The Upper Range Limit of Alien Plants Is Not in Equilibrium with Climate in the Andes of Central Chile
Abstract
:1. Introduction
2. Results
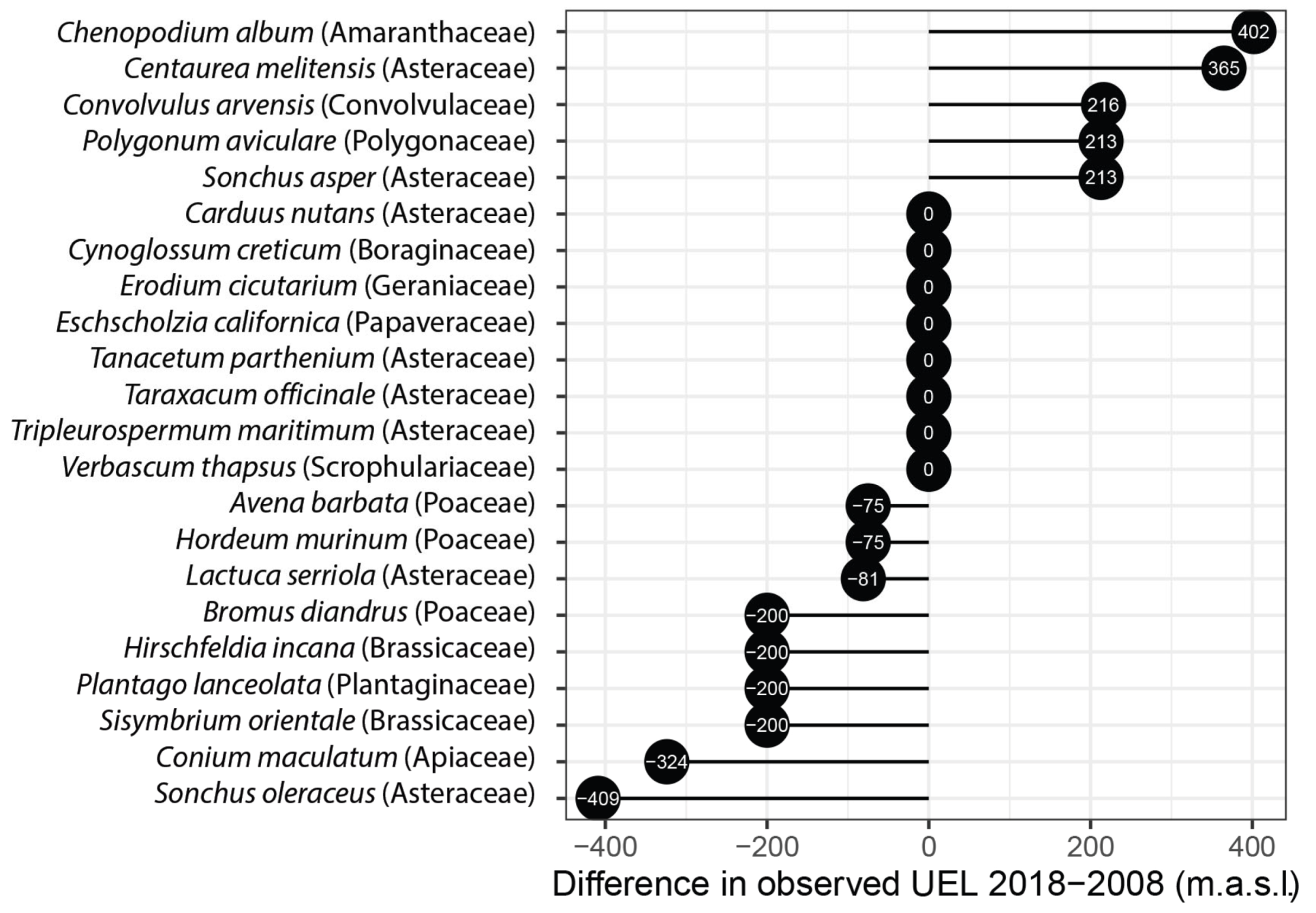
2.1. Upper Elevational Limit (UEL) Changes over Time
