Glucosinolates and Omega-3 Fatty Acids from Mustard Seeds: Phytochemistry and Pharmacology
Abstract
1. Introduction
2. Major Bioactive Compounds in Mustard Seeds: Glucosinolates and Omega-3 Fatty Acids
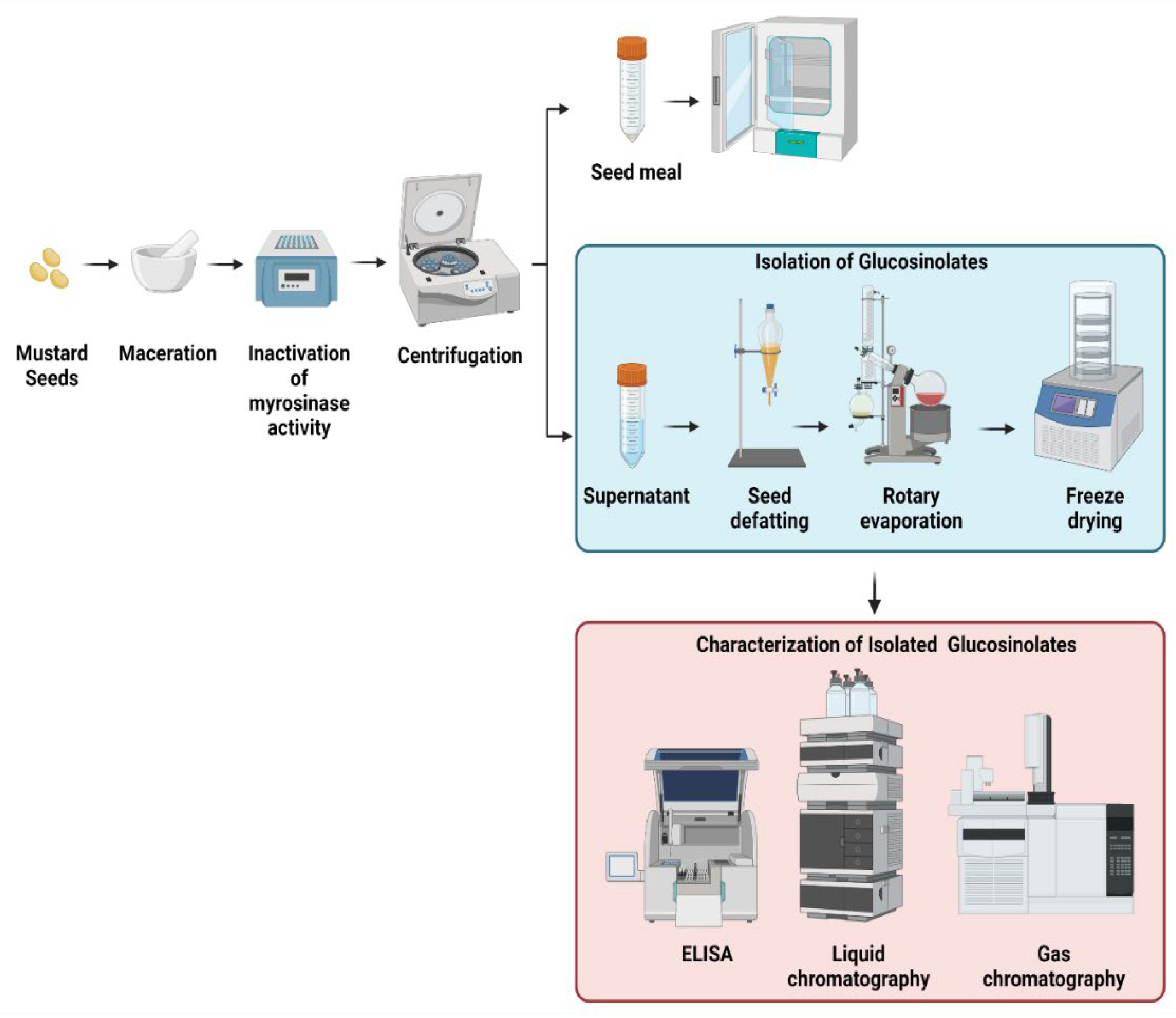
3. Major Extraction Procedures
4. Clinical Studies on Glucosinolates and Omega-3 Fatty Acids
4.1. Glucosinolates
4.2. Omega 3 Fatty Acids
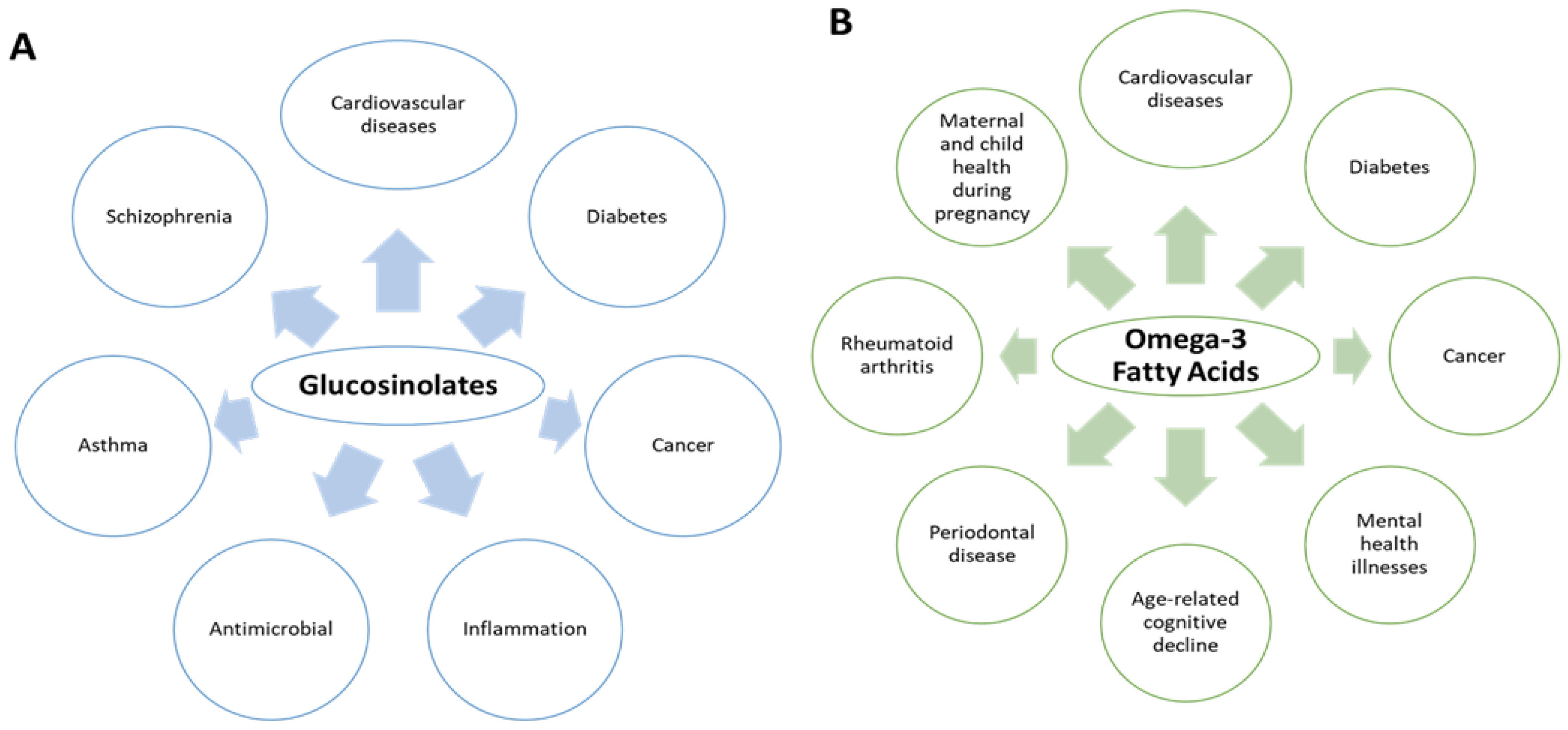
5. Pharmacological Potential of Glucosinolates and Omega-3 Fatty Acids
5.1. Glucosinolates
5.2. Omega-3 Fatty Acids
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae mustards: Traditional and agronomic uses in Australia and New Zealand. Molecules 2018, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, M.; Babu, K.N. Mustard. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 9–19. [Google Scholar]
- Melrose, J. The glucosinolates: A sulphur glucoside family of mustard anti-tumour and antimicrobial phytochemicals of potential therapeutic application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Ladak, Z.; Khairy, M.; Armstrong, E.A.; Yager, J.Y. Chapter 11-Glucosinolates: Paradoxically beneficial in fighting both brain cell death and cancer. In Nutraceuticals in Brain Health and Beyond; Ghosh, D., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 155–167. [Google Scholar]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 polyunsaturated fatty acids: Benefits and endpoints in sport. Nutrients 2019, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Grygier, A. Mustard seeds as a bioactive component of food. Food Rev. Int. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Chaddha, A.; Eagle, K.A. Omega-3 fatty acids and heart health. Circulation 2015, 132, e350–e352. [Google Scholar] [CrossRef]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.-Y. Human, animal and plant health benefits of glucosinolates and strategies for enhanced bioactivity: A systematic review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Zrybko, C.L.; Fukuda, E.K.; Rosen, R.T. Determination of glucosinolates in domestic and wild mustard by high-performance liquid chromatography with confirmation by electrospray mass spectrometry and photodiode-array detection. J. Chromatogr. A 1997, 767, 43–52. [Google Scholar] [CrossRef]
- Sharma, A.; Rai, P.; Prasad, S. GC–MS detection and determination of major volatile compounds in Brassica juncea L. leaves and seeds. Microchem. J. 2018, 138, 488–493. [Google Scholar] [CrossRef]
- Cools, K.; Terry, L.A. The effect of processing on the glucosinolate profile in mustard seed. Food Chem. 2018, 252, 343–348. [Google Scholar] [CrossRef]
- Rothlisberger, K.L.; Hons, F.M.; Gentry, T.J.; Senseman, S.A. Oilseed meal effects on the emergence and survival of crop and weed species. Appl. Environ. Soil Sci. 2012, 2012, 769357. [Google Scholar] [CrossRef][Green Version]
- Prchalová, J.; Kovařík, F.; Ševčík, R.; Čížková, H.; Rajchl, A. Characterization of mustard seeds and paste by DART ionization with time-of-flight mass spectrometry. J. Mass Spectrom. 2014, 49, 811–818. [Google Scholar] [CrossRef]
- Andini, S.; Dekker, P.; Gruppen, H.; Araya-Cloutier, C.; Vincken, J.-P. Modulation of glucosinolate composition in Brassicaceae seeds by germination and fungal elicitation. J. Agric. Food Chem. 2019, 67, 12770–12779. [Google Scholar] [CrossRef]
- Hebert, M.; Mhemdi, H.; Vorobiev, E. Selective and eco-friendly recovery of glucosinolates from mustard seeds (Brassica juncea) using process optimization and innovative pretreatment (high voltage electrical discharges). Food Bioprod. Process. 2020, 124, 11–23. [Google Scholar] [CrossRef]
- Chauhan, J.; Meena, S.; Singh, K.; Meena, M. Environmental effects on genetic parameters for oil and seed meal quality components of Indian mustard (Brassica juncea L.). Indian J. Genet. 2012, 72, 435–438. [Google Scholar]
- Cools, K.; Terry, L.A. Comparative study between extraction techniques and column separation for the quantification of sinigrin and total isothiocyanates in mustard seed. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 901, 115–118. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Budzyński, W.S.; Kijewski, Ł.; Zając, T. Biomass quality of Brassica oilseed crops in response to sulfur fertilization. Agron. J. 2015, 107, 1377–1391. [Google Scholar] [CrossRef]
- Main, M.; McCaffrey, J.; Morra, M. Insecticidal activity of Brassica juncea seed meal to the fungus gnat Bradysia impatiens Johannsen (Diptera:Sciaridae). J. Appl. Entomol. 2014, 138, 701–707. [Google Scholar] [CrossRef]
- Neubauer, C.; Hüntemann, K.; Heitmann, B.; Müller, C. Suppression of Verticillium dahliae by glucosinolate-containing seed meal amendments. Eur. J. Plant Pathol. 2015, 142, 239–249. [Google Scholar] [CrossRef]
- Borpatragohain, P.; Rose, T.J.; Liu, L.; Barkla, B.J.; Raymond, C.A.; King, G.J. Remobilization and fate of sulphur in mustard. Ann. Bot. 2019, 124, 471–480. [Google Scholar] [CrossRef]
- Popova, I.E.; Morra, M.J. Sinigrin and sinalbin quantification in mustard seed using high performance liquid chromatography–time-of-flight mass spectrometry. J. Food Compos. Anal. 2014, 35, 120–126. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Lilley, C.; Barker, A.; Ellis, S.; Wade, R.; Atkinson, H.; Urwin, P.; Redeker, K.; Hartley, S. Constant isothiocyanate-release potentials across biofumigant seeding rates. J. Agric. Food Chem. 2018, 66, 5108–5116. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.E.; Dubie, J.S.; Morra, M.J. Optimization of hydrolysis conditions for release of biopesticides from glucosinolates in Brassica juncea and Sinapis alba seed meal extracts. Ind. Crops Prod. 2017, 97, 354–359. [Google Scholar] [CrossRef]
