Abstract
Seeds from mustard (genera Brassica spp. and Sinapsis spp.), are known as a rich source of glucosinolates and omega-3 fatty acids. These compounds are widely known for their health benefits that include reducing inflammation and lowering the risk of cardiovascular diseases and cancer. This review presented a synthesis of published literature from Google Scholar, PubMed, Scopus, Sci Finder, and Web of Science regarding the different glucosinolates and omega-3 fatty acids isolated from mustard seeds. We presented an overview of extraction, isolation, purification, and structure elucidation of glucosinolates from the seeds of mustard plants. Moreover, we presented a compilation of in vitro, in vivo, and clinical studies showing the potential health benefits of glucosinolates and omega-3 fatty acids. Previous studies showed that glucosinolates have antimicrobial, antipain, and anticancer properties while omega-3 fatty acids are useful for their pharmacologic effects against sleep disorders, anxiety, cerebrovascular disease, neurodegenerative disease, hypercholesterolemia, and diabetes. Further studies are needed to investigate other naturally occurring glucosinolates and omega-3 fatty acids, improve and standardize the extraction and isolation methods from mustard seeds, and obtain more clinical evidence on the pharmacological applications of glucosinolates and omega-3 fatty acids from mustard seeds.
1. Introduction
Mustard belongs to the family Brassicaceae and is valued for its spicy and pungent dried seeds. Some of the well-known species of mustard include black mustard, Brassica nigra (L.) W. D. J. Koch, brown mustard, Brassica juncea (L.) Czerniak, Brassica rugosa Hort., Sinapis juncea L., white mustard, and Brassica hirta Moench [1]. The mustards grow best in sandy loam soils with limited rainfall. It is usually cultivated under temperate climates, but it can also be grown in tropical and subtropical regions. It is considered as one of the first domesticated crops and is commonly grown in Asia, North Africa, and Europe [2]. Mustard plants are commonly used in the food industry. White mustard is commonly used as food flavoring while black and brown mustards are generally used for their aroma. Some mustard plants such as B. alba and B. juncea are also used by traditional healers as herbal medicine to treat arthritis, colds, cough, sore throat, muscle pain, and diabetes [1].
Mustard seeds contain several bioactive compounds which include glucosinolates (GSLs) and omega-3 fatty acids [3]. GSLs are composed of three compartments: β-thioglucose, thiohydroximate-O-sulfonate, and a variable aglycone side chain derived from an α-amino acid [4]. On the other hand, omega-3 fatty acids are PUFAs that contain more than one carbon–carbon double bond in their backbone. They are widely known for their health benefits that include reducing inflammation and lowering the risk of heart diseases and cancer [5]. Additionally, consumption of glucosinolates by humans causes a positive effect on the body and have anticarcinogenic properties including contribution to the bioactive nature of the oil obtained from the mustard seeds [6]. Although these two compounds are unrelated, their presence in the mustard seeds and oils is beneficial to humans. Figure 1 summarizes the known health benefits of these compounds in humans [7,8,9,10]. This paper is an extensive review of the different GSLs and omega-3 fatty acids that can be found in mustard seeds. The review discusses the major extraction procedures to isolate these compounds and the different pharmacological applications along with the mechanism of action. Lastly, ongoing clinical studies using GSLs and omega-3 fatty acids from mustard seeds are also described in this review. All collected data have been obtained from different databases such as Google Scholar, PubMed, Scopus, Sci Finder, and Web of Science.
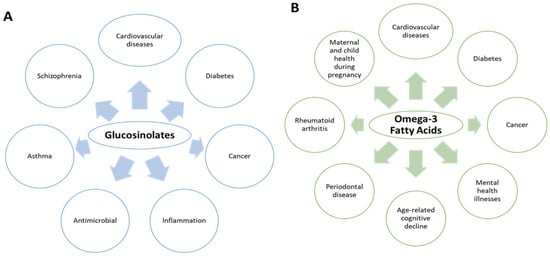
Figure 1.
Known human health benefits of glucosinolates (A) and omega-3 fatty acids (B).
2. Major Bioactive Compounds in Mustard Seeds: Glucosinolates and Omega-3 Fatty Acids
Based on the reviewed studies, several glucosinolate compounds are already isolated from mustard seeds. These include gluconapin, glucoraphanin, glucobrassicin, sinigrin, and sinalbin, to name a few (Figure 2). The major glucosinolates extracted from mustard seeds are sinigrin and sinalbin. As seen in Table 1, sinigrin is particularly abundant in Brassica juncea, while sinalbin is the chief glucosinolate in Sinapis alba. Sinigrin is responsible for the pungent taste of mustard once it is degraded by myrosinase, while sinalbin has a weaker pungent taste. The level of glucosinolate compound extracted depends on the plant part utilized. Based on the studies reviewed, the seed produces an ample amount of glucosinolate compared to other parts such as the leaves, stalk, and flower [11]. Moreover, some studies tried to investigate the different combinations of mustard species through genetic modification to increase the amount of glucosinolate content, which is not included in this review.
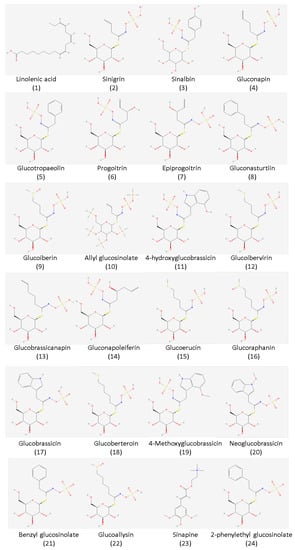
Figure 2.
Chemical structure of the most common GSLs isolated from the seeds of mustard plants. The chemical structures were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/; accessed on 20 August 2022).

Table 1.
List of major glucosinolates and omega-3 fatty acids in each mustard seed species and its separation technique.
Aside from GSLs, omega-3 fatty acids are also present in the seeds of mustard species (Table 1). Several studies isolated linolenic acid in a significant amount (16.05% of the total fatty acids) from the seeds of B. juncea [12,18]. Another study also reported the transgenic production of eicosapentaenoic acid (EPA) in B. juncea seed. EPA levels were up to 15% of total seed fatty acids [55]. These studies show that mustard seeds can potentially be tapped as natural sources of GSLs and omega-3 fatty acids. These plants can also be genetically engineered to increase their natural production of the compounds, thus providing a wide array of biological applications.
3. Major Extraction Procedures
In the extraction process of the different mustard seeds, a general procedure is observed (Figure 3). Samples from different mustard species are collected. Some studies utilized either the leaves, stalks, seedpods, or the seed of mustard to determine the glucosinolate content and to determine the fatty acid profile [11,12,20,28,29,40,56]. Generally, the samples are ground and crushed using a mortar and pestle. In some instances, maceration of the seeds is carried out with liquid nitrogen to prevent the conversion of glucosinolate into isothiocyanate. The extraction procedure is usually performed in triplicates by adding a heated or boiling polar solvent, either aqueous methanol or water. This is to inactivate the myrosinase activity. It is because myrosinase is an enzyme that catalyzes the conversion of glucosinolate into isothiocyanate [12,13]. Afterward, the extracted sample is transferred into a conical tube which is then vortexed, centrifuged, and filtered. The supernatant and the seed meal are separated. To further remove the excess solvent, extracts either undergo a rotary evaporator to remove excess methanol or lyophilized to remove excess water. Some studies used seed defatting before analysis of glucosinolate. Seed defatting is done to remove oil and other lipids in the seed. Diethyl ether or petroleum ether is usually used by employing a Soxhlet apparatus. Afterward, an aliquot of the extracts is used for the analysis of GSLs using HPLC, GC-MS, or ELISA via tetrachloropalladate solution, while GC-MS is mainly employed for omega-3 fatty acid profile.
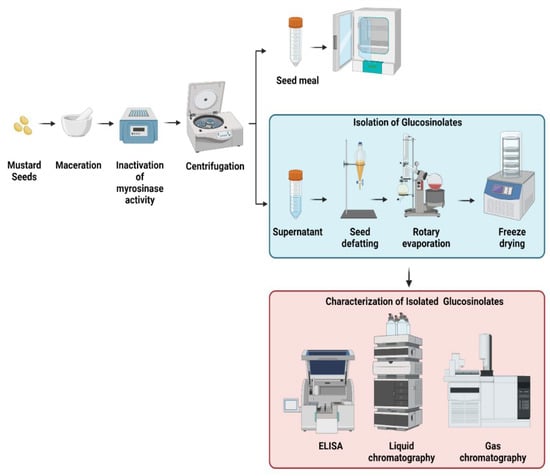
Figure 3.
Overview of the extraction, isolation, purification, and structure elucidation of glucosinolates from the seeds of mustard plants. Created with BioRender.com.
High-performance liquid chromatography (HPLC) is the standard procedure to separate and quantify glucosinolate in mustard seed [13]. In doing this, an aliquot of the sample is used and is loaded onto a DEAE Sephadex column. Afterward, it is separated using a reversed-phase C-18 column and detected using a photodiode array detector. GSLs are identified and quantified by their characteristics of being aliphatic, benzenic, and indolic [14].
