New Diterpenes with Potential Antitumoral Activity Isolated from Plants in the Years 2017–2022
Abstract
1. Introduction
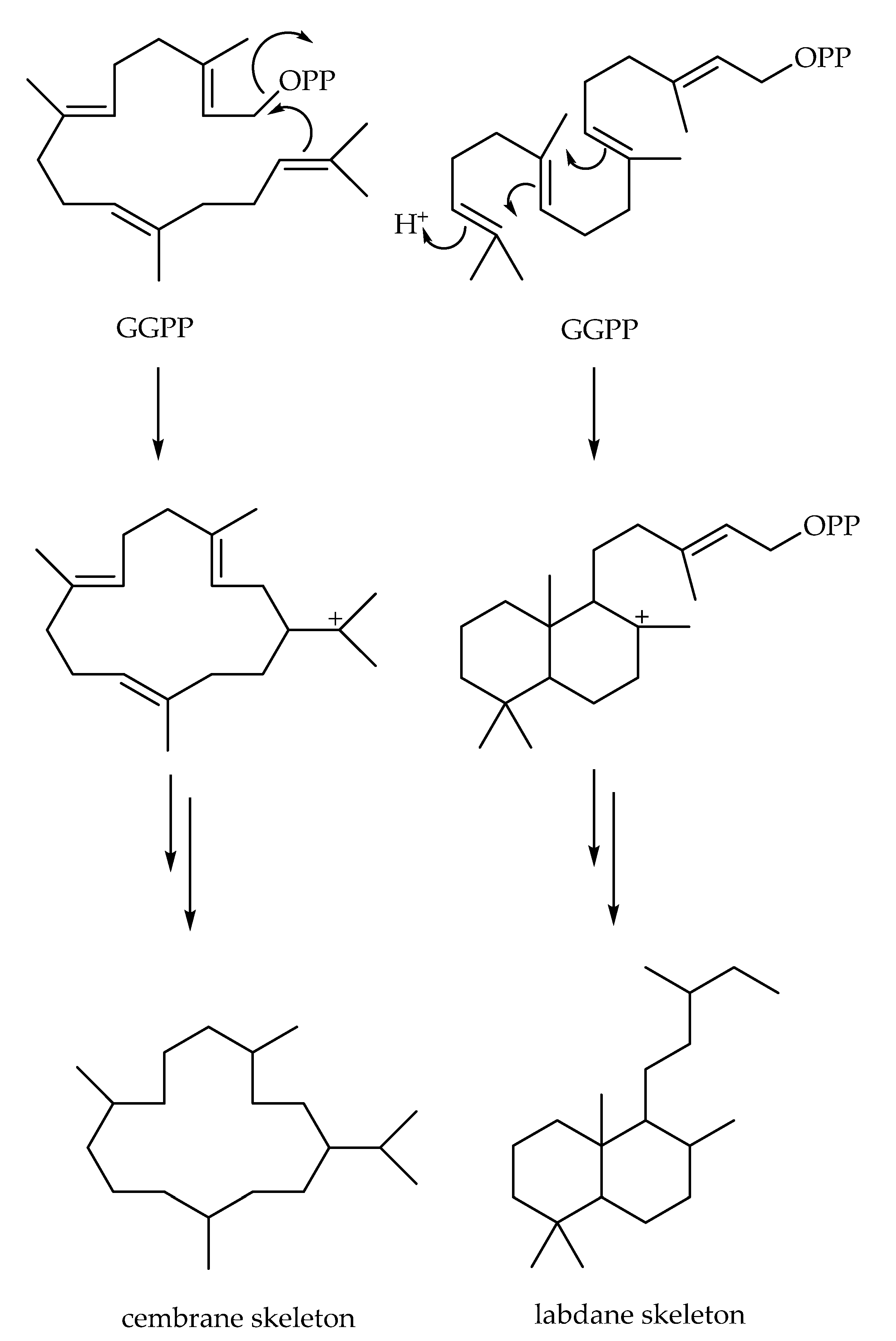
2. Monocyclic Diterpenes and Diterpenes Derived from Cembrane
2.1. Monocyclic Diterpenes
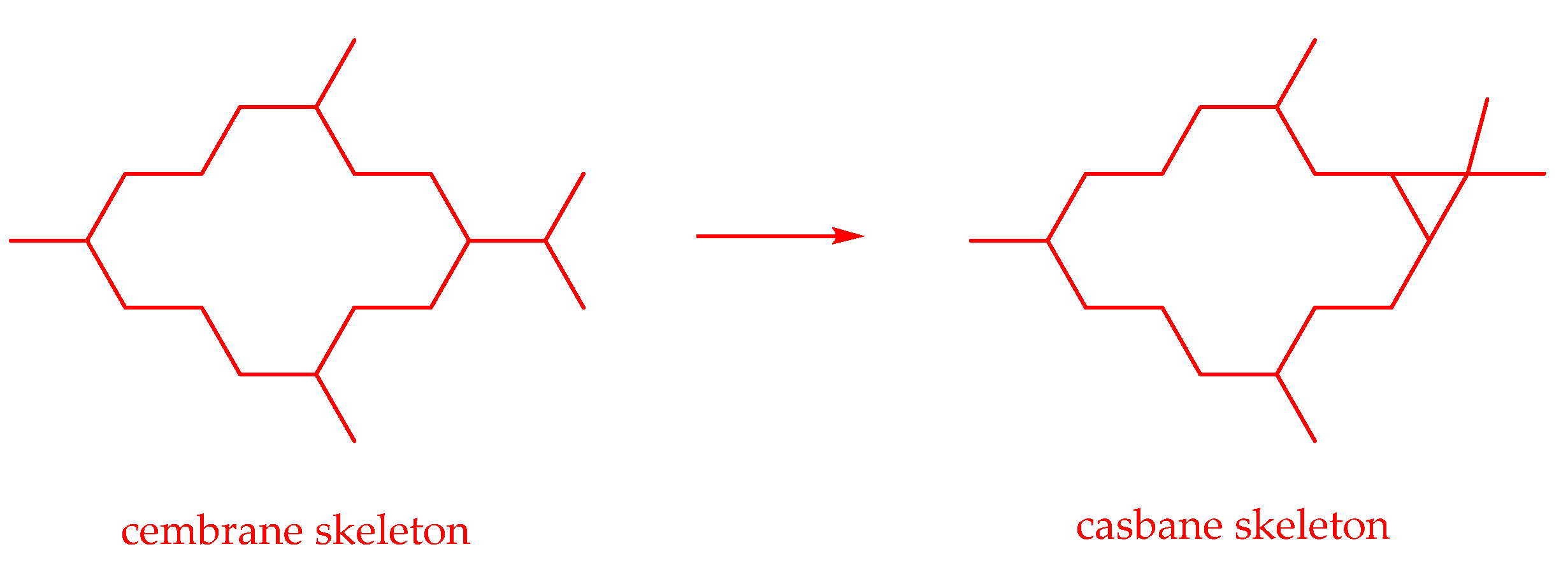
2.2. Diterpenes Derived from Cembrane
2.2.1. Casbane-Type Diterpenoids
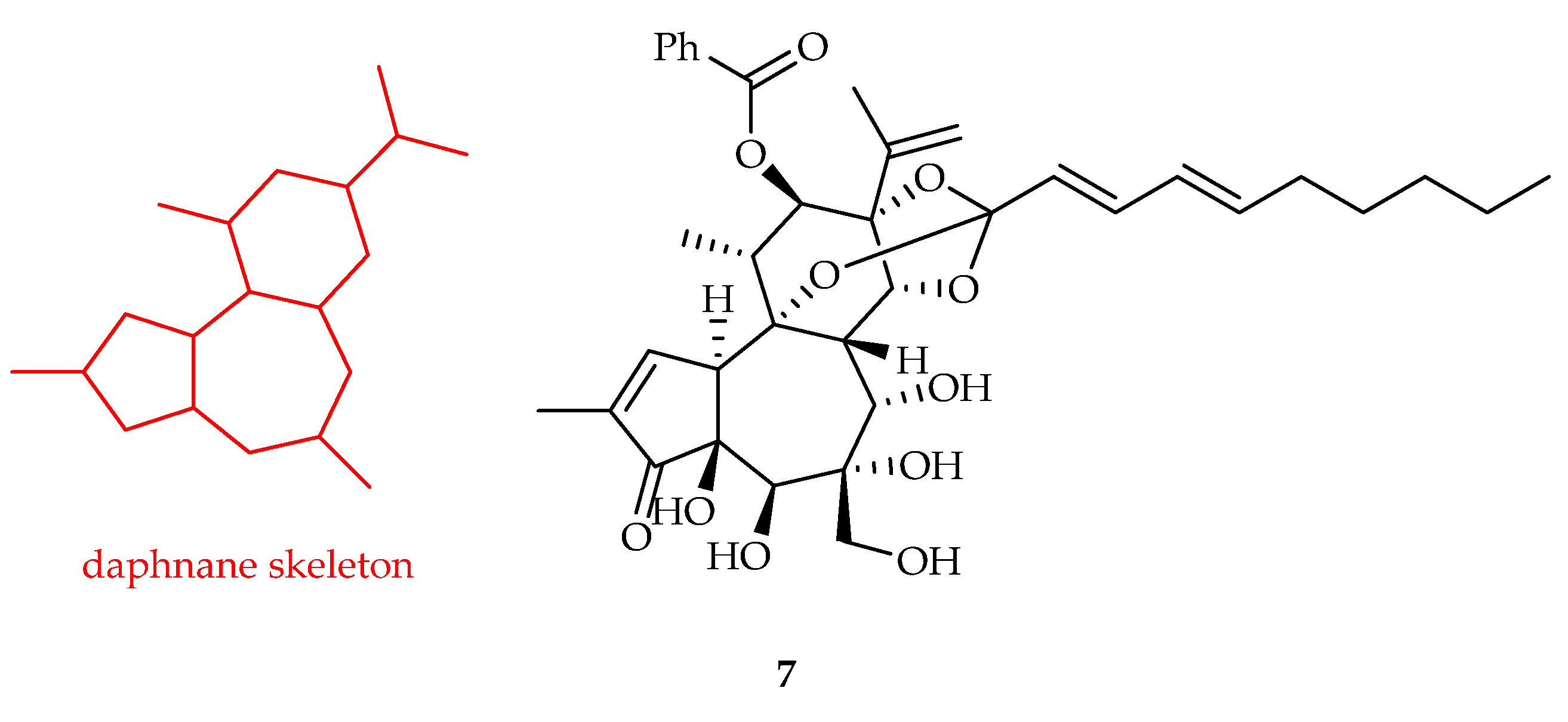
2.2.2. Daphnane-Type Diterpenoids
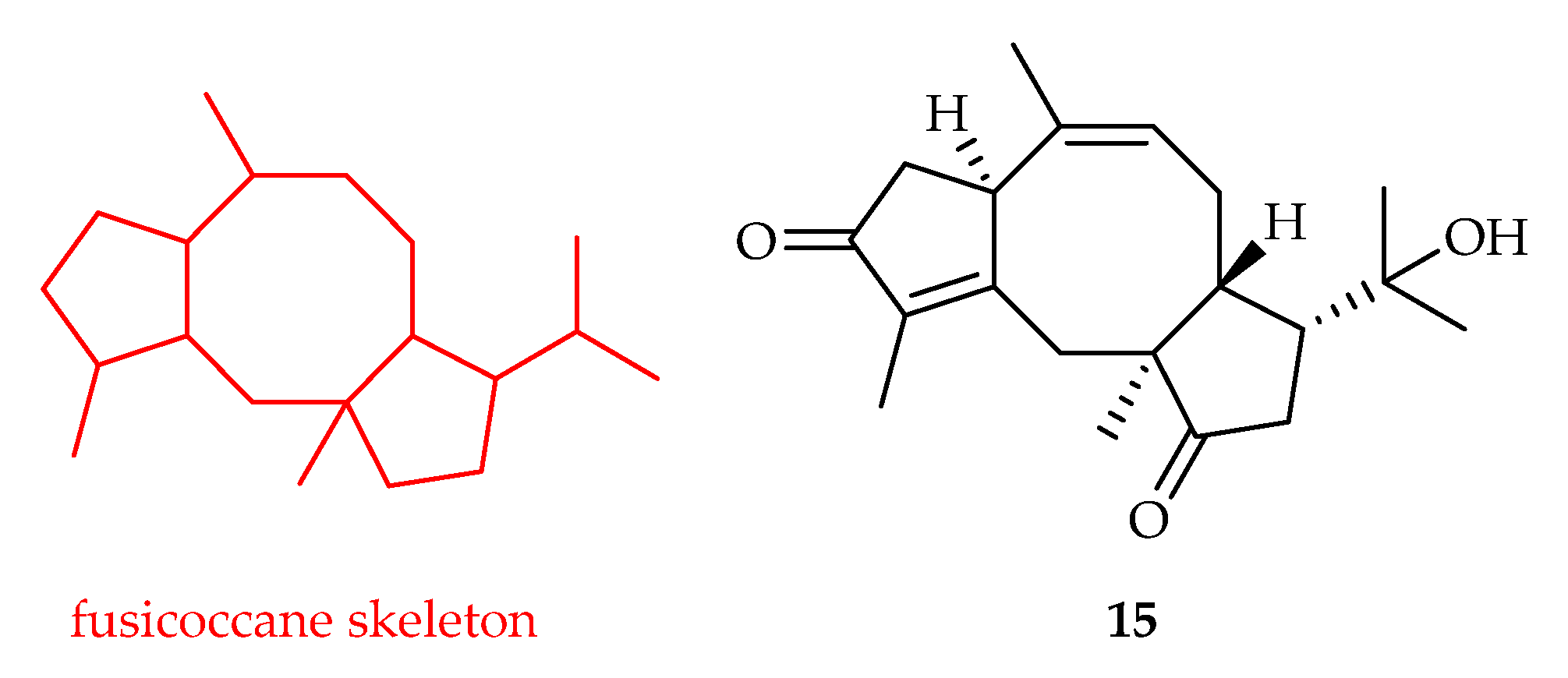
2.2.3. Fusicoccane-Type Diterpenoids
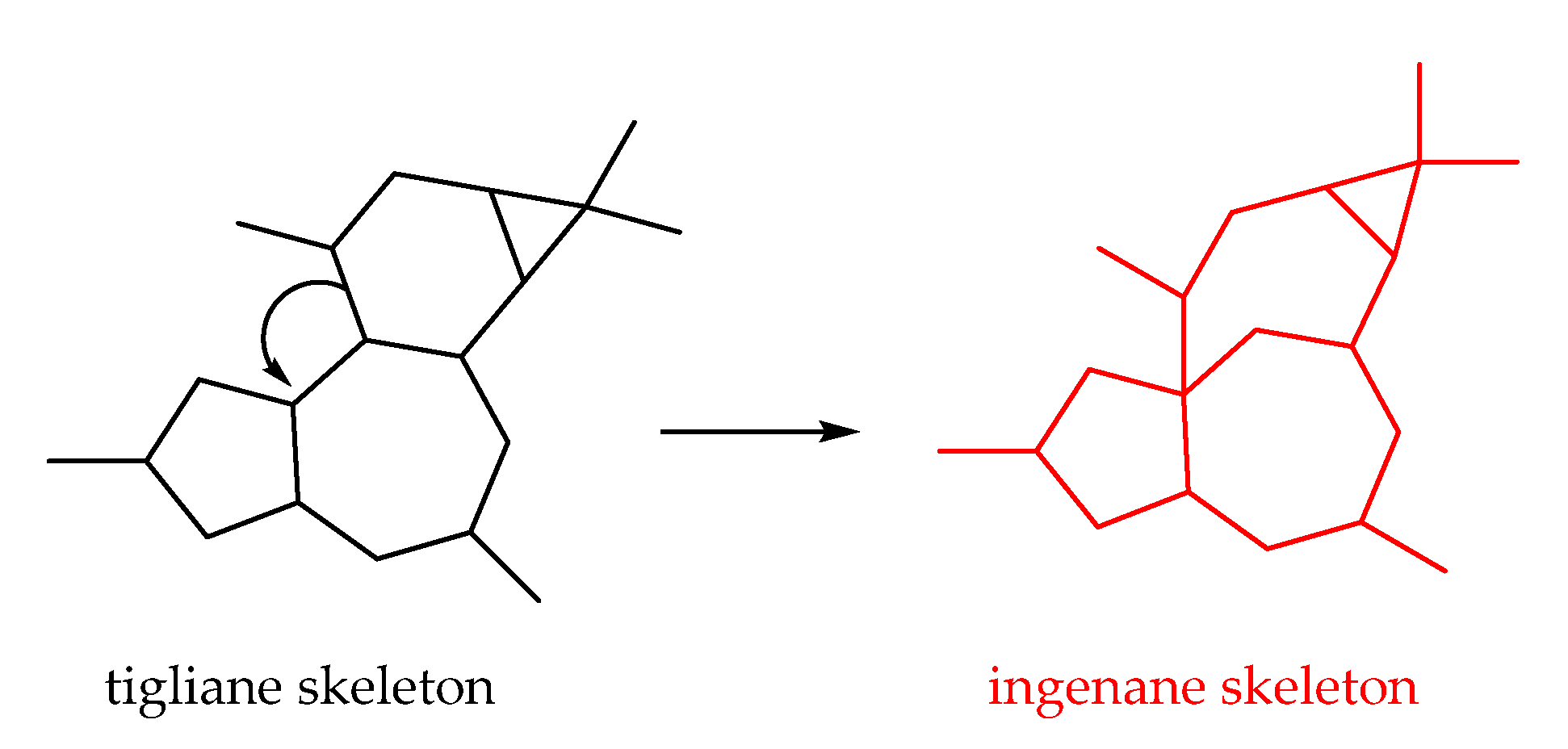
2.2.4. Ingenane-Type Diterpenoids
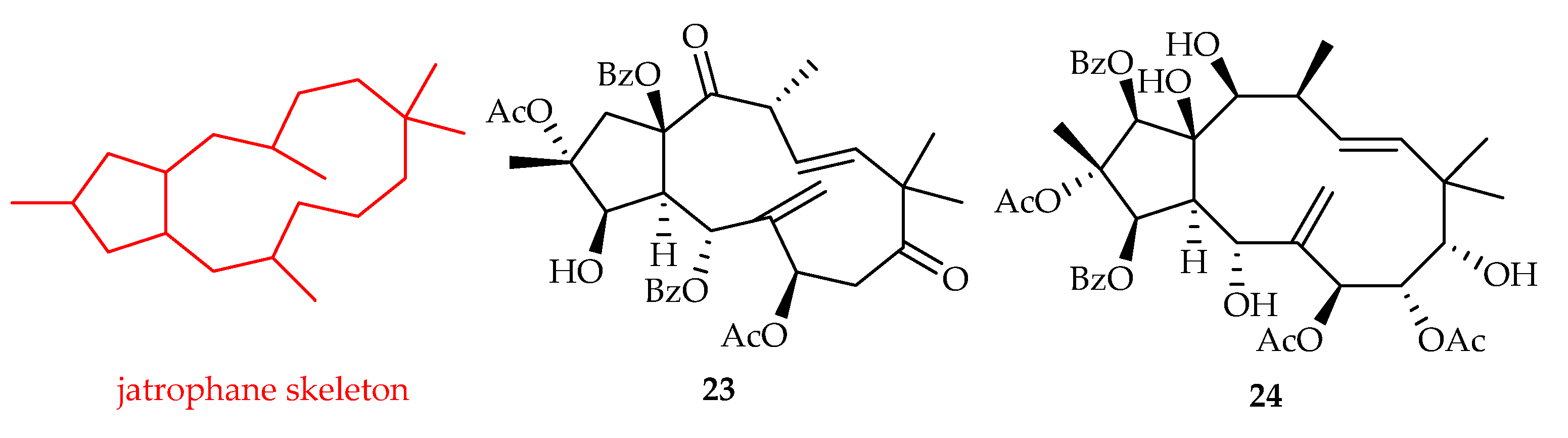
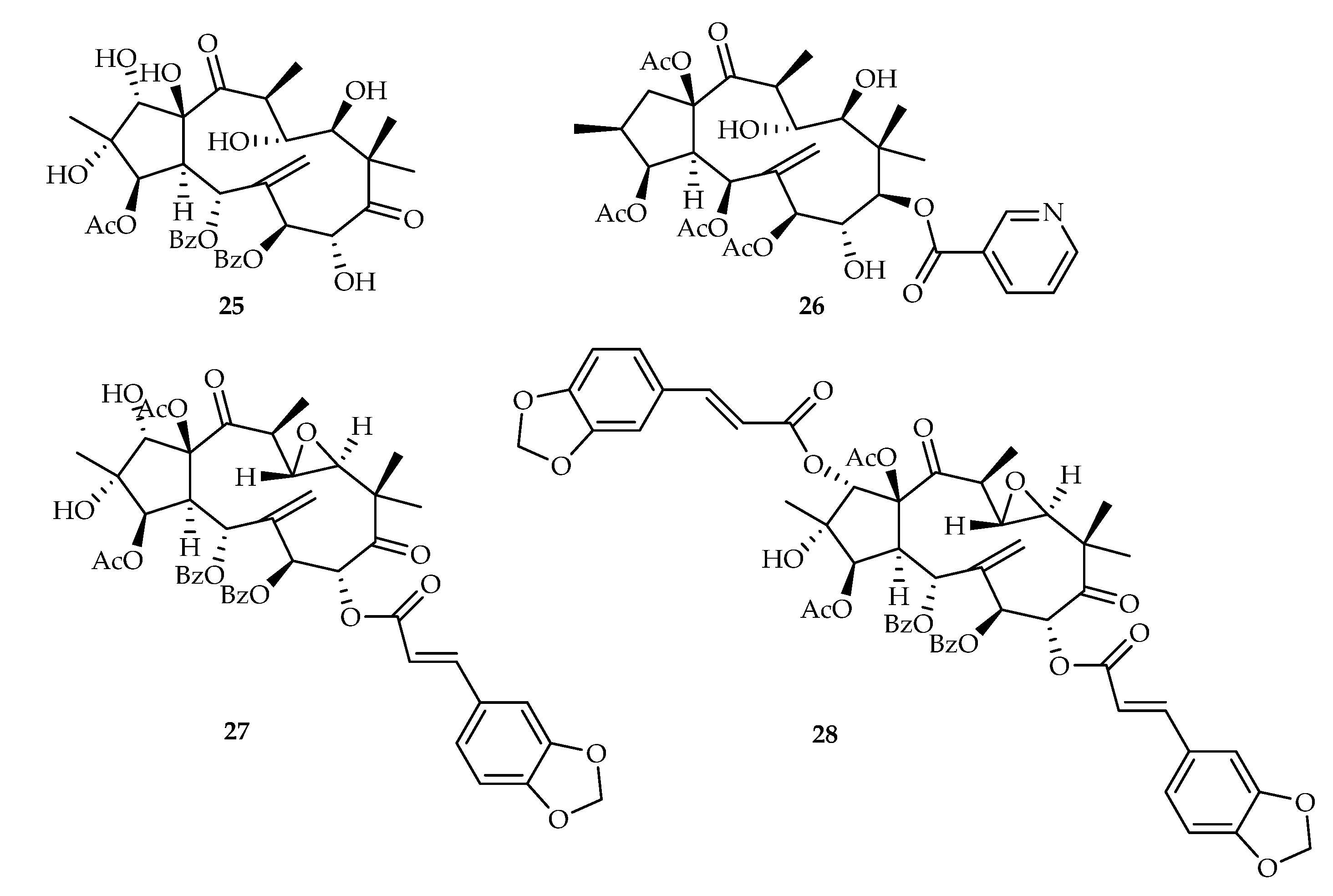
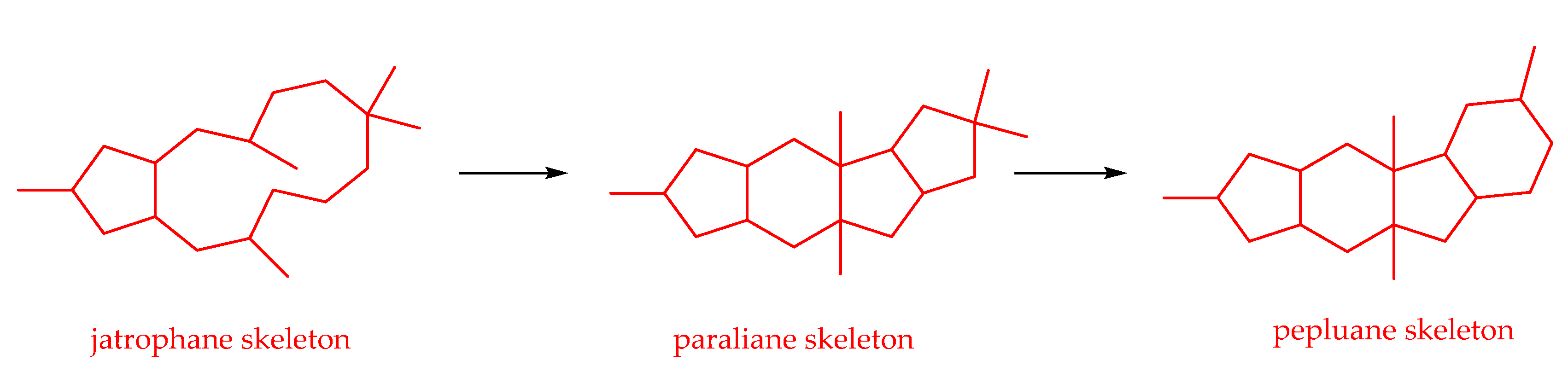
2.2.5. Jatrophane-Type Diterpenoids
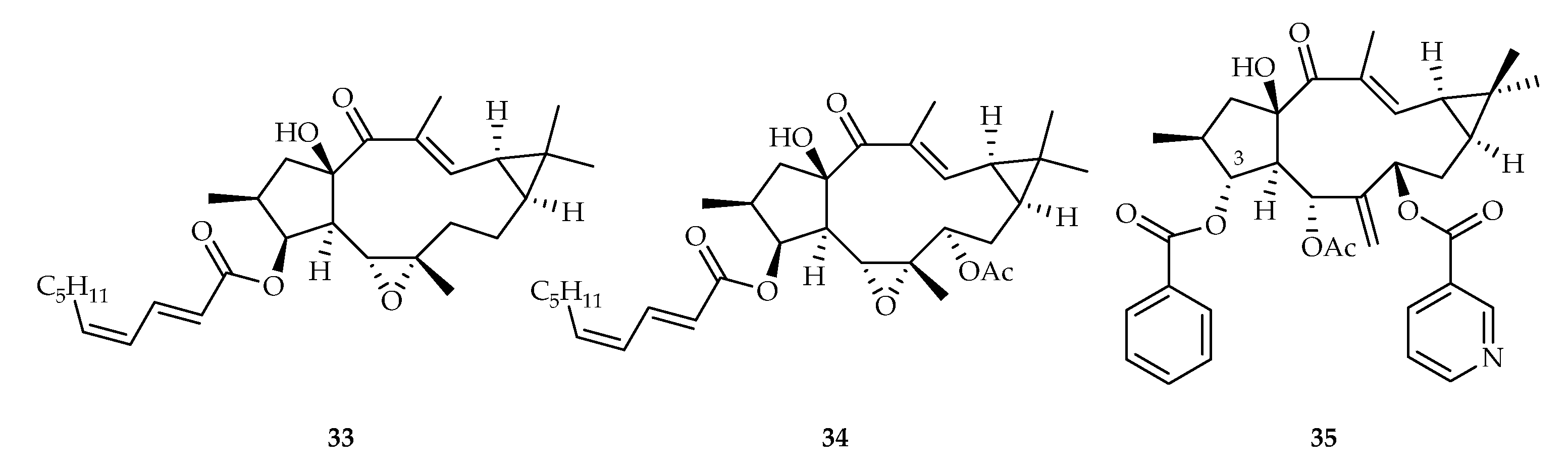
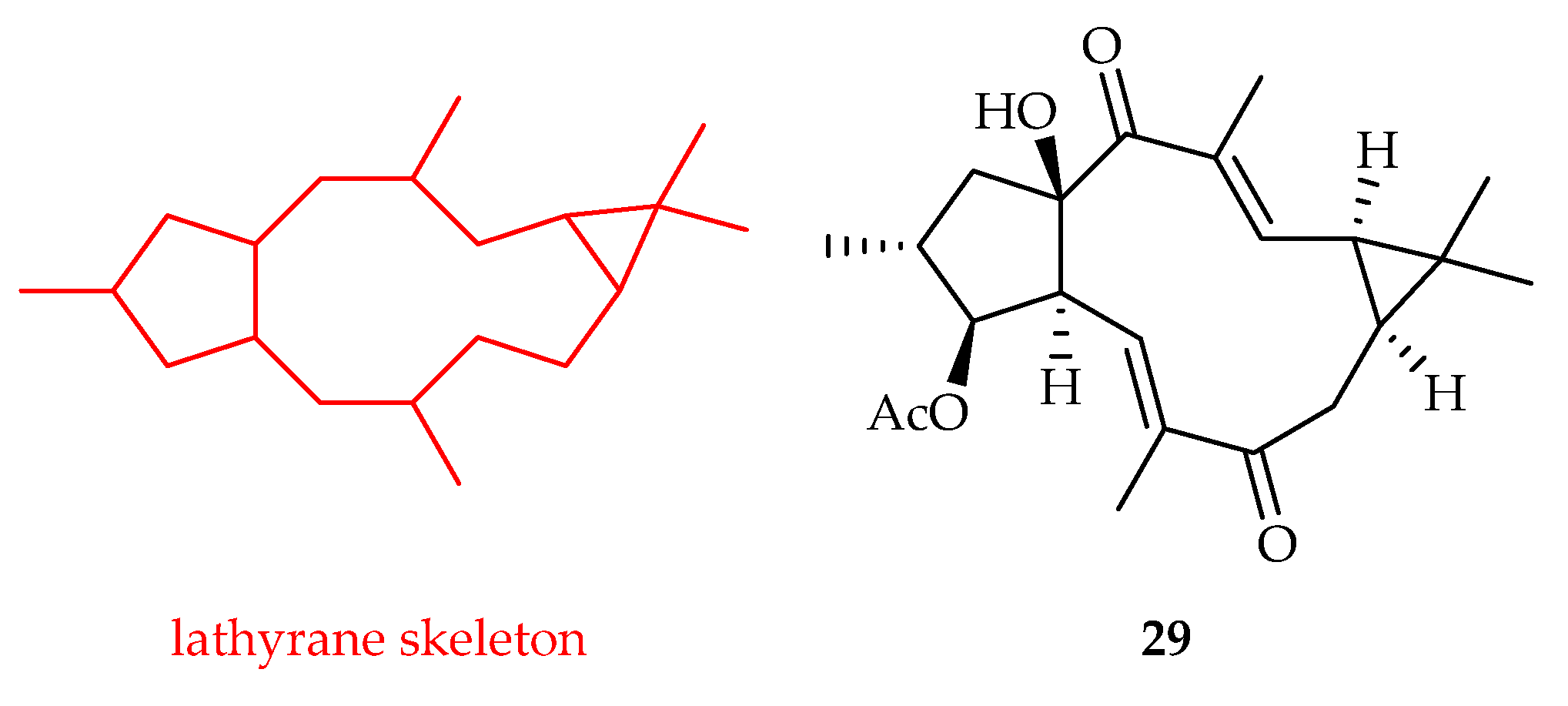
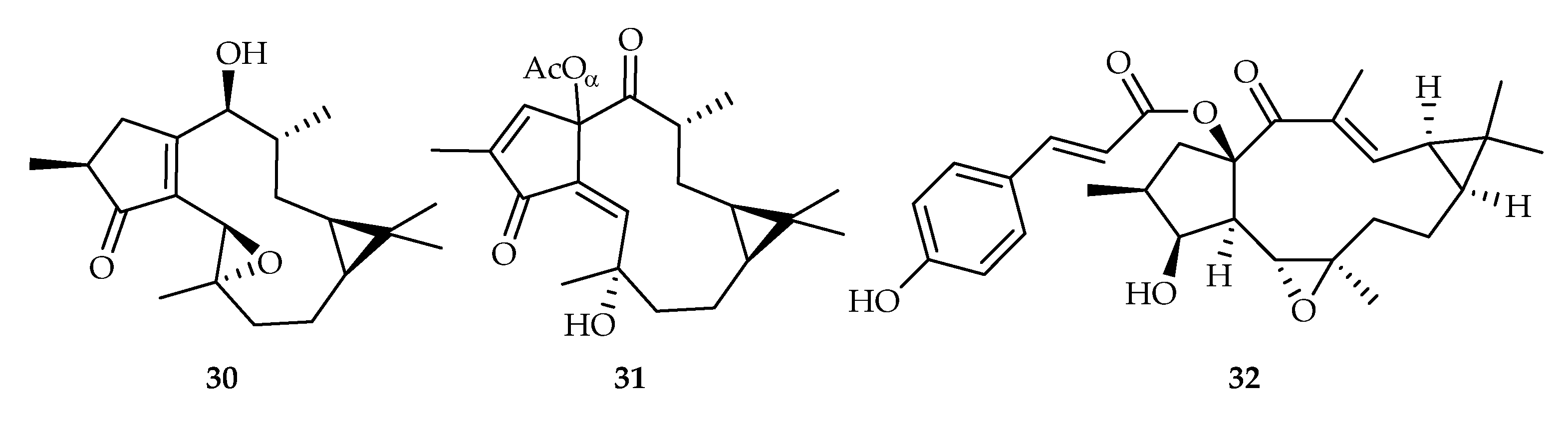
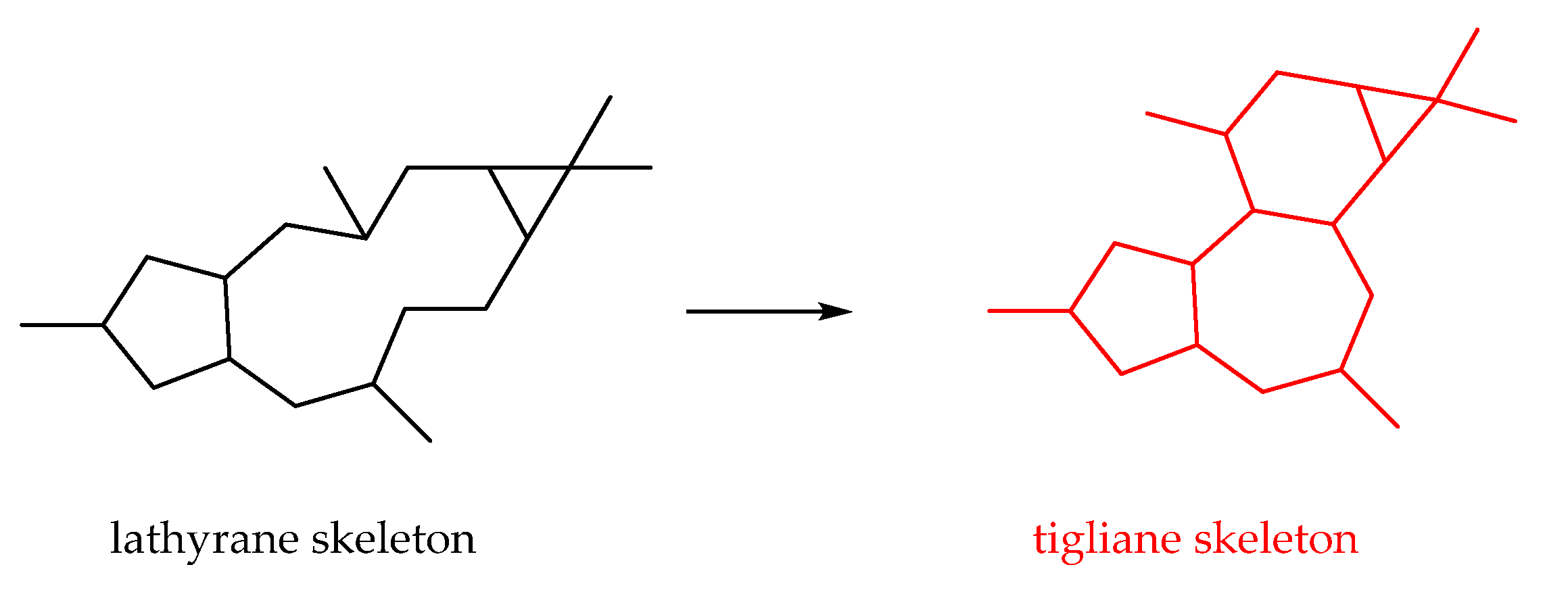
2.2.6. Lathyrane-Type Diterpenoids
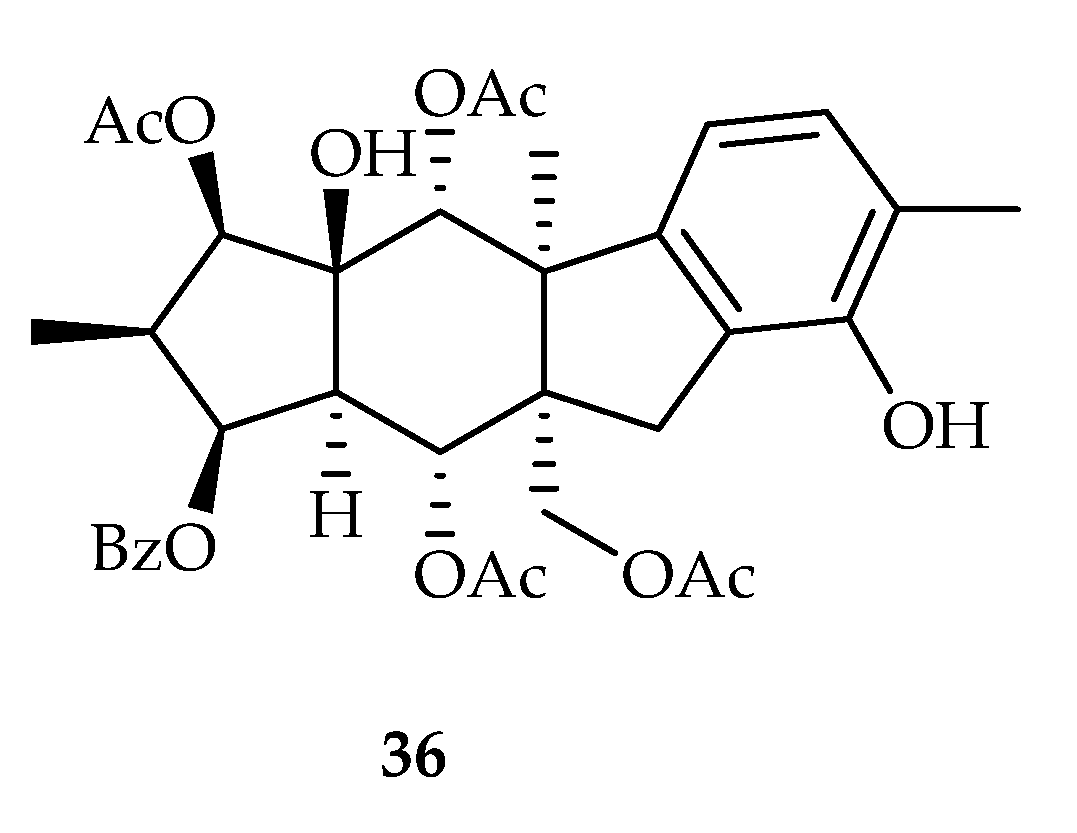
2.2.7. Pepluane-Type Diterpenoids
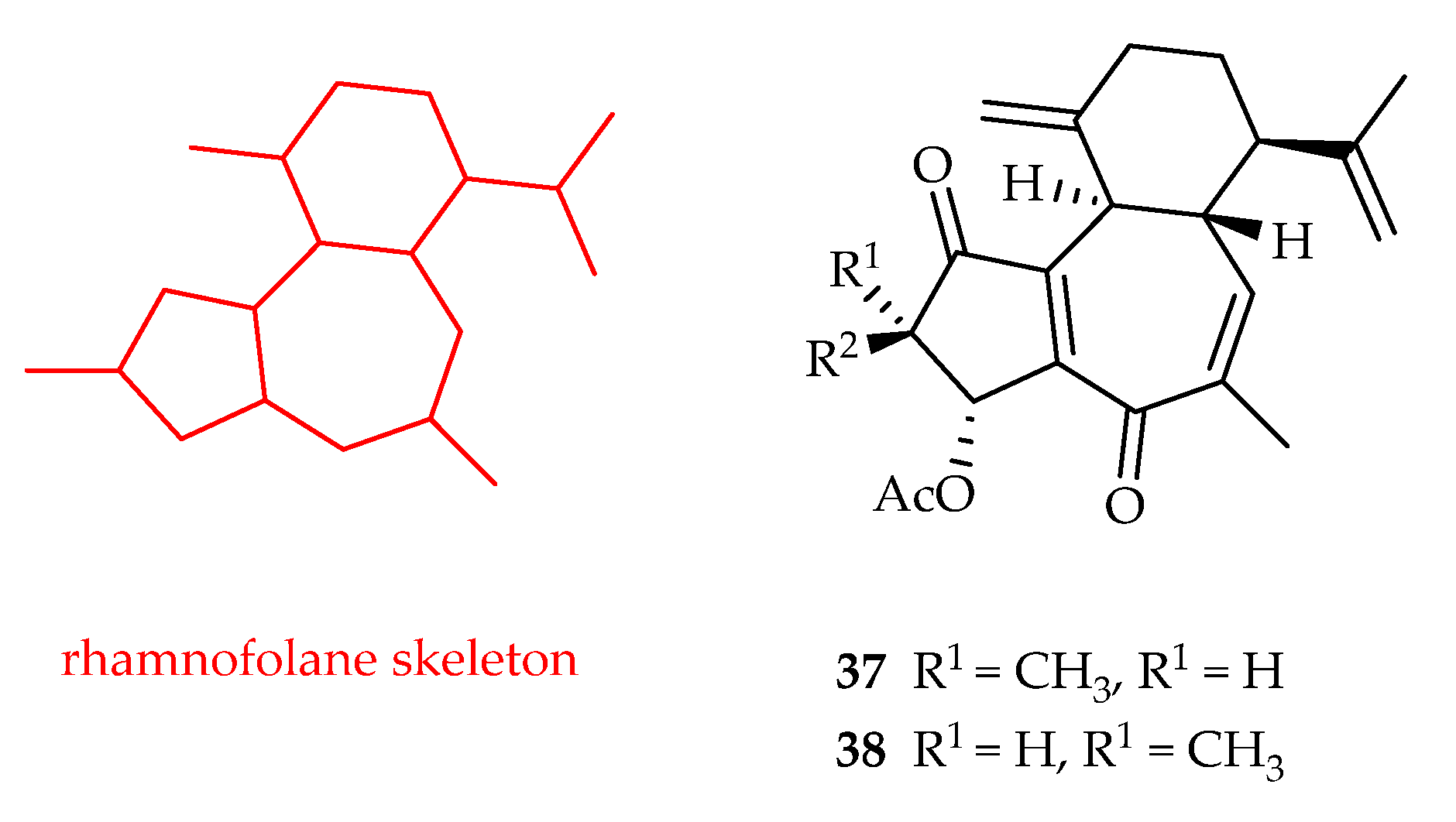
2.2.8. Rhamnofolane-Type Diterpenoids
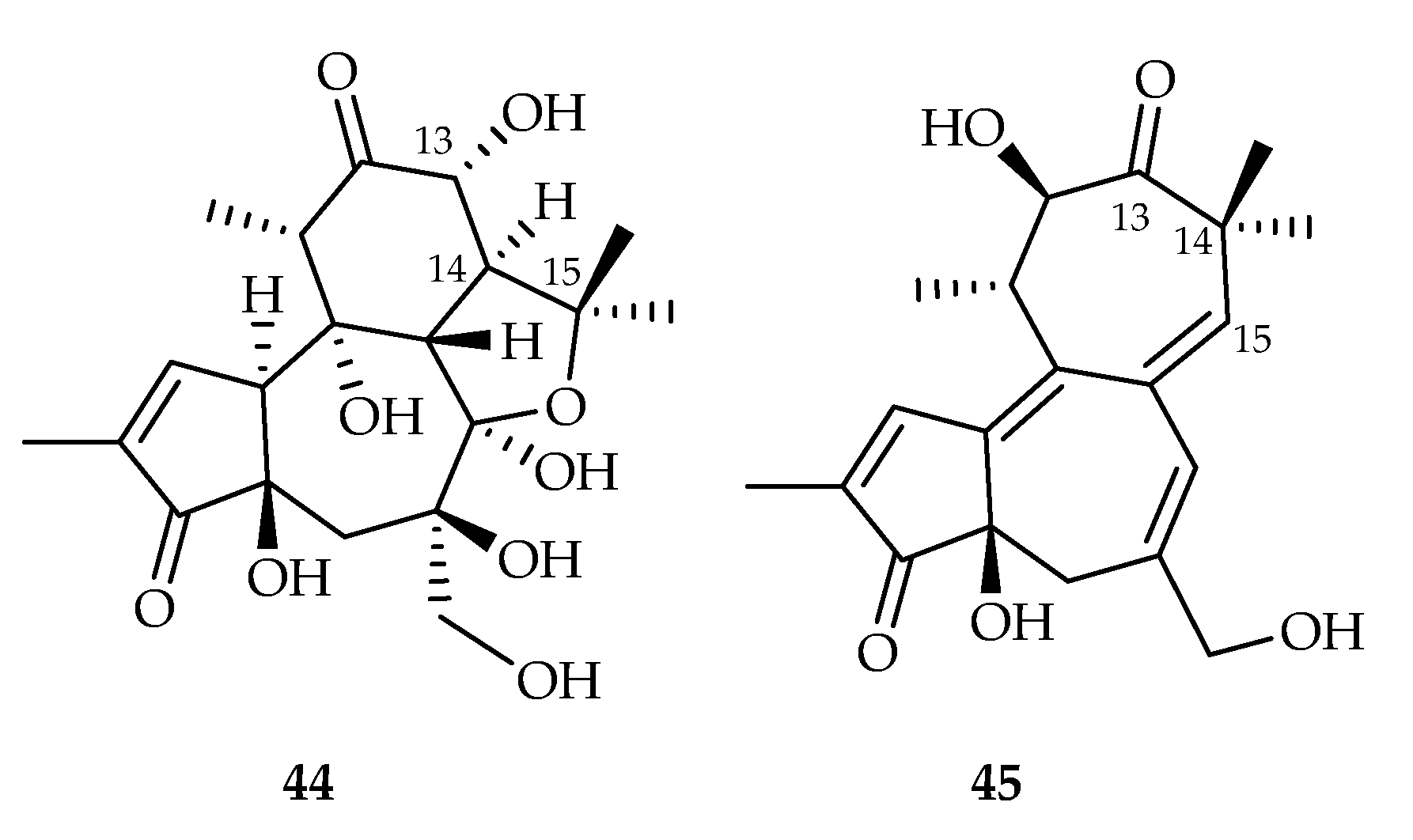
2.2.9. Tigliane-Type Diterpenoids
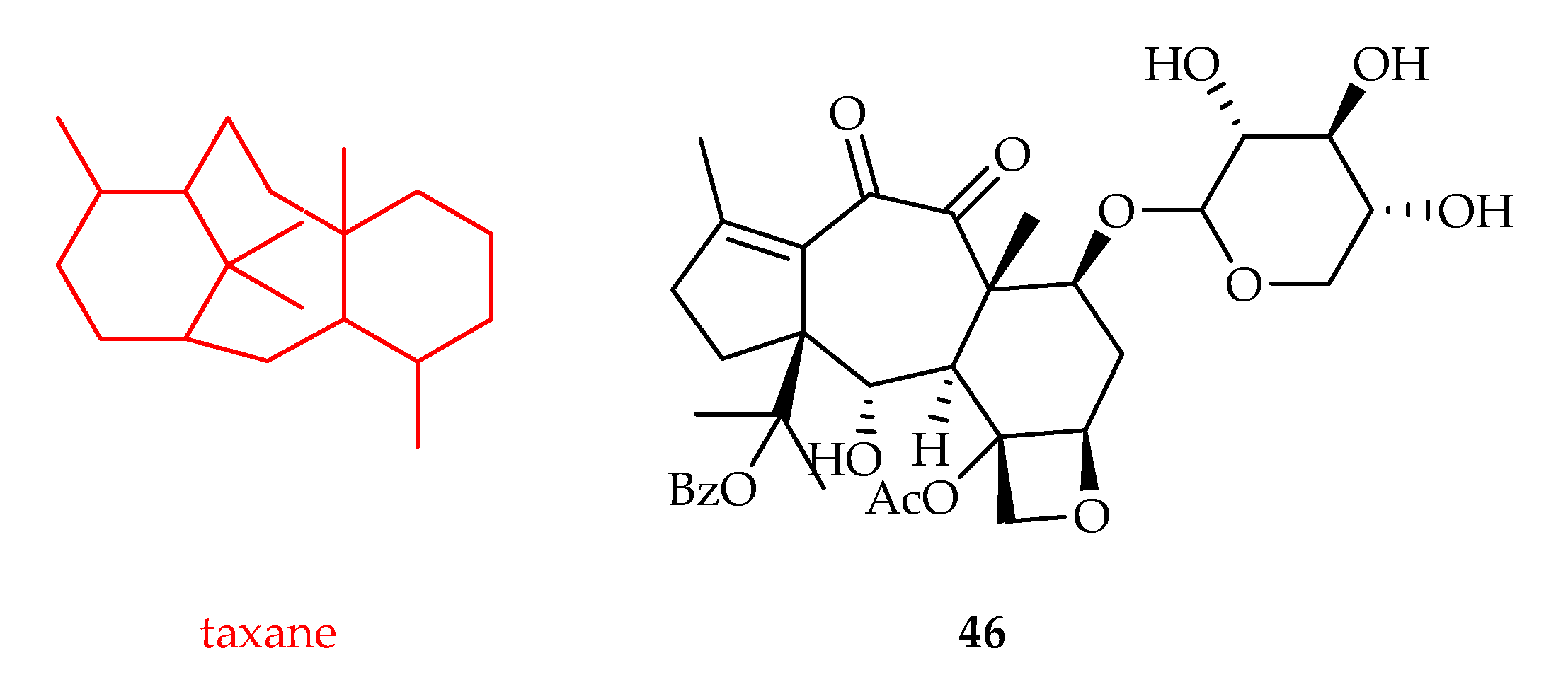
2.2.10. Taxane-Type Diterpenoids
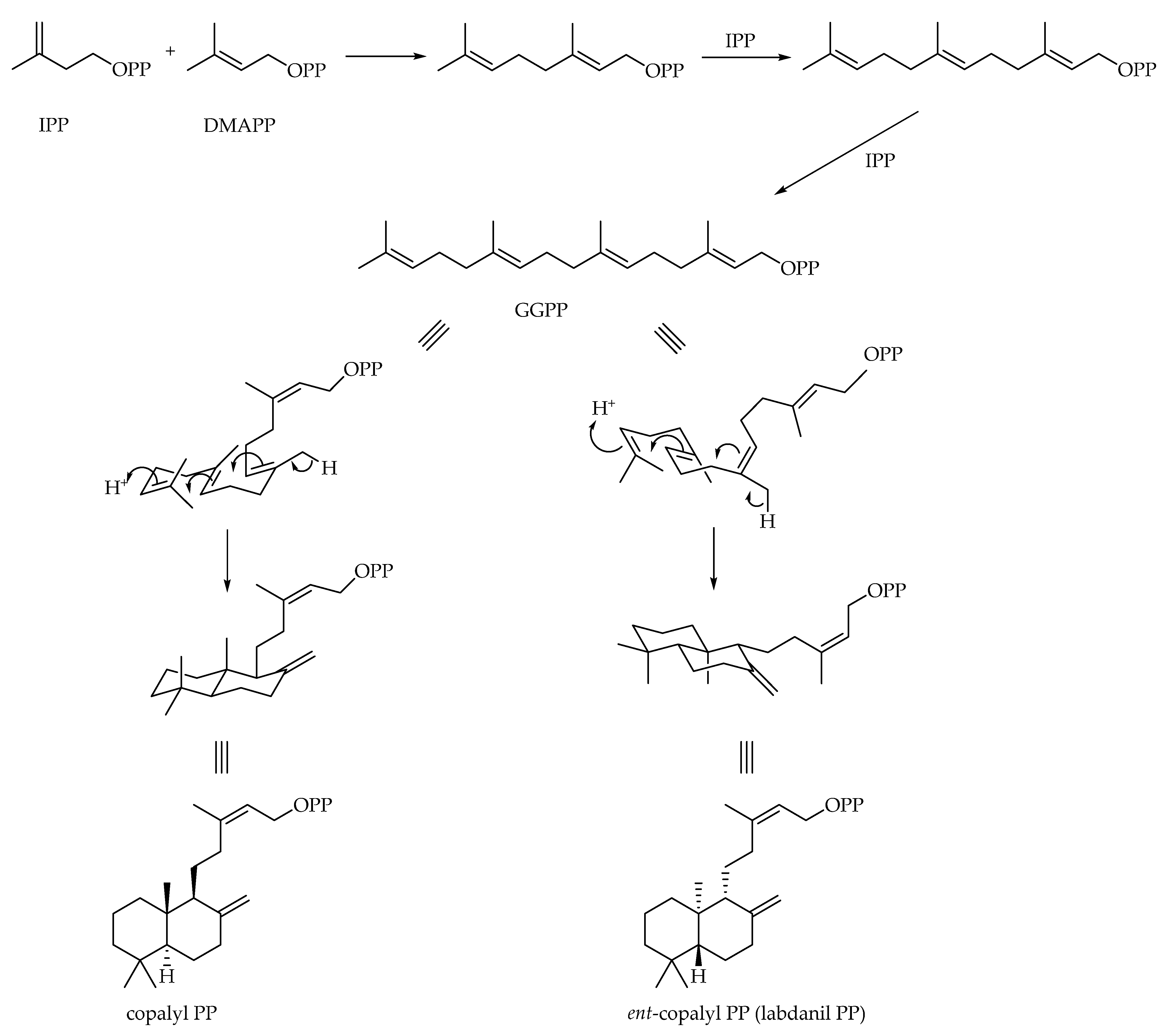
3. Labdane-Type Diterpenes
3.1. Bicyclic Diterpenes
3.1.1. Clerodane-Type Diterpenoids
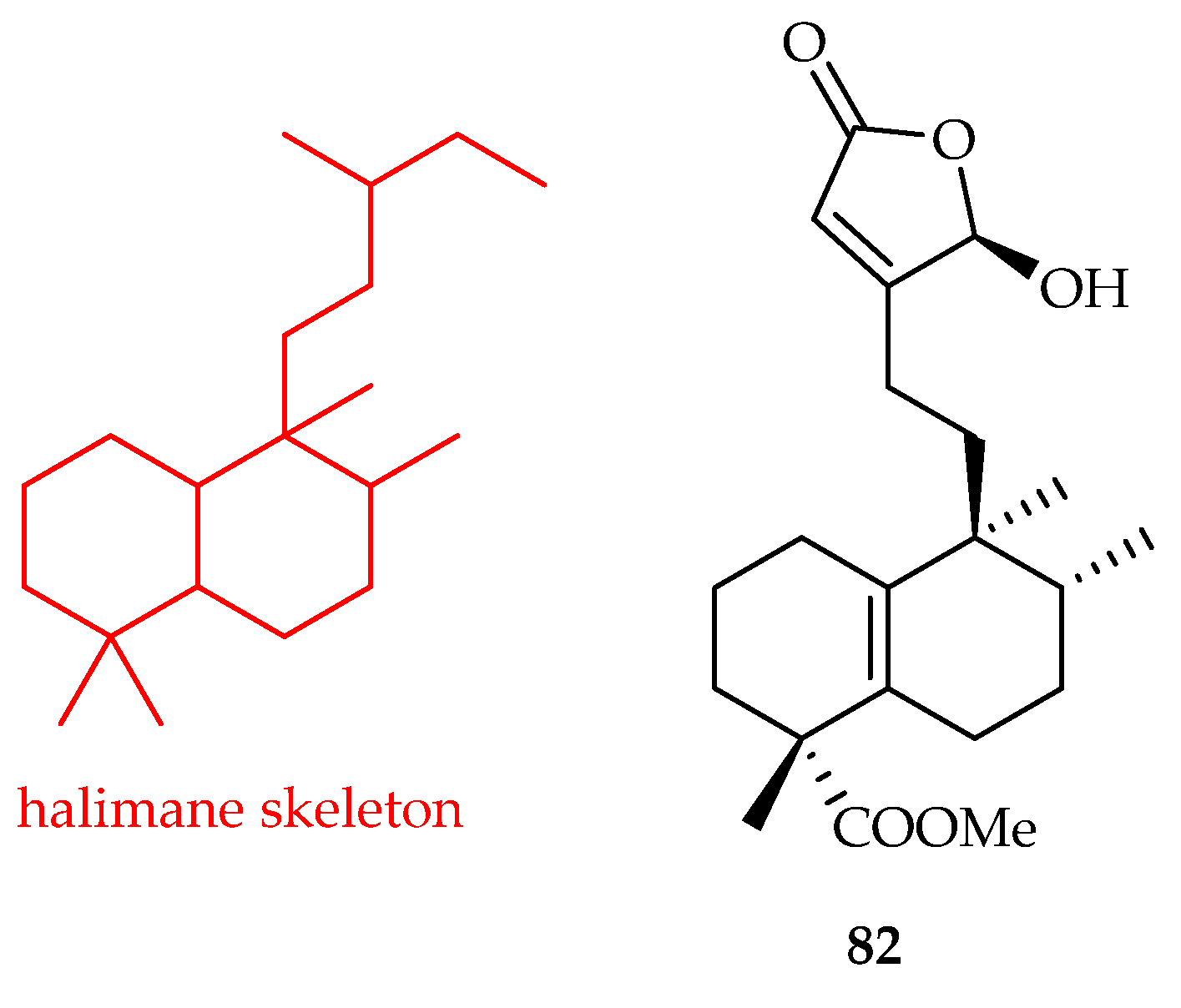
3.1.2. Halimane-Type Diterpenoids
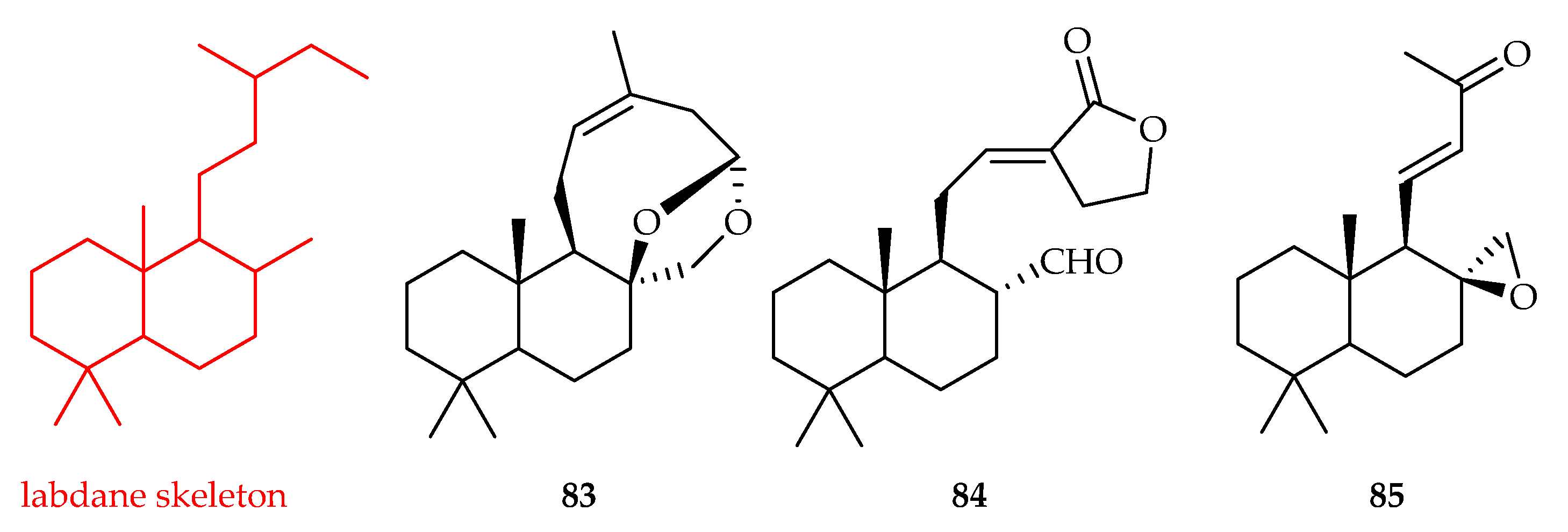
3.1.3. Labdane-Type Diterpenoids
3.2. Tricyclic Diterpenes
3.2.1. Abietane-Type Diterpenoids
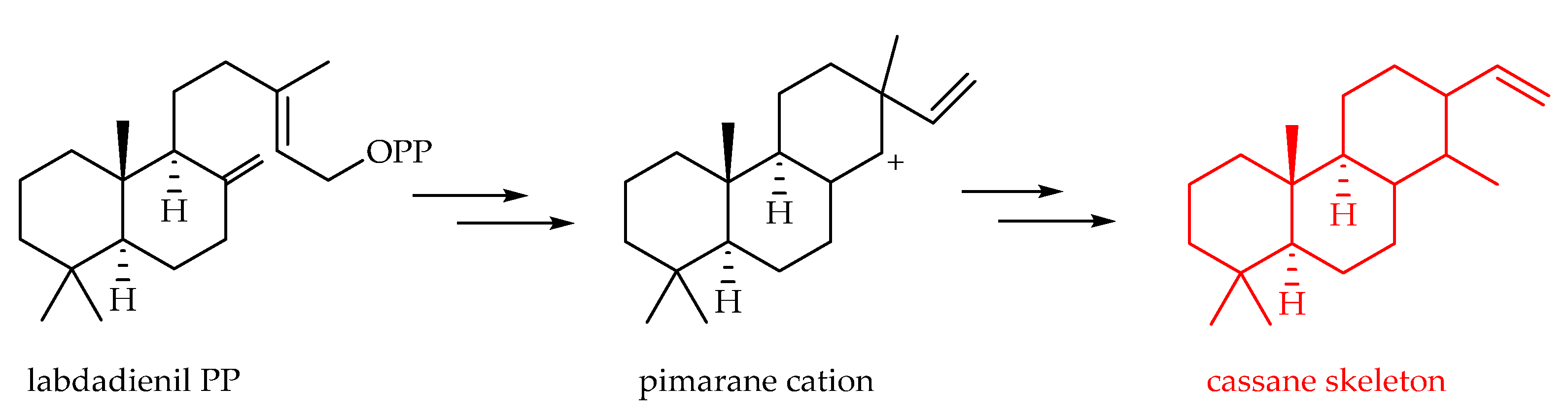
3.2.2. Cassane-Type Diterpenoids
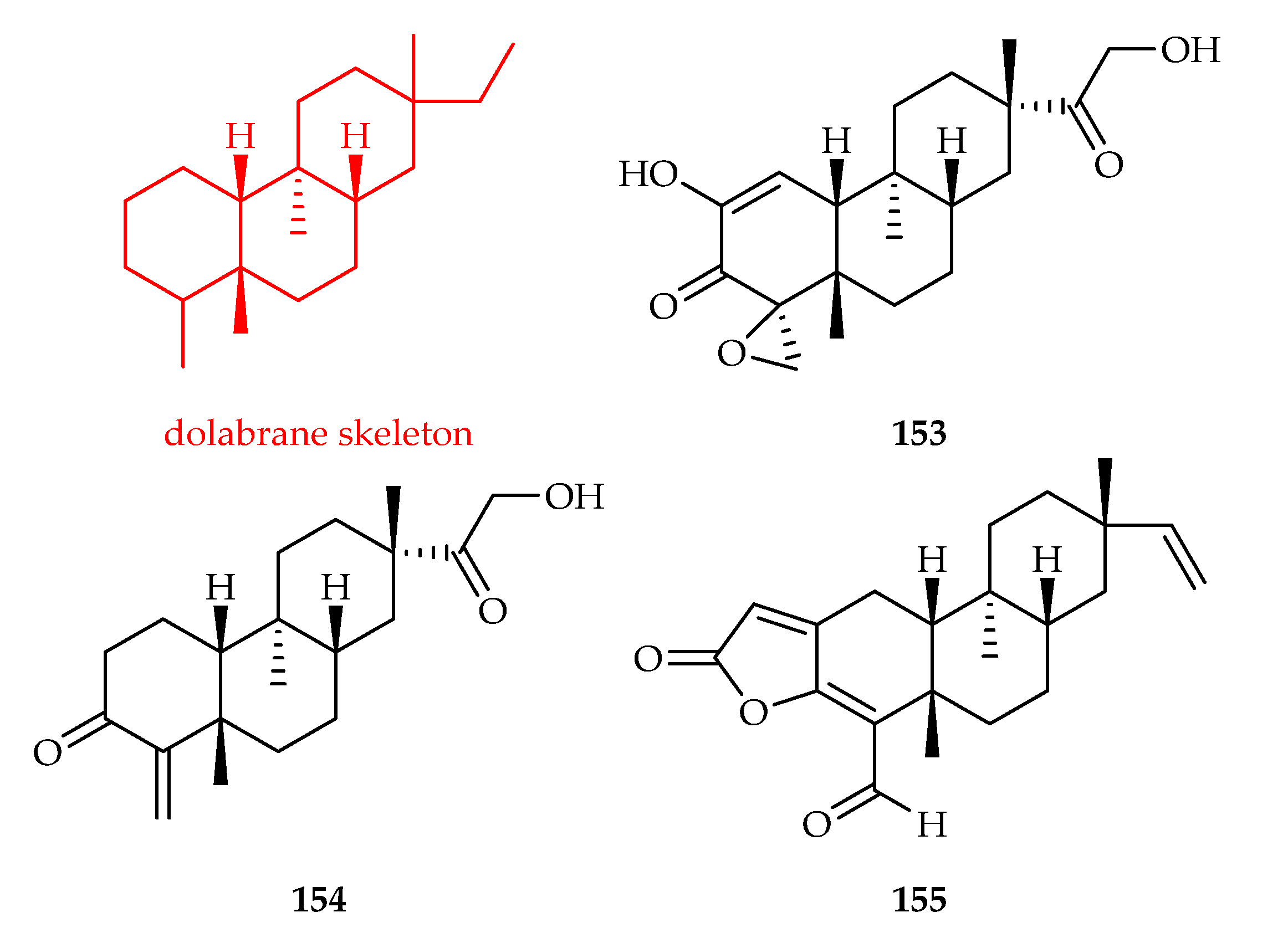
3.2.3. Dolabrane-Type Diterpenoids
