Frei, B. Quercetin affects glutathione levels and redox ratio in human aortic endothelial cells not through oxidation but formation and cellular export of quercetin-glutathione conjugates and upregulation of glutamate-cysteine ligase. Redox Biol. 2016, 9, 220–228. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Zhao, J.J.; Li, Z.Z.; Tang, G.H.; Zhao, Z.M.; Yin, S. Natural nitric oxide (NO) inhibitors from Chloranthus japonicus. Bioorg. Med. Chem. Lett. 2016, 26, 3163–3166. [Google Scholar] [CrossRef]
- Mutoh, M.; Takahashi, M.; Fukuda, K.; Matsushima-Hibiya, Y.; Mutoh, H.; Sugimura, T.; Wakabayashi, K. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 2000, 21, 959–963. [Google Scholar] [CrossRef]
- Kashyap, D.; Mittal, S.; Sak, K.; Singhal, P.; Tuli, H.S. Molecular mechanisms of action of quercetin in cancer: Recent advances. Tumour Biol. 2016, 37, 12927–12939. [Google Scholar] [CrossRef]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F.I.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Park, Y.J.; Choo, W.H.; Kim, H.R.; Chung, K.H.; Oh, S.M. Inhibitory Aromatase Effects of Flavonoids from Ginkgo Biloba Extracts on Estrogen Biosynthesis. Asian Pac. J. Cancer Prev. 2015, 16, 6317–6325. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Aspects Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Napolitano, M.; Tedesco, I.; Moccia, S.; Milito, A.; Luigi Russo, G. Neuroprotective Role of Natural Polyphenols. Curr. Top. Med. Chem. 2016, 16, 1943–1950. [Google Scholar] [CrossRef]
- Gupta, K.; Panda, D. Perturbation of microtubule polymerization by quercetin through tubulin binding: A novel mechanism of its antiproliferative activity. Biochemistry 2002, 41, 13029–13038. [Google Scholar] [CrossRef]
- Moon, S.K.; Cho, G.O.; Jung, S.Y.; Gal, S.W.; Kwon, T.K.; Lee, Y.C.; Madamanchi, N.R.; Kim, C.H. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: Role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem. Biophys. Res. Commun. 2003, 301, 1069–1078. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.; Yang, L.; Li, P. Quercetin Inhibits the Proliferation and Metastasis of Human Non-Small Cell Lung Cancer Cell Line: The Key Role of Src-Mediated Fibroblast Growth Factor-Inducible 14 (Fn14)/ Nuclear Factor kappa B (NF-κB) pathway. Med. Sci. Monit. 2020, 26, e920537-1–e920537-11. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Guo, Y.; Mah, E.; Bruno, R.S. Quercetin bioavailability is associated with inadequate plasma vitamin C status and greater plasma endotoxin in adults. Nutrition 2014, 30, 1279–1286. [Google Scholar] [CrossRef]
- Liu, K.; Zha, X.Q.; Shen, W.; Li, Q.M.; Pan, L.H.; Luo, J.P. The hydrogel of whey protein isolate coated by lotus root amylopectin enhance the stability and bioavailability of quercetin. Carbohydr. Polym. 2020, 236, 116009. [Google Scholar] [CrossRef]
- Lin, J.; Teo, L.M.; Leong, L.P.; Zhou, W. In vitro bioaccessibility and bioavailability of quercetin from the quercetin-fortified bread products with reduced glycemic potential. Food Chem. 2019, 286, 629–635. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Hussain, A.; Sameen, A.; Sahar, A.; Khan, S.; Siddique, R.; Aadil, R.M.; Xu, B. Novel extraction, rapid assessment and bioavailability improvement of quercetin: A review. Ultrason. Sonochem. 2021, 78, 1350–4177. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Borutinskaitė, V.; Virkšaitė, A.; Gudelytė, G.; Navakauskienė, R. Green tea polyphenol EGCG causes anti-cancerous epigenetic modulations in acute promyelocytic leukemia cells. Leuk. Lymphoma 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Sahadevan, R.; Singh, S.; Binoy, A.; Sadhukhan, S. Chemico-biological aspects of (-)-epigallocatechin-3-gallate (EGCG) to improve its stability, bioavailability and membrane permeability: Current status and future prospects. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–30. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res. Int. 2021, 142, 110186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation Enhances Epigallocatechin-3-Gallate Stability and Its Anti-atherogenic Bioactivities in Macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (-)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Fernández, V.A.; Toledano, L.A.; Lozano, N.P.; Tapia, E.N.; Roig, M.D.G.; Fornell, R.D.L.T.; Algar, Ó.G. Bioavailability of Epigallocatechin Gallate Administered With Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef]