On the other hand, a coupled GC with mass spectrophotometry (GC-MS) is also used in identifying the glucosinolate and omega-3 fatty acid content of the mustard seed [11]. It is because it is suitable for qualitative and quantitative analysis of volatile and semivolatile compounds [12]. It is performed by using a gas chromatograph device and mass spectrometer under a programmed setting. The spectrum of the unknown component is compared to the standard [12,33,35,54,57]. Lastly, enzyme-linked immunosorbent assay (ELISA) is also used to detect glucosinolate, which uses tetrachloropalladate solution. The total glucosinolate is estimated via the complexes that are formed between glucosinolate and the tetrachloropalladate solution. The change in the color produced is measured by the ELISA reader [18,58,59]. Currently, research advances have evolved. Different studies are discovering more ways to determine and to characterize glucosinolate and its fatty acid component in a faster and more efficient way. These include the use of near-infrared spectroscopy.
4. Clinical Studies on Glucosinolates and Omega-3 Fatty Acids
4.1. Glucosinolates
Although mustard was traditionally used in the medicine of Asian countries, only a few studies were conducted in humans to confirm the bioactivities of its seed and its main compounds. To recognize the beneficial effects of mustard seed extracts and oil action, MEDLINE® and Cochrane Collaboration Central Register of Clinical Trials databases were searched; a summary of the health benefits of GSLs and omega-3 fatty acids is presented in Table 2.

Table 2.
Importance of glucosinolates and omega-3 fatty acids with respect to health benefits.
Auriculotherapy is traditionally used in Chinese traditional medicine to treat several diseases. Kim [60] evaluated the effects of the application of white mustard seed for 4 weeks, three times a day on auricular acupressure points on the obesity index in female college students. A reduction in body weight and body mass index (BMI) was observed in all participants. Successively, Kang et al. [61] evaluated the application of white mustard seed on Meridian points on sleep and fatigue in patients undergoing chemotherapy for breast neoplasms. Results of the observational study evidenced that mustard seed application was able to reduce the level of fatigue and improve the physical and psychological conditions of participants. The positive effect of auriculotherapy with mustard seed application was confirmed by Iunes et al. [62]. Forty-four students with temporomandibular disorders and high levels of anxiety were enrolled. The subjects were divided into two groups: an auriculotherapy group and a sham group. The mustard seeds were applied to the sympathetic, brain stem, shenmen, rim, and temporomandibular points. Auriculotherapy associated with mustard seed application significantly reduced the status of anxiety and a decrease in tender points in the submandibular and mandibular regions. A reduction in temporal muscle contraction was also observed.
More recently, Cândido Dos Reis et al. [63] utilized a similar protocol to evaluate the effect on sleep disorders, anxiety, and the painful symptomatology of temporomandibular disorders. Patients between the ages of 20 and 45 years were enrolled and subjected to the treatment once a week for 8 weeks. A statistically significant reduction in sleep disorder symptoms was observed after the intervention. However, no significant difference was observed for painful temporomandibular disorders and anxiety symptoms.
Goetz et al. [64] reported the effect of mustard seed powder as a possible strategy to improve symptoms of respiratory tract infections. One hundred three participants were enrolled. The treatment consisted of footbaths with powdered mustard seeds once a day for six days. The “Herdecke Warmth Perception” (HeWEF) questionnaire was used to measure the effect of the treatment. Participants in the intervention group showed improvement of “sensation of cold”, “exhilaration,” “unwellness”, and “devotion”.
Previously, Tian et al. [65] studied the effect of chewing gum with allyl isothiocyanate, a constituent of mustard seed extract, alone and in association with zinc salts on the decrease in oral malodor. Fifteen subjects (aged 20 to 50 years) were asked to chew the trial gum for 12 minutes and the results were compared to a placebo gum. The GC analysis of their breath showed that chewing gum containing allyl isothiocyanate + zinc salts decreased the amount of volatile sulfur compounds (−89% at 1 hour after chewing ended).
Lett et al. [66] assessed the effects on glycaemic response and satiety of patients after the addition of yellow mustard bran in a potato and leek soup. In this randomized study, 10 healthy, moderately active, and nonsmoking male subjects were recruited. Results revealed the reduction in post-prandial glycaemic response after the addition of yellow mustard bran (5 g) to a soup.
The research on mustard seed oil clinical study evidenced how just one study was completed and its results are published. Summers et al. [67] reported results of a randomized controlled trial that included 500 neonates assigned to full body massage with mustard seed oil. Neonates’ skin integrity was measured over 28 days for parameters including dryness, erythema, rash, pH, stratum corneum cohesion/protein concentration, and trans epidermal water loss. Decreased skin pH was observed in the first week of life. Dryness, erythema, and rash increased during days 1–14 and then decreased by day 28. The trans epidermal water loss increased over time. The gestational age did not modify the effects of the mustard oil.
4.2. Omega 3 Fatty Acids
α-Linolenic acid (ALA) is the main abundant fatty acid of mustard seed oil. Bork et al. [68] studied associations between ALA dietary consumption and the risk of developing ischemic stroke. This Danish Diet, Cancer, and Health study involved 57,053 subjects whose ALA intake was analyzed by using a validated semiquantitative food frequency questionnaire. A total of 1859 ischemic strokes were recorded in four years of observation; however, multivariable analyses did not reveal any type of association between ALA intake and the incidence of ischemic stroke regardless of stroke subtypes.
Previously, Hennebelle et al. [69] examined the effects of an ALA-rich supplement on plasma long-chain n-3 polyunsaturated fatty acid PUFAs and ketogenic response. Results evidenced that the supplement slightly stimulated post-prandial ketogenesis. The effect of ALA on diabetes type 2 (T2DM) patients was assessed by several clinical studies; however, Jovanoski et al. [70] who conducted a systematic review and meta-analysis, concluded that diets rich in ALA did not influence parameters altered in T2DM, such as fasting blood glucose and insulin and glycated hemoglobin.
Recently, Bjornevik et al. [71] investigated the association between ALA levels and severity of multiple sclerosis (MS) in 87 patients. Results showed that ALA supplementation is a good strategy to counteract the severity of this disease. Recently, Burak et al. [72] investigated the combined effect of ALA (3.6 g/day) and quercetin (190 mg/day) administration for 8 weeks on antioxidant status, blood pressure, lipid and glucose metabolism, and biomarkers of inflammation in healthy patients. At the end of the study, data from 67 individuals with a mean age of 24.6 years were recorded. The association ALA + quercetin reduced total cholesterol, apolipoprotein B, and low-density lipoprotein cholesterol by a statistically considerable amount. However, no significant evidence was found on markers of cardiovascular disease risk, including the effect on blood pressure. This evidence was successively confirmed by Pieters et al. [73].
Some clinical studies investigated the effect of ALA in obese patients. Saito et al. [74] assessed the effects of ALA-rich triacylglycerol (ALA-TAG) and ALA-rich diacylglycerol (ALA-DAG) diet on the visceral fat area in obese patients. One hundred patients, divided into two groups, were invited to consume for twenty weeks 2.5 g/day ALA-DAG or ALA-TAG. At the end of the observational period, the BMI and visceral fat area were suggestively reduced by the ALA-DAG treatment. Moreover, ALA-DAG remarkably decreased the baseline of the fasting TAG serum concentration.
The effect of the association ALA-DAG on dietary fat oxidation in comparison with control TAG alone was assessed by Ando et al. [75]. In this intervention trial, 16 subjects were invited to consume either 2.5 g/day ALA-DAG or TAG for 14 days, separated by a 21-day washout period. Additionally, in this case, it was possible to show that ALA-DAG treatment significantly enhanced fat utilization. Successively, Egert et al. [76] analyzed and compared the effect of an energy-restricted diet on fatty acids composition of serum phospholipids in patients with metabolic syndrome. For this purpose, 81 obese or overweight patients with features of metabolic syndrome were enrolled. At the end of 26 weeks of treatment, the authors highlighted that the participants treated with a low-calorie diet high in ALA did not show alterations in the picture of serum phospholipids and did not show an increase that led to higher concentration of eicosapentaenoic acid.
GSLs are ingested in an inactive form and successively, when vegetables are cut or chewed, are converted into some degradation products such as thiocyanates, isothiocyanates, etc., by the enzyme myrosinase. They are reported to be present generally in the Brassicaceae family [78]. These compounds are particularly abundant in yellow (Sinapis alba) and Indian or brown (Brassica juncea) mustard seeds, although with qualitative and quantitative differences [17]. In fact, sinigrin, glucoiberin, epiprogoitrin, gluconapin, gluconasturtiin, and gluconeobrassicin are the main abundant compounds in B. juncea, whereas sinalbin and glucoraphanin are found in S. alba. After several epidemiological studies, it is possible to assert that the consumption of cruciferous-rich diets leads a series of beneficial effects on human health. These effects are attributed to GSLs and their breakdown products, isothiocyanates. Among them, sulforaphane, derived by the hydrolysis product of glucoraphanin, has been reported to have several beneficial effects.