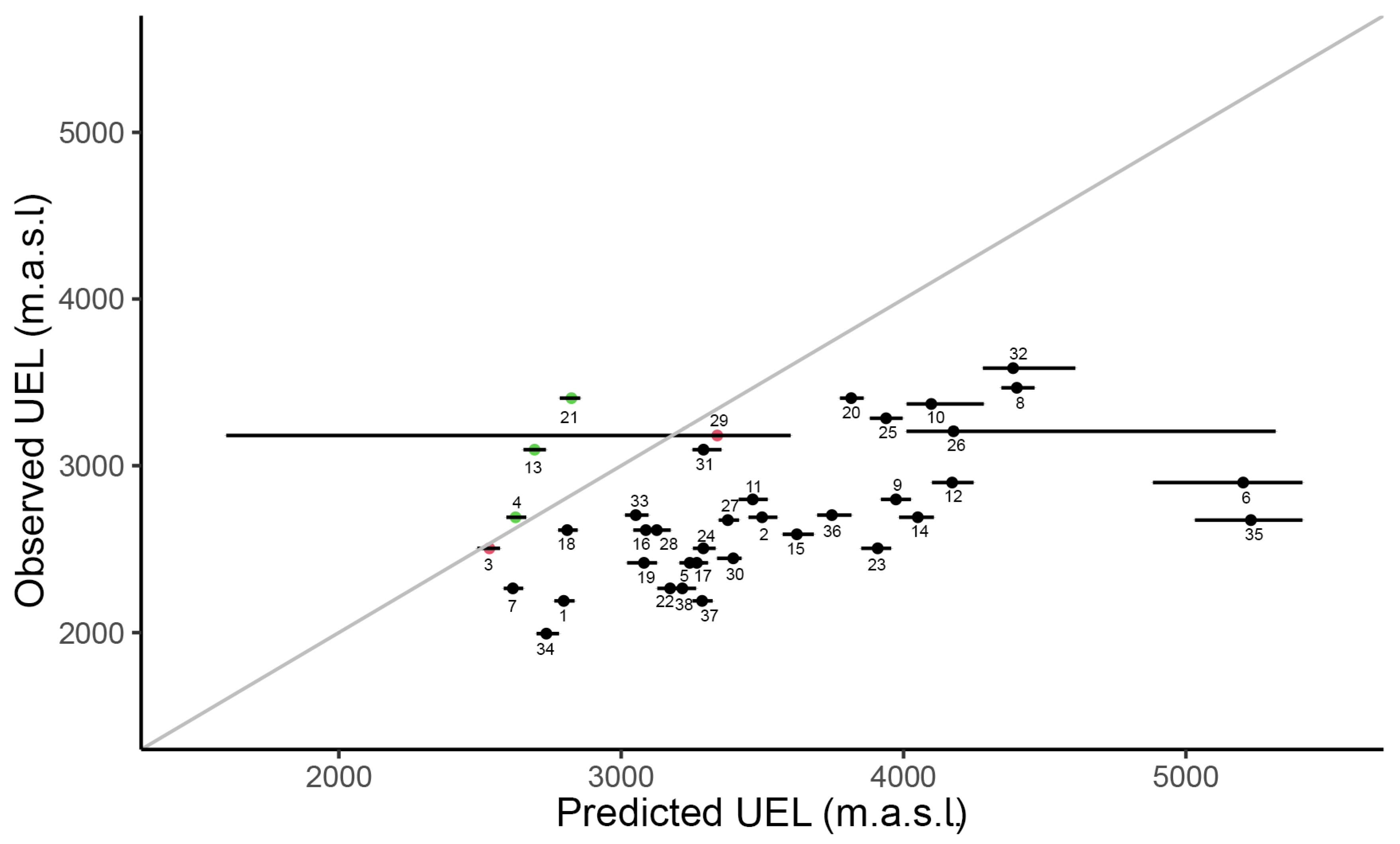
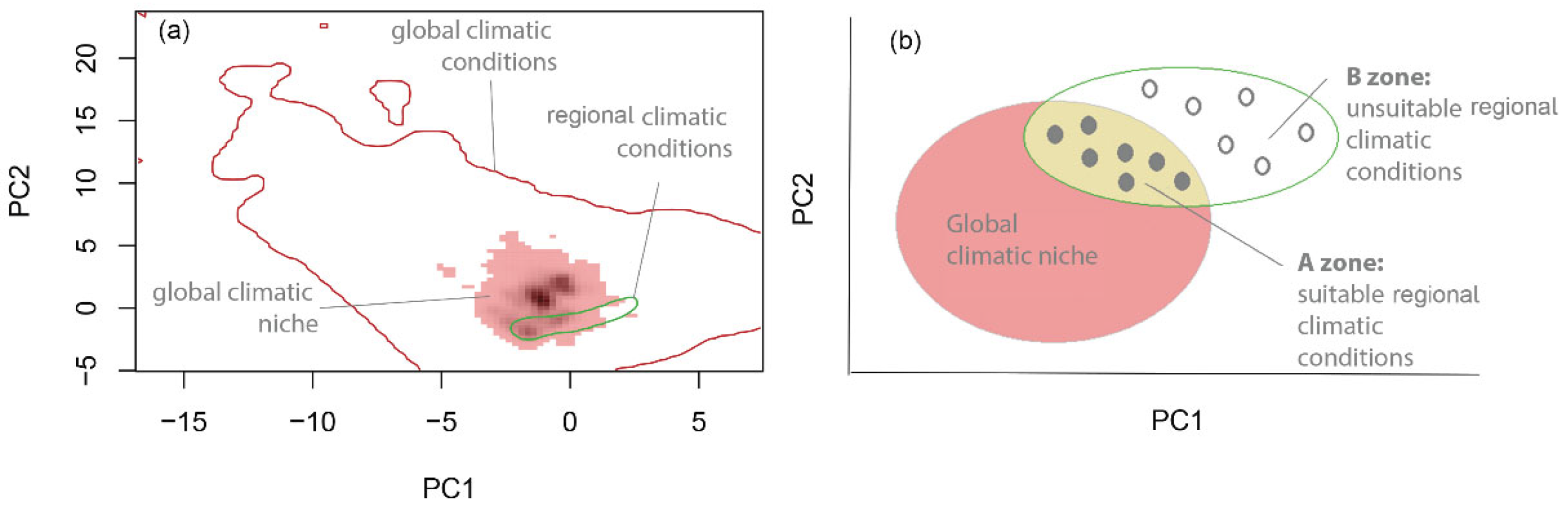
2.2. Assessing If Species’ UELs Are in Equilibrium with Climate
2.3. UEL and Species Attributes
3. Discussion
3.1. Temporal Changes
3.2. Mismatch between Observed and Predicted UEL