3.2.4. Icetexane-Type Diterpenoids
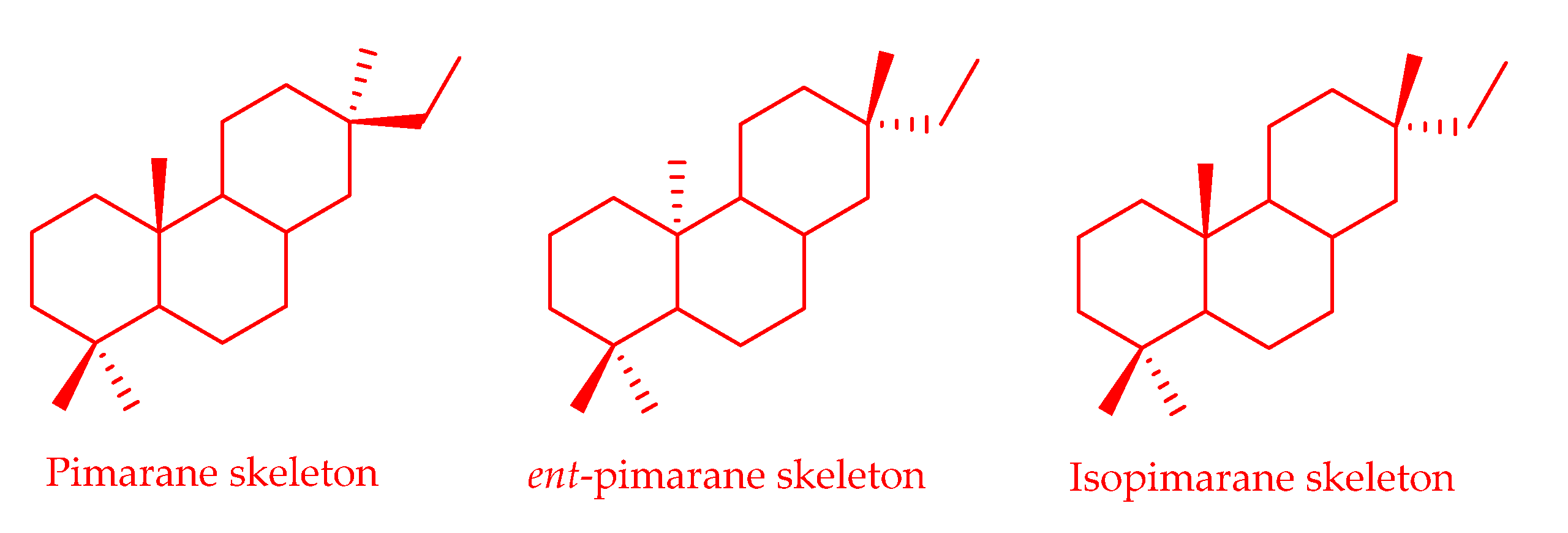
3.2.5. Pimarane-Type Diterpenoids
3.2.6. Podocarpane-Type Diterpenoids
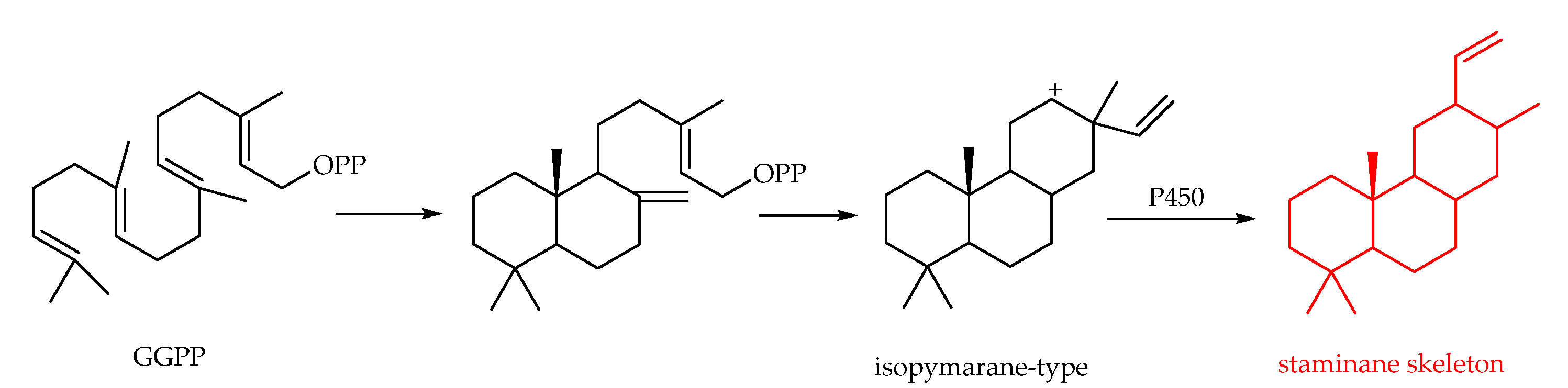
3.2.7. Staminane-Type Diterpenoids
3.3. Tetracyclic Diterpenes
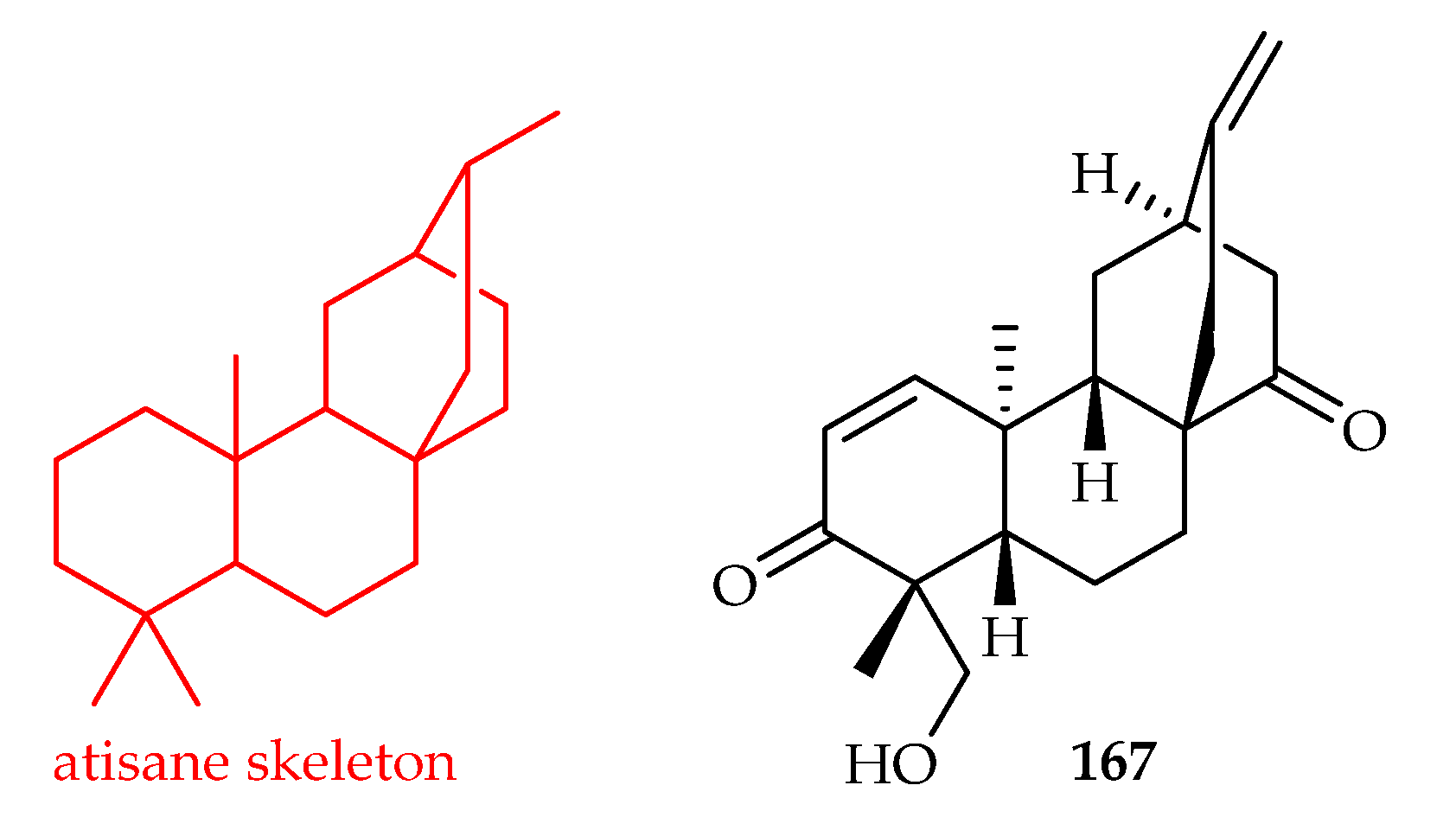
3.3.1. Atisane-Type Diterpenoids
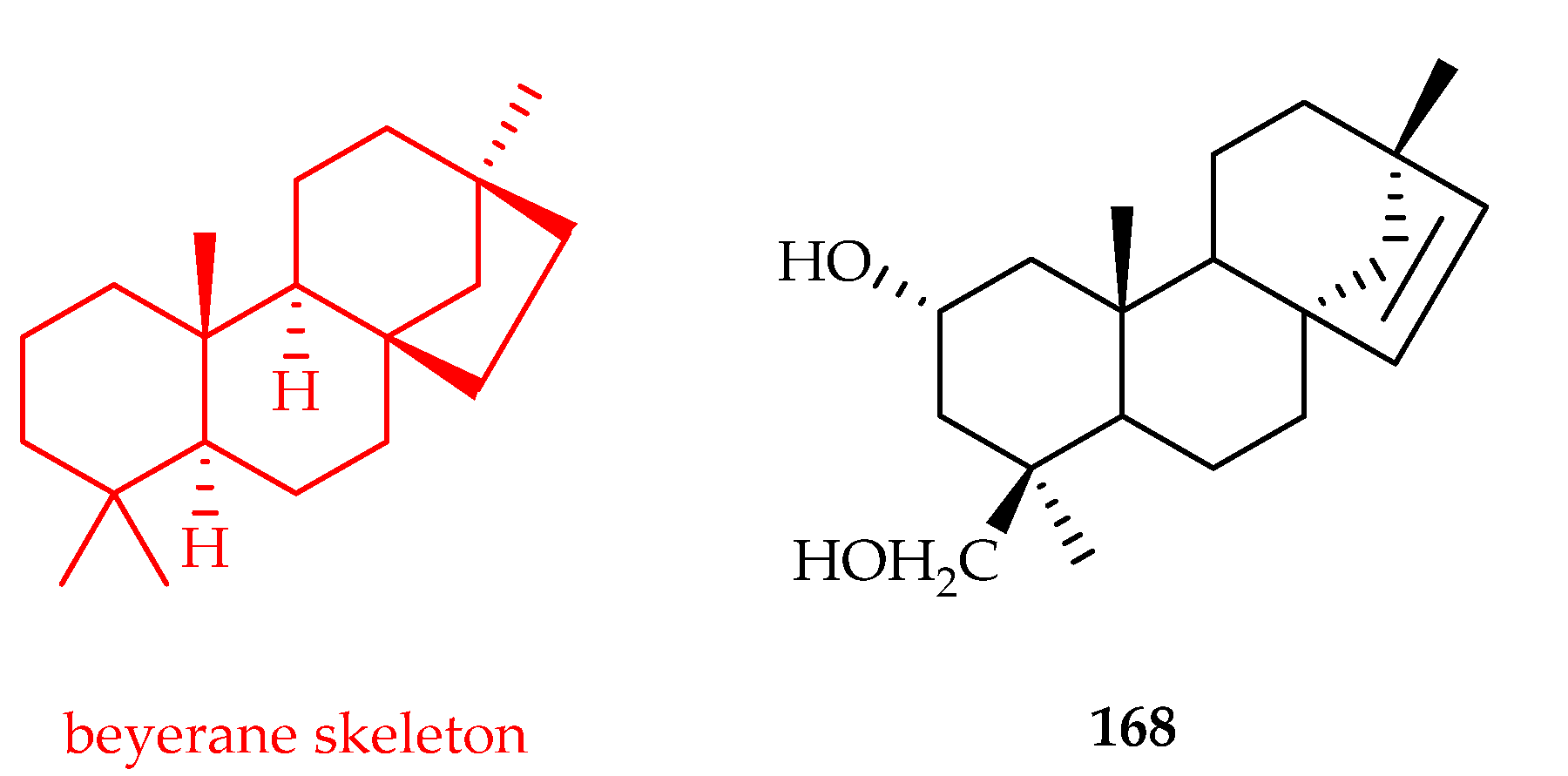
3.3.2. Beyerane-Type Diterpenoids
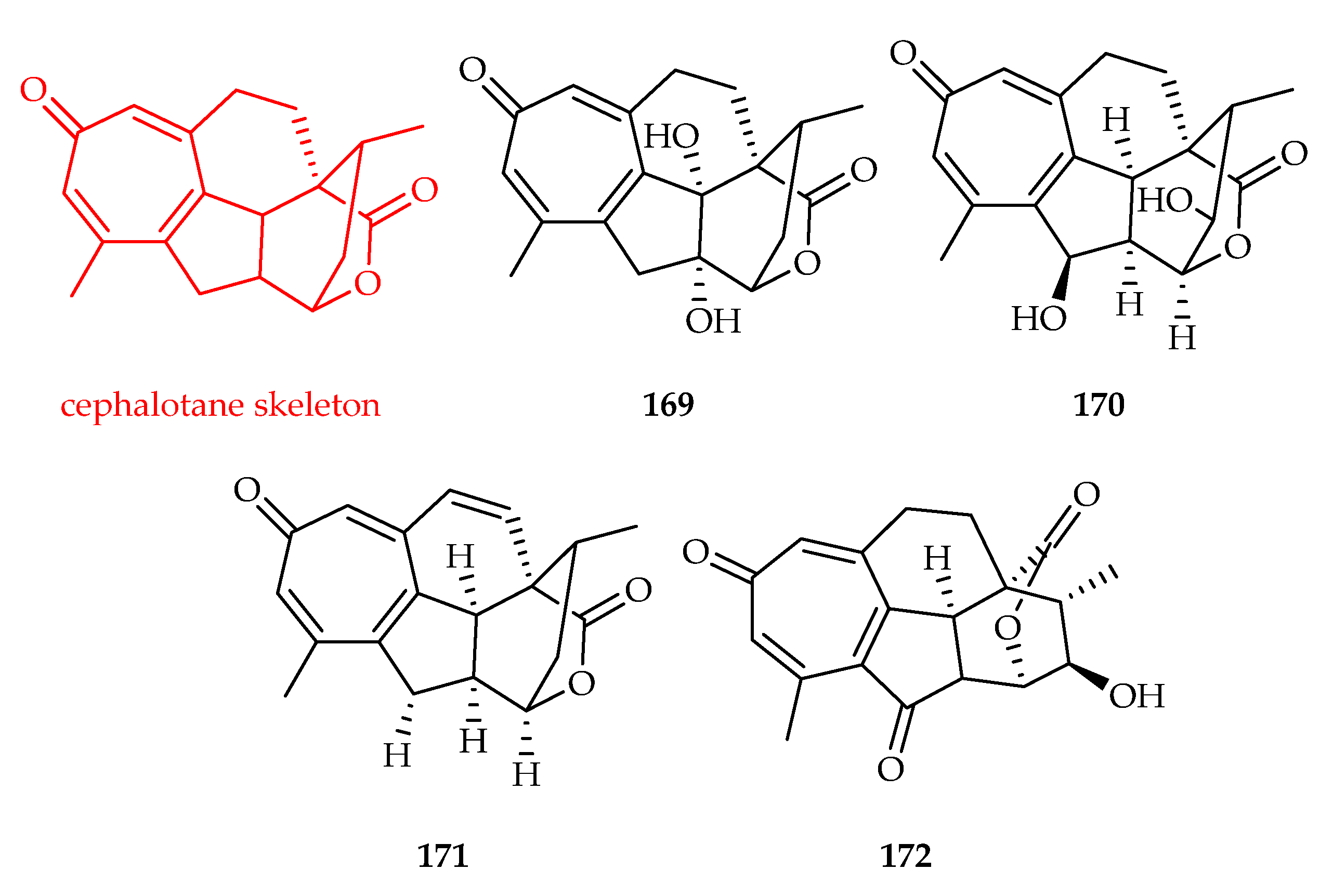
3.3.3. Cephalotane-Type Diterpenoids
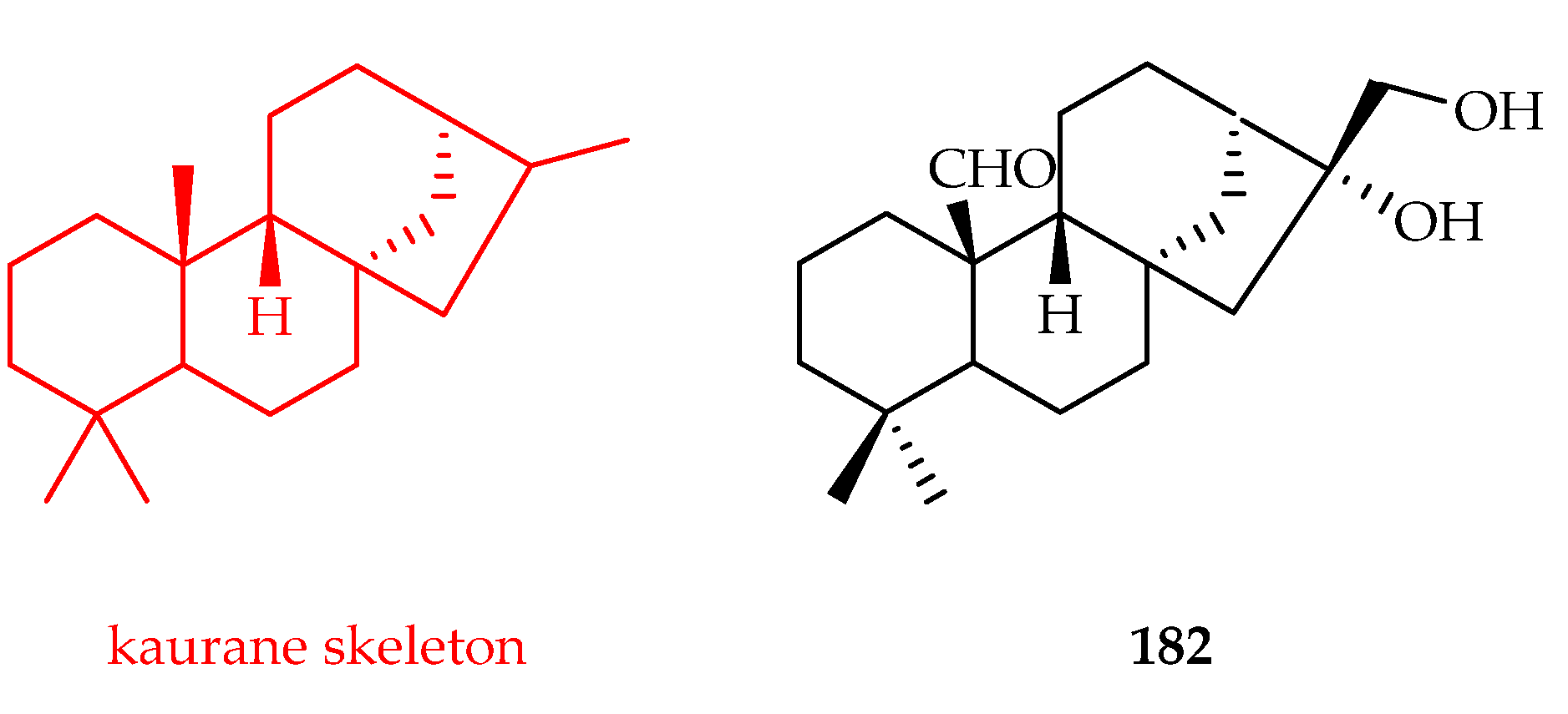
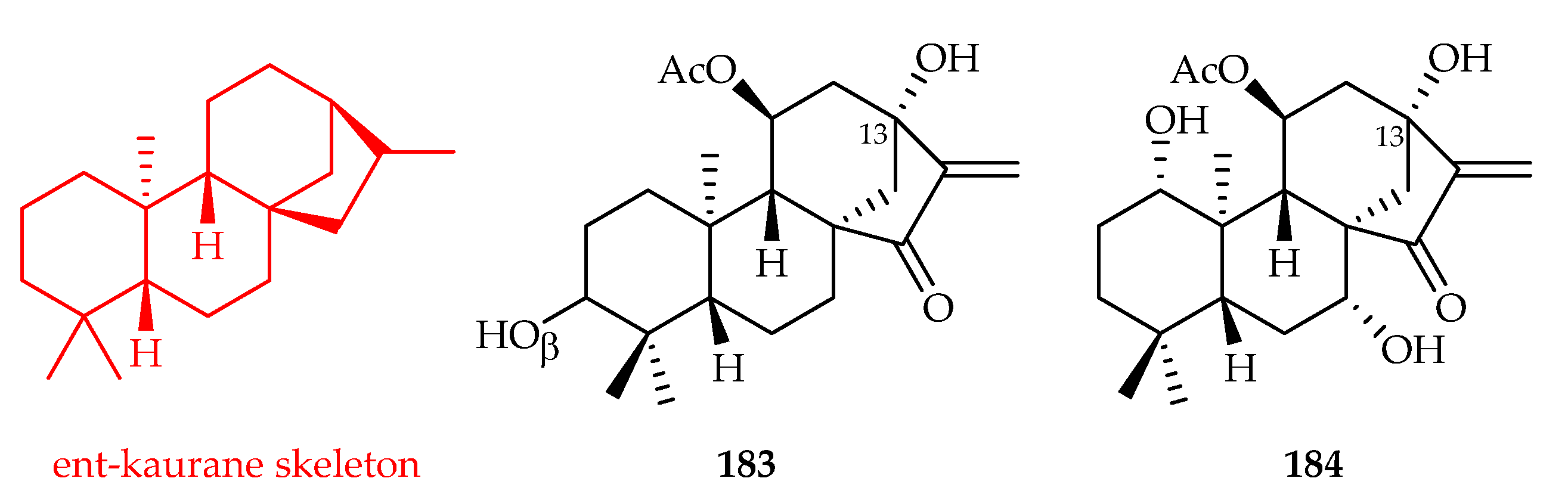
3.3.4. Kaurane-Type Diterpenoids
4. Prenylsesquiterpenes
Prenyleudesmane-Type Diterpenoids
5. Other Diterpenoid Skeletons
5.1. Norditerpenoids and Dinorditerpenoids
5.2. Vibsane-Type Diterpenoid
5.3. Other Skeletons
| Skeleton | Comp. | IC50 (<20 μM) | Cell line | Positive Control (IC50 μM) | Ref. |
|---|---|---|---|---|---|
| Monocyclic diterpenes | 1 | 13.9, 19.3 | ACHN, HeLa, | Vinblastin (28.0, 0.01) | [5] |
| 2 | 10.3, 1.6, 9.2, 11.3 | ACHN, HeLa, SMMC-7721, MCF-7 | Vinblastin (28.0, 0.01, 2.85, 7.5) | [5] | |
| 3 | 9.39, 4.68, 4.24, 12.17 | KB, MCF-7, A549, HCT116 | DOX (0.53, 0.13, 1.12, 0.39) | [6] | |
| 4 | 16.20, 15.41, 8.29 and 6.30 | KB, MCF-7, A549, HCT116 | DOX (0.53, 0.13, 1.12, 0.39) | [6] | |
| Casbane | 5 | 3, 5, 5, 4, 6, 7 | HeLa, HT29, MCF-7, MM96L, K562, NFF | - | [8] |
| 6 | 6, 5, 3, 4, 6, 6 | HeLa, HT29, MCF-7, MM96L, K562, NFF | - | [8] | |
| Daphnane | 7 | 3.0, 6.5 | SW620, RKO | 5-FU (10, >10) | [10] |
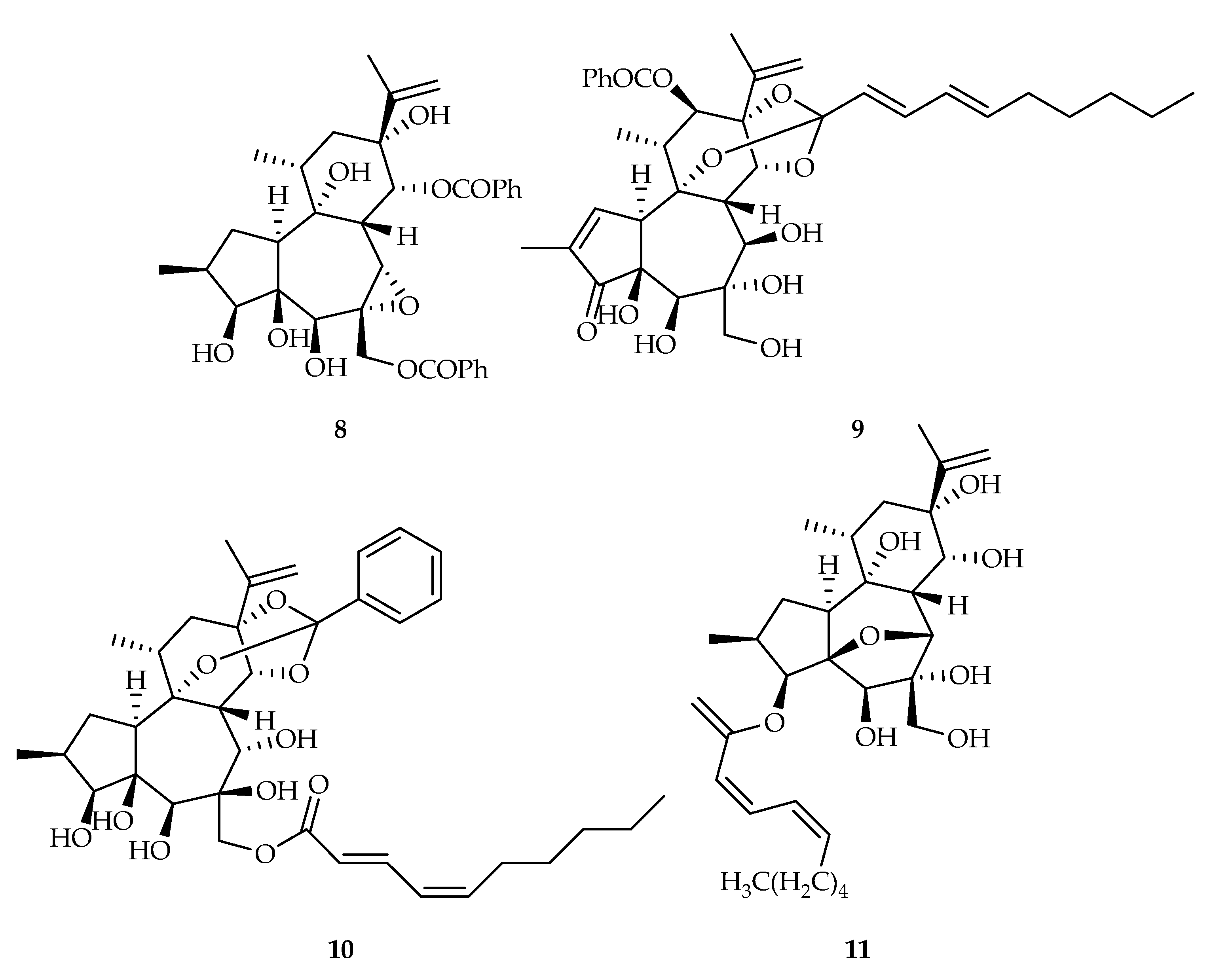
| 8 | 15.76, 18.30 | A549, MCF-7 | DDP (5.33), DOX (0.24) | [11] | |
| 9 | 9.67, 10.44, 15.57 | A549, Hep3B, MCF-7 | DDP (5.33), sorafenib (11.27), DOX (0.24) | [11] | |