- Pira, C.; Trapani, G.; Fadda, M.; Finocchiaro, C.; Bertino, E.; Coscia, A.; Ciocan, C.; Cuciureanu, M.; Hegheş, S.C.; Vranceanu, M.; et al. Comparative Study Regarding the Adherence to the Mediterranean Diet and the Eating Habits of Two Groups-The Romanian Children and Adolescents Living in Nord-West of Romania and Their Romanian Counterparts Living in Italy. Foods 2021, 10, 2045. [Google Scholar] [CrossRef]
- Hong, J.; Lambert, J.D.; Lee, S.H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein–polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Chow, H.H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef]
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins|EFSA. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95. [Google Scholar] [CrossRef]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ma, C.; Wang, H. Bilosomes as effective delivery systems to improve the gastrointestinal stability and bioavailability of epigallocatechin gallate (EGCG). Food Res. Int. 2021, 149, 110631. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases-A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Aust, O.; Ale-Agha, N.; Zhang, L.; Wollersen, H.; Sies, H.; Stahl, W. Lycopene oxidation product enhances gap junctional communication. Food Chem. Toxicol. 2003, 41, 1399–1407. [Google Scholar] [CrossRef]
- Ilic, D.; Misso, M. Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: A systematic review. Maturitas 2012, 72, 269–276. [Google Scholar] [CrossRef]
- Kolberg, M.; Pedersen, S.; Bastani, N.E.; Carlsen, H.; Blomhoff, R.; Paur, I. Tomato paste alters NF-κB and cancer-related mRNA expression in prostate cancer cells, xenografts, and xenograft microenvironment. Nutr. Cancer 2015, 67, 305–315. [Google Scholar] [CrossRef]
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef]
- Boileau, T.W.M.; Boileau, A.C.; Erdman, J.W. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med. 2002, 227, 914–919. [Google Scholar] [CrossRef]
- Moran, N.E.; Cichon, M.J.; Riedl, K.M.; Grainger, E.M.; Schwartz, S.J.; Novotny, J.A.; Erdman, J.W.; Clinton, S.K. Compartmental and noncompartmental modeling of 13C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am. J. Clin. Nutr. 2015, 102, 1436–1449. [Google Scholar] [CrossRef]
- Wang, X.D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214S–1222S. [Google Scholar] [CrossRef]
- Vitucci, D.; Amoresano, A.; Nunziato, M.; Muoio, S.; Alfieri, A.; Oriani, G.; Scalfi, L.; Frusciante, L.; Rigano, M.M.; Pucci, P.; et al. Nutritional Controlled Preparation and Administration of Different Tomato Purées Indicate Increase of β-Carotene and Lycopene Isoforms, and of Antioxidant Potential in Human Blood Bioavailability: A Pilot Study. Nutrients 2021, 13, 1336. [Google Scholar] [CrossRef]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [CrossRef]
- Lee, A.; Thurnham, D.I.; Chopra, M. Consumption of tomato products with olive oil but not sunflower oil increases the antioxidant activity of plasma. Free Radic. Biol. Med. 2000, 29, 1051–1055. [Google Scholar] [CrossRef]
- Amorim, A.D.G.N.; Vasconcelos, A.G.; Souza, J.; Oliveira, A.; Gullón, B.; de Souza de Almeida Leite, J.R.; Pintado, M. Bio-Availability, Anticancer Potential, and Chemical Data of Lycopene: An Overview and Technological Prospecting. Antioxidants 2022, 11, 360. [Google Scholar] [CrossRef]