4. Materials and Methods
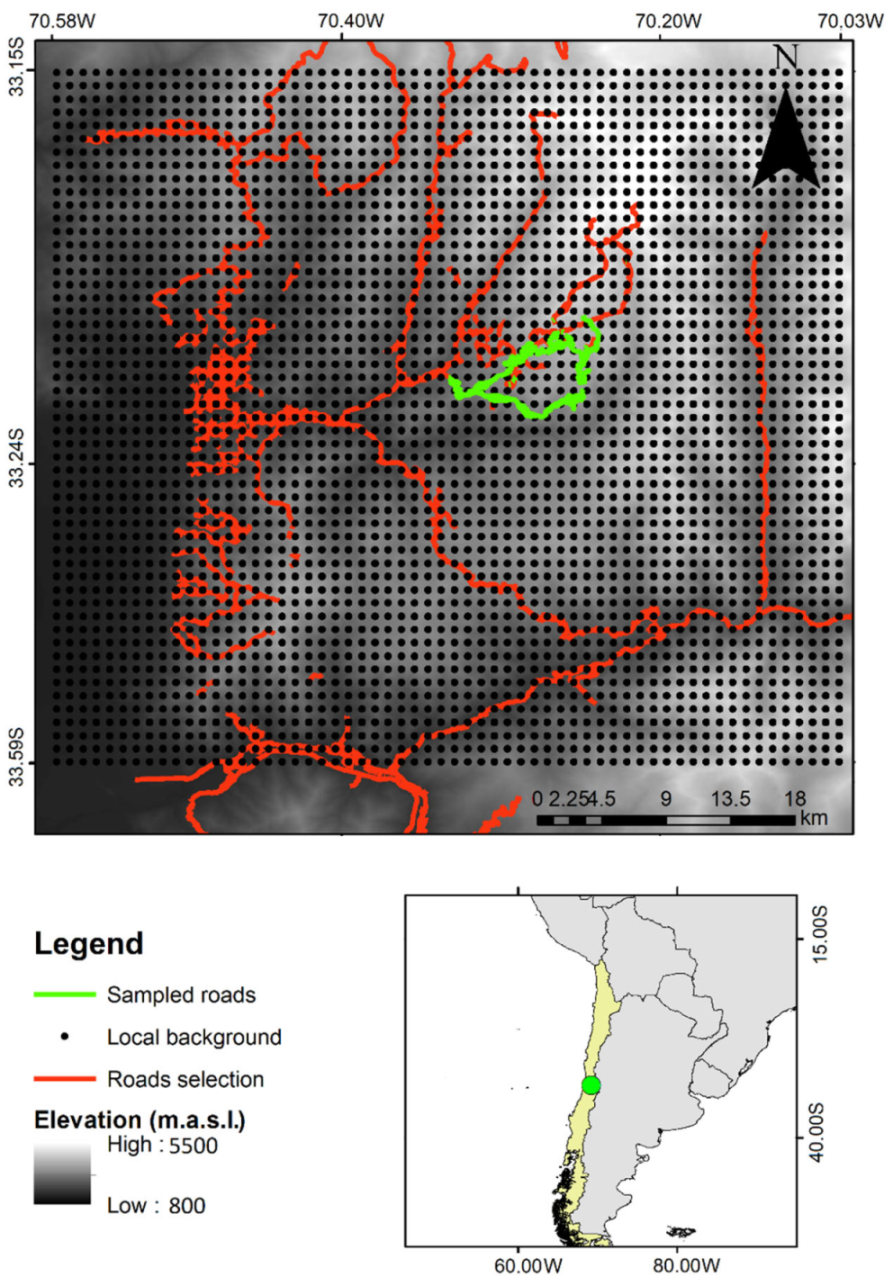
4.1. Study Area
4.2. Assessing Species Upper Elevational Limit (UEL) Changes over Time
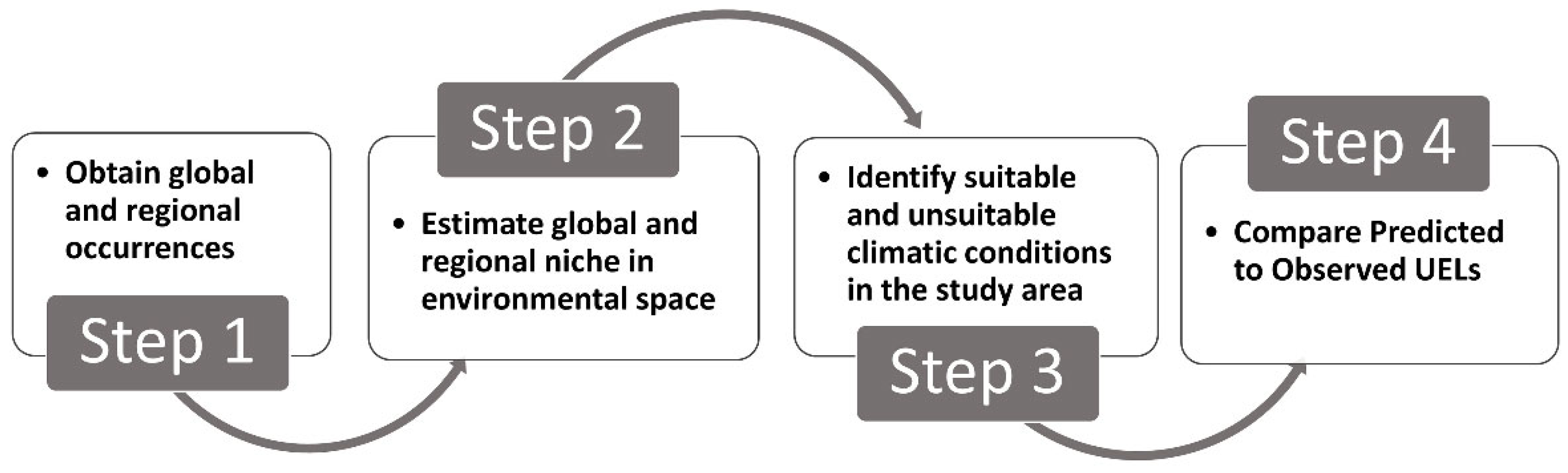
4.3. Assessing if Species’ UELs Are in Climatic Equilibrium
- Step 1.
- Obtain global and regional occurrences
- Step 2.
- Estimate global and regional niche in environmental space
- Step 3.
- Identify suitable and unsuitable climatic conditions in the study area
- Step 4.