| 10 | 9.14, 7.83, 16.55 | A549, Hep3B, MCF-7 | DDP (5.33), sorafenib (11.27), DOX (0.24) | [11] | |
| 11 | 6.84 | Hep3B | Sorafenib (11.27) | [11] | |
| 12 | 13.42 | HepG2 | Oxaliplatin (24.26) | [12] | |
| 13 | 17.59, 15.59, 14.99, 13.24 | HepG2, HL-60, K562, HeLa | DDP (7.12, 10.31, 5.63, 1.74) | [13] | |
| 14 | 11.23, 18.34, 12.82, 17.82, 5.31 | HepG2, A375, HL-60, K562, HeLa | DDP (7.12, 3.45, 10.31, 5.63, 1.74) | [13] | |
| Fusicoccane | 15 | 18.0 | HeLa | Etoposide (4.0) | [14] |
| Ingenane | 16 | 14.20, 6.19 | HepG2, DU145 | - | [16] |
| 17 | 15.82, 9.27 | MCF-7, DU145 | - | [16] | |
| 18 | 10.26 | MCF-7 | - | [16] | |
| 19 | 15.37, 15.62 | A375, HMCB | - | [17] | |
| 20 | 12.6, 11.2, 5.05, 4.72, 12.2, 11.5, 2.28, 3.83, 5.23 | HepG2, HepG2/DOX, HCT-15, HCT-15/5-FU, A549, A549/CDDP, A375, RKO, MDA-MB-231 | DOX (0.49, 6.41, 0.77, 3.94, 0.78, 4.25, 1.32, 0.93, 0.26) | [18] | |
| 21 | 5.92 | MV4-11 | Taxol (0.055) | [19] | |
| 22 | 9.8 | HeLa | Parthenolide (2.1) | [20] | |
| Jatrophane | 23 | 17.63, 10.97, 15.49 | NCI-H460, U87, U87-TxR | - | [21] |
| 24 | 9.9 | HeLa | Parthenolide (2.1) | [20] | |
| 25 | 20.19, 7.21 | HepG2, DU145 | - | [16] | |
| 26 | 15.25, 13.24, 7.24 | MCF-7, HepG2, DU145 | - | [16] | |
| 27 | 11.25, 9.47, 8.29 | MCF-7, HepG2, DU145 | - | [16] | |
| 28 | 6.29, 10.07, 4.19 | MCF-7, HepG2, DU145 | - | [16] | |
| Latyrane | 29 | 8.08, 14.64 | Saos-2, MG-63 | 5-FU (19.01, 25.00) | [22] |
| 30 | 18.4, 15.8 | A549, HeLa | DOX (1.23, 0.52) | [23] | |
| 31 | 12.5 | A549 | DOX (0.52) | [23] | |
| 32 | 11.3 | C4-2B | DOX (0.23) | [24] | |
| 33 | 12.29 | MV4-11 | Taxol (0.055) | [19] | |
| 34 | 14.80 | MV4-11 | Taxol (0.055) | [19] | |
| 35 | 9.43, 13.22 | MCF-7, HepG2 | DDP (16.55, 2.29) | [25] | |