- Crowe-White, K.M.; Voruganti, V.S.; Talevi, V.; Dudenbostel, T.; Nagabooshanam, V.A.; Locher, J.L.; Ellis, A.C. Variation of Serum Lycopene in Response to 100% Watermelon Juice: An Exploratory Analysis of Genetic Variants in a Randomized Controlled Crossover Study. Curr. Dev. Nutr. 2020, 4, nzaa102. [Google Scholar] [CrossRef] [PubMed]
- Zubair, N.; Kooperberg, C.; Liu, J.; Di, C.; Peters, U.; Neuhouser, M.L. Genetic variation predicts serum lycopene concentrations in a multiethnic population of postmenopausal women. J. Nutr. 2015, 145, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Perry, J.R.B.; Matteini, A.; Perola, M.; Tanaka, T.; Silander, K.; Rice, N.; Melzer, D.; Murray, A.; Cluett, C.; et al. Common variation in the β-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2008, 84, 123–133. [Google Scholar] [CrossRef] [PubMed]
| Natural Source | Epigenetic Modulation | Gene Targets | Biological Effects | Micro RNAs Regulated | Cancer Types | References |
|---|---|---|---|---|---|---|
| Curcumin | ||||||
| Turmeric | DNMT1 DNMT3b DNMT3a HDAC1 HDAC4 HDAC7 | P65, Sp1, CDK, Her2, NrF2, STAT3, BAX, p38, p53 VEGF IL6 IL23 IL1-β | Chemoprevention, cell growth inhibition, cell-cycle arrest Apoptosis, angiogenesis inhibition | miR-15a↑ miR-16↑ miR-22↑ miR-26a↑ miR-34a↑ miR-145↑ miR-146a↑ miR-200b↑ miR-200c↑ miR-203 ↑ let7↑ miR-19a,b↓ miR-21↓ miR-27a↓ miR-130a↓ miR-186↓ | AML Breast Prostate Colon Lung | [19,20,21,22,23,24,25,26,27,28,29,30,31] |
| Resveratrol | ||||||
| Black grapes, red wine, plum, peanuts, berries, cocoa powder, dark chocolate | DNMT HDAC | p53 p300 p16 CDK AP1 EGR1 STAT1 STAT3 SIRT1 MAPK Bcl2 hTERT MTA1 | Cell growth inhibition, cell-cycle arrest Apoptosis Chemopreventive | miR34a↑ miR 663↑ miR 141↑ miR 200↑ miR17↓ miR25↓ miR92a-2↓ | Colon Breast Prostate Lung | [32,33,34,35,36,37,38,39] |
| Sulforaphane | ||||||
| Broccoli Cauliflower Cabbage Brussels sprout | DNMT1 DNMT3a DNMT3b HDCA1, 2,3,8 | p21 p27 CDKN hTERT EGFR Cyclin D2 Nrf2 | Chemopreventive Cell-cycle arrest Apoptosis Cell growth inhibition | miR-let-7a-e↑ miR-15a↑ miR-16↑ miR-27b↑ miR-30e↑ miR-31↑ miR-34a↑ miR-124↑ miR-200a-b-c↑ miR-219-5p↑ miR-320↑ miR-19a↓ miR-19b↓ miR-92a-2↓ miR-106a↓ miR-181a↓ miR-181b↓ miR-210-3p↓ miR-221↓ miR-495↓ | Prostate Breast Lung | [40,41,42,43,44,45,46,47,48] |
| Astaxanthin | ||||||
| Algae, yeast, salmon, trout, krill, shrimp, and crayfish | DNMT1 DNMT3a DNMT3b | MMP2 ZEB1 EMT EGFR XPC Rad51 NQO1 NRF2/ KEAP1 | Chemopreventive Apoptosis Cell growth inhibition Cell proliferation inhibition | miR-29a-3p↑ miR-200a↑ miR-375↑ miR-478b↑ miR-221↓ | Pancreatic Lung Prostate Skin | [49,50,51,52,53,54,55] |
| Quercetin | ||||||
| Onion, apple, citrus fruits, raspberries Grapes Olives Tomatoes | DNMT3a DNMT3b HDAC1 DNMT1 | p53 CD1 p21 PLAU ERK1/2 KRAS BRCA1 BRCA2 IGF1 IGFBP3 JNK AR Bcl2 JAK | Cell growth inhibition Cell proliferation inhibition Chemopreventive Apoptosis Cell-cycle arrest | miR-let-7↑ miR-146a↑ miR-15a↑ miR-16↑ miR-26↑ miR-142-3p↑ miR-200b-3p↑ miR-217↑ miR-330↑ miR-27a miR-21 miR-19b miR-155 miR-148c | Breast Prostate Colon Ovarian Gastric Pancreatic Lung Leukemia | [56,57,58,59,60,61,62,63,64,65,66] |
| EGCG | ||||||
| Green tea, carob flour, apples, pistachios, prunes, peaches, avocados | DNMT1 DNMT3a DNMT3b HDCA1 | GSTP1 CDX2 BMP2 TIMP3 MMP2 MMP9 IGF, IGF1, IGFBP-3 VEGF p53 Bcl2 | Cell growth inhibition Cell proliferation inhibition Chemopreventive Apoptosis Cell-cycle arrest Angiogenesis decreases | miR-16↑ miR-210↑ miR-330↑ miR-21↓ miR-98-5p↓ | Liver Breast Prostate Lung Bladder Gastric Colon | [67,68,69,70,71] |
| Lycopene | ||||||
| Tomatoes Apricots Guava Papaya Watermelon Pink grapefruit | DNMT3a | GSTP1 AKT2 CDK2 CDK4 p53 CCND1 CCND3 | Cell growth inhibition Chemopreventive Cell-cycle arrest Apoptosis | miR-let-7f-1 ↑ | Prostate cancer Breast cancer | [72,73,74,75] |
| Plant-Derived Bioactive Compound | Type of Cancer | Primary Outcome Measures | Clinical Trial Identifier |