- Popova, I.E.; Morra, M.J. Simultaneous quantification of sinigrin, sinalbin, and anionic glucosinolate hydrolysis products in Brassica juncea and Sinapis alba seed extracts using ion chromatography. J. Agric. Food Chem. 2014, 62, 10687–10693. [Google Scholar] [CrossRef]
- Borpatragohain, P.; Rose, T.J.; Liu, L.; Raymond, C.A.; Barkla, B.J.; King, G.J. Seed glucosinolate yield is maximized by higher rates of sulfur nutrition than required for seed yield in condiment mustard (Brassica juncea L.). PLoS ONE 2019, 14, e0213429. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Dhingra, O.D.; Lima, A.O.; Jham, G.N.; Berhow, M.A.; Holloway, R.K.; Vaughn, S.F. Glucosinolate content and nematicidal activity of Brazilian wild mustard tissues against Meloidogyne incognita in tomato. Plant Soil 2011, 341, 155–164. [Google Scholar] [CrossRef]
- Sukovata, L.; Jaworski, T.; Kolk, A. Efficacy of Brassica juncea granulated seed meal against Melolontha grubs. Ind. Crops Prod. 2015, 70, 260–265. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Separation of sinigrin from Indian mustard (Brassica juncea L.) seed using macroporous ion-exchange resin. Korean J. Chem. Eng. 2012, 29, 396–403. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Optimization of ultrasonic-stimulated solvent extraction of sinigrin from Indian mustard seed (Brassica Juncea L.) using response surface methodology. Phytochem. Anal. 2011, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wanasundara, J.P.; Abeysekara, S.J.; McIntosh, T.C.; Falk, K.C. Solubility differences of major storage proteins of Brassicaceae oilseeds. J. Am. Oil Chem. Soc. 2012, 89, 869–881. [Google Scholar] [CrossRef]
- Font, R.; Del Río, M.; Fernández-Martínez, J.M.; de Haro-Bailón, A. Use of near-infrared spectroscopy for screening the individual and total glucosinolate contents in Indian mustard seed (Brassica juncea L. Czern. & Coss.). J. Agric. Food Chem. 2004, 52, 3563–3569. [Google Scholar] [PubMed]
- Velíšek, J.; Mikulcova, R.; Mikova, K.; Woldie, K.; Link, J.; Davídek, J. Chemometric investigation of mustard seed. LWT-Food Sci. Technol. 1995, 28, 620–624. [Google Scholar] [CrossRef]
- Fabre, N.; Bon, M.; Moulis, C.; Fouraste, I.; Stanislas, E. Three glucosinolates from seeds of Brassica juncea. Phytochemistry 1997, 45, 525–527. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, S.; Sukhija, P.; Munshi, S. Accumulation of glucosinolates in developing mustard (Brassica juncea L.) seeds in response to sulphur application. Plant Sci. 1990, 66, 181–184. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Nicolas, M.E.; Bennett, R.N.; Premier, R.R.; Eagling, D.R.; Taylor, P.W. Determination of sinigrin and glucoraphanin in Brassica species using a simple extraction method combined with ion-pair HPLC analysis. Sci. Hortic. 2002, 96, 27–41. [Google Scholar] [CrossRef]
- Palmer, M.; Sang, J.; Oram, R.; Tran, D.; Salisbury, P. Variation in seed glucosinolate concentrations of Indian mustard (Brassica juncea (L.) Czern.+ Coss.). Aust. J. Exp. Agric. 1988, 28, 779–782. [Google Scholar] [CrossRef]
- Palmer, M.V.; Yeung, S.P.; Sang, J.P. Glucosinolate content of seedlings, tissue cultures, and regenerant plants of Brassica juncea (Indian mustard). J. Agric. Food Chem. 1987, 35, 262–265. [Google Scholar] [CrossRef]
- Szmigielska, A.M.; Schoenau, J.J. Use of anion-exchange membrane extraction for the high-performance liquid chromatographic analysis of mustard seed glucosinolates. J. Agric. Food Chem. 2000, 48, 5190–5194. [Google Scholar] [CrossRef]
- Velasco, L.; Becker, H.C. Variability for seed glucosinolates in a germplasm collection of the genus Brassica. Genet. Resour. Crop Evol. 2000, 47, 231–238. [Google Scholar] [CrossRef]
- Sodhi, Y.; Mukhopadhyay, A.; Arumugam, N.; Verma, J.; Gupta, V.; Pental, D.; Pradhan, A. Genetic analysis of total glucosinolate in crosses involving a high glucosinolate Indian variety and a low glucosinolate line of Brassica juncea. Plant Breed. 2002, 121, 508–511. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef]
- Ciska, E.; Honke, J.; Kozłowska, H. Effect of light conditions on the contents of glucosinolates in germinating seeds of white mustard, red radish, white radish, and rapeseed. J. Agric. Food Chem. 2008, 56, 9087–9093. [Google Scholar] [CrossRef]
- Lazzeri, L.; Tacconi, R.; Palmieri, S. In vitro activity of some glucosinolates and their reaction products toward a population of the nematode Heterodera schachtii. J. Agric. Food Chem. 1993, 41, 825–829. [Google Scholar] [CrossRef]
- Toribio, A.; Nuzillard, J.-M.; Renault, J.-H. Strong ion-exchange centrifugal partition chromatography as an efficient method for the large-scale purification of glucosinolates. J. Chromatogr. A 2007, 1170, 44–51. [Google Scholar] [CrossRef]
- Toribio, A.; Nuzillard, J.M.; Pinel, B.; Boudesocque, L.; Lafosse, M.; De La Poype, F.; Renault, J.H. Pilot-scale ion-exchange centrifugal partition chromatography: Purification of sinalbin from white mustard seeds. J. Sep. Sci. 2009, 32, 1801–1807. [Google Scholar] [CrossRef]
- Gohain, B.; Kumar, P.; Malhotra, B.; Augustine, R.; Pradhan, A.K.; Bisht, N.C. A comprehensive Vis-NIRS equation for rapid quantification of seed glucosinolate content and composition across diverse Brassica oilseed chemotypes. Food Chem. 2021, 354, 129527. [Google Scholar] [CrossRef]