| Pepluane | 36 | 5.8 | HeLa | Parthenolide (2.1) | [20] |
| Rhamnofolane | 37 | 8.21, 6.71, 13.8, 19.1 | A549, MDAMB231, HepG2, HepG2-DOX | DOX (1.23, 0.34, 0.76, 30.5) | [23] |
| 38 | 2.69, 4.22, 6.44, 4.20, 5.80 | A549, HeLa, MDAMB231, HepG2, HepG2-DOX | DOX (1.23, 0.52, 0.34, 0.76, 30.5) | [23] | |
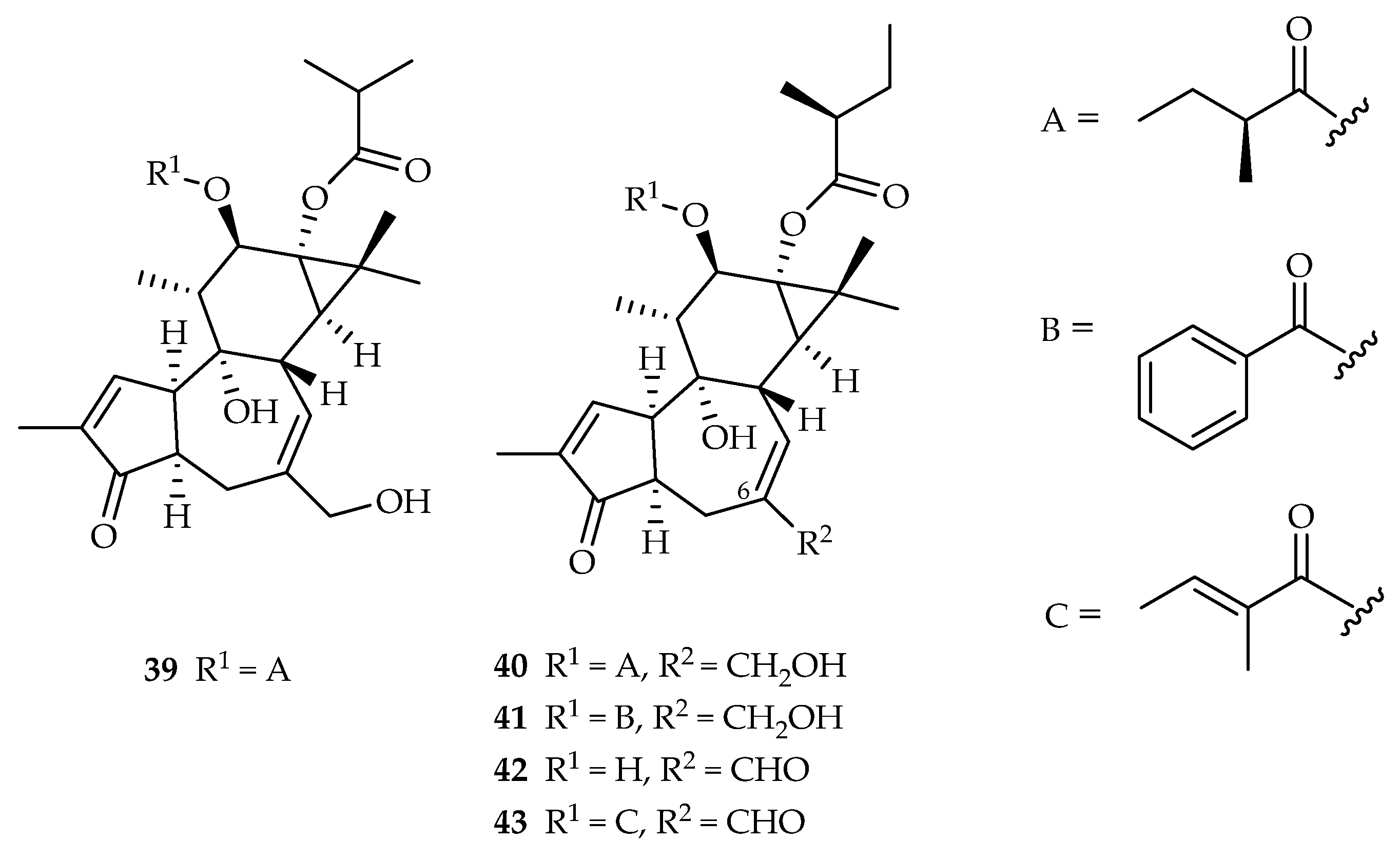
| Tigliane | 39 | 3.7 | A549 | ADR (0.4) | [28] |
| 40 | 4.6, 18.9 | A549, HL-60 | ADR (0.4, 0.08) | [28] | |
| 41 | 4.1 | A549 | ADR (0.4) | [28] | |
| 42 | 0.9 | A549 | ADR (0.4) | [28] | |
| 43 | 1.3, 2.4 | A549, HL-60 | ADR (0.4, 0.08) | [28] | |
| 44 | 0.20, 17.60 | K562, MCF-7 | Paclitaxel (3.67, 7.56) | [29] | |
| 45 | 0.21 | K562 | Paclitaxel (3.67) | [29] | |
| Taxane | 46 | 4.9 | HeLa | Paclitaxel (0.0014) | [30] |
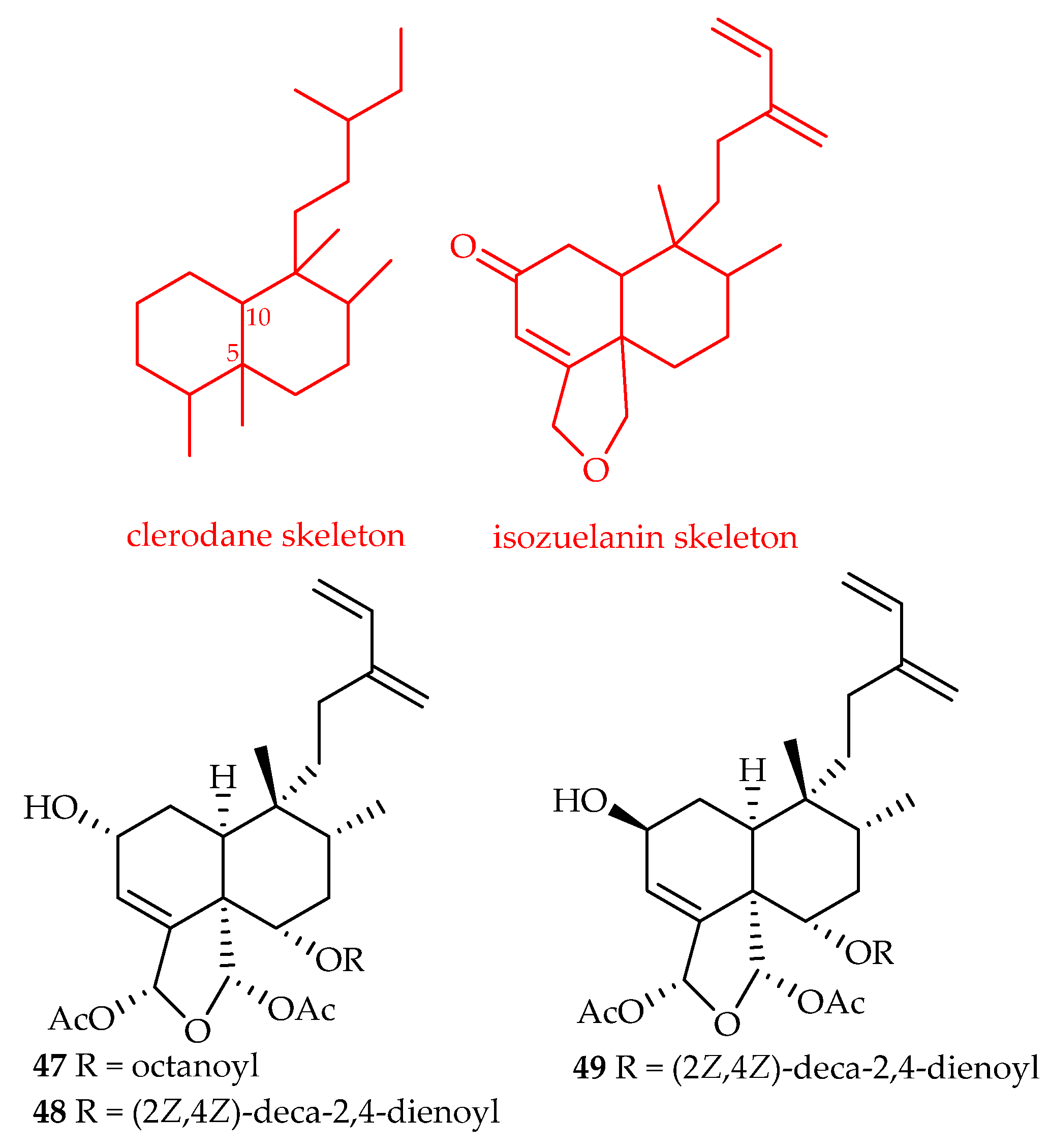
| Clerodane | 47 | 4.7, 5.1, 4.9, 5.0, 4.9 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (6.2, 8.8, 10.4, 6.3, 1926.0) | [32] |
| 48 | 4.6, 4.8, 4.8, 4.8, 4.9 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (6.2, 8.8, 10.4, 6.3, 1926.0) | [32] | |
| 49 | 4.8, 5.1, 5.2, 4.9, 4.8 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (6.2, 8.8, 10.4, 6.3, 1926.0) | [32] | |
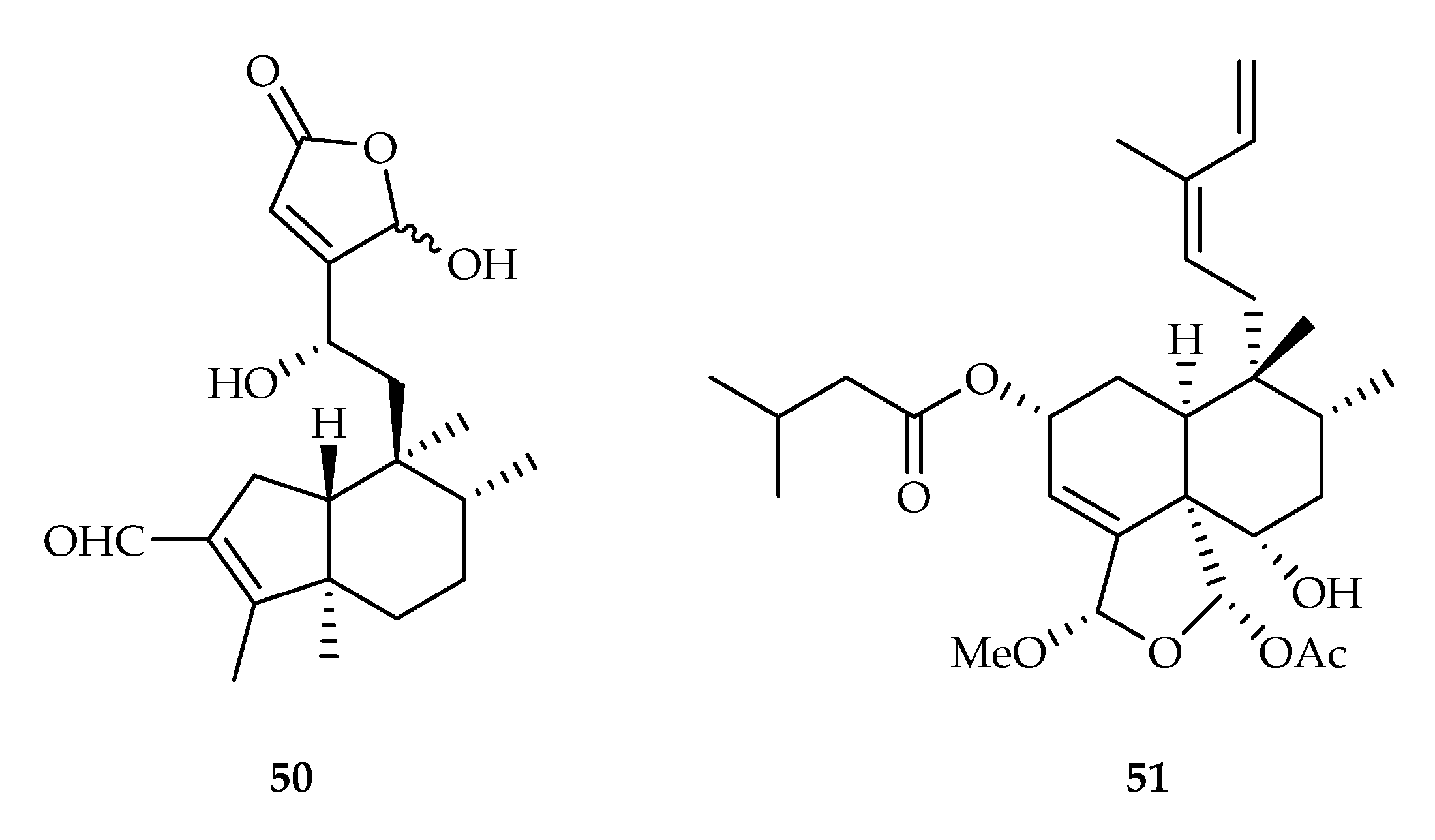
| 50 | 10.0, 6.4, 11.0, 9.9, 11.4 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (5.8, 6.4, 8.5, 5.0, 1421.9) | [33] | |
| 51 | 12.84, 12.43, 9.46, 11.21, 11.21 | BT474, Chago-K1, HepG2, KATO-III, SW-620 | DOX (0.64, 0.47, 0.07, 0.85, 0.10) | [34] | |
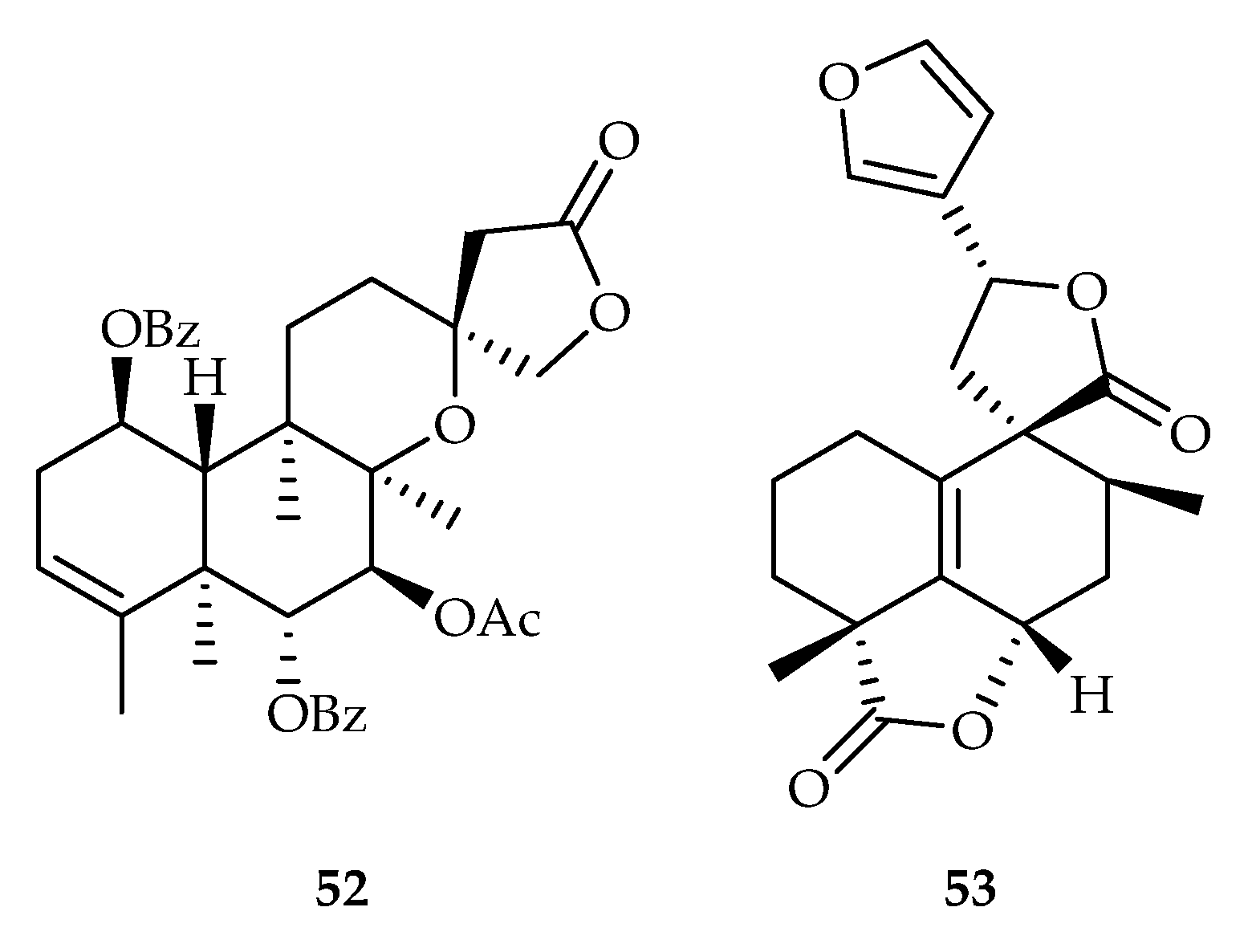
| 52 | 10.4, 15.3 | A549, HL-60 | DDP (7.8, 3.4) | [35] | |
| 53 | 17.91 | Hep3B | 5-FU (10.53) | [36] | |
| 54 | 4.4 | A549 | DDP (-) | [37] | |
| 55 | 4.6 | A549 | DDP (-) | [37] | |