- Compare predicted to observed UELs
4.4. UEL and Species Attributes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, J.M.; Kueffer, C.; Daehler, C.C.; Edwards, P.J.; Pauchard, A.; Seipel, T.; MIREN Consortium; Arevalo, J.; Cavieres, L.; Dietz, H.; et al. Assembly of Nonnative Floras along Elevational Gradients Explained by Directional Ecological Filtering. Proc. Natl. Acad. Sci. USA 2011, 108, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Dietz, H.; Billeter, R.; Buschmann, H.; Edwards, P.J. Altitudinal Distribution of Alien Plant Species in the Swiss Alps. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 173–183. [Google Scholar] [CrossRef]
- McDougall, K.L.; Alexander, J.M.; Haider, S.; Pauchard, A.; Walsh, N.G.; Kueffer, C. Alien Flora of Mountains: Global Comparisons for the Development of Local Preventive Measures against Plant Invasions: Alien Flora of Mountains. Divers. Distrib. 2011, 17, 103–111. [Google Scholar] [CrossRef]
- Barni, E.; Bacaro, G.; Falzoi, S.; Spanna, F.; Siniscalco, C. Establishing Climatic Constraints Shaping the Distribution of Alien Plant Species along the Elevation Gradient in the Alps. Plant Ecol. 2012, 213, 757–767. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Koide, D.; Yoshida, K.; Daehler, C.C.; Mueller-Dombois, D. An Upward Elevation Shift of Native and Non-Native Vascular Plants over 40 Years on the Island of Hawai’i. J. Veg. Sci. 2017, 28, 939–950. [Google Scholar] [CrossRef]
- Pauchard, A.; Kueffer, C.; Dietz, H.; Daehler, C.C.; Alexander, J.; Edwards, P.J.; Arévalo, J.R.; Cavieres, L.A.; Guisan, A.; Haider, S.; et al. Ain’t No Mountain High Enough: Plant Invasions Reaching New Elevations. Front. Ecol. Environ. 2009, 7, 479–486. [Google Scholar] [CrossRef]
- Tanaka, T.; Sato, T. Contemporary Patterns and Temporal Changes in Alien Plant Species Richness along an Elevational Gradient in Central Japan. Plant Ecol. Evol. 2016, 149, 177–188. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Delgado, J.D.; Otto, R.; Naranjo, A.; Salas, M.; Fernández-Palacios, J.M. Distribution of Alien vs. Native Plant Species in Roadside Communities along an Altitudinal Gradient in Tenerife and Gran Canaria (Canary Islands). Perspect. Plant Ecol. Evol. Syst. 2005, 7, 185–202. [Google Scholar] [CrossRef]
- Steyn, C.; Greve, M.; Robertson, M.P.; Kalwij, J.M.; le Roux, P.C. Alien Plant Species That Invade High Elevations Are Generalists: Support for the Directional Ecological Filtering Hypothesis. J. Veg. Sci. 2017, 28, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Körner, C.; Ohsawa, M. Mountain Systems. In Ecosystems and Human Well-Being: Wetlands and Water; Millennium Ecosystem Assessment, Ed.; World Resources Institute: Washington, DC, USA, 2005; ISBN 1-56973-597-2. [Google Scholar]
- Spehn, E.M.; Rudmann-Maurer, K.; Körner, C. Mountain Biodiversity. Plant Ecol. Divers. 2011, 4, 301–302. [Google Scholar] [CrossRef]
- Noroozi, J.; Talebi, A.; Doostmohammadi, M.; Rumpf, S.B.; Linder, H.P.; Schneeweiss, G.M. Hotspots within a Global Biodiversity Hotspot—Areas of Endemism Are Associated with High Mountain Ranges. Sci. Rep. 2018, 8, 10345. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Lembrechts, J.J.; Cavieres, L.A.; Daehler, C.; Haider, S.; Kueffer, C.; Liu, G.; McDougall, K.; Milbau, A.; Pauchard, A.; et al. Plant Invasions into Mountains and Alpine Ecosystems: Current Status and Future Challenges. Alp. Bot. 2016, 126, 89–103. [Google Scholar] [CrossRef]
- Joshi, S.; Shrestha, B.B.; Shrestha, L.; Rashid, I.; Adkins, S. Plant Invasions in Mountains. In Global Plant Invasions; Clements, D.R., Upadhyaya, M.K., Joshi, S., Shrestha, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-89683-6. [Google Scholar]
- Araújo, M.B.; Pearson, R.G. Equilibrium of Species’ Distributions with Climate. Ecography 2005, 28, 693–695. [Google Scholar] [CrossRef]
- Kalwij, J.M.; Robertson, M.P.; van Rensburg, B.J. Annual Monitoring Reveals Rapid Upward Movement of Exotic Plants in a Montane Ecosystem. Biol. Invasions 2015, 17, 3517–3529. [Google Scholar] [CrossRef]