- Xin, H.; Khan, N.A.; Falk, K.C.; Yu, P. Mid-infrared spectral characteristics of lipid molecular structures in Brassica carinata seeds: Relationship to oil content, fatty acid and glucosinolate profiles, polyphenols, and condensed tannins. J. Agric. Food Chem. 2014, 62, 7977–7988. [Google Scholar] [CrossRef]
- Matthäus, B.; Angelini, L.G. Anti-nutritive constituents in oilseed crops from Italy. Ind. Crops Prod. 2005, 21, 89–99. [Google Scholar] [CrossRef]
- Mnzava, N.; Olsson, K. Studies on tropical vegetables. Part 1: Seed amino, fatty acid and glucosinolate profile of ethiopian mustards (Brassica carinata Braun). Food Chem. 1990, 35, 229–235. [Google Scholar] [CrossRef]
- Montaut, S.; Blažević, I.; Ruščić, M.; Rollin, P. LC–MS profiling of glucosinolates in the seeds of Brassica elongata Ehrh., and of the two stenoendemic B. botteri Vis and B. cazzae Ginzb. & Teyber. Nat. Prod. Res. 2017, 31, 58–62. [Google Scholar]
- Horn, P.J.; Vaughan, J.G. Seed glucosinolates of fourteen wild Brassica species. Phytochemistry 1983, 22, 465–470. [Google Scholar] [CrossRef]
- Wu, G.; Truksa, M.; Datla, N.; Vrinten, P.; Bauer, J.; Zank, T.; Cirpus, P.; Heinz, E.; Qiu, X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005, 23, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Dungey, S.G.; Sang, J.P.; Rothnie, N.E.; Palmer, M.V.; Burke, D.G.; Knox, R.B.; Williams, E.G.; Hilliard, E.P.; Salisbury, P.A. Glucosinolates in the pollen of rapeseed and Indian mustard. Phytochemistry 1988, 27, 815–817. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Chauhan, J.; Bhadauria, V.; Singh, M.; Singh, K.; KUMAR, A. QualiSy characteristics and their interrelationships in Indian rapeseed-mustard (Brassiea sp.) varieties. Indian J. Agric. Sci. 2007, 77, 616–620. [Google Scholar]
- Singh, B.K.; Bala, M.; Rai, P.K. Fatty acid composition and seed meal characteristics of Brassica and allied genera. Natl. Acad. Sci. Lett. 2014, 37, 219–226. [Google Scholar] [CrossRef]
- Kim, D.W. The Effects of Auricular Acupressure Using Sinapsis alba on Female College Students’ Obesity Indices and Self-Efficacy; Dongeui University Press: Busan, Korea, 2011. [Google Scholar]
- Kang, M.; Kim, Y.K.; Shin, J.S.; Yeo, H.N. Effects of semen sinapsis albae acupressure on fatigue and sleep related chemotherapy in patients with breast cancer. J. Korean Clin. Nurs. Res. 2015, 21, 180–187. [Google Scholar]
- Iunes, D.H.; Chaves, É.D.C.L.; Moura, C.D.C.; Côrrea, B.; Carvalho, L.C.; Silva, A.M.; de Carvalho, E.C. Role of auriculotherapy in the treatment of temporomandibular disorders with anxiety in university students. Evid.-Based Complement. Altern. Med. 2015, 2015, 430143. [Google Scholar] [CrossRef]
- Cândido dos Reis, A.; Theodoro de Oliveira, T.; Larissa Vidal, C.; Cristina Borsatto, M.; da Costa Valente, M.L. Effect of auricular acupuncture on the reduction of symptoms related to sleep disorders, anxiety and temporomandibular disorder (TMD). Altern. Ther. Health Med. 2021, 27, 22–26. [Google Scholar]
- Goetz, K.; Hinz, A.; Steinhäuser, J.; von Rath, U. Use of mustard seed footbaths for respiratory tract infections: A pilot study. Evid.-Based Complement. Altern. Med. 2020, 2020, 5648560. [Google Scholar] [CrossRef]
- Tian, M.; Hanley, A.B.; Dodds, M.; Yaegaki, K. Chewing gum containing allyl isothiocyanate from mustard seed extract is effective in reducing volatile sulfur compounds responsible for oral malodor. Am. J. Dent. 2013, 26, 180–184. [Google Scholar]
- Lett, A.M.; Thondre, P.S.; Rosenthal, A.J. Yellow mustard bran attenuates glycaemic response of a semi-solid food in young healthy men. Int. J. Food Sci. Nutr. 2013, 64, 140–146. [Google Scholar] [CrossRef]
- Summers, A.; Visscher, M.O.; Khatry, S.K.; Sherchand, J.B.; LeClerq, S.C.; Katz, J.; Tielsch, J.M.; Mullany, L.C. Impact of sunflower seed oil versus mustard seed oil on skin barrier function in newborns: A community-based, cluster-randomized trial. BMC Pediatrics 2019, 19, 512. [Google Scholar] [CrossRef]
- Bork, C.S.; Venø, S.K.; Lundbye-Christensen, S.; Jakobsen, M.U.; Tjønneland, A.; Schmidt, E.B.; Overvad, K. Dietary intake of α-linolenic acid is not appreciably associated with risk of ischemic stroke among middle-aged Danish men and women. J. Nutr. 2018, 148, 952–958. [Google Scholar] [CrossRef]
- Hennebelle, M.; Courchesne-Loyer, A.; St-Pierre, V.; Vandenberghe, C.; Castellano, C.-A.; Fortier, M.; Tessier, D.; Cunnane, S.C. Preliminary evaluation of a differential effect of an α-linolenate-rich supplement on ketogenesis and plasma ω-3 fatty acids in young and older adults. Nutrition 2016, 32, 1211–1216. [Google Scholar] [CrossRef]
- Jovanovski, E.; Li, D.; Ho, H.V.T.; Djedovic, V.; Marques, A.d.C.R.; Shishtar, E.; Mejia, S.B.; Sievenpiper, J.L.; de Souza, R.J.; Duvnjak, L. The effect of alpha-linolenic acid on glycemic control in individuals with type 2 diabetes: A systematic review and meta-analysis of randomized controlled clinical trials. Medicine 2017, 96, e6531. [Google Scholar] [CrossRef]
- Bjornevik, K.; Myhr, K.-M.; Beiske, A.; Bjerve, K.S.; Holmøy, T.; Hovdal, H.; Midgard, R.; Riise, T.; Wergeland, S.; Torkildsen, Ø. α-Linolenic acid is associated with MRI activity in a prospective cohort of multiple sclerosis patients. Mult. Scler. J. 2019, 25, 987–993. [Google Scholar] [CrossRef]
- Burak, C.; Wolffram, S.; Zur, B.; Langguth, P.; Fimmers, R.; Alteheld, B.; Stehle, P.; Egert, S. Effect of alpha-linolenic acid in combination with the flavonol quercetin on markers of cardiovascular disease risk in healthy, non-obese adults: A randomized, double-blinded placebo-controlled crossover trial. Nutrition 2019, 58, 47–56. [Google Scholar] [CrossRef]