Recently, researchers discovered that mustard seeds contain a more resistant form of myrosinase, which is why adding mustard to broccoli increases the formation of sulforaphane. This compound can inhibit Salmonella and E. coli growth in the small intestine. To demonstrate the synergy between broccoli and mustard, Okunade et al. [77] measured the urinary concentration of sulforaphane N-acetyl-l-cysteine, a metabolite of sulforaphane in twelve healthy adults after ingesting cooked broccoli (200 g), with and without brown mustard powder (1 g). The results showed the addition of mustard increases the bioavailability of sulforaphane by more than four times as the N-acetyl-l-cysteine sulforaphane excreted was 9.8 versus 44.7 μmol per g of creatinine for participants who consumed cooked broccoli alone and in association with powdered mustard seeds, respectively.
5. Pharmacological Potential of Glucosinolates and Omega-3 Fatty Acids
Mustard seeds are characterized by the presence of secondary metabolites [79,80] mainly including phenolic compounds, GSLs, and omega-3 PUFAs that have attracted the attention of numerous researchers. Herein, we report their pharmacological potential highlighting the mechanisms of action.
5.1. Glucosinolates
GSLs are converted into several products of degradation including thiocyanates and isothiocyanates (ITCs). These molecules are demonstrated to possess different biological properties including protection against pathogens and anticarcinogenic effects by their ability to inhibit the formation of exogenous or endogenous carcinogens. As reported in Table 3, in vivo studies showed that several GSLs hydrolysis products, in particular ITCs, have cytotoxic activity against different cancer cells and protective properties against chemical-carcinogen-induced cancer [81,82,83,84,85,86,87,88]. Rose et al. [89] investigated the ability of 4-methysulfinylbutyl and 7-methylsulphinylheptyl ITCs, extracted from Rorippa nasturtium-aquaticum and Brassica oleracea, to suppress in vitro the potential invasivity of the MDA-MB-231 tumor cell line and to inhibit metalloproteinase 9.

Table 3.
Antiproliferative activity of GSLs hydrolyzed compounds (isothiocyanates, ITCs).
Several studies showed that sulforaphane (SFN) is one of the most promising anticancer agents. SFN inhibited PC-3 (human prostate) cancer cells proliferation by inducing apoptosis and also prevented the mammary tumorigenesis induced by 9,10-dimethyl-1,2-benzanthracene [102,108]. Moreover, in rats, SFN considerably inhibited the formation of azoxymethane-induced colonic aberrant crypt foci formation in rats The proapoptotic activity of SNF can be attributed to its ability to downregulate Bcl-2, activate caspases-3, -8, and -9, and upregulate Bax [102].
Kan et al. [122] showed that SFN inhibited several cancers-associated signaling pathways, such as P53, phosphorylated nuclear factor-κB, caspase-3, phosphorylated AKT, B-cell lymphoma 2 (Bcl-2), P27, Bcl-2-associated X protein, cMyc, Cyclin-D 1, and cytochrome c, and decreased the levels of expression of epidermal growth factor receptor 2 (HER2) in the human ovarian cancer cell line. Interestingly, SFN acted in synergism with cisplatin to enhance apoptosis and inhibit cancer cell proliferation. The ability of SFN to suppress cancer growth was confirmed by xenograft experiments in vivo [122].
Recently, the effects of ITCs (SFN and PEITC) on DNA damage and replication in PC-3 tumor cells, prostate epithelial cells (PNT2), and normal fibroblasts (HDFa) were analyzed [123]. Both SFN and PEITC inhibited the replication of DNA, followed by double-strand breaks (DSB), which were more marked in PC-3 cells. The selective antiproliferative effects demonstrated by SFN and PEITC toward investigated tumor cell lines derived from less effective DNA repair in these cell lines in comparison to the normally used cell lines.
The inhibition of these enzymes promotes cells protection against DNA damage produced by different carcinogens and reactive oxygen species. The nuclear factor erythroid 2 related factors 2/antioxidant response element pathway is the main determinant of the gene induction of enzymes of phase II. Among GSL hydrolysis products, ITCs are shown to be strong inducers of phase II enzymes activity by increasing the transcription of genes that contain ARE [124]. SFN is an active inducer of enzymes of phase II. This ITC showed indirect antioxidant activity probably related to the induction of quinine reductase, heme-oxygenase, and glutathione transferases [125].
ITCs not only exhibited antioxidant activity through the upregulation of ARE-driven genes, but also demonstrated to be potent activators of NrF2 and to decrease the inflammatory responses via the NFκB pathway [126]. PEITC and 8-methylsulfinyloctyl isothiocyanate (MSO) were examined for their potential ability to modulate the inflammatory response in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages by assessment of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression [118]. Both PEITC and MSO iNOS protein and COX-2 expression levels are in association with the inactivation of NFκB. As demonstrated for other ITCs, Boyanapalli et al. [127] have shown that the anti-inflammatory activity of PEITC is also linked to its interaction with the NrF2 pathway.
Several studies showed that the ITC erucin induced HO-1 expression through p38 signaling and NrF2 via ERK1/2, p38-MAPK, and JNK pathways [128]. Previously, Yehuda et al. [129] also reported the ability of erucin to decrease the transcription of proinflammatory agents, such as TNF-α, IL-1, and IL-12, in THP-1 cells treated with LPS. Moreover, erucin also demonstrated its remarkable anti-inflammatory effects in LPS-stimulated macrophages through the inhibition of NFκB signaling [130].
Numerous works concerning the antimicrobial activity of GSLs are present in the literature and several studies are evidenced as in part responsible for the antimicrobial properties of GSLs and their hydrolysis products. In this regard, some mechanisms are proposed. Among them, Kojima [131] proposed that these compounds can determine the obstruction of the synthesis of ATP in bacteria by the uncoupler action of oxidative phosphorylation in mitochondria. Moreover, GSL hydrolysis products can act by inactivating several bacteria enzymes through the oxidative breakdown of the S–S– bridges [132].
Borges et al. [133] investigated the antibacterial activity of 2-phenylethylisothiocyanate (PEITC) and allylisothiocyanate (AITC) against Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Pseudomonas aeruginosa, finding an MIC (minimal inhibitory concentration) of 100 μg /mL against all tested bacteria. These results agree with those reported in other works. Pang et al. [134] demonstrated that AITC possesses antimicrobial effects against P. aeruginosa (ATCC 10145, 15442, and 27853), extending the shelf-life of catfish fillets. AITC exhibited MIC values of 50, 100, and 200 µg/mL against E. coli, L. monocytogenes, and S. aureus, respectively [135]. Successively, Luciano and Holley [136] revealed MIC in the range 25.5–510 µg/mL with the raising of pH for AITC against E. coli O157:H7.
Conrad et al. [137] studied a mixture of AITC, PEITC, and benzyl-ITC against E. coli, P. aeruginosa, Haemophilus influenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Proteus vulgaris, S. aureus, Serratia marcescens, S. pyogenes, and Streptococcus pneumoniae. For Gram-positive bacteria, the ITCs MBC (minimum bactericidal concentration) was > 1000 µg/mL. The same results were found for PEITC against P. aeruginosa and E. coli. Considering the MIC and MBC results, AITC and PEITC may be considered as nonspecific antimicrobial agents on both Gram-positive and Gram-negative bacteria. Indeed, the presence, along with the cytoplasmic membrane, of an outer membrane in Gram-negative bacteria, did not increase the antimicrobial resistance of P. aeruginosa and E. coli.
Helicobacter pylori infection increases the risk for developing gastric cancer. The hydrolytic product of glucoraphanin, namely sulforaphane (SFN), demonstrated potent bacteriostatic effects against three standard strains and 45 clinical isolates of H. pylori. Additionally, short-term exposure to SFN removed H. pylori from the Hep-2 cell line. In another work, the administration of SFN for five days eliminated H. pylori from eight out of eleven xenografts of human gastric tissue implanted in immunocompromised mice [138]. Aires et al. also showed the potential antibacterial activity of GSLs and their hydrolysis products against bacteria isolated from the human intestinal tract [139]. In this work, the most effective GSLs hydrolysis products were ITCs with benzyl-ITC and sulforaphane as the most active growth inhibitors. Indole-3-acetonitrile showed inhibitory activity against Gram-negative bacteria, while indole-3-carbinol exhibited inhibitory activity against Gram-positive microorganisms but not against Gram-negative bacteria.
Generally, ITCs are more effective than other GSLs hydrolysis products, and aromatic ITCs are more active compared to aliphatic ITCs. ITC can react nonenzymatically with amino and thiol groups to form thioureas and dithiocarbamates, respectively, compounds that can contribute to the antibacterial properties of ITCs by inhibiting enzymes and/or essential proteins and increasing oxidation, consequently leading to bacterial cell death [124,140]. Moreover, ITC, interacting with cytochrome P-450 enzymes, can be oxidized and produce ITCs more reactive than the parent compounds [141].
Mustard oils show inhibitory activity against fungi [142,143,144]. This activity may be related to the presence of allyl and phenethyl ITCs, although, of course, each compound shows a specific activity and the activity ranges differ with changes in the ITCs substituent groups [142]. Kojima [131], using three different Saccharomyces cerevisiae strains, described the ability of ITCs to stop coupling between reactions of phosphorylation and electron transport, consequently blocking ATP synthesis. Studies have demonstrated fungicidal activity of 2-propenyl-ITC against pear P. expansum; allyl-ITC against strawberry Botrytis cinerea, nectarine and peach Monilinia laxa, and pizza crust Aspergillus parasiticus; and benzyl-ITC against tomato A. alternata, cotton Phymatotrichopsis omnivora, and grapes, soybeans, green coffee, and peanuts Aspergillus ochraceus [145,146,147,148,149,150,151].