|---|---|---|---|
| Curcumin | Breast cancer | Tumor proliferation rate | NCT03980509 |
| Sulforaphane | Lung cancer | Prevention of lung cancer in former smokers/bronchial dysplasia index | NCT03232138 |
| Quercetin | Squamous cell carcinoma | Prevention of squamous cell carcinoma in patients with Fanconi anemia/reduction in buccal micronuclei | NCT03476330 |
| Epigallocatechin-3-gallate | Colorectal cancer | Change in methylation from baseline when compared to the control arm | NCT02891538 |
| Lycopene | Metastatic colorectal cancer and skin toxicity | Skin toxicity reduction in metastatic colorectal cancer submitted to therapy with panitumumab | NCT03167268 |
| Mixture of carotenoids, indole-3-carbinol, curcumin, EGCG, caffeine, resveratrol, lycopene, genistein, phytoestrogens | Breast and ovarian cancer syndrome | DNA damage change | NCT05306002 |
| Quercetin’s Functions | References |
|---|---|
| Ability to restore tocopherol after its transformation into tocopheryl radical. | [202] |
| Ability to protect the endogenous antioxidant enzymatic systems, catalase (CAT), superoxide dismutase (SOD2), glutathione peroxidase (GPX), and glutathione reductase (GR). | [203] |
| Ability to eliminate superoxide anion and limit nitric oxide biosynthesis during inflammatory processes. | [204] |
| Ability to inhibit proinflammatory pathways such as those focused on the action of 5-lipoxygenase, which would otherwise lead to the possible excessive biosynthesis of leukotriene mediators of inflammation and phospholipase A2, which generates arachidonic acid and, in turn, favors the biosynthesis of inflammatory prostaglandins. | [205] |
| Inhibition of multiple cellular enzymes such as tyrosine kinase (TK) including growth factor receptor EGFR, calcium-phospho-lipid-dependent protein kinase (PKC), and ornithine decarboxylase (ODC), which produces polyamines known to be involved in cell proliferation and phosphoinositide kinases PI3K and PI4P-5K, involved in the proliferative responses triggered by the mitogenic pathways of signal transduction. For these last two properties, quercetin has been extensively studied in oncology, in particular with reference to the mechanisms of cell proliferation and carcinogenesis. | [206,207] |
| Mimics aromatase inhibitors. | [208] |
| Antiplatelet and cardioprotective action that limits its use in the case of concomitant intake by the patient of anticoagulant drugs such as dicoumarols. | [209] |
| Neuroprotective and neurotrophic action as an adjuvant therapy in the case of neurodegenerative diseases and the prevention of the same in subjects with increased susceptibility. | [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrânceanu, M.; Galimberti, D.; Banc, R.; Dragoş, O.; Cozma-Petruţ, A.; Hegheş, S.-C.; Voştinaru, O.; Cuciureanu, M.; Stroia, C.M.; Miere, D.; et al. The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression. Plants 2022, 11, 2524. https://doi.org/10.3390/plants11192524
Vrânceanu M, Galimberti D, Banc R, Dragoş O, Cozma-Petruţ A, Hegheş S-C, Voştinaru O, Cuciureanu M, Stroia CM, Miere D, et al. The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression. Plants. 2022; 11(19):2524. https://doi.org/10.3390/plants11192524
Chicago/Turabian StyleVrânceanu, Maria, Damiano Galimberti, Roxana Banc, Ovidiu Dragoş, Anamaria Cozma-Petruţ, Simona-Codruţa Hegheş, Oliviu Voştinaru, Magdalena Cuciureanu, Carmina Mariana Stroia, Doina Miere, and et al. 2022. "The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression" Plants 11, no. 19: 2524. https://doi.org/10.3390/plants11192524
APA StyleVrânceanu, M., Galimberti, D., Banc, R., Dragoş, O., Cozma-Petruţ, A., Hegheş, S.-C., Voştinaru, O., Cuciureanu, M., Stroia, C. M., Miere, D., & Filip, L. (2022). The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression. Plants, 11(19), 2524. https://doi.org/10.3390/plants11192524