- Pieters, D.J.; Zock, P.L.; Fuchs, D.; Mensink, R.P. Effect of α-linolenic acid on 24-h ambulatory blood pressure in untreated high-normal and stage I hypertensive subjects. Br. J. Nutr. 2019, 121, 155–163. [Google Scholar] [CrossRef]
- Saito, S.; Mori, A.; Osaki, N.; Katsuragi, Y. Diacylglycerol enhances the effects of alpha-linolenic acid against visceral fat: A double-blind randomized controlled trial. Obesity 2017, 25, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Saito, S.; Yamanaka, N.; Suzuki, C.; Ono, T.; Osaki, N.; Katsuragi, Y. Alpha linolenic acid-enriched diacylglycerol consumption enhances dietary fat oxidation in healthy subjects: A randomized double-blind controlled trial. J. Oleo Sci. 2017, 66, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Baxheinrich, A.; Lee-Barkey, Y.H.; Tschoepe, D.; Stehle, P.; Stratmann, B.; Wahrburg, U. Effects of a hypoenergetic diet rich in α-linolenic acid on fatty acid composition of serum phospholipids in overweight and obese patients with metabolic syndrome. Nutrition 2018, 49, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Okunade, O.; Niranjan, K.; Ghawi, S.K.; Kuhnle, G.; Methven, L. Supplementation of the diet by exogenous myrosinase via mustard seeds to increase the bioavailability of sulforaphane in healthy human subjects after the consumption of cooked broccoli. Mol. Nutr. Food Res. 2018, 62, 1700980. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Engels, C.; Schieber, A.; Gänzle, M.G. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MS n and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Conaway, C.; Yang, Y.; Chung, F. Isothiocyanates as cancer chemopreventive agents: Their biological activities and metabolism in rodents and humans. Curr. Drug Metab. 2002, 3, 233–255. [Google Scholar] [CrossRef]
- Hecht, S.S. Inhibition of carcinogenesis by isothiocyanates. Drug Metab. Rev. 2000, 32, 395–411. [Google Scholar] [CrossRef]
- Jiao, D.; Smith, T.J.; Yang, C.S.; Pittman, B.; Desai, D.; Amin, S.; Chung, F.L. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis 1997, 18, 2143–2147. [Google Scholar] [CrossRef][Green Version]
- Morse, M.A.; Wang, C.-X.; Stoner, G.D.; Mandal, S.; Conran, P.B.; Amin, S.G.; Hecht, S.S.; Chung, F.-L. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989, 49, 549–553. [Google Scholar]
- Morse, M.A.; Eklind, K.I.; Hecht, S.S.; Jordan, K.G.; Choi, C.-I.; Desai, D.H.; Amin, S.G.; Chung, F.-L. Structure-activity relationships for inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone lung tumorigenesis by arylalkyl isothiocyanates in A/J mice. Cancer Res. 1991, 51, 1846–1850. [Google Scholar]
- Stoner, G.D.; Morrissey, D.T.; Heur, Y.-H.; Daniel, E.M.; Galati, A.J.; Wagner, S.A. Inhibitory effects of phenetyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 1991, 51, 2063–2068. [Google Scholar]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 131, 3027S–3033S. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Conaway, C.C.; Chiao, J.; Wang, C.-X.; Amin, S.; Whysner, J.; Dai, W.; Reinhardt, J.; Chung, F.-L. Inhibition of benzo (a) pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002, 62, 2–7. [Google Scholar]
- Rose, P.; Huang, Q.; Ong, C.N.; Whiteman, M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2005, 209, 105–113. [Google Scholar] [CrossRef]
- Kirlin, W.G.; Cai, J.; DeLong, M.J.; Patten, E.J.; Jones, D.P. Dietary compounds that induce cancer preventive phase 2 enzymes activate apoptosis at comparable doses in HT29 colon carcinoma cells. J. Nutr. 1999, 129, 1827–1835. [Google Scholar] [CrossRef][Green Version]
- Srivastava, S.K.; Singh, S.V. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis 2004, 25, 1701–1709. [Google Scholar] [CrossRef]
- Kuroiwa, Y.; Nishikawa, A.; Kitamura, Y.; Kanki, K.; Ishii, Y.; Umemura, T.; Hirose, M. Protective effects of benzyl isothiocyanate and sulforaphane but not resveratrol against initiation of pancreatic carcinogenesis in hamsters. Cancer Lett. 2006, 241, 275–280. [Google Scholar] [CrossRef]
- Bonnesen, C.; Eggleston, I.M.; Hayes, J.D. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001, 61, 6120–6130. [Google Scholar]
- Lui, V.W.; Wentzel, A.L.; Xiao, D.; Lew, K.L.; Singh, S.V.; Grandis, J.R. Requirement of a carbon spacer in benzyl isothiocyanate-mediated cytotoxicity and MAPK activation in head and neck squamous cell carcinoma. Carcinogenesis 2003, 24, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Manesh, C.; Kuttan, G. Effect of naturally occurring isothiocyanates in the inhibition of cyclophosphamide-induced urotoxicity. Phytomedicine 2005, 12, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Xiao, D.; Lew, K.L.; Hershberger, P.; Kokkinakis, D.M.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis 2003, 24, 1665–1670. [Google Scholar] [CrossRef]
- Xiao, D.; Srivastava, S.K.; Lew, K.L.; Zeng, Y.; Hershberger, P.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G 2/M arrest and inducing apoptosis. Carcinogenesis 2003, 24, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Thornalley, P.J. Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem. Pharmacol. 2000, 60, 221–231. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo [a] pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, I.; Skupinska, K.; Kasprzycka-Guttman, T. Sulforaphane and 2-oxohexyl isothiocyanate induce cell growth arrest and apoptosis in L-1210 leukemia and ME-18 melanoma cells. Oncol. Rep. 2003, 10, 2045–2050. [Google Scholar] [CrossRef]