Recently, Zhang et al. [152] evaluated the antifungal activity against A. alternata of 2-phenylethyl-ITC (2-PEITC) in pear fruit; 2-PEITC remarkably inhibited A. alternata spore germination and mycelial growth and significantly decreased the expansion of black spot rot on pears that had been treated with A. alternata. Choi et al. [153] assessed the antifungal activity ITCs to find natural antifungal agents against pathogenic dermal fungi. ITCs inhibited the growth of Epidermophyton floccosum, Trichophyton mentagrophytes, T. rubrum, and Microsporum canis pathogenic dermal fungi with minimum fungicidal concentrations of 200 µg/mL.
5.2. Omega-3 Fatty Acids
In recent decades, research on n-3 PUFAs has grown exponentially. In fact, n-3 PUFAs have been shown to play a critical role in neuronal cell function and in immune and inflammatory reactions, and many studies have revealed the benefits of n-3 PUFAs in diabetes mellitus, obesity, cardiovascular disease, atherosclerosis, dyslipidemia, metabolic syndrome, hypertension, neurological/neuropsychiatric disorders, osteoporosis, and renal diseases [5,154].
Several review articles have reported the existing knowledge on the chemistry, bioavailability, dietary sources, potential deficiency states, and biological properties of n-3 PUFAs [5,10,154,155,156,157,158,159]. Recently, Oppedisano et al. [159] described the antioxidant and anti-inflammatory properties of n-3 PUFAs and their role in preventing and/or treating cardiovascular diseases. In fact, several research reports have noted the ability of n-3 PUFAs to decrease endothelial cell apoptosis and oxidative stress-related mitochondrial dysfunction through the increased activity of endogenous antioxidant enzymes, and to counteract the release of proinflammatory cytokines in the myocardium and vascular tissues, thus restoring the activity of the myocardium and the integrity of vascular tissues. However, further studies involving large numbers of patients are necessary to confirm their potential use to reduce and/or to treat cardiovascular diseases.
McGlory et al. [158] analyzed available literature data on the potential enhancement of skeletal muscle anabolism by n-3 PUFAs intake. An increase in strength and muscle mass in healthy older people following supplementation with n-3 PUFA was also observed in subjects who experienced a loss of muscle mass due to prolonged immobility. EPA and DHA incorporation into membrane phospholipids is found as the principal means by which n-3 PUFAs positively impact skeletal muscle.
The incorporation of these n-3 PUFAs into membrane phospholipids has been proven to lead to a reduction in the expression of some factors that regulate muscle protein breakdown, the enhancement of mitochondrial respiration kinetics, and the rate of synthesis of muscle proteins. However, how EPA and DHA incorporation into membrane phospholipids can modify these processes is not yet known. Of considerable interest is the potential for n-3 PUFAs to counteract the atrophy of muscles and to stimulate recovery from periods of muscle disuse. Studies have been carried out, but much additional research must be performed before drawing conclusions concerning the effectiveness of n-3 PUFAs intake on musculoskeletal health. Some important questions to be answered concern, in particular, the possibility to discern, given their independent biological actions, the independent role of EPA and DHA in producing modifications in skeletal muscle plasticity. Another factor of interest is the evaluation of potential off-target effects of increased intake of n-3 PUFAs and whether there are negative consequences in other vital processes.
Avallone et al. [160] critically described clinical trials and epidemiological studies that evaluated the impact on neurodegenerative disorders, mainly on Alzheimer’s disease (AD) and Parkinson’s disease (PD), by dietary intake of n-3 PUFAs that represent potentially interesting agents for the treatment of these diseases. Two important studies, namely, “Nurses’ Health Study” (1984–2000) and “Health Professionals Follow-Up Study” (1986–2002), analyzed the association between the potential risk of PD and dietary lifestyle. Two dietary styles, namely the prudent diet (high consumption of fish, vegetables, and fruit) and the Western diet (high consumption of red meats, refined grains, and high-fat dairy products) have been recognized and compared. The prudent diet was demonstrated to significantly reduce the risk of PD, while the Western diet did not [161]. Additionally, in The Rotterdam Study, PUFAs consumption was related to a lower PD risk [162]. Data obtained in The Rotterdam Study, successively confirmed by other studies, revealed that the consumption of n-3 PUFAs, oils rich in n-3 PUFAs, fatty fish, or a diet with high consumption of fish, vegetables, and fruit, is connected to a reduction in the potential risk of occurrence of AD [163,164,165].
The protective activity against AD by n-3 PUFAs was investigated in the RBMVECs (rat brain microvascular endothelial) cell line [166]. This study demonstrated that the activity of catalase, superoxide dismutase, and glutathione peroxidase was improved, and ROS and lipid peroxidation was reduced after incubation of cells with n-3 PUFAs. A reduction in the amount of apoptotic RBMVECs was also described.
Most of the studies have focused on investigating mixtures of n-3 PUFAs and not individual fatty acids. In recent years, evidence for the effects of DHA, DPA, and EPA has grown. For example, with neurodegenerative diseases, such as AD, the focus of several studies has been on DHA because of its essential role in the growth and functional development of the brain. Different effects, including the modulation of key properties such as membrane fluidity, permeability, compression, fusion, and protein activity, have been described for DHA [167,168,169].
Increasing the phosphatidylserine levels of neuronal membranes may affect neuronal survival through the phosphoinositide 3-kinase/serine/threonine-protein kinase pathway [170]. DHA exerts an important role in modulating phosphatidylserine synthesis [171,172]. The dietary intake of EPA and DHA similarly improved the levels of brain phospholipids [173].
6. Conclusions and Future Perspectives
In summary, this review shows the different GSLs and omega-3 fatty acids from mustard seeds, extraction procedures from mustard seeds, and preclinical and clinical studies supporting the use of these compounds in improving human health. Previous in vitro, animal, and human studies showed that these compounds may be further developed as potential treatments for infections, cancer, diabetes, and metabolic syndrome. However, further studies are still needed to investigate action mechanisms of these naturally occurring GSLs and omega-3 fatty acids, together with their safety and efficacy. Since these compounds are being developed for pharmacologic use in humans, there is also a need to improve and standardize the extraction, isolation, and characterization methods for GSLs and omega-3 fatty acids from mustard seeds. This will also be useful in the quality control of these compounds for large-scale production and commercialization.
Author Contributions
Conceptualization, J.K.P.; methodology, J.K.P.; validation, J.K.P. and G.D.; formal analysis, J.K.P., G.D., O.A.G.T. and R.T.; investigation, J.K.P., G.D., O.A.G.T., R.T., J.A.H.R. and M.R.L.; resources, J.K.P., O.A.G.T. and R.T.; writing—original draft preparation, J.K.P., G.D., O.A.G.T., R.T., J.A.H.R. and M.R.L.; writing—review and editing, J.K.P., G.D., O.A.G.T., R.T. and H.S.S.; visualization, J.K.P.; supervision, J.K.P.; project administration, J.K.P.; funding acquisition, J.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), Republic of Korea.
Data Availability Statement
All data are presented in the form of tables and figures within the manuscript.