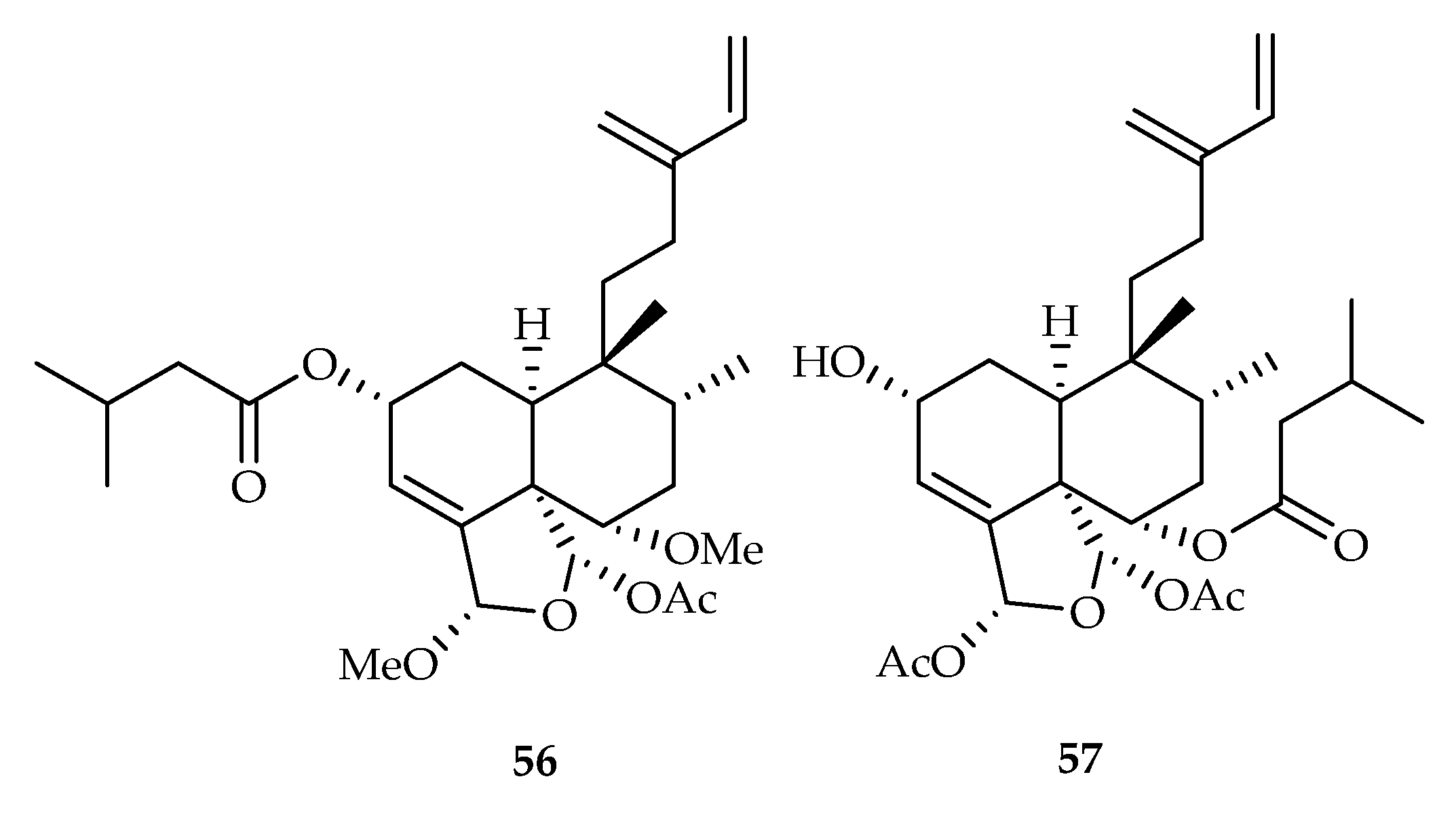
| 56 | 19.0, 15.8 | A549, HeLa | Etoposide (12.5, 36.1) | [38] | |
| 57 | 8.7, 5.3, 8.1 | A549, HeLa, HepG2 | Etoposide (12.5, 36.1, 2.5) | [38] | |
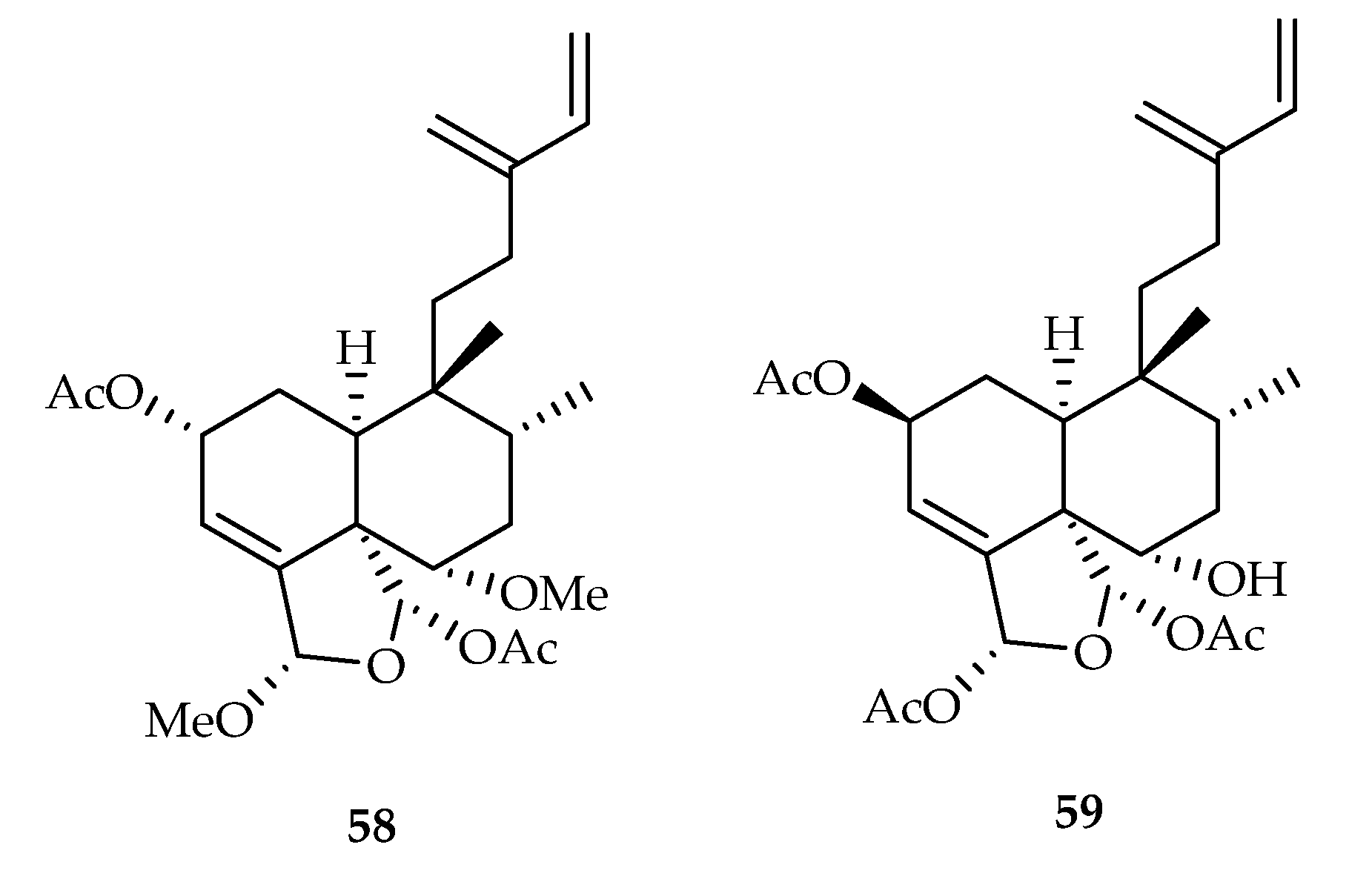
| 58 | 17.9 | HeLa | Etoposide (25.8) | [39] | |
| 59 | 9.7, 10.9, 12.4, 7.2 | HepG2, A549, HeLa, K562 | Etoposide (16.0, 10.4, 36.1, 17.9) | [40] | |
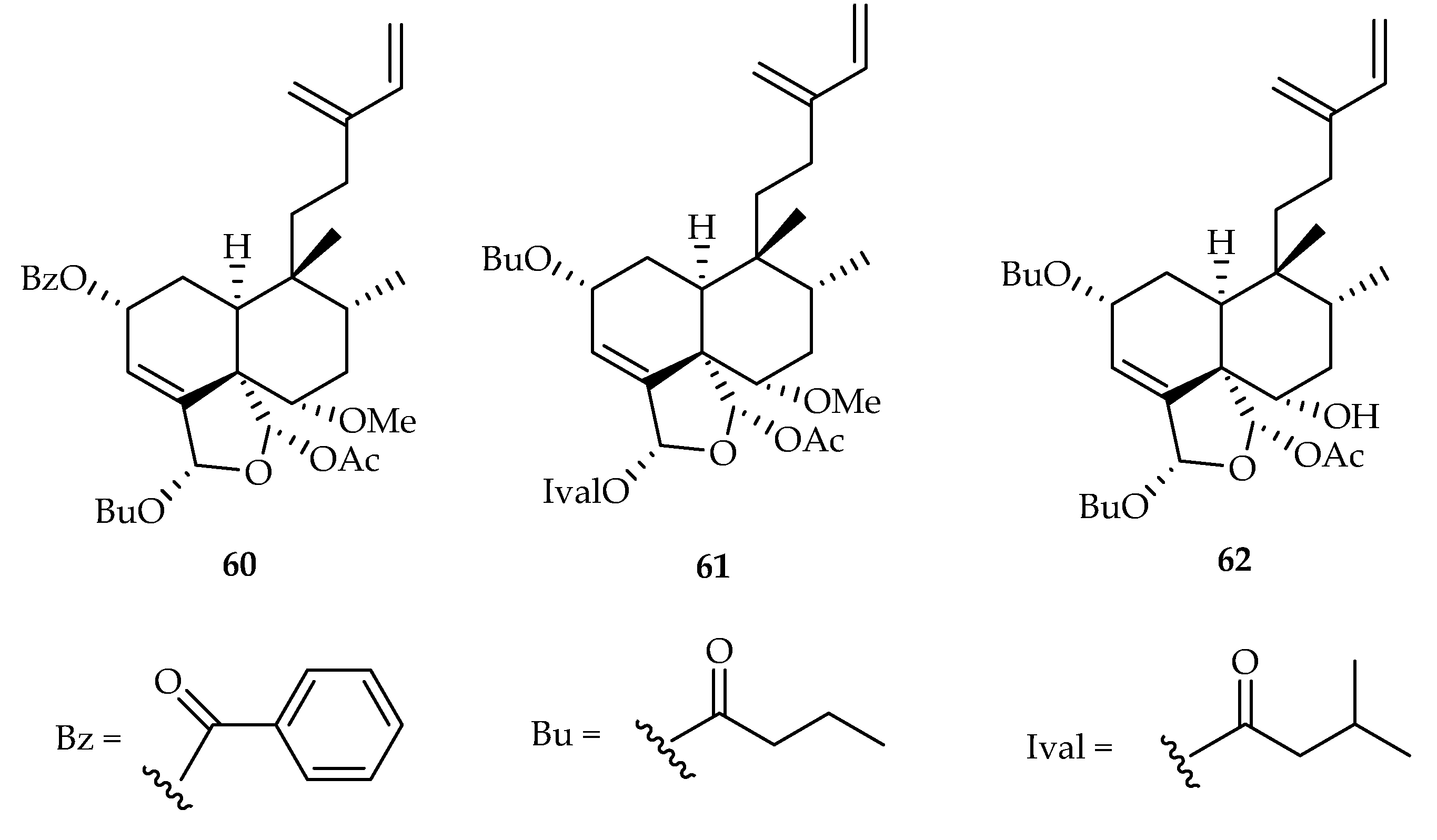
| 60 | 19.7, 12.1 | A549, HeLa | Etoposide (16.5, 25.8) | [41] | |
| 61 | 18.3, 9.0 | A549, HeLa | Etoposide (16.5, 25.8) | [41] | |
| 62 | 10.2, 5.3, 10.7 | A549, HeLa, HepG2 | Etoposide (16.5, 25.8, 16.0) | [41] | |
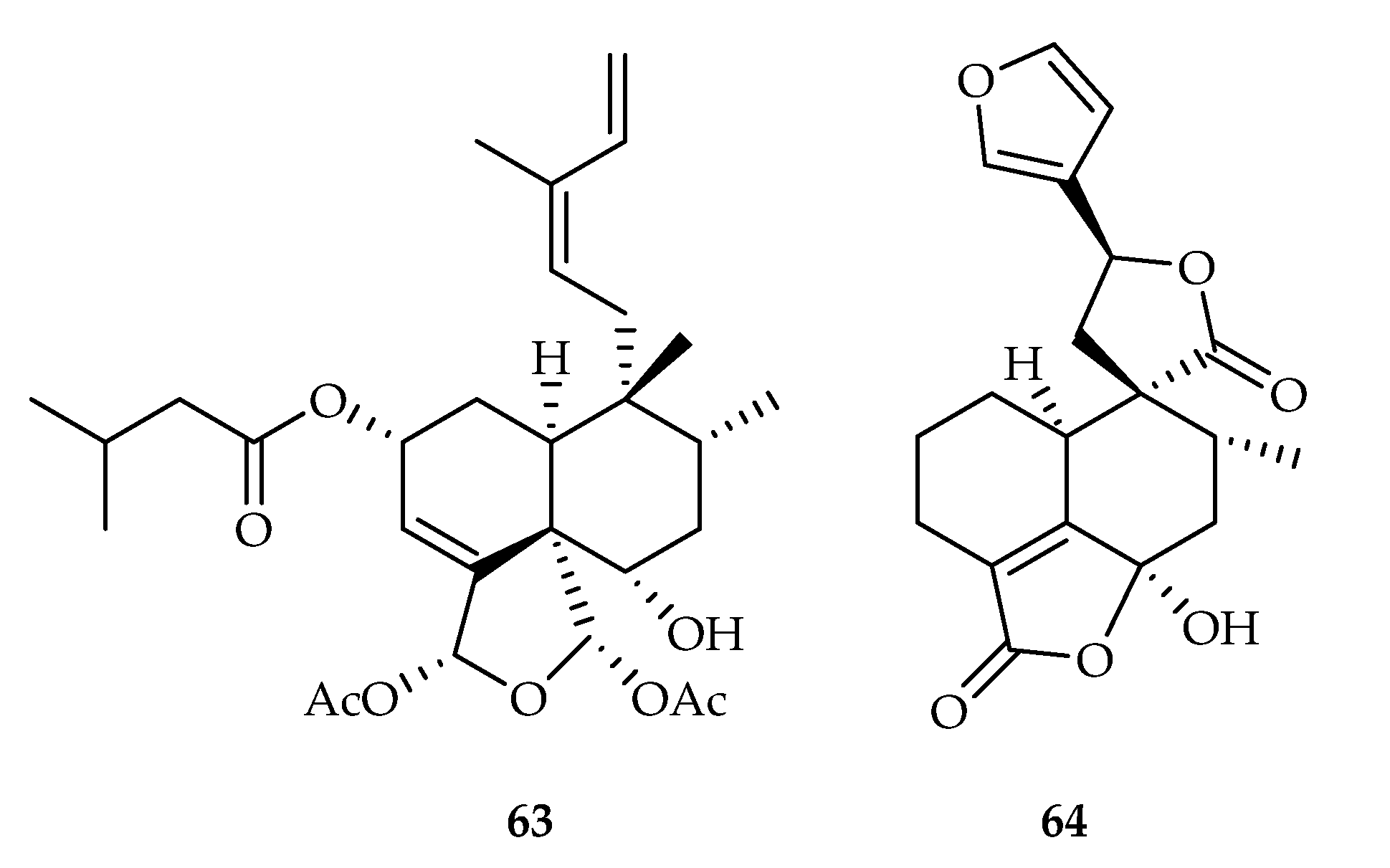
| 63 | 1.9, 4.6 | A549, HepG2 | Homoharringtonine (0.0316, 0.0345) | [42] | |
| 64 | 12.26 | T24 | Gemcitabine (6.50) | [43] | |
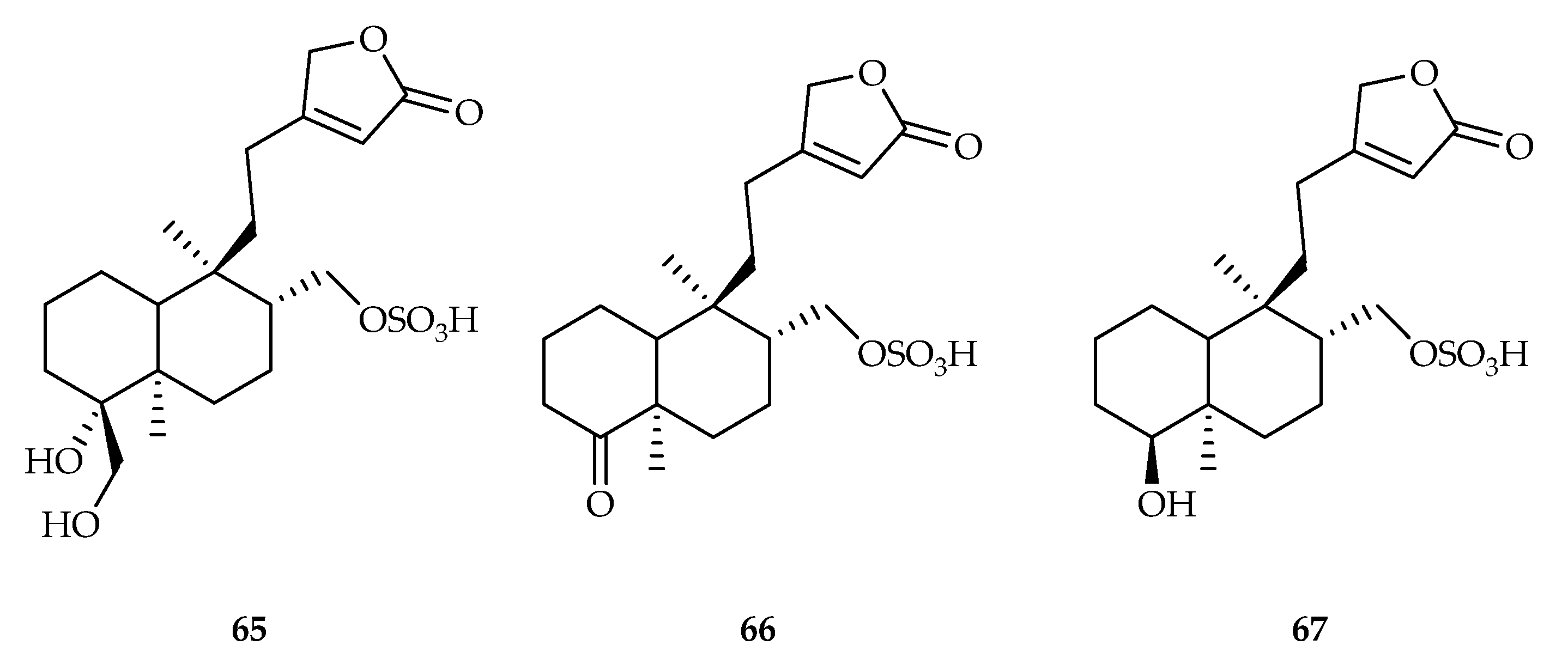
| 65 | 11.6, 7.1, 9.3 | HeLa, PANC-1, A549 | Etoposide (3.8, 5.2, 9.9) | [44] | |
| 66 | 9.4, 5.6, 6.8 | HeLa, PANC-1, A549 | Etoposide (3.8, 5.2, 9.9) | [44] | |
| 67 | 17.2, 9.8, 12.5 | HeLa, PANC-1, A549 | Etoposide (3.8, 5.2, 9.9) | [44] | |
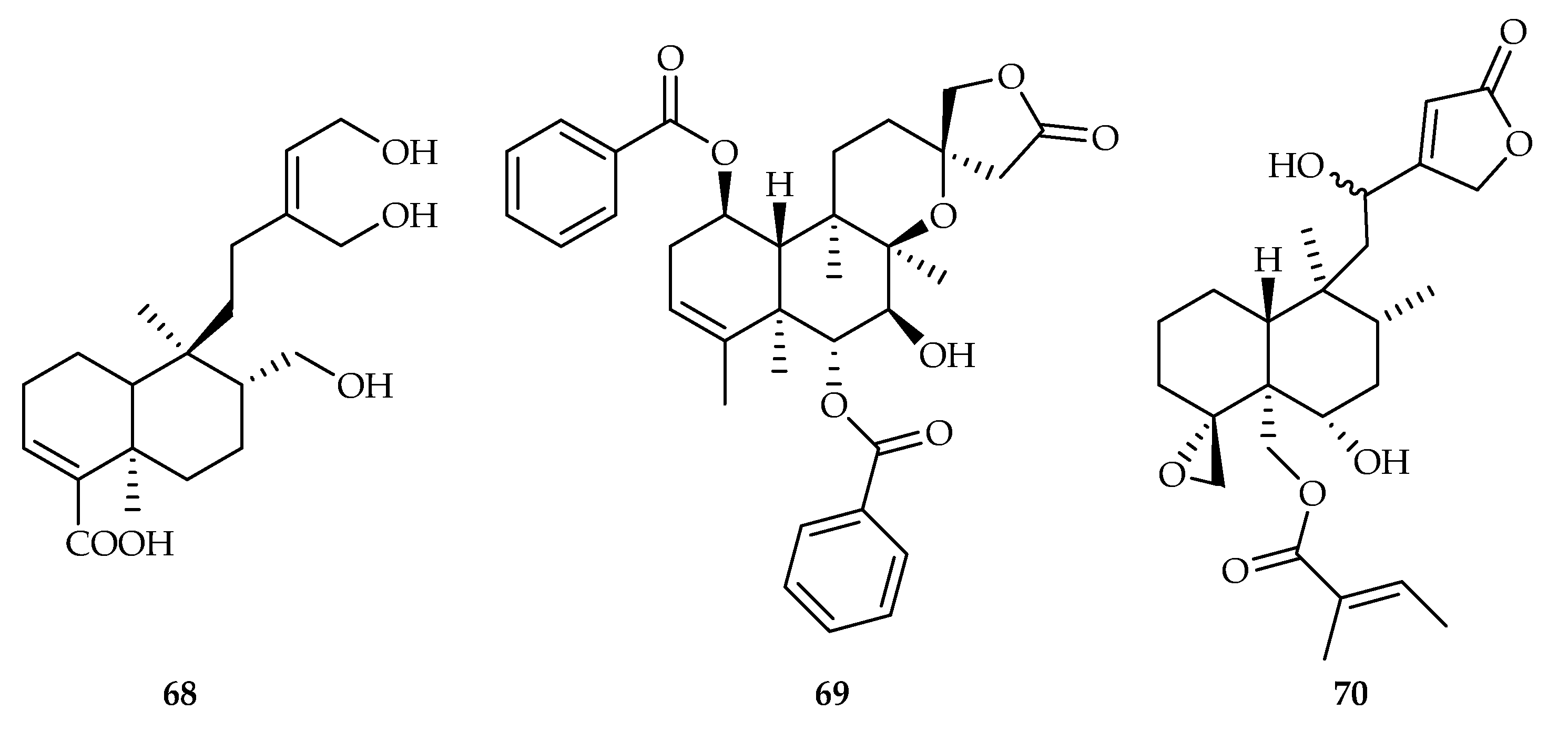
| 68 | 5.6 | ECC-1 | - | [45] | |
| 69 | 17.9 | SGC-7901 | DDP (16.7) | [46] | |
| 70 | 19.7 | HepG2 | DOX (0.38) | [47] | |
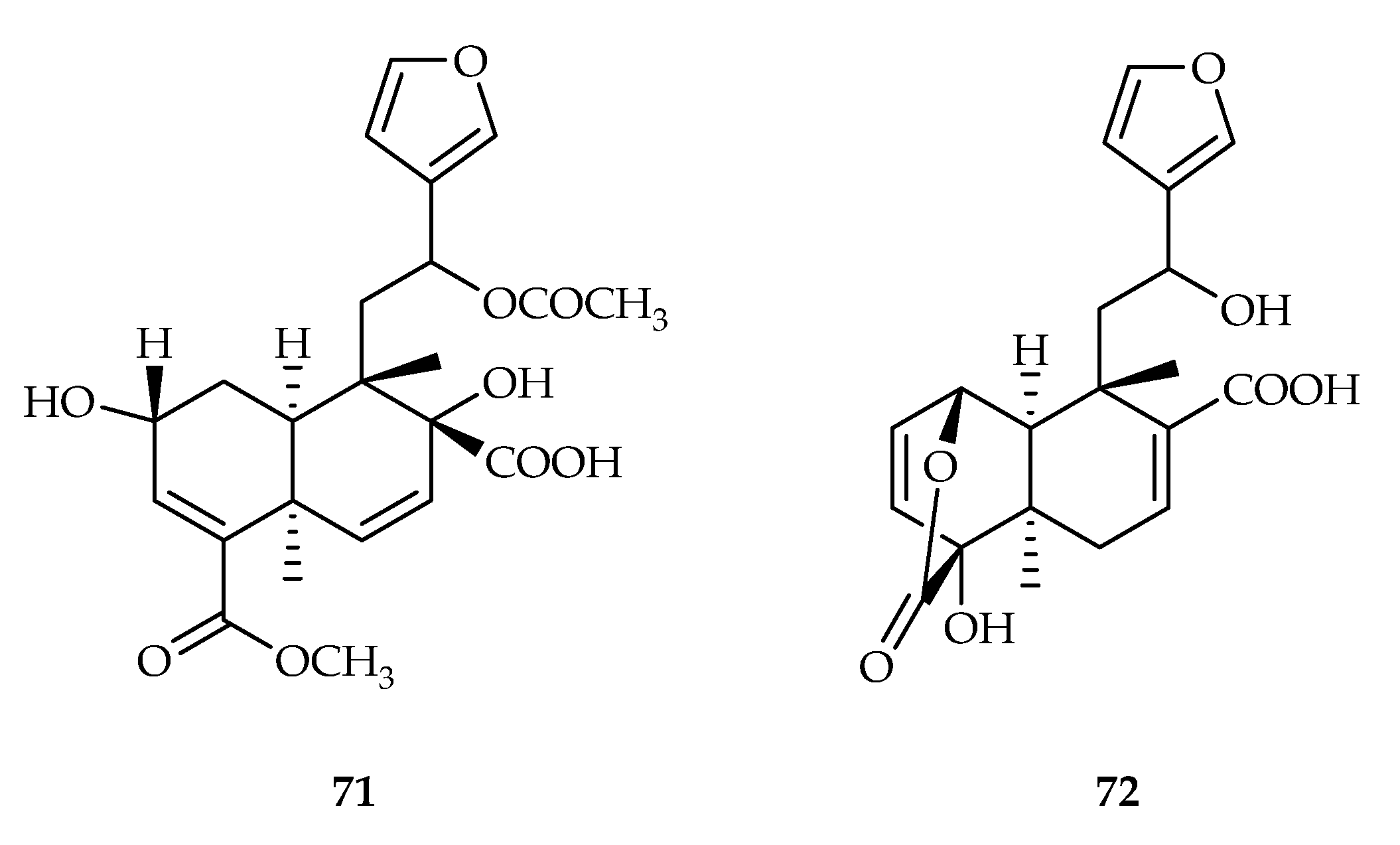
| 71 | 10 | MDA MB 231 | - | [48] | |
| 72 | 7.8 | MDA MB 231 | - | [48] | |
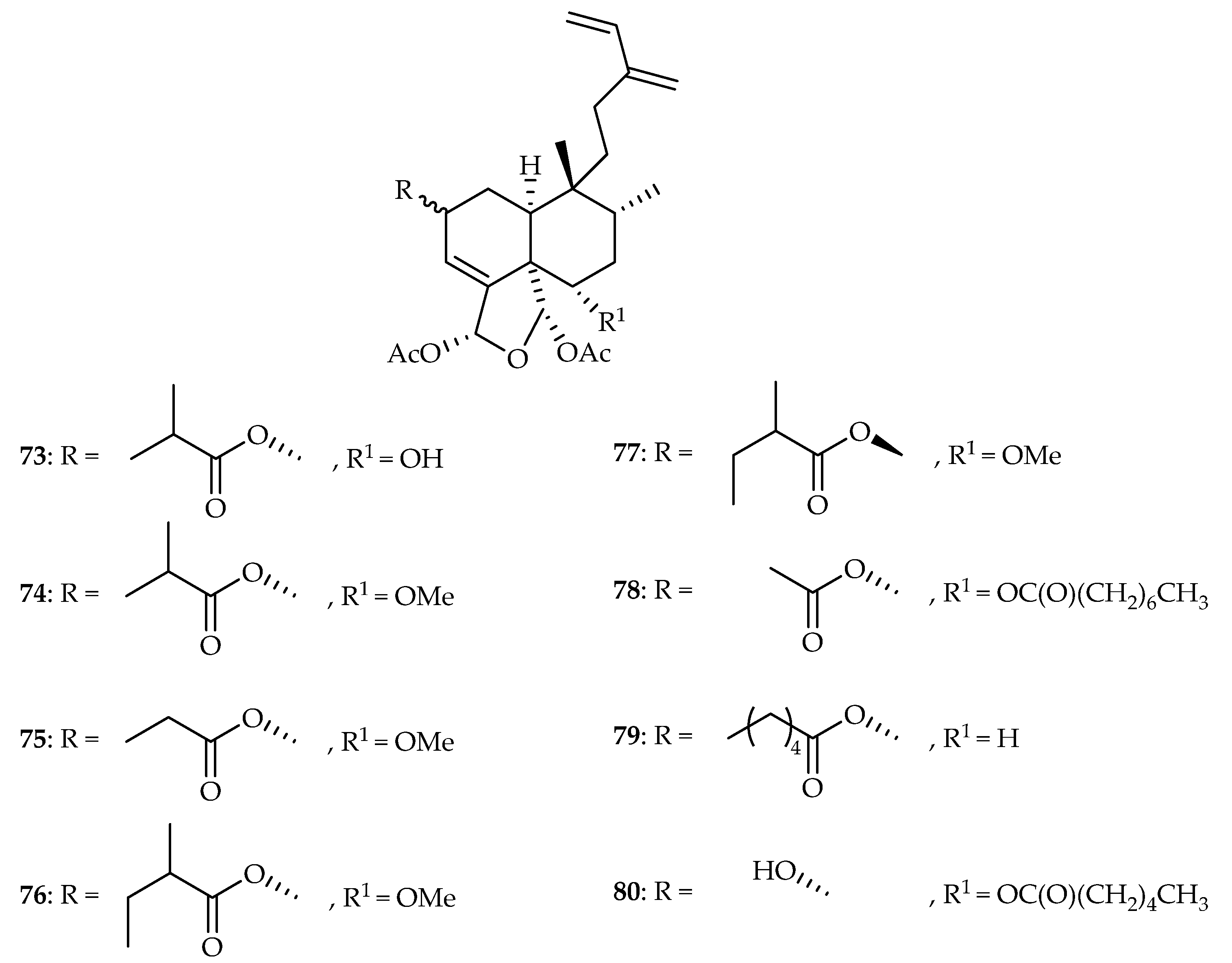
| 73 | 0.66, 0.48, 0.68, 0.56, 0.98 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (0.0065, 0.0084, 0.0121, 0.0071, 2.21) | [49] | |
| 74 | 0.47, 0.49, 0.50, 0.45, 0.49 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (0.0065, 0.0084, 0.0121, 0.0071, 2.21) | [49] | |