- Kim, B.-R.; Hu, R.; Keum, Y.-S.; Hebbar, V.; Shen, G.; Nair, S.S.; Kong, A.T. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003, 63, 7520–7525. [Google Scholar]
- Singh, A.V.; Xiao, D.; Lew, K.L.; Dhir, R.; Singh, S.V. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis 2004, 25, 83–90. [Google Scholar] [CrossRef]
- Gingras, D.; Gendron, M.; Boivin, D.; Moghrabi, A.; Théorêt, Y.; Béliveau, R. Induction of medulloblastoma cell apoptosis by sulforaphane, a dietary anticarcinogen from Brassica vegetables. Cancer Lett. 2004, 203, 35–43. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Ahmed, T.; Chung, F.-L.; Conaway, C.; Chiao, J.-W. Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention. Int. J. Oncol. 2004, 24, 187–192. [Google Scholar] [CrossRef]
- Fimognari, C.; Nüsse, M.; Cesari, R.; Iori, R.; Cantelli-Forti, G.; Hrelia, P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis 2002, 23, 581–586. [Google Scholar] [CrossRef]
- Gamet-Payrastre, L.; Li, P.; Lumeau, S.; Cassar, G.; Dupont, M.-A.; Chevolleau, S.; Gasc, N.; Tulliez, J.; Tercé, F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000, 60, 1426–1433. [Google Scholar]
- Chung, F.-L.; Conaway, C.C.; Rao, C.; Reddy, B.S. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis 2000, 21, 2287–2291. [Google Scholar] [CrossRef]
- Zhang, Y.; Kensler, T.W.; Cho, C.-G.; Posner, G.H.; Talalay, P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef]
- Chiao, J.; Chung, F.; Krzeminski, J.; Amin, S.; Arshad, R.; Ahmed, T.; Conaway, C. Modulation of growth of human prostate cancer cells by the N-acetylcysteine conjugate of phenethyl isothiocyanate. Int. J. Oncol. 2000, 16, 1215–1224. [Google Scholar] [CrossRef]
- Xiao, D.; Singh, S.V. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002, 62, 3615–3619. [Google Scholar] [PubMed]
- Chen, Y.-R.; Han, J.; Kori, R.; Kong, A.-N.T.; Tan, T.-H. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J. Biol. Chem. 2002, 277, 39334–39342. [Google Scholar] [CrossRef]
- Nishikawa, A.; Morse, M.A.; Chung, F.-L. Inhibitory effects of 2-mercaptoethane sulfonate and 6-phenylhexyl isothiocyanate on urinary bladder tumorigenesis in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Cancer Lett. 2003, 193, 11–16. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kim, I.-W.; Hu, R.; Kong, A.-N.T. Modulatory properties of various natural chemopreventive agents on the activation of NF-κB signaling pathway. Pharm. Res. 2004, 21, 661–670. [Google Scholar] [CrossRef]
- Johnson, C.R.; Chun, J.; Bittman, R.; Jarvis, W.D. Intrinsic cytotoxicity and chemomodulatory actions of novel phenethylisothiocyanate sphingoid base derivatives in HL-60 human promyelocytic leukemia cells. J. Pharmacol. Exp. Ther. 2004, 309, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Thomson, S.J.; King, M.J.; Turnbull, C.I.; Midwinter, R.G.; Hampton, M.B. The chemopreventive agent phenethyl isothiocyanate sensitizes cells to Fas-mediated apoptosis. Carcinogenesis 2004, 25, 765–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, X.; Kassie, F.; Mersch-Sundermann, V. Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds. Mutat. Res./Rev. Mutat. Res. 2005, 589, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Satyan, K.; Swamy, N.; Dizon, D.S.; Singh, R.; Granai, C.O.; Brard, L. Phenethyl isothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: Role of caspase and MAPK activation. Gynecol. Oncol. 2006, 103, 261–270. [Google Scholar] [CrossRef]
- Rose, P.; Won, Y.K.; Ong, C.N.; Whiteman, M. β-Phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide 2005, 12, 237–243. [Google Scholar] [CrossRef]
- Singh, R.K.; Lange, T.S.; Kim, K.; Zou, Y.; Lieb, C.; Sholler, G.L.; Brard, L. Effect of indole ethyl isothiocyanates on proliferation, apoptosis, and MAPK signaling in neuroblastoma cell lines. Bioorg. Med. Chem. Lett. 2007, 17, 5846–5852. [Google Scholar] [CrossRef][Green Version]
- Yu, R.; Mandlekar, S.; Harvey, K.J.; Ucker, D.S.; Kong, A.T. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998, 58, 402–408. [Google Scholar]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Kan, S.F.; Wang, J.; Sun, G.X. Sulforaphane regulates apoptosis-and proliferation-related signaling pathways and synergizes with cisplatin to suppress human ovarian cancer. Int. J. Mol. Med. 2018, 42, 2447–2458. [Google Scholar] [CrossRef]
- Hać, A.; Brokowska, J.; Rintz, E.; Bartkowski, M.; Węgrzyn, G.; Herman-Antosiewicz, A. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur. J. Nutr. 2020, 59, 1421–1432. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef]
- Sita, G.; Hrelia, P.; Tarozzi, A.; Morroni, F. Isothiocyanates are promising compounds against oxidative stress, neuroinflammation and cell death that may benefit neurodegeneration in Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 1454. [Google Scholar] [CrossRef]
- Boyanapalli, S.S.; Paredes-Gonzalez, X.; Fuentes, F.; Zhang, C.; Guo, Y.; Pung, D.; Saw, C.L.L.; Kong, A.-N.T. Nrf2 knockout attenuates the anti-inflammatory effects of phenethyl isothiocyanate and curcumin. Chem. Res. Toxicol. 2014, 27, 2036–2043. [Google Scholar] [CrossRef]
- Wagner, A.E.; Sturm, C.; Piegholdt, S.; Wolf, I.M.; Esatbeyoglu, T.; De Nicola, G.R.; Iori, R.; Rimbach, G. Myrosinase-treated glucoerucin is a potent inducer of the Nrf2 target gene heme oxygenase 1—Studies in cultured HT-29 cells and mice. J. Nutr. Biochem. 2015, 26, 661–666. [Google Scholar] [CrossRef]