Acknowledgments
Authors are grateful to their respective institutions for their support. J.K. Patra G. Das and H.S. Shin are grateful to Dongguk University, Republic of Korea, for its support. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae mustards: Traditional and agronomic uses in Australia and New Zealand. Molecules 2018, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, M.; Babu, K.N. Mustard. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 9–19. [Google Scholar]
- Melrose, J. The glucosinolates: A sulphur glucoside family of mustard anti-tumour and antimicrobial phytochemicals of potential therapeutic application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Ladak, Z.; Khairy, M.; Armstrong, E.A.; Yager, J.Y. Chapter 11-Glucosinolates: Paradoxically beneficial in fighting both brain cell death and cancer. In Nutraceuticals in Brain Health and Beyond; Ghosh, D., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 155–167. [Google Scholar]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 polyunsaturated fatty acids: Benefits and endpoints in sport. Nutrients 2019, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Grygier, A. Mustard seeds as a bioactive component of food. Food Rev. Int. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Chaddha, A.; Eagle, K.A. Omega-3 fatty acids and heart health. Circulation 2015, 132, e350–e352. [Google Scholar] [CrossRef]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.-Y. Human, animal and plant health benefits of glucosinolates and strategies for enhanced bioactivity: A systematic review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Zrybko, C.L.; Fukuda, E.K.; Rosen, R.T. Determination of glucosinolates in domestic and wild mustard by high-performance liquid chromatography with confirmation by electrospray mass spectrometry and photodiode-array detection. J. Chromatogr. A 1997, 767, 43–52. [Google Scholar] [CrossRef]
- Sharma, A.; Rai, P.; Prasad, S. GC–MS detection and determination of major volatile compounds in Brassica juncea L. leaves and seeds. Microchem. J. 2018, 138, 488–493. [Google Scholar] [CrossRef]
- Cools, K.; Terry, L.A. The effect of processing on the glucosinolate profile in mustard seed. Food Chem. 2018, 252, 343–348. [Google Scholar] [CrossRef]
- Rothlisberger, K.L.; Hons, F.M.; Gentry, T.J.; Senseman, S.A. Oilseed meal effects on the emergence and survival of crop and weed species. Appl. Environ. Soil Sci. 2012, 2012, 769357. [Google Scholar] [CrossRef][Green Version]
- Prchalová, J.; Kovařík, F.; Ševčík, R.; Čížková, H.; Rajchl, A. Characterization of mustard seeds and paste by DART ionization with time-of-flight mass spectrometry. J. Mass Spectrom. 2014, 49, 811–818. [Google Scholar] [CrossRef]
- Andini, S.; Dekker, P.; Gruppen, H.; Araya-Cloutier, C.; Vincken, J.-P. Modulation of glucosinolate composition in Brassicaceae seeds by germination and fungal elicitation. J. Agric. Food Chem. 2019, 67, 12770–12779. [Google Scholar] [CrossRef]
- Hebert, M.; Mhemdi, H.; Vorobiev, E. Selective and eco-friendly recovery of glucosinolates from mustard seeds (Brassica juncea) using process optimization and innovative pretreatment (high voltage electrical discharges). Food Bioprod. Process. 2020, 124, 11–23. [Google Scholar] [CrossRef]
- Chauhan, J.; Meena, S.; Singh, K.; Meena, M. Environmental effects on genetic parameters for oil and seed meal quality components of Indian mustard (Brassica juncea L.). Indian J. Genet. 2012, 72, 435–438. [Google Scholar]
- Cools, K.; Terry, L.A. Comparative study between extraction techniques and column separation for the quantification of sinigrin and total isothiocyanates in mustard seed. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 901, 115–118. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Budzyński, W.S.; Kijewski, Ł.; Zając, T. Biomass quality of Brassica oilseed crops in response to sulfur fertilization. Agron. J. 2015, 107, 1377–1391. [Google Scholar] [CrossRef]
- Main, M.; McCaffrey, J.; Morra, M. Insecticidal activity of Brassica juncea seed meal to the fungus gnat Bradysia impatiens Johannsen (Diptera:Sciaridae). J. Appl. Entomol. 2014, 138, 701–707. [Google Scholar] [CrossRef]
- Neubauer, C.; Hüntemann, K.; Heitmann, B.; Müller, C. Suppression of Verticillium dahliae by glucosinolate-containing seed meal amendments. Eur. J. Plant Pathol. 2015, 142, 239–249. [Google Scholar] [CrossRef]
- Borpatragohain, P.; Rose, T.J.; Liu, L.; Barkla, B.J.; Raymond, C.A.; King, G.J. Remobilization and fate of sulphur in mustard. Ann. Bot. 2019, 124, 471–480. [Google Scholar] [CrossRef]
- Popova, I.E.; Morra, M.J. Sinigrin and sinalbin quantification in mustard seed using high performance liquid chromatography–time-of-flight mass spectrometry. J. Food Compos. Anal. 2014, 35, 120–126. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Lilley, C.; Barker, A.; Ellis, S.; Wade, R.; Atkinson, H.; Urwin, P.; Redeker, K.; Hartley, S. Constant isothiocyanate-release potentials across biofumigant seeding rates. J. Agric. Food Chem. 2018, 66, 5108–5116. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.E.; Dubie, J.S.; Morra, M.J. Optimization of hydrolysis conditions for release of biopesticides from glucosinolates in Brassica juncea and Sinapis alba seed meal extracts. Ind. Crops Prod. 2017, 97, 354–359. [Google Scholar] [CrossRef]
- Popova, I.E.; Morra, M.J. Simultaneous quantification of sinigrin, sinalbin, and anionic glucosinolate hydrolysis products in Brassica juncea and Sinapis alba seed extracts using ion chromatography. J. Agric. Food Chem. 2014, 62, 10687–10693. [Google Scholar] [CrossRef]
- Borpatragohain, P.; Rose, T.J.; Liu, L.; Raymond, C.A.; Barkla, B.J.; King, G.J. Seed glucosinolate yield is maximized by higher rates of sulfur nutrition than required for seed yield in condiment mustard (Brassica juncea L.). PLoS ONE 2019, 14, e0213429. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Dhingra, O.D.; Lima, A.O.; Jham, G.N.; Berhow, M.A.; Holloway, R.K.; Vaughn, S.F. Glucosinolate content and nematicidal activity of Brazilian wild mustard tissues against Meloidogyne incognita in tomato. Plant Soil 2011, 341, 155–164. [Google Scholar] [CrossRef]
- Sukovata, L.; Jaworski, T.; Kolk, A. Efficacy of Brassica juncea granulated seed meal against Melolontha grubs. Ind. Crops Prod. 2015, 70, 260–265. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Separation of sinigrin from Indian mustard (Brassica juncea L.) seed using macroporous ion-exchange resin. Korean J. Chem. Eng. 2012, 29, 396–403. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Optimization of ultrasonic-stimulated solvent extraction of sinigrin from Indian mustard seed (Brassica Juncea L.) using response surface methodology. Phytochem. Anal. 2011, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wanasundara, J.P.; Abeysekara, S.J.; McIntosh, T.C.; Falk, K.C. Solubility differences of major storage proteins of Brassicaceae oilseeds. J. Am. Oil Chem. Soc. 2012, 89, 869–881. [Google Scholar] [CrossRef]
- Font, R.; Del Río, M.; Fernández-Martínez, J.M.; de Haro-Bailón, A. Use of near-infrared spectroscopy for screening the individual and total glucosinolate contents in Indian mustard seed (Brassica juncea L. Czern. & Coss.). J. Agric. Food Chem. 2004, 52, 3563–3569. [Google Scholar] [PubMed]
- Velíšek, J.; Mikulcova, R.; Mikova, K.; Woldie, K.; Link, J.; Davídek, J. Chemometric investigation of mustard seed. LWT-Food Sci. Technol. 1995, 28, 620–624. [Google Scholar] [CrossRef]
- Fabre, N.; Bon, M.; Moulis, C.; Fouraste, I.; Stanislas, E. Three glucosinolates from seeds of Brassica juncea. Phytochemistry 1997, 45, 525–527. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, S.; Sukhija, P.; Munshi, S. Accumulation of glucosinolates in developing mustard (Brassica juncea L.) seeds in response to sulphur application. Plant Sci. 1990, 66, 181–184. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Nicolas, M.E.; Bennett, R.N.; Premier, R.R.; Eagling, D.R.; Taylor, P.W. Determination of sinigrin and glucoraphanin in Brassica species using a simple extraction method combined with ion-pair HPLC analysis. Sci. Hortic. 2002, 96, 27–41. [Google Scholar] [CrossRef]
- Palmer, M.; Sang, J.; Oram, R.; Tran, D.; Salisbury, P. Variation in seed glucosinolate concentrations of Indian mustard (Brassica juncea (L.) Czern.+ Coss.). Aust. J. Exp. Agric. 1988, 28, 779–782. [Google Scholar] [CrossRef]
- Palmer, M.V.; Yeung, S.P.; Sang, J.P. Glucosinolate content of seedlings, tissue cultures, and regenerant plants of Brassica juncea (Indian mustard). J. Agric. Food Chem. 1987, 35, 262–265. [Google Scholar] [CrossRef]