| 75 | 4.60, 4.95, 4.94, 5.19, 4.92 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (0.0065, 0.0084, 0.0121, 0.0071, 2.21) | [49] | |
| 76 | 5.04, 4.90, 5.82, 5.23, 5.19 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (0.0065, 0.0084, 0.0121, 0.0071, 2.21) | [49] | |
| 77 | 4.75, 3.31, 4.65, 4.25, 4.76 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (0.0065, 0.0084, 0.0121, 0.0071, 2.21) | [49] | |
| 78 | 5.98, 4.93, 6.39, 5.16, 5.03 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (0.0065, 0.0084, 0.0121, 0.0071, 2.21) | [49] | |
| 79 | 2.29, 0.49, 0.69, 0.56, 0.61 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (0.0065, 0.0084, 0.0121, 0.0071, 2.21) | [49] | |
| 80 | 4.76, 4.73, 5.19, 4.74, 4.88 | A549, MDA-MB-231, MCF-7, KB, KB-VIN | Paclitaxel (0.0065, 0.0084, 0.0121, 0.0071, 2.21) | [49] | |
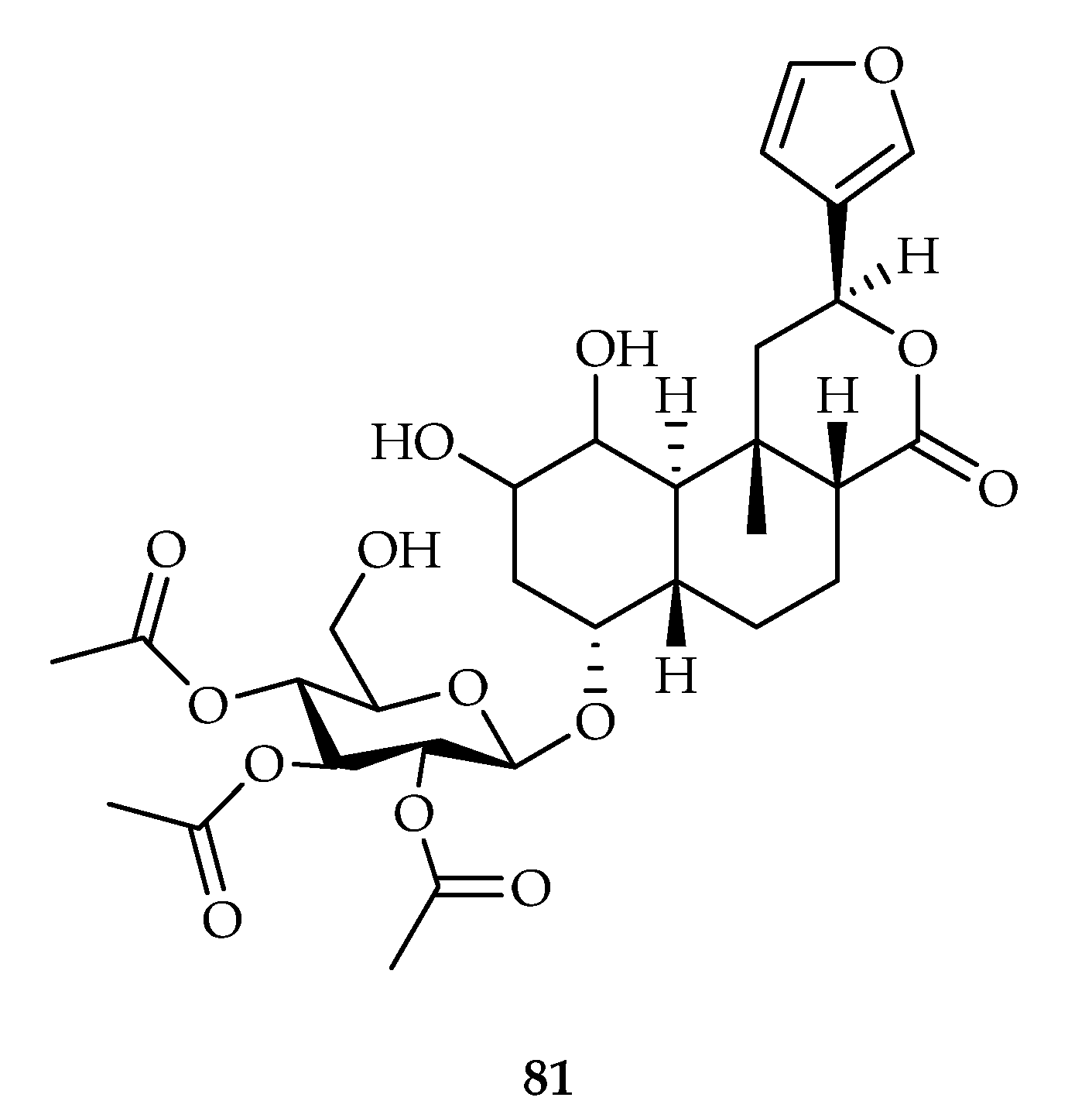
| 81 | 8, 10.4, 14.8 | HCT-116, PC-3, MDA-MB-435 | 5-FU (52), Mitomycin-C (63) | [50] | |
| Halimane | 82 | 5.2, 11.8 | A549, HL-60 | DDP (7.8, 3.4) | [51] |
| Labdane | 83 | 4.4 | theophylline-stimulated murine B16 melanoma 4A5 cells | Arbutin (174) | [52] |
| 84 | 8.6 | theophylline-stimulated murine B16 melanoma 4A5 cells | Arbutin (174) | [52] | |
| 85 | 4.6 | theophylline-stimulated murine B16 melanoma 4A5 cells | Arbutin (174) | [52] | |
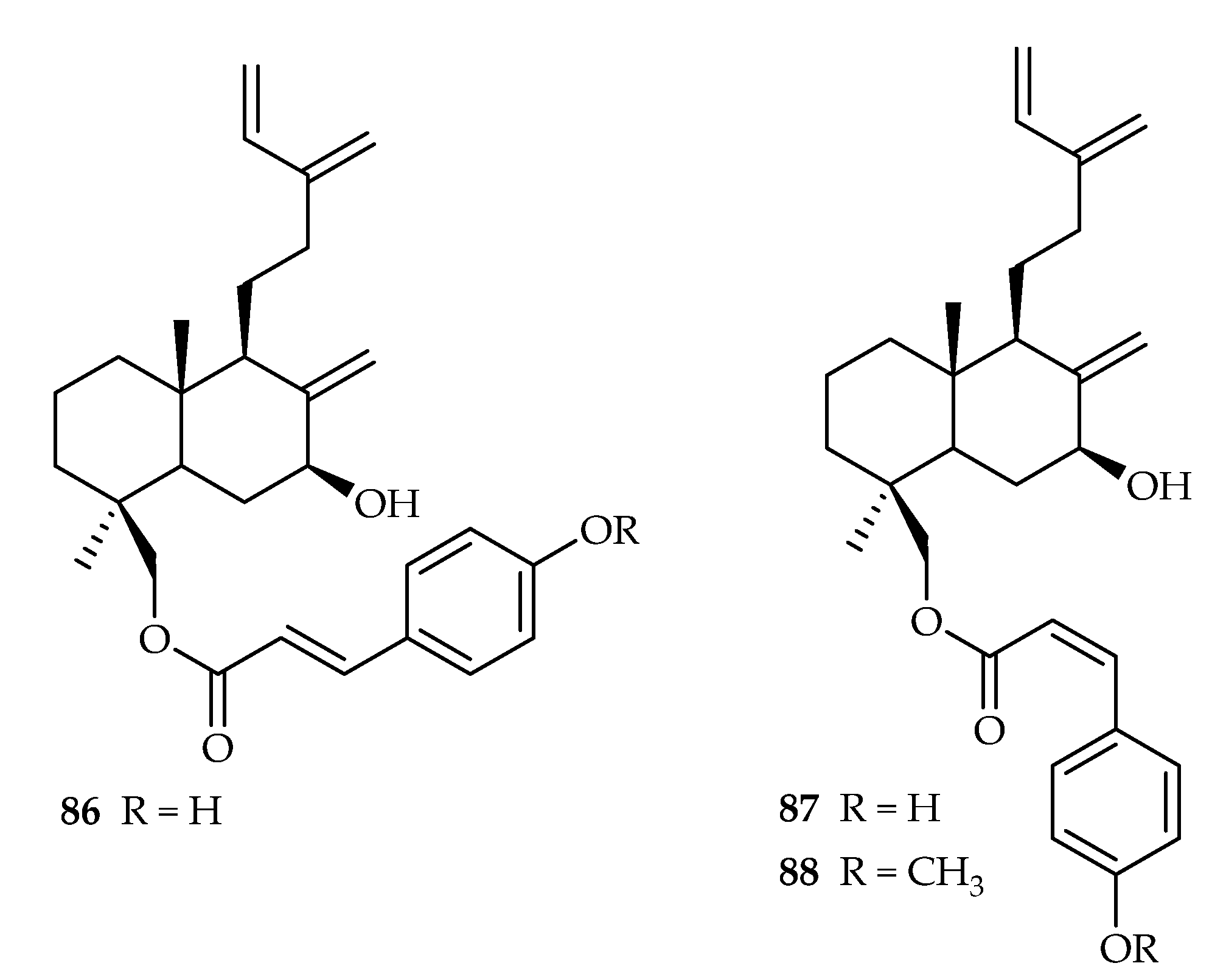
| 86 | 2.22 | L5178Y | Kahalalide F (4.30) | [53] | |
| 87 | 1.42 | L5178Y | Kahalalide F (4.30) | [53] | |
| 88 | 12.9 | L5178Y | Kahalalide F (4.30) | [53] | |
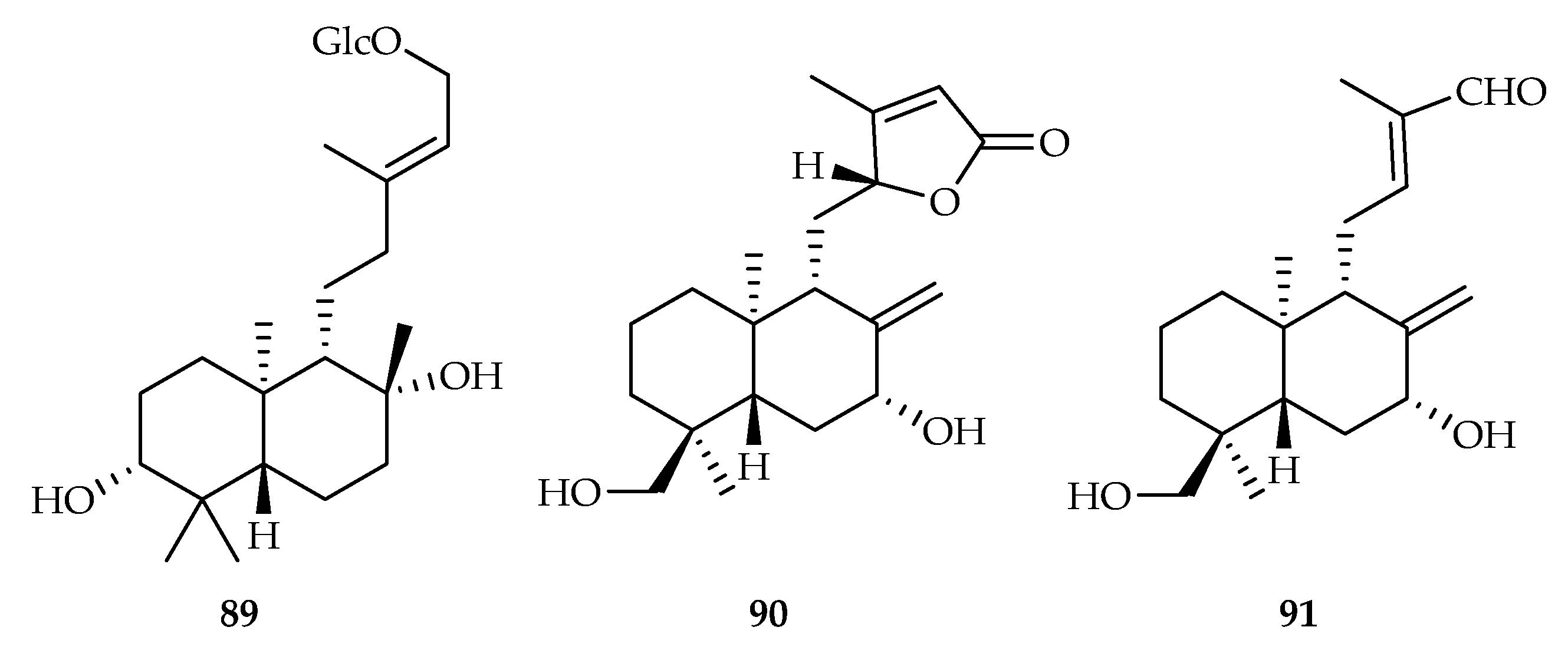
| 89 | 17.7 | p388D1 | Taxol (0.03) | [54] | |
| 90 | 1.6 | MDA-MB231 | Paclitaxel (0.003) | [55] | |
| 91 | 1.5 | MDA-MB231 | Paclitaxel (0.003) | [55] | |
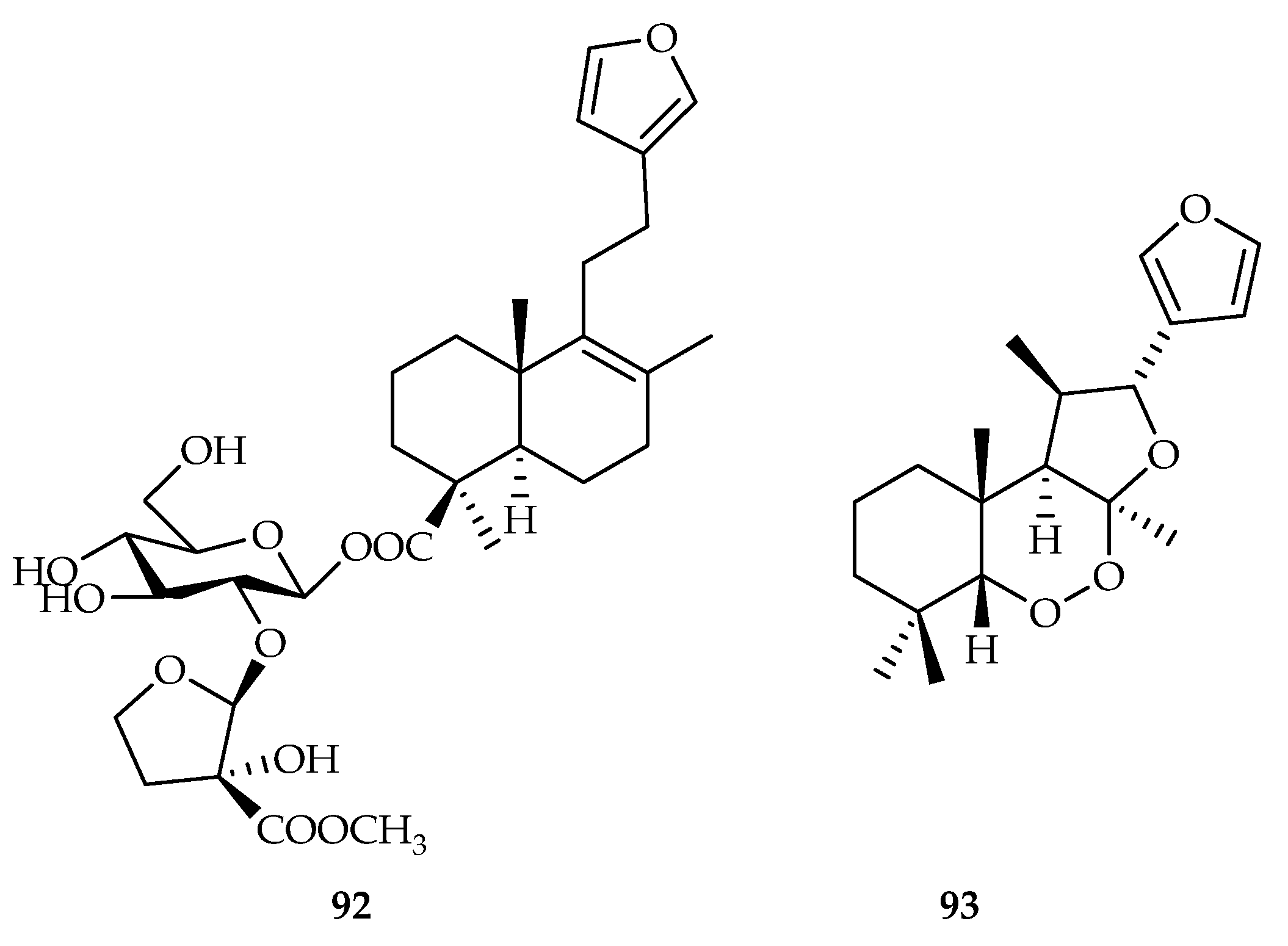