- Yehuda, H.; Soroka, Y.; Zlotkin-Frušić, M.; Gilhar, A.; Milner, Y.; Tamir, S. Isothiocyanates inhibit psoriasis-related proinflammatory factors in human skin. Inflamm. Res. 2012, 61, 735–742. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, K.W.; Park, J.H.Y. Erucin exerts anti-inflammatory properties in murine macrophages and mouse skin: Possible mediation through the inhibition of NFκB signaling. Int. J. Mol. Sci. 2013, 14, 20564–20577. [Google Scholar] [CrossRef]
- Kojima, M. Studies on the effects of isothiocyanates and their analogues on microorganisms. I. Effect of isothiocyanates on the oxygen uptake of yeasts. J. Ferment. Technol. 1971, 49, 740–746. [Google Scholar]
- Zsolnai, T. Antimicrobial effects of thiocyanates, isothiocyanates and potential isothiocyanate forming substances. Arzneimittel-Forschung 1971, 21, 121–127. [Google Scholar]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef]
- Pang, Y.-H.; Sheen, S.; Zhou, S.; Liu, L.; Yam, K.L. Antimicrobial effects of allyl isothiocyanate and modified atmosphere on Pseduomonas aeruginosa in fresh catfish fillet under abuse temperatures. J. Food Sci. 2013, 78, M555–M559. [Google Scholar] [CrossRef]
- Kyung, K.; Fleming, H. Antimicrobial activity-of sulfur compounds derived from cabbage. J. Food Prot. 1997, 60, 67–71. [Google Scholar] [CrossRef]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157: H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef]
- Conrad, A.; Biehler, D.; Nobis, T.; Richter, H.; Engels, I.; Biehler, K.; Frank, U. Broad spectrum antibacterial activity of a mixture of isothiocyanates from nasturtium (Tropaeoli majoris herba) and horseradish (Armoraciae rusticanae radix). Drug Res. 2013, 63, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Haristoy, X.; Angioi-Duprez, K.; Duprez, A.; Lozniewski, A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob. Agents Chemother. 2003, 47, 3982–3984. [Google Scholar] [CrossRef]
- Aires, A.; Mota, V.; Saavedra, M.; Rosa, E.; Bennett, R. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Song, L.; Morrison, J.J.; Botting, N.P.; Thornalley, P.J. Analysis of glucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC–MS/MS. Anal. Biochem. 2005, 347, 234–243. [Google Scholar] [CrossRef]
- Dhingra, O.; Costa, M.; Silva, G., Jr. Potential of allyl isothiocyanate to control Rhizoctonia solani seedling damping off and seedling blight in transplant production. J. Phytopathol. 2004, 152, 352–357. [Google Scholar] [CrossRef]
- Gamliel, A.; Stapleton, J. Characterization of antifungal volatile compounds evolved from solarized soil amended with cabbage residues. Phytopathology 1993, 83, 899–905. [Google Scholar] [CrossRef]
- Greenhalgh, J.; Mitchell, N. The involvement of flavour volatiles in the resistance to downy mildew of wild and cultivated forms of Brassica oleracea. N. Phytol. 1976, 77, 391–398. [Google Scholar] [CrossRef]
- Clemente, I.; Aznar, M.; Nerin, C. Effect of an active label based on benzyl isothiocyanate on the morphology and ochratoxins production of Aspergillus ochraceus. Food Res. Int. 2017, 101, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Wang, A.; Engledow, A.; Hollister, E.; Rothlisberger, K.; Matocha, J.; Zuberer, D.; Provin, T.; Hons, F.; Gentry, T. Inhibition of the germination and growth of Phymatotrichopsis omnivora (cotton root rot) by oilseed meals and isothiocyanates. Appl. Soil Ecol. 2011, 49, 68–75. [Google Scholar] [CrossRef]
- Mari, M.; Leoni, O.; Bernardi, R.; Neri, F.; Palmieri, S. Control of brown rot on stonefruit by synthetic and glucosinolate-derived isothiocyanates. Postharvest Biol. Technol. 2008, 47, 61–67. [Google Scholar] [CrossRef]
- Mari, M.; Leoni, O.; Iori, R.; Cembali, T. Antifungal vapour-phase activity of allyl-isothiocyanate against Penicillium expansum on pears. Plant Pathol. 2002, 51, 231–236. [Google Scholar] [CrossRef]
- Quiles, J.M.; Manyes, L.; Luciano, F.; Manes, J.; Meca, G. Influence of the antimicrobial compound allyl isothiocyanate against the Aspergillus parasiticus growth and its aflatoxins production in pizza crust. Food Chem. Toxicol. 2015, 83, 222–228. [Google Scholar] [CrossRef]
- Troncoso-Rojas, R.; Sánchez-Estrada, A.; Ruelas, C.; García, H.S.; Tiznado-Hernández, M. Effect of benzyl isothiocyanate on tomato fruit infection development by Alternaria alternata. J. Sci. Food Agric. 2005, 85, 1427–1434. [Google Scholar] [CrossRef]
- Ugolini, L.; Martini, C.; Lazzeri, L.; D’Avino, L.; Mari, M. Control of postharvest grey mould (Botrytis cinerea Per.: Fr.) on strawberries by glucosinolate-derived allyl-isothiocyanate treatments. Postharvest Biol. Technol. 2014, 90, 34–39. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Bi, Y.; Wang, T.; Dong, Y.; Yang, Q.; Zhang, T. 2-Phenylethyl isothiocyanate exerts antifungal activity against Alternaria alternata by affecting membrane integrity and mycotoxin production. Toxins 2020, 12, 124. [Google Scholar] [CrossRef]
- Choi, K.-D.; Kim, H.-Y.; Shin, I.-S. Antifungal activity of isothiocyanates extracted from horseradish (Armoracia rusticana) root against pathogenic dermal fungi. Food Sci. Biotechnol. 2017, 26, 847–852. [Google Scholar] [CrossRef]
- Yashodhara, B.; Umakanth, S.; Pappachan, J.; Bhat, S.; Kamath, R.; Choo, B. Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar] [CrossRef]
- Aucoin, M.; Cooley, K.; Knee, C.; Fritz, H.; Balneaves, L.G.; Breau, R.; Fergusson, D.; Skidmore, B.; Wong, R.; Seely, D. Fish-derived omega-3 fatty acids and prostate cancer: A systematic review. Integr. Cancer Ther. 2017, 16, 32–62. [Google Scholar] [CrossRef]