- Szmigielska, A.M.; Schoenau, J.J. Use of anion-exchange membrane extraction for the high-performance liquid chromatographic analysis of mustard seed glucosinolates. J. Agric. Food Chem. 2000, 48, 5190–5194. [Google Scholar] [CrossRef]
- Velasco, L.; Becker, H.C. Variability for seed glucosinolates in a germplasm collection of the genus Brassica. Genet. Resour. Crop Evol. 2000, 47, 231–238. [Google Scholar] [CrossRef]
- Sodhi, Y.; Mukhopadhyay, A.; Arumugam, N.; Verma, J.; Gupta, V.; Pental, D.; Pradhan, A. Genetic analysis of total glucosinolate in crosses involving a high glucosinolate Indian variety and a low glucosinolate line of Brassica juncea. Plant Breed. 2002, 121, 508–511. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef]
- Ciska, E.; Honke, J.; Kozłowska, H. Effect of light conditions on the contents of glucosinolates in germinating seeds of white mustard, red radish, white radish, and rapeseed. J. Agric. Food Chem. 2008, 56, 9087–9093. [Google Scholar] [CrossRef]
- Lazzeri, L.; Tacconi, R.; Palmieri, S. In vitro activity of some glucosinolates and their reaction products toward a population of the nematode Heterodera schachtii. J. Agric. Food Chem. 1993, 41, 825–829. [Google Scholar] [CrossRef]
- Toribio, A.; Nuzillard, J.-M.; Renault, J.-H. Strong ion-exchange centrifugal partition chromatography as an efficient method for the large-scale purification of glucosinolates. J. Chromatogr. A 2007, 1170, 44–51. [Google Scholar] [CrossRef]
- Toribio, A.; Nuzillard, J.M.; Pinel, B.; Boudesocque, L.; Lafosse, M.; De La Poype, F.; Renault, J.H. Pilot-scale ion-exchange centrifugal partition chromatography: Purification of sinalbin from white mustard seeds. J. Sep. Sci. 2009, 32, 1801–1807. [Google Scholar] [CrossRef]
- Gohain, B.; Kumar, P.; Malhotra, B.; Augustine, R.; Pradhan, A.K.; Bisht, N.C. A comprehensive Vis-NIRS equation for rapid quantification of seed glucosinolate content and composition across diverse Brassica oilseed chemotypes. Food Chem. 2021, 354, 129527. [Google Scholar] [CrossRef]
- Xin, H.; Khan, N.A.; Falk, K.C.; Yu, P. Mid-infrared spectral characteristics of lipid molecular structures in Brassica carinata seeds: Relationship to oil content, fatty acid and glucosinolate profiles, polyphenols, and condensed tannins. J. Agric. Food Chem. 2014, 62, 7977–7988. [Google Scholar] [CrossRef]
- Matthäus, B.; Angelini, L.G. Anti-nutritive constituents in oilseed crops from Italy. Ind. Crops Prod. 2005, 21, 89–99. [Google Scholar] [CrossRef]
- Mnzava, N.; Olsson, K. Studies on tropical vegetables. Part 1: Seed amino, fatty acid and glucosinolate profile of ethiopian mustards (Brassica carinata Braun). Food Chem. 1990, 35, 229–235. [Google Scholar] [CrossRef]
- Montaut, S.; Blažević, I.; Ruščić, M.; Rollin, P. LC–MS profiling of glucosinolates in the seeds of Brassica elongata Ehrh., and of the two stenoendemic B. botteri Vis and B. cazzae Ginzb. & Teyber. Nat. Prod. Res. 2017, 31, 58–62. [Google Scholar]
- Horn, P.J.; Vaughan, J.G. Seed glucosinolates of fourteen wild Brassica species. Phytochemistry 1983, 22, 465–470. [Google Scholar] [CrossRef]
- Wu, G.; Truksa, M.; Datla, N.; Vrinten, P.; Bauer, J.; Zank, T.; Cirpus, P.; Heinz, E.; Qiu, X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005, 23, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Dungey, S.G.; Sang, J.P.; Rothnie, N.E.; Palmer, M.V.; Burke, D.G.; Knox, R.B.; Williams, E.G.; Hilliard, E.P.; Salisbury, P.A. Glucosinolates in the pollen of rapeseed and Indian mustard. Phytochemistry 1988, 27, 815–817. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Chauhan, J.; Bhadauria, V.; Singh, M.; Singh, K.; KUMAR, A. QualiSy characteristics and their interrelationships in Indian rapeseed-mustard (Brassiea sp.) varieties. Indian J. Agric. Sci. 2007, 77, 616–620. [Google Scholar]
- Singh, B.K.; Bala, M.; Rai, P.K. Fatty acid composition and seed meal characteristics of Brassica and allied genera. Natl. Acad. Sci. Lett. 2014, 37, 219–226. [Google Scholar] [CrossRef]
- Kim, D.W. The Effects of Auricular Acupressure Using Sinapsis alba on Female College Students’ Obesity Indices and Self-Efficacy; Dongeui University Press: Busan, Korea, 2011. [Google Scholar]
- Kang, M.; Kim, Y.K.; Shin, J.S.; Yeo, H.N. Effects of semen sinapsis albae acupressure on fatigue and sleep related chemotherapy in patients with breast cancer. J. Korean Clin. Nurs. Res. 2015, 21, 180–187. [Google Scholar]
- Iunes, D.H.; Chaves, É.D.C.L.; Moura, C.D.C.; Côrrea, B.; Carvalho, L.C.; Silva, A.M.; de Carvalho, E.C. Role of auriculotherapy in the treatment of temporomandibular disorders with anxiety in university students. Evid.-Based Complement. Altern. Med. 2015, 2015, 430143. [Google Scholar] [CrossRef]
- Cândido dos Reis, A.; Theodoro de Oliveira, T.; Larissa Vidal, C.; Cristina Borsatto, M.; da Costa Valente, M.L. Effect of auricular acupuncture on the reduction of symptoms related to sleep disorders, anxiety and temporomandibular disorder (TMD). Altern. Ther. Health Med. 2021, 27, 22–26. [Google Scholar]
- Goetz, K.; Hinz, A.; Steinhäuser, J.; von Rath, U. Use of mustard seed footbaths for respiratory tract infections: A pilot study. Evid.-Based Complement. Altern. Med. 2020, 2020, 5648560. [Google Scholar] [CrossRef]
- Tian, M.; Hanley, A.B.; Dodds, M.; Yaegaki, K. Chewing gum containing allyl isothiocyanate from mustard seed extract is effective in reducing volatile sulfur compounds responsible for oral malodor. Am. J. Dent. 2013, 26, 180–184. [Google Scholar]
- Lett, A.M.; Thondre, P.S.; Rosenthal, A.J. Yellow mustard bran attenuates glycaemic response of a semi-solid food in young healthy men. Int. J. Food Sci. Nutr. 2013, 64, 140–146. [Google Scholar] [CrossRef]
- Summers, A.; Visscher, M.O.; Khatry, S.K.; Sherchand, J.B.; LeClerq, S.C.; Katz, J.; Tielsch, J.M.; Mullany, L.C. Impact of sunflower seed oil versus mustard seed oil on skin barrier function in newborns: A community-based, cluster-randomized trial. BMC Pediatrics 2019, 19, 512. [Google Scholar] [CrossRef]
- Bork, C.S.; Venø, S.K.; Lundbye-Christensen, S.; Jakobsen, M.U.; Tjønneland, A.; Schmidt, E.B.; Overvad, K. Dietary intake of α-linolenic acid is not appreciably associated with risk of ischemic stroke among middle-aged Danish men and women. J. Nutr. 2018, 148, 952–958. [Google Scholar] [CrossRef]
- Hennebelle, M.; Courchesne-Loyer, A.; St-Pierre, V.; Vandenberghe, C.; Castellano, C.-A.; Fortier, M.; Tessier, D.; Cunnane, S.C. Preliminary evaluation of a differential effect of an α-linolenate-rich supplement on ketogenesis and plasma ω-3 fatty acids in young and older adults. Nutrition 2016, 32, 1211–1216. [Google Scholar] [CrossRef]
- Jovanovski, E.; Li, D.; Ho, H.V.T.; Djedovic, V.; Marques, A.d.C.R.; Shishtar, E.; Mejia, S.B.; Sievenpiper, J.L.; de Souza, R.J.; Duvnjak, L. The effect of alpha-linolenic acid on glycemic control in individuals with type 2 diabetes: A systematic review and meta-analysis of randomized controlled clinical trials. Medicine 2017, 96, e6531. [Google Scholar] [CrossRef]
- Bjornevik, K.; Myhr, K.-M.; Beiske, A.; Bjerve, K.S.; Holmøy, T.; Hovdal, H.; Midgard, R.; Riise, T.; Wergeland, S.; Torkildsen, Ø. α-Linolenic acid is associated with MRI activity in a prospective cohort of multiple sclerosis patients. Mult. Scler. J. 2019, 25, 987–993. [Google Scholar] [CrossRef]
- Burak, C.; Wolffram, S.; Zur, B.; Langguth, P.; Fimmers, R.; Alteheld, B.; Stehle, P.; Egert, S. Effect of alpha-linolenic acid in combination with the flavonol quercetin on markers of cardiovascular disease risk in healthy, non-obese adults: A randomized, double-blinded placebo-controlled crossover trial. Nutrition 2019, 58, 47–56. [Google Scholar] [CrossRef]
- Pieters, D.J.; Zock, P.L.; Fuchs, D.; Mensink, R.P. Effect of α-linolenic acid on 24-h ambulatory blood pressure in untreated high-normal and stage I hypertensive subjects. Br. J. Nutr. 2019, 121, 155–163. [Google Scholar] [CrossRef]
- Saito, S.; Mori, A.; Osaki, N.; Katsuragi, Y. Diacylglycerol enhances the effects of alpha-linolenic acid against visceral fat: A double-blind randomized controlled trial. Obesity 2017, 25, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Saito, S.; Yamanaka, N.; Suzuki, C.; Ono, T.; Osaki, N.; Katsuragi, Y. Alpha linolenic acid-enriched diacylglycerol consumption enhances dietary fat oxidation in healthy subjects: A randomized double-blind controlled trial. J. Oleo Sci. 2017, 66, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Baxheinrich, A.; Lee-Barkey, Y.H.; Tschoepe, D.; Stehle, P.; Stratmann, B.; Wahrburg, U. Effects of a hypoenergetic diet rich in α-linolenic acid on fatty acid composition of serum phospholipids in overweight and obese patients with metabolic syndrome. Nutrition 2018, 49, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Okunade, O.; Niranjan, K.; Ghawi, S.K.; Kuhnle, G.; Methven, L. Supplementation of the diet by exogenous myrosinase via mustard seeds to increase the bioavailability of sulforaphane in healthy human subjects after the consumption of cooked broccoli. Mol. Nutr. Food Res. 2018, 62, 1700980. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Engels, C.; Schieber, A.; Gänzle, M.G. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MS n and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Conaway, C.; Yang, Y.; Chung, F. Isothiocyanates as cancer chemopreventive agents: Their biological activities and metabolism in rodents and humans. Curr. Drug Metab. 2002, 3, 233–255. [Google Scholar] [CrossRef]