| 92 | 7.5 | MCF-7 | DDP (22.2) | [56] | |
| 93 | 8.0, 19.7 | HepG2, XWLC-05 | DDP (3.0, 4.3) | [57] | |
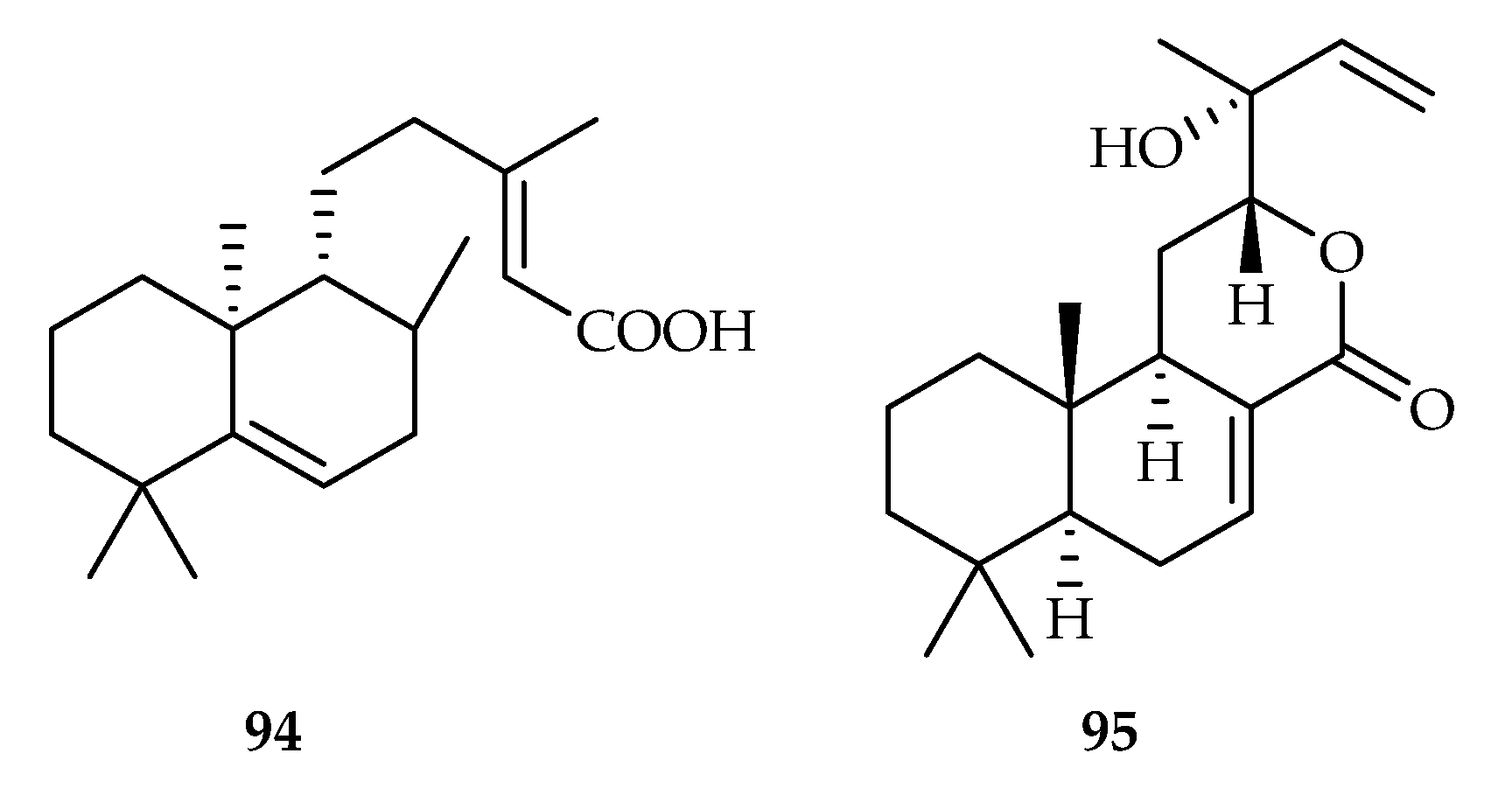
| 94 | 11.7, 17.6 | ACP01, A549 | DOX (8.3, 3.1) | [58] | |
| 95 | 2.8 | HL-60 | ADR (0.02) | [59] | |
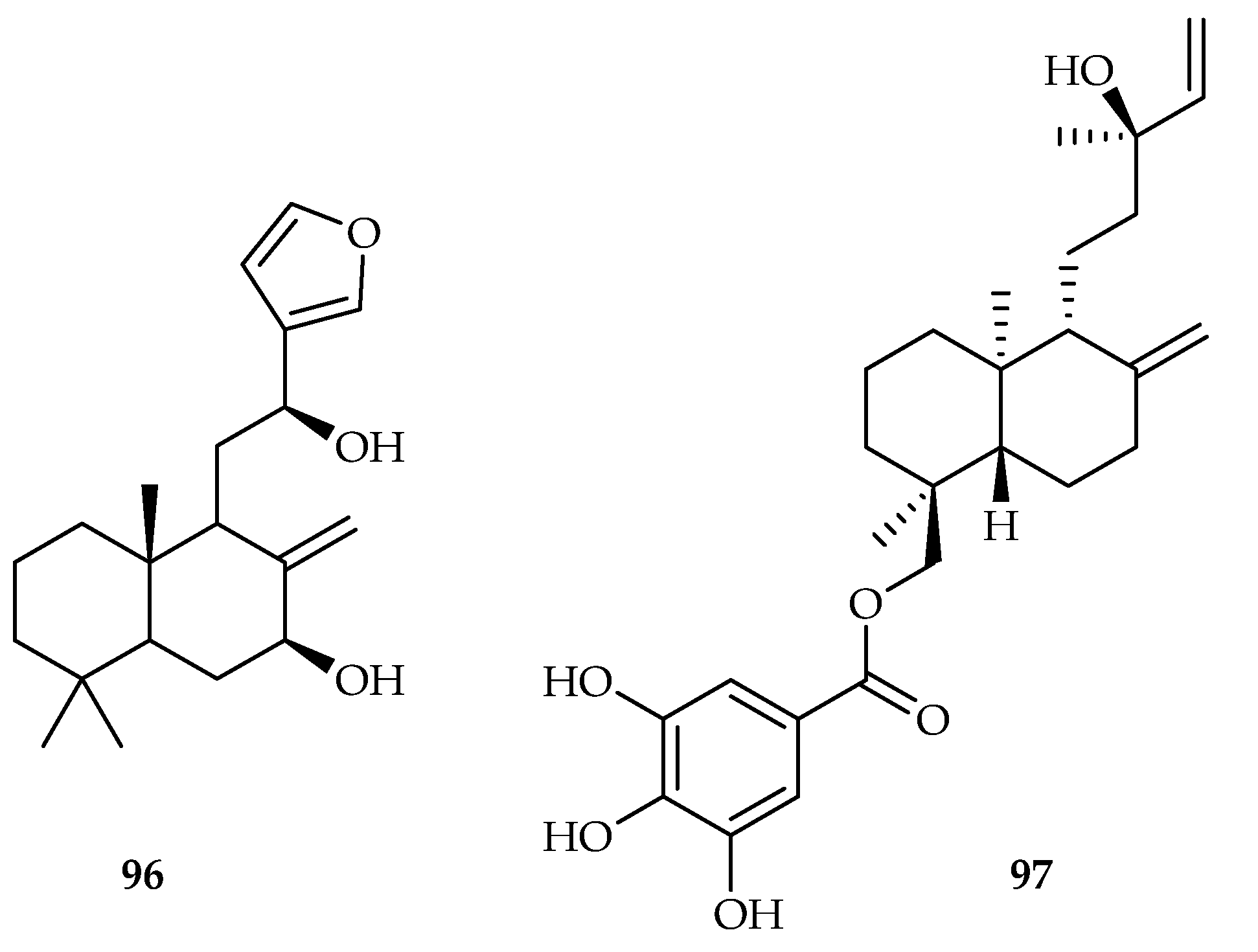
| 96 | 13.49 | A549 | Paclitaxel (6.2) | [60] | |
| 97 | 5.92 | U937 | Paclitaxel (0.001) | [61] | |
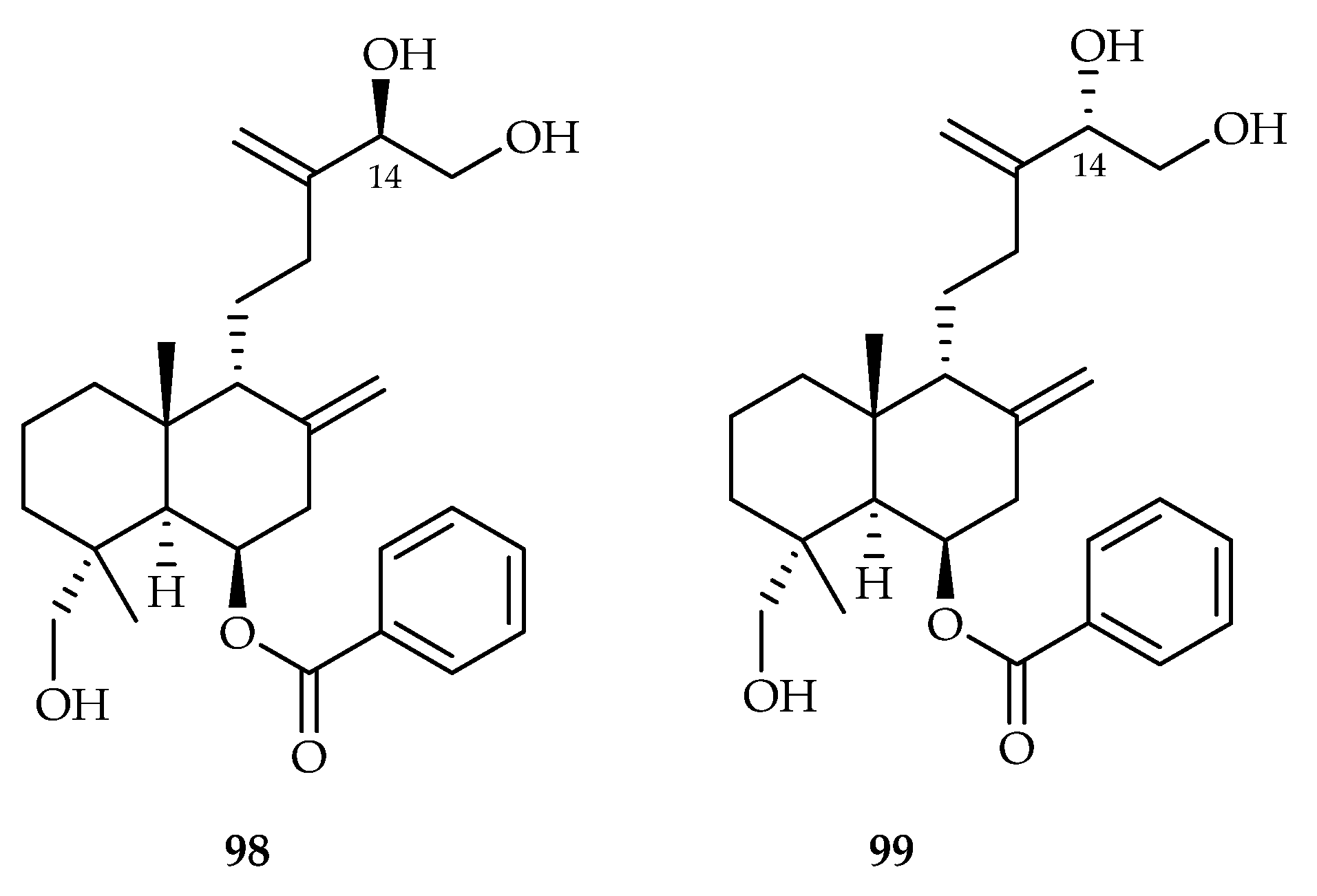
| 98 | 10.9, 14.6, 18.2 | MCF-7, MDA-MB231, A549 | SAHA (14.2, 6.91, 3.85) | [62] | |
| 99 | 7.63, 13.5, 15.3 | MCF-7, MDA-MB231, A549 | SAHA (14.2, 6.91, 3.85) | [62] | |
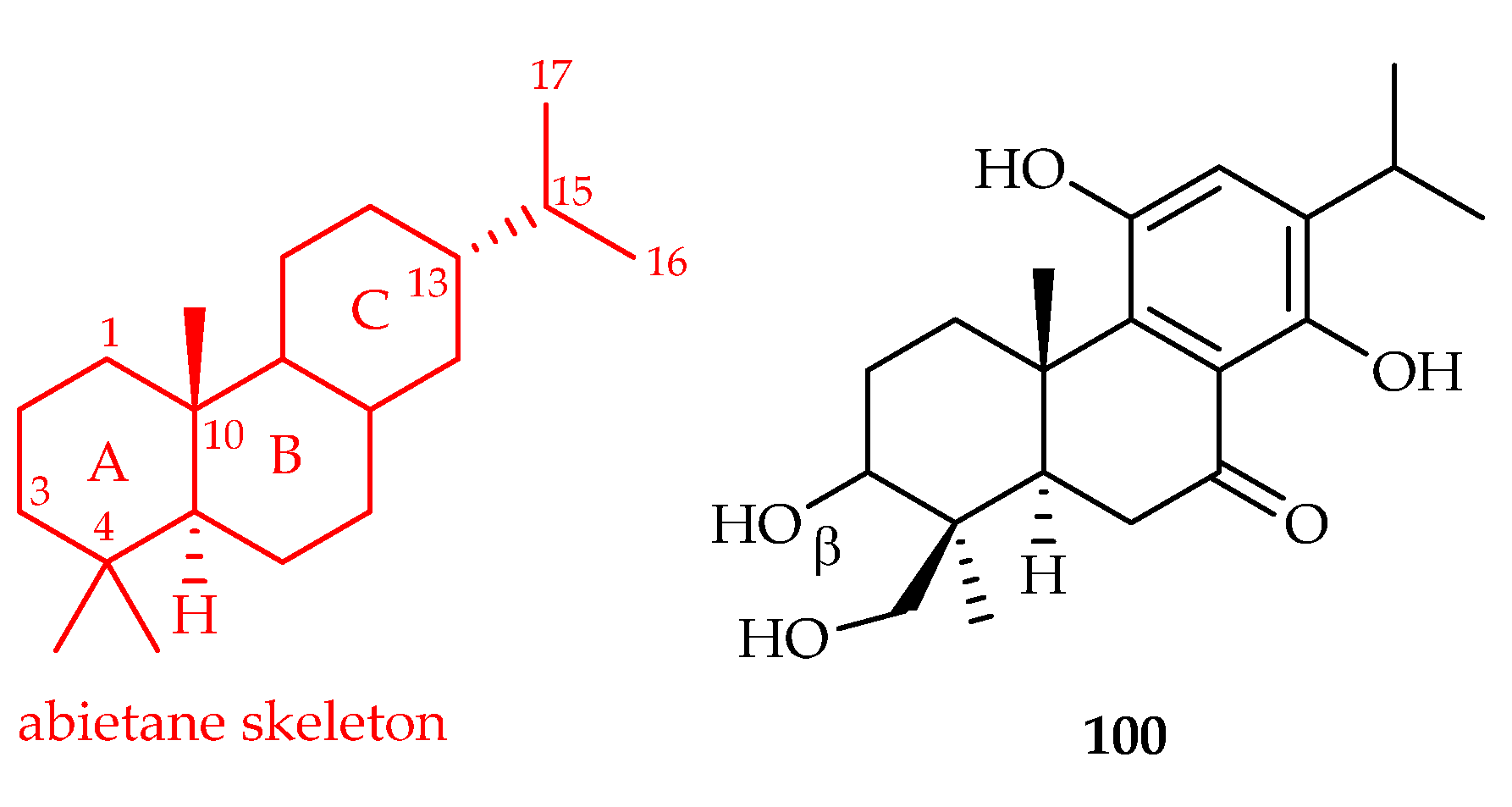
| Abietane | 100 | 5.88, 11.74 | A2780, HepG2 | Taxol (0.006, 0.003) | [63] |
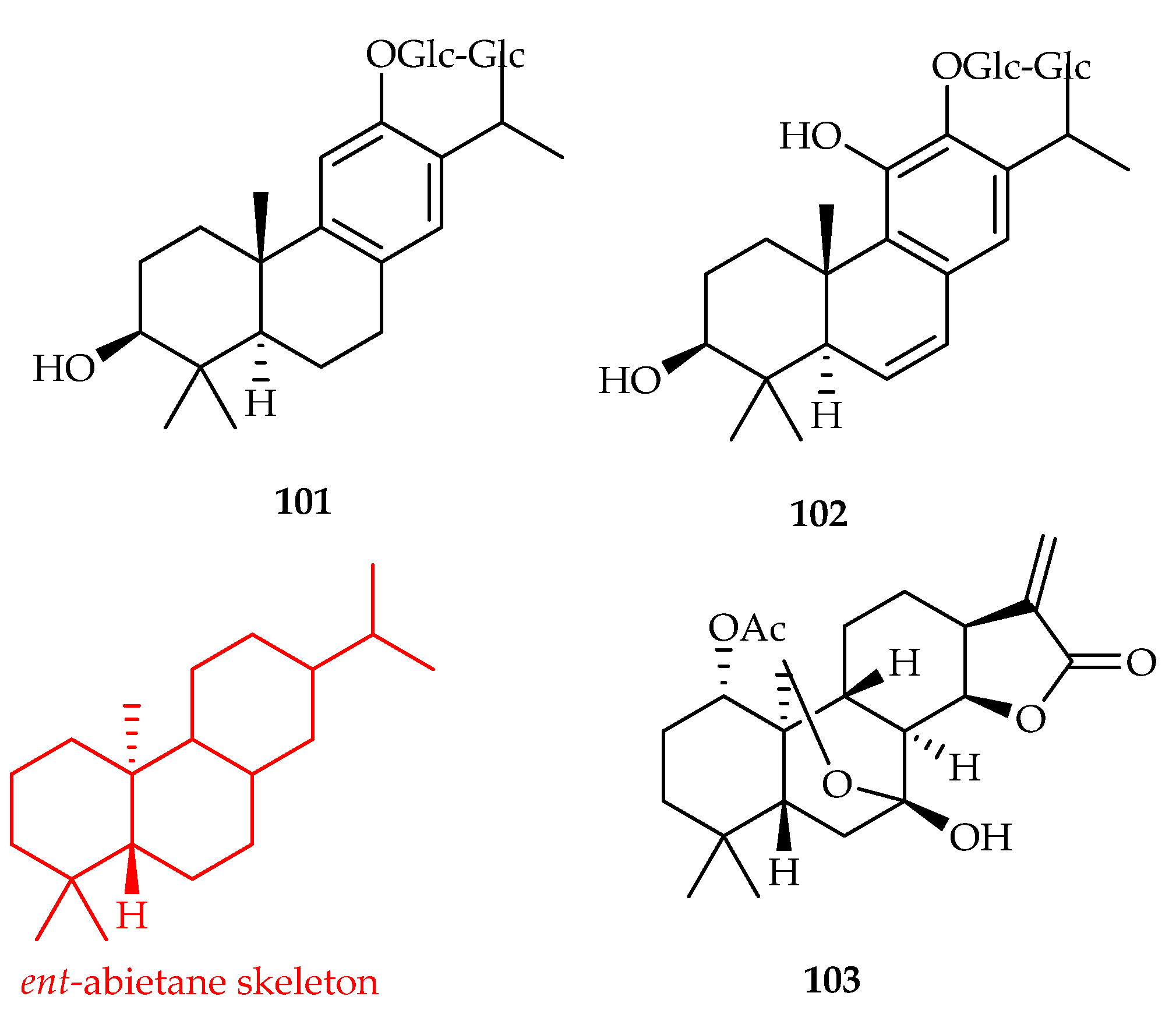
| 101 | 0.37, 7.85, 1.45, 8.72, 0.23 | HepG2, NB4, HeLa, MCF-7, HL-60 | DDP (0.2, 0.03, 0.05, 0.12, 0.18) | [64] | |
| 102 | 1.27, 1.02, 0.35, 0.96, 5.17 | NB4, HeLa, K562, MCF-7, HL-60 | DDP (0.03, 0.05, 0.2, 0.12, 0.18) | [64] | |
| 103 | 9.4–20.4 | HL-60, SMMC-7721, A549, MCF-7, SW480 | DDP (1.9–18.3) | [65] | |
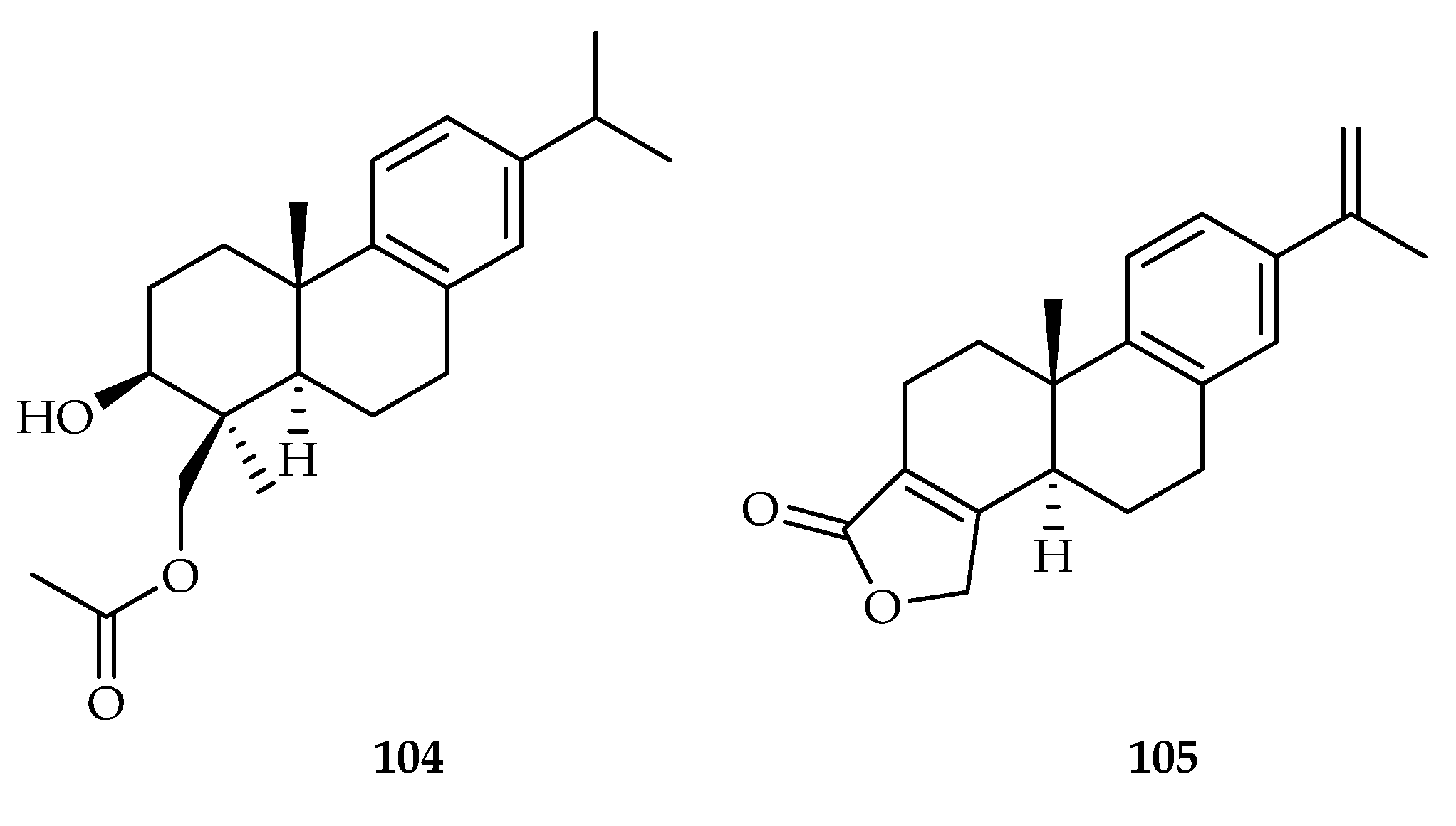
| 104 | 6.3, 12.7, 7.9 | LUPF045, OVPF038, OVPF008 | Paclitaxel (11.2, 1.4, 1.1) | [66] | |
| 105 | 9.0, 11.7, 19.3, 16.8 | LUPF045, LUPF003, OVPF038, OVPF008 | Paclitaxel (11.2, 11.0, 1.4, 1.1) | [66] | |
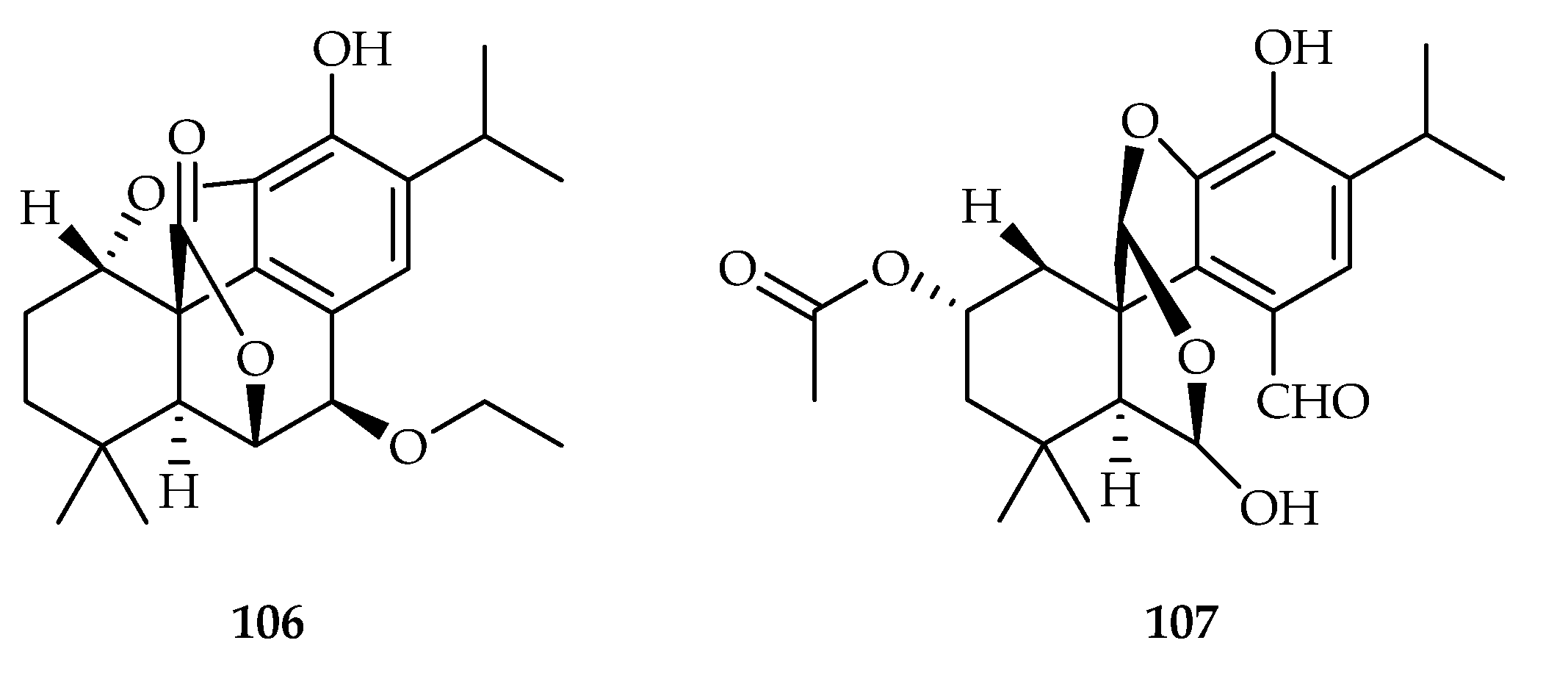
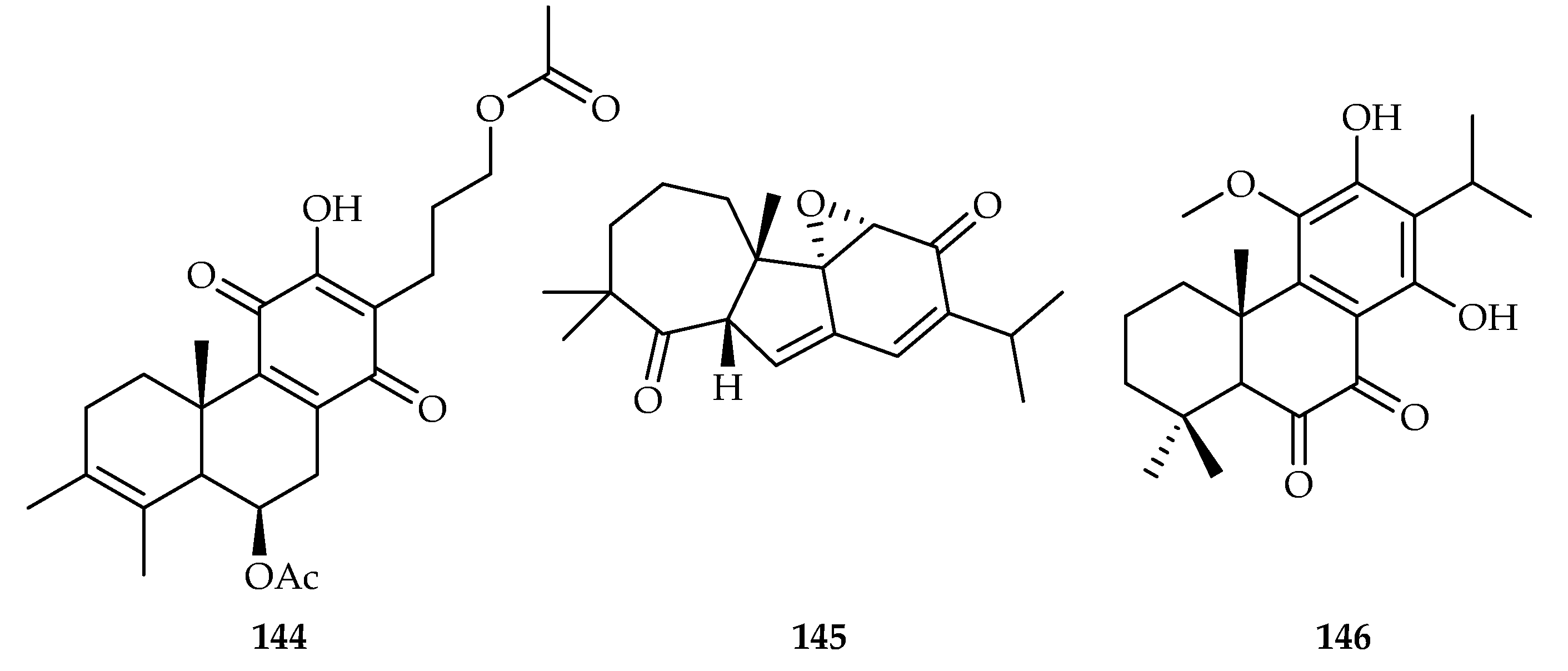
| 106 | 9.65 | CaCo2 | ADR (0.348) | [67] | |
| 107 | 17.86 | CaCo2 | ADR (0.348) | [67] | |
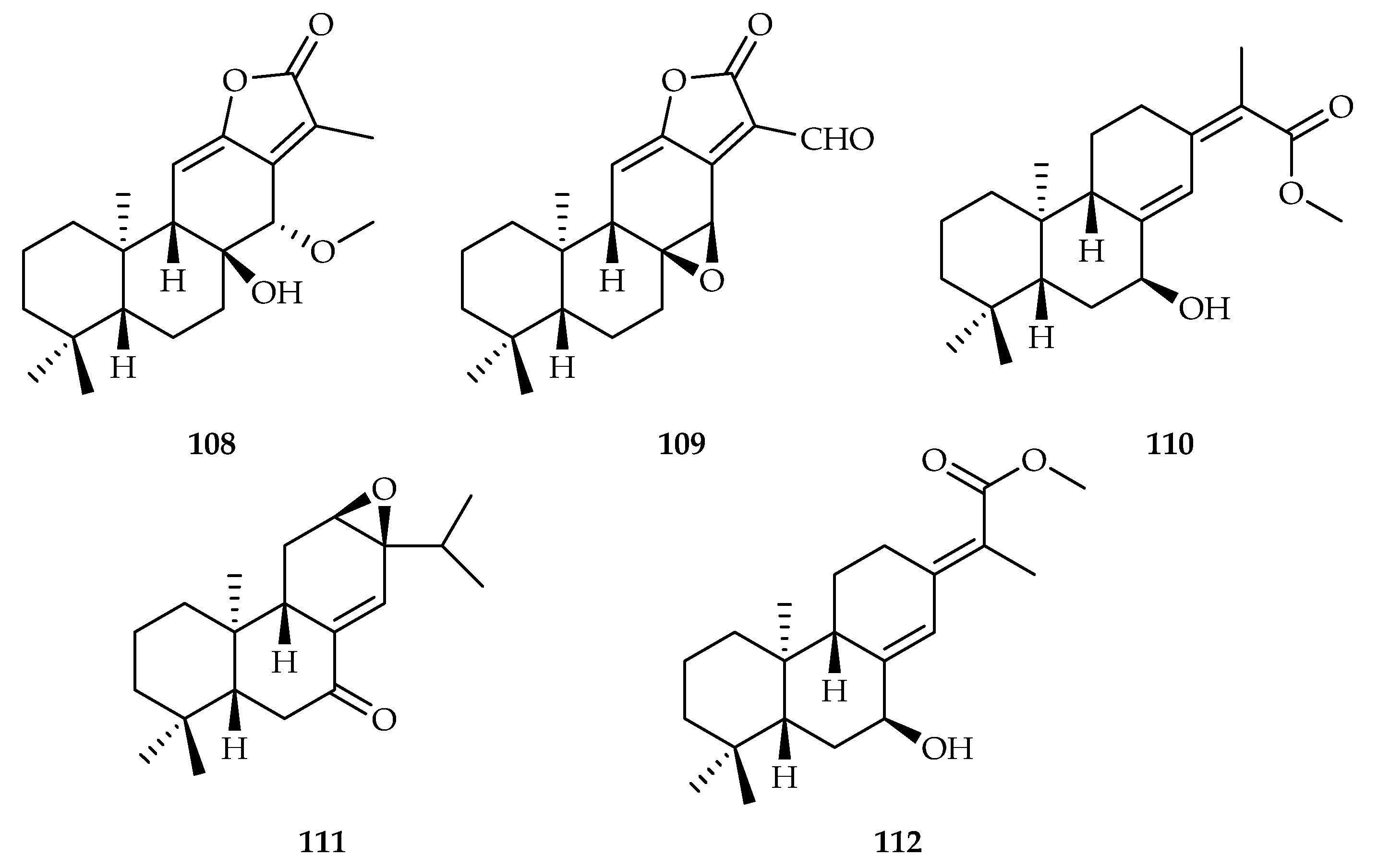
| 108 | 9.18, 9.70, 18.3, 16.2 | C4-2B, C4-2B/ENZR, HCT-15, RKO | DOX (0.12, 0.34, 0.56, 0.87) | [68] | |
| 109 | 13.4, 11.1 | C4-2B, C4-2B/ENZR | DOX (0.12, 0.34) | [68] | |