- Bozzatello, P.; Brignolo, E.; De Grandi, E.; Bellino, S. Supplementation with omega-3 fatty acids in psychiatric disorders: A review of literature data. J. Clin. Med. 2016, 5, 67. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The influence of omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 fatty acids and neurodegenerative diseases: New evidence in clinical trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef]
- De Lau, L.; Bornebroek, M.; Witteman, J.; Hofman, A.; Koudstaal, P.; Breteler, M. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam study. Neurology 2005, 64, 2040–2045. [Google Scholar] [CrossRef]
- Kalmijn, S.; Launer, L.J.; Ott, A.; Witteman, J.C.; Hofman, A.; Breteler, M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997, 42, 776–782. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; He, J.; Wang, L.; Tian, Z.; Wang, C. Protective effects of omega-3 fatty acids against Alzheimer’s disease in rat brain endothelial cells. Brain Behav. 2018, 8, e01037. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Stillwell, W.; Shaikh, S.R.; Zerouga, M.; Siddiqui, R.; Wassall, S.R. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod. Nutr. Dev. 2005, 45, 559–579. [Google Scholar] [CrossRef]
- Wassall, S.R.; Stillwell, W. Docosahexaenoic acid domains: The ultimate non-raft membrane domain. Chem. Phys. Lipids 2008, 153, 57–63. [Google Scholar] [CrossRef]
- Akbar, M.; Calderon, F.; Wen, Z.; Kim, H.-Y. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA 2005, 102, 10858–10863. [Google Scholar] [CrossRef]
- Garcia, M.C.; Ward, G.; Ma, Y.C.; Salem, N., Jr.; Kim, H.Y. Effect of docosahexaenoic acid on the synthesis of phosphatidylserine in rat brain microsomes and C6 glioma cells. J. Neurochem. 1998, 70, 24–30. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef]
- Cansev, M.; Wurtman, R. Chronic administration of docosahexaenoic acid or eicosapentaenoic acid, but not arachidonic acid, alone or in combination with uridine, increases brain phosphatide and synaptic protein levels in gerbils. Neuroscience 2007, 148, 421–431. [Google Scholar] [CrossRef]
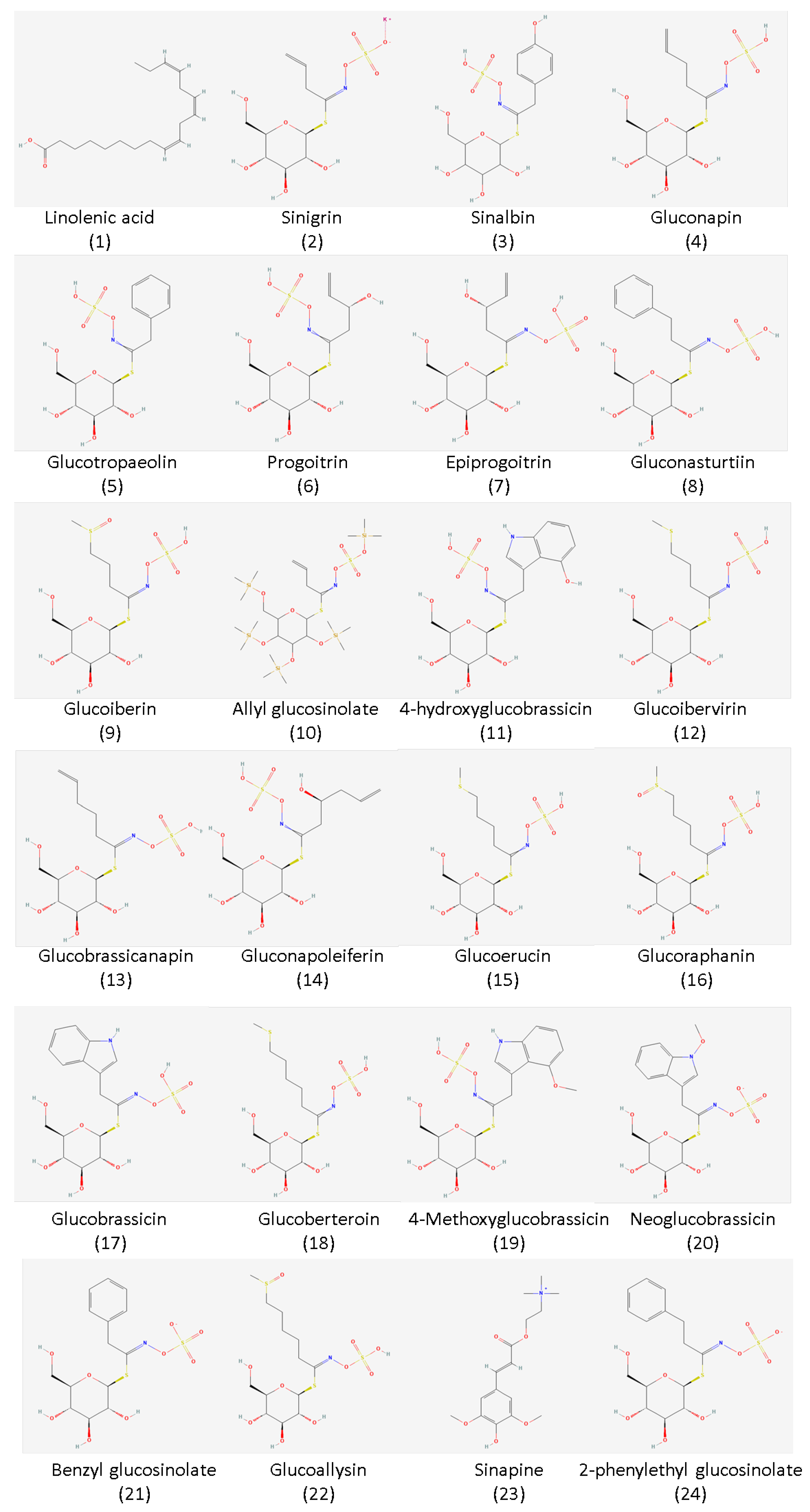
| Glucosinolate Compound * | Plant Material | Isolation Technique | Reference |
|---|---|---|---|
| Brassica juncea | |||
| (1) | Seed | GC-MS | [12] |
| (2) | Seed | HPLC | [13] |
| (2) | Seed meal | HPLC | [14] |
| (2) | Seed | HPLC DART-MS | [15] |
| (3) | Seed | RP-UHPLC-PDA-ESI-MSn | [16] |
| (2) (4) | Seed | Process optimization and innovative pretreatment (high voltage electrical discharges) | [17] |
| (1) | Seed meal | ELISA at 405 nm (tetrachloropalladate solution) | [18] |
| (2) | Seed | HPLC | [19] |
| (2) | Roots and stubble, straw, seed | HPLC | [20] |
| (2) | Seed meal | HPLC-MS | [21] |
| (2) | Seed meal | HPLC | [22] |
| (2); (4) | Seed | HPLC | [23] |
| (2) | Seed | HPLC-TOF-MS | [24] |
| (2) | Stem Leaves | HPLC | [25] |
| (2) | Seed Seed meal | HPLC/UV | [26] |
| (2) | Seed | HPLC/UV Ion chromatography HPLC/MS | [27] |
| (2); (4); (5); (6); (7); (8); (9) | Seed Stalk | HPLC-MS | [28] |
| (2) | Leaves Seed meal | HPLC HPLC-MS | [29] |
| (2) | Seed meal | HPLC | [30] |
| (2) | Seed | HPLC | [31] |
| (2) | Seed | HPLC | [32] |
| (10) | Seed meal | GC | [33] |
| (2); (4); (11) | Seed | Near-infrared spectroscopy | [34] |
| (2); (4); (6); (12) | Seed | GC | [35] |
| (3) | Seed | HPLC | [36] |
| (2); (4); (6); (13); (14) | Seed | GC | [37] |
| (2); (16) | Seed | Ion-pair HPLC | [38] |
| (2); (16) | Seed | HPLC | [39] |
| (1) | Seed Leaves | HPLC | [40] |
| (2) | Seed | HPLC | [41] |
| (2); (4); (6); (9); (11); (15) | Seed | NIRS HPLC | [42] |
| (2); (6) | Flowers, seed pods, seeds, leaves, stems, stalks, roots | HPLC | [11] |
| (2); (4); (13) | Seed | HPLC | [43] |
| Sinapis alba | |||
| (2) | Seed meal | HPLC | [14] |
| (3) | Seed | HPLC DART-MS | [15] |
| (3) | Seed | RP-UHPLC-PDA-ESI-MSn | [16] |
| (2); (4); (5); (7); (8); (9); (11); (16); (17); (18); (19); (20) | Seed | HPLC-PDA-ESI-MSn | [44] |
| (3) | Roots and stubble, straw, seed | HPLC | [20] |
| (2); (3) | Seed meal | HPLC | [22] |
| (3) | Seed | HPLC-TOF-MS | [24] |
| (3) | Seed Seed meal | HPLC/UV | [26] |
| (3) | Seed | HPLC/UV; Ion chromatography; HPLC/MS | [27] |
| (10); (21) | Seed meal | GC | [33] |
| (2); (4); (6); (12) | Seed | GC | [35] |
| (2); (3) | Seed | HPLC | [45] |
| (3) | Seed | HPLC | [46] |
| (3); (16) | Seed | Strong ion-exchange displacement centrifugal partition chromatography (SIX-CPC) HPLC | [47] |
| (2) | Seed | HPLC | [41] |
| (3) | Seed | Ion-exchange centrifugal partition chromatography | [48] |
| Brassica nigra | |||
| (2); (4); (8); (9); (11); (13); (16); (17); (19); (22) | Seed meal | HPLC | [49] |
| (2); (3) | Seed | HPLC DART-MS | [15] |
| (2); (4); (6); (12) | Seed | GC | [35] |
| (2); (16) | Seed | Ion-pair HPLC | [38] |
| (2); (4); (6); (9); (11); (15) | Seed | NIRS HPLC | [42] |
| Brassica carinata | |||
| (2); (4); (8); (11); (13); (16); (17); (19); (22) | Seed meal | HPLC | [49] |
| (2); (3) | Seed meal | HPLC | [22] |
| (4); (10) | Seed | Fourier transform infrared spectroscopy | [50] |
| (23) | Seed | HPLC | [51] |
| (2); (4); (6) | Seed | HPLC | [52] |
| (2) | Seed | HPLC | [46] |
| (2); (4); (6); (9); (11) | Seed | NIRS HPLC | [42] |
| Brassica elongata | |||
| (6) | Seed | LC-MS | [53] |
| (24) | Seed | GC | [54] |
| (2); (4); (6); (9); (11); (15) | Seed | NIRS HPLC | [42] |
| Brassica hirta | |||
| (2); (3); (6) | Flowers, seed pods, seeds, leaves, stems, stalks, roots | HPLC | [11] |