- Hecht, S.S. Inhibition of carcinogenesis by isothiocyanates. Drug Metab. Rev. 2000, 32, 395–411. [Google Scholar] [CrossRef]
- Jiao, D.; Smith, T.J.; Yang, C.S.; Pittman, B.; Desai, D.; Amin, S.; Chung, F.L. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis 1997, 18, 2143–2147. [Google Scholar] [CrossRef][Green Version]
- Morse, M.A.; Wang, C.-X.; Stoner, G.D.; Mandal, S.; Conran, P.B.; Amin, S.G.; Hecht, S.S.; Chung, F.-L. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989, 49, 549–553. [Google Scholar]
- Morse, M.A.; Eklind, K.I.; Hecht, S.S.; Jordan, K.G.; Choi, C.-I.; Desai, D.H.; Amin, S.G.; Chung, F.-L. Structure-activity relationships for inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone lung tumorigenesis by arylalkyl isothiocyanates in A/J mice. Cancer Res. 1991, 51, 1846–1850. [Google Scholar]
- Stoner, G.D.; Morrissey, D.T.; Heur, Y.-H.; Daniel, E.M.; Galati, A.J.; Wagner, S.A. Inhibitory effects of phenetyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 1991, 51, 2063–2068. [Google Scholar]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 131, 3027S–3033S. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Conaway, C.C.; Chiao, J.; Wang, C.-X.; Amin, S.; Whysner, J.; Dai, W.; Reinhardt, J.; Chung, F.-L. Inhibition of benzo (a) pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002, 62, 2–7. [Google Scholar]
- Rose, P.; Huang, Q.; Ong, C.N.; Whiteman, M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2005, 209, 105–113. [Google Scholar] [CrossRef]
- Kirlin, W.G.; Cai, J.; DeLong, M.J.; Patten, E.J.; Jones, D.P. Dietary compounds that induce cancer preventive phase 2 enzymes activate apoptosis at comparable doses in HT29 colon carcinoma cells. J. Nutr. 1999, 129, 1827–1835. [Google Scholar] [CrossRef][Green Version]
- Srivastava, S.K.; Singh, S.V. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis 2004, 25, 1701–1709. [Google Scholar] [CrossRef]
- Kuroiwa, Y.; Nishikawa, A.; Kitamura, Y.; Kanki, K.; Ishii, Y.; Umemura, T.; Hirose, M. Protective effects of benzyl isothiocyanate and sulforaphane but not resveratrol against initiation of pancreatic carcinogenesis in hamsters. Cancer Lett. 2006, 241, 275–280. [Google Scholar] [CrossRef]
- Bonnesen, C.; Eggleston, I.M.; Hayes, J.D. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001, 61, 6120–6130. [Google Scholar]
- Lui, V.W.; Wentzel, A.L.; Xiao, D.; Lew, K.L.; Singh, S.V.; Grandis, J.R. Requirement of a carbon spacer in benzyl isothiocyanate-mediated cytotoxicity and MAPK activation in head and neck squamous cell carcinoma. Carcinogenesis 2003, 24, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Manesh, C.; Kuttan, G. Effect of naturally occurring isothiocyanates in the inhibition of cyclophosphamide-induced urotoxicity. Phytomedicine 2005, 12, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Xiao, D.; Lew, K.L.; Hershberger, P.; Kokkinakis, D.M.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis 2003, 24, 1665–1670. [Google Scholar] [CrossRef]
- Xiao, D.; Srivastava, S.K.; Lew, K.L.; Zeng, Y.; Hershberger, P.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G 2/M arrest and inducing apoptosis. Carcinogenesis 2003, 24, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Thornalley, P.J. Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem. Pharmacol. 2000, 60, 221–231. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo [a] pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, I.; Skupinska, K.; Kasprzycka-Guttman, T. Sulforaphane and 2-oxohexyl isothiocyanate induce cell growth arrest and apoptosis in L-1210 leukemia and ME-18 melanoma cells. Oncol. Rep. 2003, 10, 2045–2050. [Google Scholar] [CrossRef]
- Kim, B.-R.; Hu, R.; Keum, Y.-S.; Hebbar, V.; Shen, G.; Nair, S.S.; Kong, A.T. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003, 63, 7520–7525. [Google Scholar]
- Singh, A.V.; Xiao, D.; Lew, K.L.; Dhir, R.; Singh, S.V. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis 2004, 25, 83–90. [Google Scholar] [CrossRef]
- Gingras, D.; Gendron, M.; Boivin, D.; Moghrabi, A.; Théorêt, Y.; Béliveau, R. Induction of medulloblastoma cell apoptosis by sulforaphane, a dietary anticarcinogen from Brassica vegetables. Cancer Lett. 2004, 203, 35–43. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Ahmed, T.; Chung, F.-L.; Conaway, C.; Chiao, J.-W. Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention. Int. J. Oncol. 2004, 24, 187–192. [Google Scholar] [CrossRef]
- Fimognari, C.; Nüsse, M.; Cesari, R.; Iori, R.; Cantelli-Forti, G.; Hrelia, P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis 2002, 23, 581–586. [Google Scholar] [CrossRef]
- Gamet-Payrastre, L.; Li, P.; Lumeau, S.; Cassar, G.; Dupont, M.-A.; Chevolleau, S.; Gasc, N.; Tulliez, J.; Tercé, F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000, 60, 1426–1433. [Google Scholar]
- Chung, F.-L.; Conaway, C.C.; Rao, C.; Reddy, B.S. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis 2000, 21, 2287–2291. [Google Scholar] [CrossRef]
- Zhang, Y.; Kensler, T.W.; Cho, C.-G.; Posner, G.H.; Talalay, P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef]
- Chiao, J.; Chung, F.; Krzeminski, J.; Amin, S.; Arshad, R.; Ahmed, T.; Conaway, C. Modulation of growth of human prostate cancer cells by the N-acetylcysteine conjugate of phenethyl isothiocyanate. Int. J. Oncol. 2000, 16, 1215–1224. [Google Scholar] [CrossRef]
- Xiao, D.; Singh, S.V. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002, 62, 3615–3619. [Google Scholar] [PubMed]
- Chen, Y.-R.; Han, J.; Kori, R.; Kong, A.-N.T.; Tan, T.-H. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J. Biol. Chem. 2002, 277, 39334–39342. [Google Scholar] [CrossRef]
- Nishikawa, A.; Morse, M.A.; Chung, F.-L. Inhibitory effects of 2-mercaptoethane sulfonate and 6-phenylhexyl isothiocyanate on urinary bladder tumorigenesis in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Cancer Lett. 2003, 193, 11–16. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kim, I.-W.; Hu, R.; Kong, A.-N.T. Modulatory properties of various natural chemopreventive agents on the activation of NF-κB signaling pathway. Pharm. Res. 2004, 21, 661–670. [Google Scholar] [CrossRef]
- Johnson, C.R.; Chun, J.; Bittman, R.; Jarvis, W.D. Intrinsic cytotoxicity and chemomodulatory actions of novel phenethylisothiocyanate sphingoid base derivatives in HL-60 human promyelocytic leukemia cells. J. Pharmacol. Exp. Ther. 2004, 309, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Thomson, S.J.; King, M.J.; Turnbull, C.I.; Midwinter, R.G.; Hampton, M.B. The chemopreventive agent phenethyl isothiocyanate sensitizes cells to Fas-mediated apoptosis. Carcinogenesis 2004, 25, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kassie, F.; Mersch-Sundermann, V. Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds. Mutat. Res./Rev. Mutat. Res. 2005, 589, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Satyan, K.; Swamy, N.; Dizon, D.S.; Singh, R.; Granai, C.O.; Brard, L. Phenethyl isothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: Role of caspase and MAPK activation. Gynecol. Oncol. 2006, 103, 261–270. [Google Scholar] [CrossRef]
- Rose, P.; Won, Y.K.; Ong, C.N.; Whiteman, M. β-Phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide 2005, 12, 237–243. [Google Scholar] [CrossRef]
- Singh, R.K.; Lange, T.S.; Kim, K.; Zou, Y.; Lieb, C.; Sholler, G.L.; Brard, L. Effect of indole ethyl isothiocyanates on proliferation, apoptosis, and MAPK signaling in neuroblastoma cell lines. Bioorg. Med. Chem. Lett. 2007, 17, 5846–5852. [Google Scholar] [CrossRef][Green Version]
- Yu, R.; Mandlekar, S.; Harvey, K.J.; Ucker, D.S.; Kong, A.T. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998, 58, 402–408. [Google Scholar]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Kan, S.F.; Wang, J.; Sun, G.X. Sulforaphane regulates apoptosis-and proliferation-related signaling pathways and synergizes with cisplatin to suppress human ovarian cancer. Int. J. Mol. Med. 2018, 42, 2447–2458. [Google Scholar] [CrossRef]