- Seipel, T.; Alexander, J.M.; Edwards, P.J.; Kueffer, C. Range Limits and Population Dynamics of Non-Native Plants Spreading along Elevation Gradients. Perspect. Plant Ecol. Evol. Syst. 2016, 20, 46–55. [Google Scholar] [CrossRef]
- Hargreaves, A.L.; Samis, K.E.; Eckert, C.G. Are Species’ Range Limits Simply Niche Limits Writ Large? A Review of Transplant Experiments beyond the Range. Am. Nat. 2014, 183, 157–173. [Google Scholar] [CrossRef]
- Barros, A.; Pickering, C.M. Non-Native Plant Invasion in Relation to Tourism Use of Aconcagua Park, Argentina, the Highest Protected Area in the Southern Hemisphere. Mt. Res. Dev. 2014, 34, 13–26. [Google Scholar] [CrossRef]
- Johnston, F.M.; Pickering, C.M. Alien Plants in the Australian Alps. Mt. Res. Dev. 2001, 21, 284–291. [Google Scholar] [CrossRef]
- Marini, L.; Gaston, K.J.; Prosser, F.; Hulme, P.E. Contrasting Response of Native and Alien Plant Species Richness to Environmental Energy and Human Impact along Alpine Elevation Gradients. Glob. Ecol. Biogeogr. 2009, 18, 652–661. [Google Scholar] [CrossRef]
- Paiaro, V.; Cabido, M.; Pucheta, E. Altitudinal Distribution of Native and Alien Plant Species in Roadside Communities from Central Argentina: Plant Species Distribution along Roadsides. Austral. Ecol. 2011, 36, 176–184. [Google Scholar] [CrossRef]
- Corcos, D.; Nascimbene, J.; Campesan, M.; Donadello, D.; Segat, V.; Marini, L. Establishment Dynamics of Native and Exotic Plants after Disturbance along Roadsides. Appl. Veg. Sci. 2020, 23, 277–284. [Google Scholar] [CrossRef]
- Kalwij, J.M.; Robertson, M.P.; van Rensburg, B.J. Human Activity Facilitates Altitudinal Expansion of Exotic Plants along a Road in Montane Grassland, South Africa. Appl. Veg. Sci. 2008, 11, 491–498. [Google Scholar] [CrossRef]
- von der Lippe, M.; Bullock, J.M.; Kowarik, I.; Knopp, T.; Wichmann, M. Human-Mediated Dispersal of Seeds by the Airflow of Vehicles. PLoS ONE 2013, 8, e52733. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Milbau, A.; Nijs, I. Alien Roadside Species More Easily Invade Alpine than Lowland Plant Communities in a Subarctic Mountain Ecosystem. PLoS ONE 2014, 9, e89664. [Google Scholar] [CrossRef]
- Van Der Ree, R.; Smith, D.J.; Grilo, C. The Ecological Effects of Linear Infrastructure and Traffic: Challenges and Opportunities of Rapid Global Growth. In Handbook of Road Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1-118-56816-8. [Google Scholar]
- Matteodo, M.; Wipf, S.; Stöckli, V.; Rixen, C.; Vittoz, P. Elevation Gradient of Successful Plant Traits for Colonizing Alpine Summits under Climate Change. Environ. Res. Lett. 2013, 8, 024043. [Google Scholar] [CrossRef]
- Siefert, A.; Lesser, M.R.; Fridley, J.D. How Do Climate and Dispersal Traits Limit Ranges of Tree Species along Latitudinal and Elevational Gradients?: Latitudinal and Elevational Range Limits. Glob. Ecol. Biogeogr. 2015, 24, 581–593. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V. Residence Time Determines the Distribution of Alien Plants. In Invasive Plants: Ecological and Agricultural Aspects; Inderjit, Ed.; Birkhäuser-Verlag: Basel, Switzerland, 2005; pp. 77–96. ISBN 978-3-7643-7137-1. [Google Scholar]
- Wilson, J.R.U.; Richardson, D.M.; Rouget, M.; Procheş, Ş.; Amis, M.A.; Henderson, L.; Thuiller, W. Residence Time and Potential Range: Crucial Considerations in Modelling Plant Invasions: Range Size of Invasive Plants. Divers. Distrib. 2007, 13, 11–22. [Google Scholar] [CrossRef]
- Fuentes, N.; Pauchard, A.; Sánchez, P.; Esquivel, J.; Marticorena, A. A New Comprehensive Database of Alien Plant Species in Chile Based on Herbarium Records. Biol. Invasions 2013, 15, 847–858. [Google Scholar] [CrossRef]