| Mustard Seed/Compound Source | Biological Activity | References |
|---|---|---|
| White mustard seed | Auriculotherapy Reduces body weight and body mass index | [60] |
| White mustard seed | Reduces fatigue Improves the physical and psychological condition | [61] |
| White mustard seed | Auriculotherapy Reduces anxiety and temporomandibular muscle contraction | [62,63] |
| Mustard seed powder | Improves respiratory tract infections | [64] |
| Mustard seed extract/Allyl isothiocyanate | Reduces volatile sulfur compound causing oral malodor | [65] |
| Yellow mustard bran | Reduces postprandial glycemic response | [66] |
| Mustard seed oil | Effect on the epidermal integrity | [67] |
| Mustard seed oil/α-Linolenic acid (ALA) | Association of ALA intake and ischemic stroke | [68] |
| ALA | Stimulates postprandial ketogenesis | [69] |
| ALA | No effect in fasting blood glucose and insulin and glycated hemoglobin | [70] |
| ALA | Reduces the severity of multiple sclerosis | [71] |
| ALA + quercetin | Decreases total cholesterol, LDL, apolipoprotein B | [72,73] |
| ALA-rich triacylglycerol (ALA-TAG) ALA-rich diacylglycerol (ALA-DAG) | Reduction in BMI and visceral fat with ALA-DAG | [74] |
| ALA-rich diacylglycerol (ALA-DAG) | Enhances fat utilization | [75] |
| ALA | Effect of ALA-rich diet on the fatty-acid composition of serum phospholipids in obese patients affected by metabolic syndrome | [76] |
| Sinapis alba (yellow mustard)/Glucoraphanin | Inhibits Salmonella and E. coli growth | [77] |
| Compounds | Cell Lines/In Vivo Models | Activity | Reference |
|---|---|---|---|
| Benzyl-ITCs | HT29 colon carcinoma cells | Apoptosis induction | [90] |
| BxPC-3 cells | Cell cycle arrest, apoptotic induction, inhibition of NF-κB | [91] | |
| Hamsters | Protection against pancreatic carcinogenesis initiation | [92] | |
| Caco-2 and LS-174 cells | Growth inhibition | [93] | |
| HNSCC head and neck squamous cell carcinoma cell line | Activation of PARP cleavage and caspase-3 | [94] | |
| Allyl-ITCs | Swiss albino mice | Inhibition of cyclophophamide-induced urotoxicity | [95] |
| PC-3 xenografts | Growth inhibition | [96] | |
| LNCaP cells | Apoptosis induction and growth inhibition by G2/M arrest | [97] | |
| Human myeloblastic leukemia-1 cells | Inhibition of HL60 (p53-) and (p53þ) | [98] | |
| 4-Methylsulfinylbutyl-ITCs | Hamsters | Protective activity against pancreatic carcinogenesis initiation | [92] |
| MDA-MB-231 cells | Growth inhibition | [89] | |
| Mice | Benzo(a)pyrene-induced forestomach cancer inhibition | [99] | |
| L-1210 and ME-18 cells | Growth inhibition and induction of apoptosis | [100] | |
| HepG2 cells | Growth inhibition | [101] | |
| PC-3 cells | Caspases-mediated apoptosis | [102] | |
| Medulloblastoma cells | Caspases-mediated apoptosis | [103] | |
| DU-145 cells | Growth inhibition | [104] | |
| LNCaP cells | Growth inhibition | [93] | |
| Human T-cell leukemia | Induction of apoptosis and cell cycle arrest | [105] | |
| HT29 cells | Growth inhibition | [106] | |
| F344 rats | Azoxymethane-induced colonic crypt foci inhibition | [107] | |
| Phenylethyl-ITCs | F344 rats | Tumorigenicity and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct inhibition | [84] |
| Rats | Azoxymethane-induced colonic crypt foci inhibition | [108] | |
| DU-145 and LNCaP cells | Enhancement of p21 protein and G0–G1 arrest | [109] | |
| F344 rats | Azoxymethane-induced colonic crypt foci inhibition | [107] | |
| p53-deficient PC-3 cells | Apoptosis induction | [110] | |
| LNCaP cells | Apoptosis induction | [111] | |
| Rats | Urinary bladder tumorigenesis inhibition | [112] | |
| HT29 cells | Caspase-3 activation and Inhibition of NF-κB activity | [113] | |
| HL60 cells | Protein kinase C inhibition | [114] | |
| Leukemia and human bladder carcinoma cells | Growth inhibition | [115] | |
| Rats | 4-(Methylnitrosamino)-1(3-pyridyl)-1-butone-induced pulmonary neoplasia | [116] | |
| Ovarian cancer cells | Apoptosis induction | [117] | |
| 7-Methylsulfinylheptyl-ITCs | MDA-MB-231 cells | Suppression of activity | [118] |
| Indole ethyl-ITCs | SH-S454, SMS-KCNR, SK-N-SH, IMR-32 cells | Anti-proliferative and apoptotic effects | [119] |
| Phenylmethyl-ITCs | HeLa cells | Caspase-3 activation | [120] |
| Phenyl-ITCs | Swiss albino mice | Cyclophophamide-induced urotoxicity inhibition | [95] |
| Phenylbenzyl-ITCs | HeLa cells | Caspase-3 activation | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, G.; Tantengco, O.A.G.; Tundis, R.; Robles, J.A.H.; Loizzo, M.R.; Shin, H.S.; Patra, J.K. Glucosinolates and Omega-3 Fatty Acids from Mustard Seeds: Phytochemistry and Pharmacology. Plants 2022, 11, 2290. https://doi.org/10.3390/plants11172290
Das G, Tantengco OAG, Tundis R, Robles JAH, Loizzo MR, Shin HS, Patra JK. Glucosinolates and Omega-3 Fatty Acids from Mustard Seeds: Phytochemistry and Pharmacology. Plants. 2022; 11(17):2290. https://doi.org/10.3390/plants11172290
Chicago/Turabian StyleDas, Gitishree, Ourlad Alzeus G. Tantengco, Rosa Tundis, Joyce Ann H. Robles, Monica Rosa Loizzo, Han Seung Shin, and Jayanta Kumar Patra. 2022. "Glucosinolates and Omega-3 Fatty Acids from Mustard Seeds: Phytochemistry and Pharmacology" Plants 11, no. 17: 2290. https://doi.org/10.3390/plants11172290
APA StyleDas, G., Tantengco, O. A. G., Tundis, R., Robles, J. A. H., Loizzo, M. R., Shin, H. S., & Patra, J. K. (2022). Glucosinolates and Omega-3 Fatty Acids from Mustard Seeds: Phytochemistry and Pharmacology. Plants, 11(17), 2290. https://doi.org/10.3390/plants11172290