- Hać, A.; Brokowska, J.; Rintz, E.; Bartkowski, M.; Węgrzyn, G.; Herman-Antosiewicz, A. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur. J. Nutr. 2020, 59, 1421–1432. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef]
- Sita, G.; Hrelia, P.; Tarozzi, A.; Morroni, F. Isothiocyanates are promising compounds against oxidative stress, neuroinflammation and cell death that may benefit neurodegeneration in Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 1454. [Google Scholar] [CrossRef]
- Boyanapalli, S.S.; Paredes-Gonzalez, X.; Fuentes, F.; Zhang, C.; Guo, Y.; Pung, D.; Saw, C.L.L.; Kong, A.-N.T. Nrf2 knockout attenuates the anti-inflammatory effects of phenethyl isothiocyanate and curcumin. Chem. Res. Toxicol. 2014, 27, 2036–2043. [Google Scholar] [CrossRef]
- Wagner, A.E.; Sturm, C.; Piegholdt, S.; Wolf, I.M.; Esatbeyoglu, T.; De Nicola, G.R.; Iori, R.; Rimbach, G. Myrosinase-treated glucoerucin is a potent inducer of the Nrf2 target gene heme oxygenase 1—Studies in cultured HT-29 cells and mice. J. Nutr. Biochem. 2015, 26, 661–666. [Google Scholar] [CrossRef]
- Yehuda, H.; Soroka, Y.; Zlotkin-Frušić, M.; Gilhar, A.; Milner, Y.; Tamir, S. Isothiocyanates inhibit psoriasis-related proinflammatory factors in human skin. Inflamm. Res. 2012, 61, 735–742. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, K.W.; Park, J.H.Y. Erucin exerts anti-inflammatory properties in murine macrophages and mouse skin: Possible mediation through the inhibition of NFκB signaling. Int. J. Mol. Sci. 2013, 14, 20564–20577. [Google Scholar] [CrossRef]
- Kojima, M. Studies on the effects of isothiocyanates and their analogues on microorganisms. I. Effect of isothiocyanates on the oxygen uptake of yeasts. J. Ferment. Technol. 1971, 49, 740–746. [Google Scholar]
- Zsolnai, T. Antimicrobial effects of thiocyanates, isothiocyanates and potential isothiocyanate forming substances. Arzneimittel-Forschung 1971, 21, 121–127. [Google Scholar]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef]
- Pang, Y.-H.; Sheen, S.; Zhou, S.; Liu, L.; Yam, K.L. Antimicrobial effects of allyl isothiocyanate and modified atmosphere on Pseduomonas aeruginosa in fresh catfish fillet under abuse temperatures. J. Food Sci. 2013, 78, M555–M559. [Google Scholar] [CrossRef]
- Kyung, K.; Fleming, H. Antimicrobial activity-of sulfur compounds derived from cabbage. J. Food Prot. 1997, 60, 67–71. [Google Scholar] [CrossRef]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157: H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef]
- Conrad, A.; Biehler, D.; Nobis, T.; Richter, H.; Engels, I.; Biehler, K.; Frank, U. Broad spectrum antibacterial activity of a mixture of isothiocyanates from nasturtium (Tropaeoli majoris herba) and horseradish (Armoraciae rusticanae radix). Drug Res. 2013, 63, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Haristoy, X.; Angioi-Duprez, K.; Duprez, A.; Lozniewski, A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob. Agents Chemother. 2003, 47, 3982–3984. [Google Scholar] [CrossRef]
- Aires, A.; Mota, V.; Saavedra, M.; Rosa, E.; Bennett, R. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Song, L.; Morrison, J.J.; Botting, N.P.; Thornalley, P.J. Analysis of glucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC–MS/MS. Anal. Biochem. 2005, 347, 234–243. [Google Scholar] [CrossRef]
- Dhingra, O.; Costa, M.; Silva, G., Jr. Potential of allyl isothiocyanate to control Rhizoctonia solani seedling damping off and seedling blight in transplant production. J. Phytopathol. 2004, 152, 352–357. [Google Scholar] [CrossRef]
- Gamliel, A.; Stapleton, J. Characterization of antifungal volatile compounds evolved from solarized soil amended with cabbage residues. Phytopathology 1993, 83, 899–905. [Google Scholar] [CrossRef]
- Greenhalgh, J.; Mitchell, N. The involvement of flavour volatiles in the resistance to downy mildew of wild and cultivated forms of Brassica oleracea. N. Phytol. 1976, 77, 391–398. [Google Scholar] [CrossRef]
- Clemente, I.; Aznar, M.; Nerin, C. Effect of an active label based on benzyl isothiocyanate on the morphology and ochratoxins production of Aspergillus ochraceus. Food Res. Int. 2017, 101, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Wang, A.; Engledow, A.; Hollister, E.; Rothlisberger, K.; Matocha, J.; Zuberer, D.; Provin, T.; Hons, F.; Gentry, T. Inhibition of the germination and growth of Phymatotrichopsis omnivora (cotton root rot) by oilseed meals and isothiocyanates. Appl. Soil Ecol. 2011, 49, 68–75. [Google Scholar] [CrossRef]
- Mari, M.; Leoni, O.; Bernardi, R.; Neri, F.; Palmieri, S. Control of brown rot on stonefruit by synthetic and glucosinolate-derived isothiocyanates. Postharvest Biol. Technol. 2008, 47, 61–67. [Google Scholar] [CrossRef]
- Mari, M.; Leoni, O.; Iori, R.; Cembali, T. Antifungal vapour-phase activity of allyl-isothiocyanate against Penicillium expansum on pears. Plant Pathol. 2002, 51, 231–236. [Google Scholar] [CrossRef]
- Quiles, J.M.; Manyes, L.; Luciano, F.; Manes, J.; Meca, G. Influence of the antimicrobial compound allyl isothiocyanate against the Aspergillus parasiticus growth and its aflatoxins production in pizza crust. Food Chem. Toxicol. 2015, 83, 222–228. [Google Scholar] [CrossRef]
- Troncoso-Rojas, R.; Sánchez-Estrada, A.; Ruelas, C.; García, H.S.; Tiznado-Hernández, M. Effect of benzyl isothiocyanate on tomato fruit infection development by Alternaria alternata. J. Sci. Food Agric. 2005, 85, 1427–1434. [Google Scholar] [CrossRef]
- Ugolini, L.; Martini, C.; Lazzeri, L.; D’Avino, L.; Mari, M. Control of postharvest grey mould (Botrytis cinerea Per.: Fr.) on strawberries by glucosinolate-derived allyl-isothiocyanate treatments. Postharvest Biol. Technol. 2014, 90, 34–39. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Bi, Y.; Wang, T.; Dong, Y.; Yang, Q.; Zhang, T. 2-Phenylethyl isothiocyanate exerts antifungal activity against Alternaria alternata by affecting membrane integrity and mycotoxin production. Toxins 2020, 12, 124. [Google Scholar] [CrossRef]
- Choi, K.-D.; Kim, H.-Y.; Shin, I.-S. Antifungal activity of isothiocyanates extracted from horseradish (Armoracia rusticana) root against pathogenic dermal fungi. Food Sci. Biotechnol. 2017, 26, 847–852. [Google Scholar] [CrossRef]
- Yashodhara, B.; Umakanth, S.; Pappachan, J.; Bhat, S.; Kamath, R.; Choo, B. Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar] [CrossRef]
- Aucoin, M.; Cooley, K.; Knee, C.; Fritz, H.; Balneaves, L.G.; Breau, R.; Fergusson, D.; Skidmore, B.; Wong, R.; Seely, D. Fish-derived omega-3 fatty acids and prostate cancer: A systematic review. Integr. Cancer Ther. 2017, 16, 32–62. [Google Scholar] [CrossRef]
- Bozzatello, P.; Brignolo, E.; De Grandi, E.; Bellino, S. Supplementation with omega-3 fatty acids in psychiatric disorders: A review of literature data. J. Clin. Med. 2016, 5, 67. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The influence of omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 fatty acids and neurodegenerative diseases: New evidence in clinical trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef]
- De Lau, L.; Bornebroek, M.; Witteman, J.; Hofman, A.; Koudstaal, P.; Breteler, M. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam study. Neurology 2005, 64, 2040–2045. [Google Scholar] [CrossRef]
- Kalmijn, S.; Launer, L.J.; Ott, A.; Witteman, J.C.; Hofman, A.; Breteler, M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997, 42, 776–782. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; He, J.; Wang, L.; Tian, Z.; Wang, C. Protective effects of omega-3 fatty acids against Alzheimer’s disease in rat brain endothelial cells. Brain Behav. 2018, 8, e01037. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Stillwell, W.; Shaikh, S.R.; Zerouga, M.; Siddiqui, R.; Wassall, S.R. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod. Nutr. Dev. 2005, 45, 559–579. [Google Scholar] [CrossRef]
- Wassall, S.R.; Stillwell, W. Docosahexaenoic acid domains: The ultimate non-raft membrane domain. Chem. Phys. Lipids 2008, 153, 57–63. [Google Scholar] [CrossRef]
- Akbar, M.; Calderon, F.; Wen, Z.; Kim, H.-Y. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA 2005, 102, 10858–10863. [Google Scholar] [CrossRef]
- Garcia, M.C.; Ward, G.; Ma, Y.C.; Salem, N., Jr.; Kim, H.Y. Effect of docosahexaenoic acid on the synthesis of phosphatidylserine in rat brain microsomes and C6 glioma cells. J. Neurochem. 1998, 70, 24–30. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef]
- Cansev, M.; Wurtman, R. Chronic administration of docosahexaenoic acid or eicosapentaenoic acid, but not arachidonic acid, alone or in combination with uridine, increases brain phosphatide and synaptic protein levels in gerbils. Neuroscience 2007, 148, 421–431. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).