- Linder, H.P.; Lehmann, C.E.R.; Archibald, S.; Osborne, C.P.; Richardson, D.M. Global Grass (Poaceae) Success Underpinned by Traits Facilitating Colonization, Persistence and Habitat Transformation: Grass Success. Biol. Rev. 2018, 93, 1125–1144. [Google Scholar] [CrossRef] [Green Version]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A Climate Dynamics Perspective. Int. J. Clim. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Larson, C.D.; Pollnac, F.W.; Schmitz, K.; Rew, L.J. Climate Change and Micro-Topography Are Facilitating the Mountain Invasion by a Non-Native Perennial Plant Species. NeoBiota 2021, 65, 23. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Pauchard, A.; Lenoir, J.; Nuñez, M.A.; Geron, C.; Ven, A.; Bravo-Monasterio, P.; Teneb, E.; Nijs, I.; Milbau, A. Disturbance Is the Key to Plant Invasions in Cold Environments. Proc. Natl. Acad. Sci. USA 2016, 113, 14061–14066. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lillo, E.; Lembrechts, J.J.; Cavieres, L.A.; Jiménez, A.; Haider, S.; Barros, A.; Pauchard, A. Anthropogenic Factors Overrule Local Abiotic Variables in Determining Non-Native Plant Invasions in Mountains. Biol. Invasions 2021, 23, 3671–3686. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Deshaies, D.; Lieffers, V.J. Disturbance Facilitates Rapid Range Expansion of Aspen into Higher Elevations of the Rocky Mountains under a Warming Climate: Disturbance and Range Expansion of Aspen. J. Biogeogr. 2009, 37, 68–76. [Google Scholar] [CrossRef]
- Quiroz, C.L.; Cavieres, L.A.; Pauchard, A. Assessing the Importance of Disturbance, Site Conditions, and the Biotic Barrier for Dandelion Invasion in an Alpine Habitat. Biol. Invasions 2011, 13, 2889–2899. [Google Scholar] [CrossRef]
- Alexander, J.M.; Poll, M.; Dietz, H.; Edwards, P.J. Contrasting Patterns of Genetic Variation and Structure in Plant Invasions of Mountains. Divers. Distrib. 2009, 15, 502–512. [Google Scholar] [CrossRef]
- Taylor, D.R.; Keller, S.R. Historical Range Expansion Determines the Phylogenetic Diversity Introduced during Contemporary Species Invasion: Historical Effects on Invasion Genetics. Evolution 2007, 61, 334–345. [Google Scholar] [CrossRef]
- Badano, E.I.; Villarroel, E.; Bustamante, R.O.; Marquet, P.A.; Cavieres, L.A. Ecosystem Engineering Facilitates Invasions by Exotic Plants in High-Andean Ecosystems. J. Ecol. 2007, 95, 682–688. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the Relationship between Niche and Distribution. Ecol. Lett. 2000, 3, 349–361. [Google Scholar] [CrossRef]
- Holt, R.D.; Keitt, T. Alternative Causes for Range Limits: A Metapopulation Perspective. Ecol. Lett. 2000, 3, 41–47. [Google Scholar] [CrossRef]
- Pulliam, H.R. Sources, Sinks, and Population Regulation. Am. Nat. 1988, 132, 652–661. [Google Scholar] [CrossRef]
- Cavieres, L.; Peñaloza, A.; Kalin Arroyo, M. Altitudinal Vegetation Belts in the High-Andes of Central Chile (33°S). Rev. Chil. Hist. Nat. 2000, 73, 331–344. [Google Scholar] [CrossRef]
- Di Castri, F.; Hajek, E.R. Bioclimatología de Chile; Vicerrectoría Académica de la Universidad Católica de Chile: Santiago, Chile, 1976. [Google Scholar]
- Santibáñez, F.; Uribe, J. Atlas Agroclimático de La V Región y Región Metropolitana; Universidad de Chile: Santiago, Chile, 1990. [Google Scholar]
- Rozzi, R.; Molina, J.; Miranda, P. Mioroclima y Periodos de Floración En Laderas de Exposición Ecuatorial y Polar En Los Andes de Chile Central. Rev. Chil. Hist. Nat. 1989, 62, 75–84. [Google Scholar]
- Cavieres, L.; Arroyo, M. Bancos de semillas en Phacelia secunda J.F. Gmelin (Hydrophyllaceae ): Variación altitudinal en los Andes de Chile central (33°S). Rev. Chil. Hist. Nat. 1999, 72, 569–577. [Google Scholar]
- Haider, S.; Lembrechts, J.; McDougall, K.; Pauchard, A.; Alexander, J.M.; Barros, A.; Cavieres, L.; Rashid, I.; Rew, L.; Aleksanyan, A.; et al. Think Globally, Measure Locally: The MIREN Standardized Protocol for Monitoring Species Distributions along Elevation Gradients. Ecol. Evol. 2022, 12, e8590. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring Ecological Niche Overlap from Occurrence and Spatial Environmental Data: Measuring Niche Overlap. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Soft. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; d’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A. Ecospat: An R Package to Support Spatial Analyses and Modeling of Species Niches and Distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Jarvis, A.H.I.; Reuter, A.; Nelsol, A.; Guevara, E. Hole-Field Seamless SRTM Data. International Centre for Tropical Agriculture (CIAT). 2018. Available online: https://srtm.csi.cgiar.org/srtmdata/org (accessed on 3 August 2021).
- Huisman, J.; Olff, H.; Fresco, L.F.M. A Hierarchical Set of Models for Species Response Analysis. J. Veg. Sci. 1993, 4, 37–46. [Google Scholar] [CrossRef]
- Jansen, F.; Oksanen, J. How to Model Species Responses along Ecological Gradients—Huisman-Olff-Fresco Models Revisited. J. Veg. Sci. 2013, 24, 1108–1117. [Google Scholar] [CrossRef]
- Heegaard, E. The Outer Border and Central Border for Species–Environmental Relationships Estimated by Non-Parametric Generalised Additive Models. Ecol. Model. 2002, 157, 131–139. [Google Scholar] [CrossRef]
| Response Variable | Variable | Df | Sum Sq | Mean Sq | F Value | p-Value |
|---|---|---|---|---|---|---|
| (a) Observed UEL difference between years (2018–2008) | Residence time | 1 | 13 | 13 | 0.0002 | 0.9878 |
| Dispersal mode | 2 | 87,625 | 43,812 | 0.8483 | 0.4505 | |
| Life span | 2 | 77,297 | 38,648 | 0.7483 | 0.4925 | |
| Residuals | 13 | 671,395 | 51,646 | |||
| (b) Standardized difference between observed and predicted UEL | Residence time | 1 | 0.00522 | 0.0052192 | 0.2572 | 0.6162 |
| Dispersal mode | 2 | 0.01949 | 0.0097462 | 0.4802 | 0.6238 | |
| Life span | 2 | 0.00644 | 0.0032198 | 0.1586 | 0.8541 | |
| Residuals | 27 | 0.54799 | 0.0202959 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goncalves, E.; Herrera, I.; Alexander, J.; Duarte, M.; Cavieres, L.A.; Morales-Salinas, L.; Bustamante, R.O. The Upper Range Limit of Alien Plants Is Not in Equilibrium with Climate in the Andes of Central Chile. Plants 2022, 11, 2345. https://doi.org/10.3390/plants11182345
Goncalves E, Herrera I, Alexander J, Duarte M, Cavieres LA, Morales-Salinas L, Bustamante RO. The Upper Range Limit of Alien Plants Is Not in Equilibrium with Climate in the Andes of Central Chile. Plants. 2022; 11(18):2345. https://doi.org/10.3390/plants11182345
Chicago/Turabian StyleGoncalves, Estefany, Ileana Herrera, Jake Alexander, Milen Duarte, Lohengrin A. Cavieres, Luis Morales-Salinas, and Ramiro O. Bustamante. 2022. "The Upper Range Limit of Alien Plants Is Not in Equilibrium with Climate in the Andes of Central Chile" Plants 11, no. 18: 2345. https://doi.org/10.3390/plants11182345
APA StyleGoncalves, E., Herrera, I., Alexander, J., Duarte, M., Cavieres, L. A., Morales-Salinas, L., & Bustamante, R. O. (2022). The Upper Range Limit of Alien Plants Is Not in Equilibrium with Climate in the Andes of Central Chile. Plants, 11(18), 2345. https://doi.org/10.3390/plants11182345