| 110 | 17.7, 15.2 | C4-2B, C4-2B/ENZR, | DOX (0.12, 0.34) | [68] | |
| 111 | 9.23, 15.1 | C4-2B, C4-2B/ENZR, | DOX (0.12, 0.34) | [68] | |
| 112 | 7.39, 9.20, 19.0 | C4-2B, C4-2B/ENZR, HCT-15 | DOX (0.12, 0.34, 0.56) | [68] | |
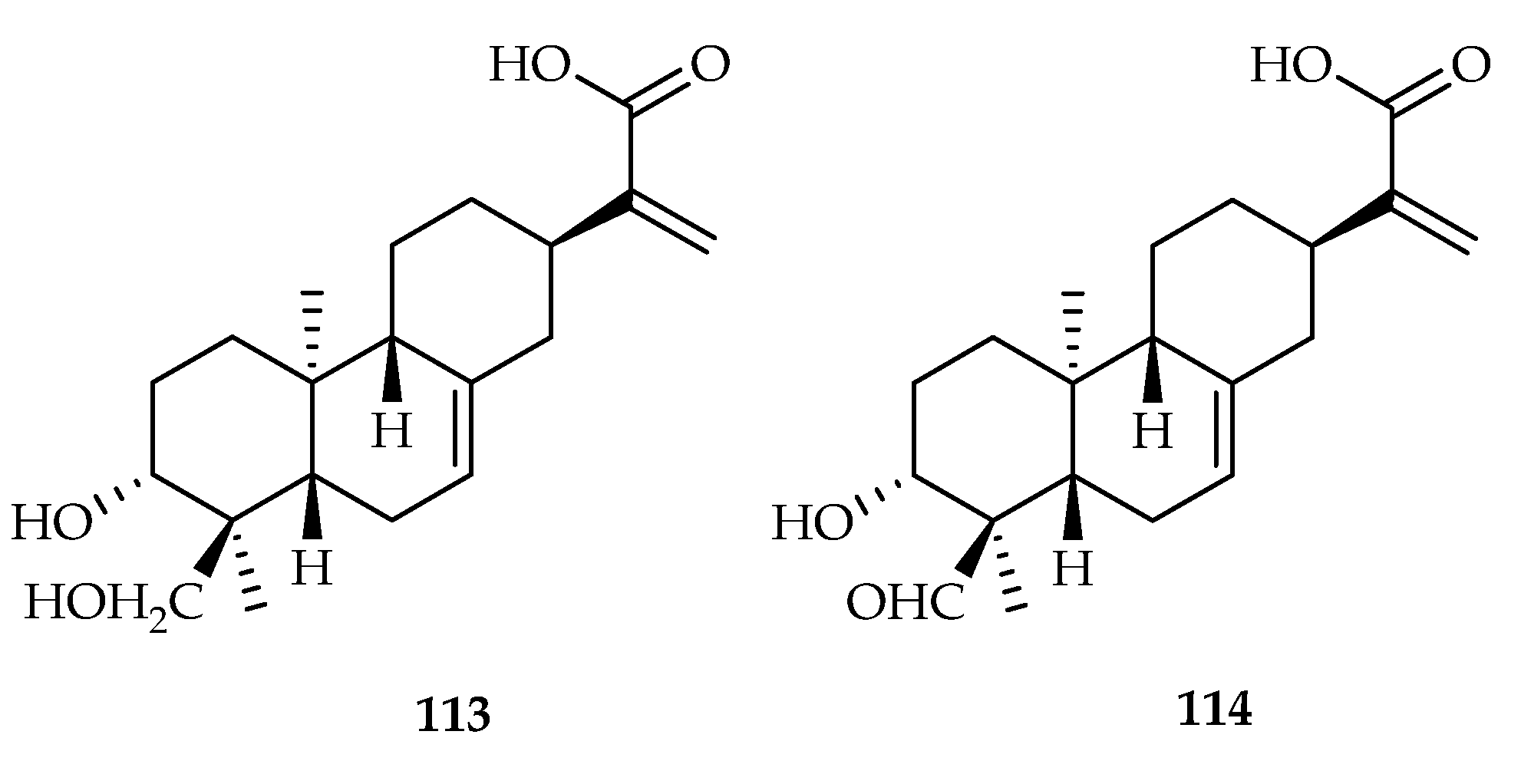
| 113 | 12.10, 15.95 | MM-231, Hep3B | DDP (3.82, 2.97) | [70] | |
| 114 | 9.12, 8.50 | MM-231, Hep3B | DDP (3.82, 2.97) | [70] | |
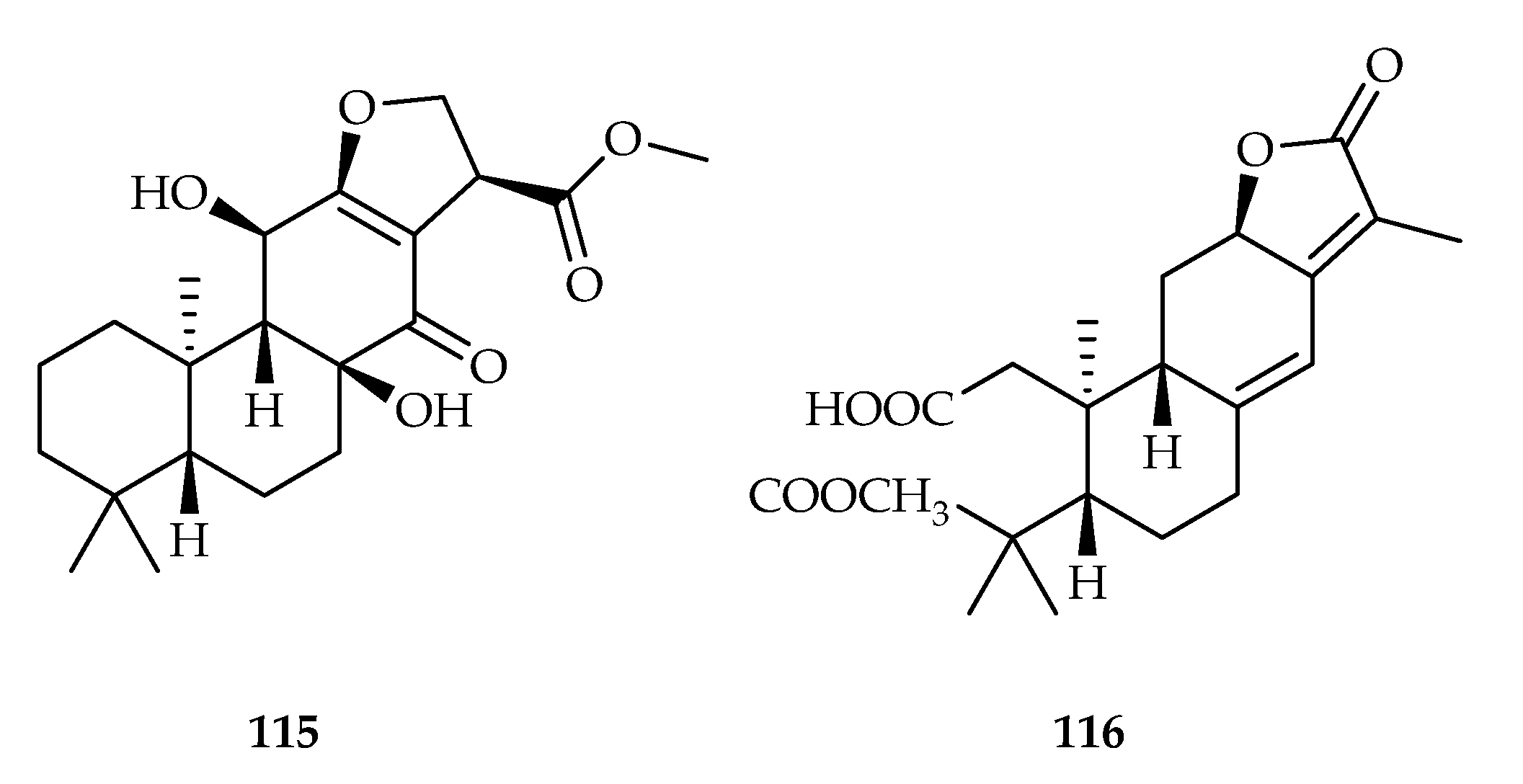
| 115 | 15.47 | HeLa | DDP (11.34) | [71] | |
| 116 | 3.75, 9.31 | HeLa, MCF-7 | DDP (11.34, 25.14) | [71] | |
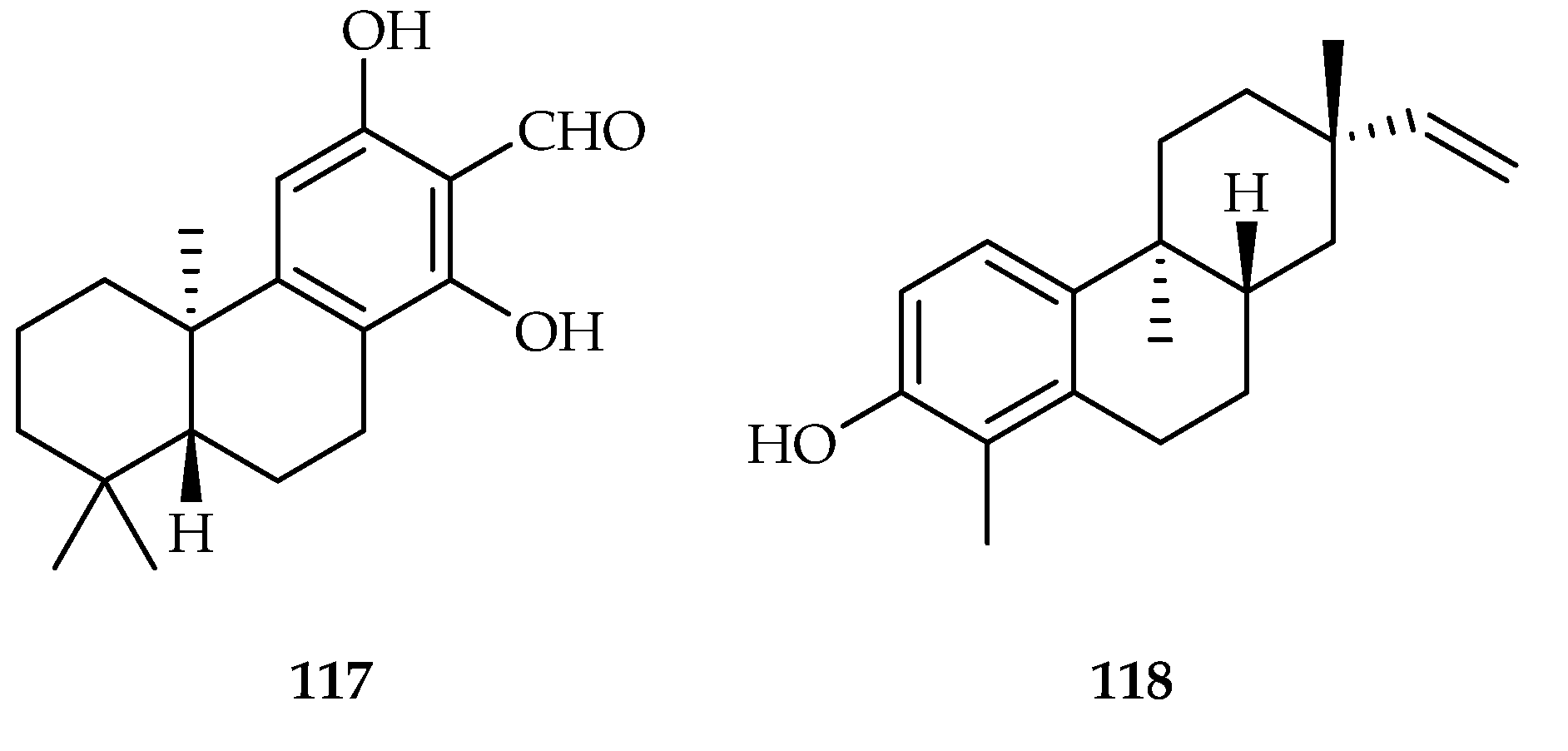
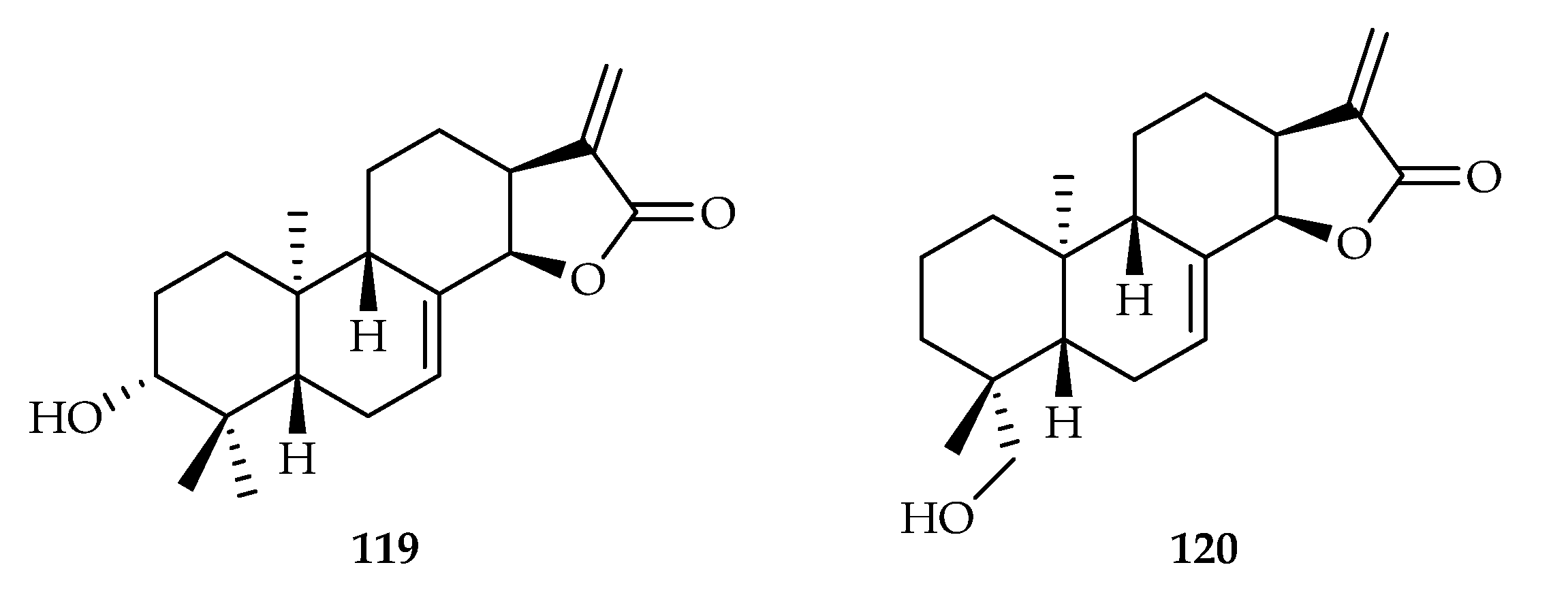
| 117 | 4.6, 11.5, 16.4 | HeLa, H460, Namalwa | DOX (8.2, 0.7, 70.1) | [72] | |
| 118 | 9.5, 17.4, 13.3 | HeLa, H460, Namalwa | DOX (8.2, 0.7, 70.1) | [72] | |
| 119 | 15.7, 19.8, 13.2 | HL-60, MCF-7, SW-480 | DDP (2.2, 10.5, 12.7) | [73] | |
| 120 | 15.6, 17.4, 17.0, 16.6, 10.1 | HL-60, SMMC-7721, A549, MCF-7, SW480 | DDP (2.2, 17.3, 15.6, 10.5, 12.7) | [73] | |
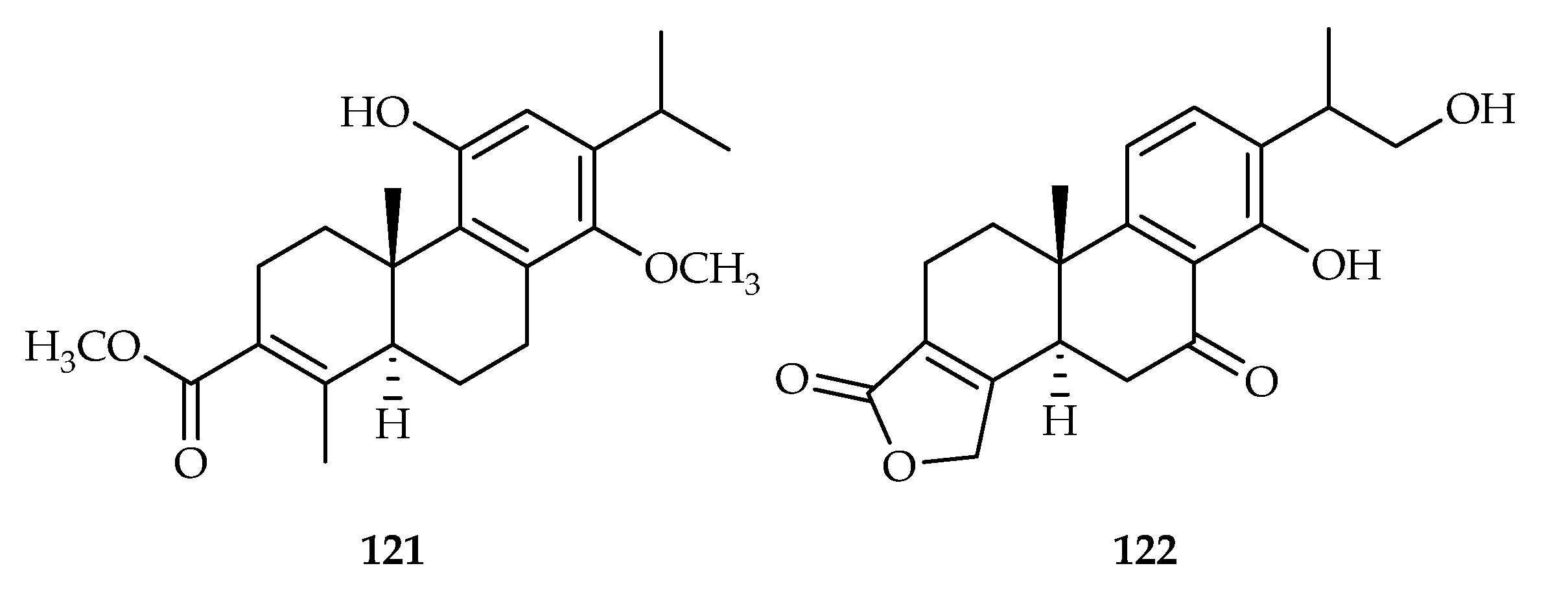
| 121 | 0.58, 1.36, 5.82, 2.06, 4.21 | HL-60, SMMC-7721, A549, MCF-7, SW-480 | DDP (1.56, 12.32, 15.34, 20.58, 24.26) | [74] | |
| 122 | 0.82, 2.65, 5.64, 6.26, 8.53 | HL-60, SMMC-7721, A549, MCF-7, SW-480 | DDP (1.49, 13.32, 11.54, 24.59, 28.32) | [75] | |
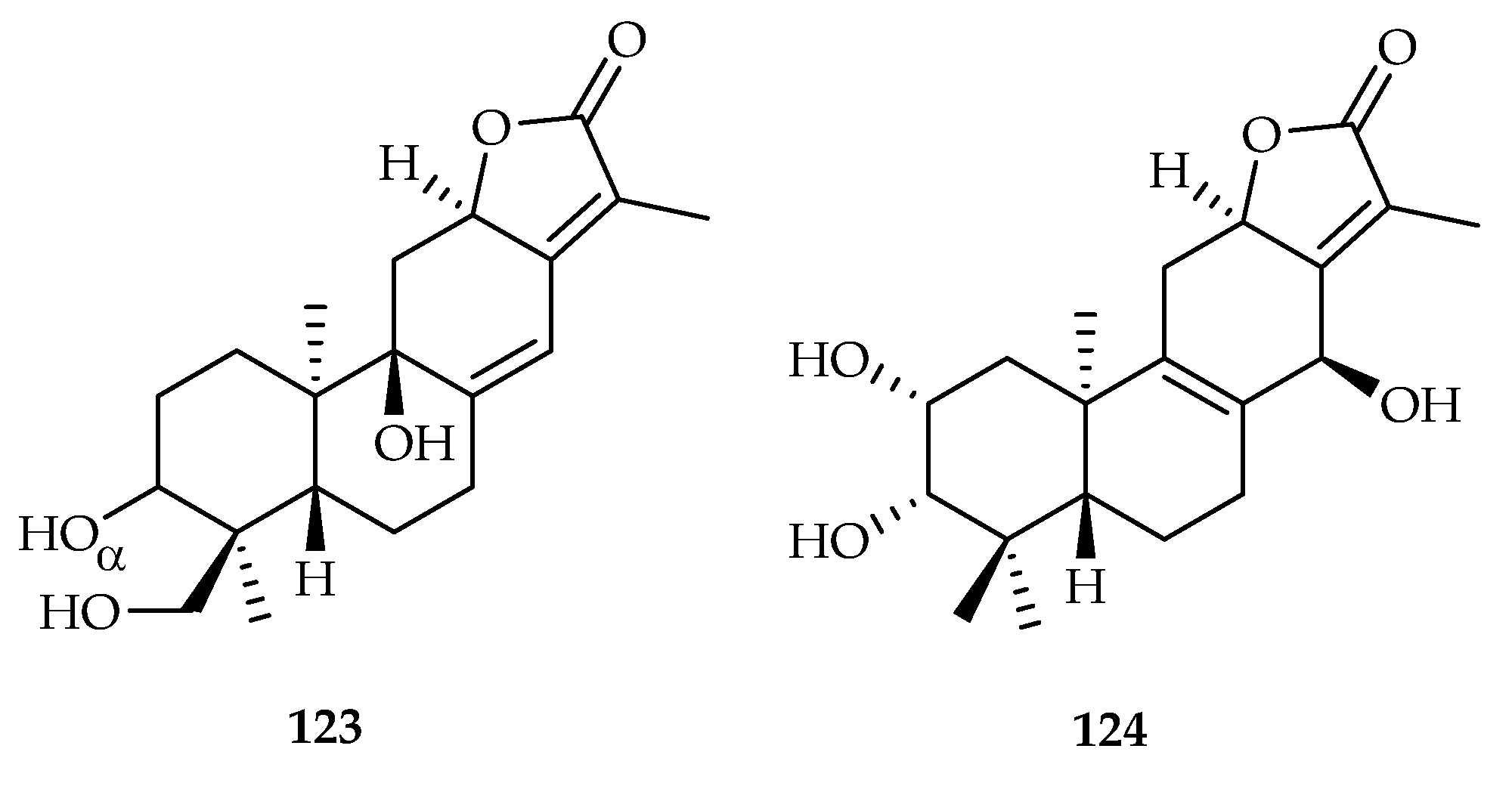
| 123 | 9.5, 10.7 | MCF-7, PANC-1 | Paclitaxel (0.65), gemcitabine (0.059) | [76] | |
| 124 | 9.8, 10.3 | MCF-7, PANC-1 | Paclitaxel (0.65), gemcitabine (0.059) | [76] | |
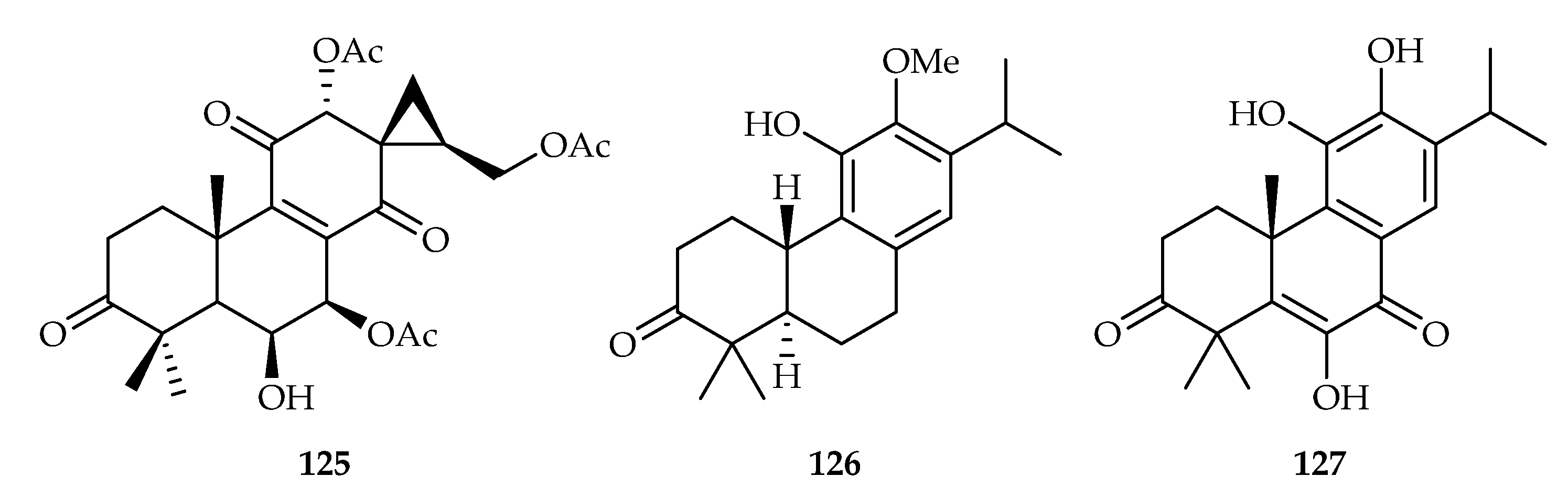
| 125 | 17.9 | MCF-7 | 5-FU (3.6) | [77] | |
| 126 | 4.3, 2.8, 4.5 | MDA-MB-231, MCF-7, HeLa | DOX (0.48, 0.36, 0.82) | [78] | |
| 127 | 20.02 | MDA-MB-231 | DDP (20.27) | [79] | |
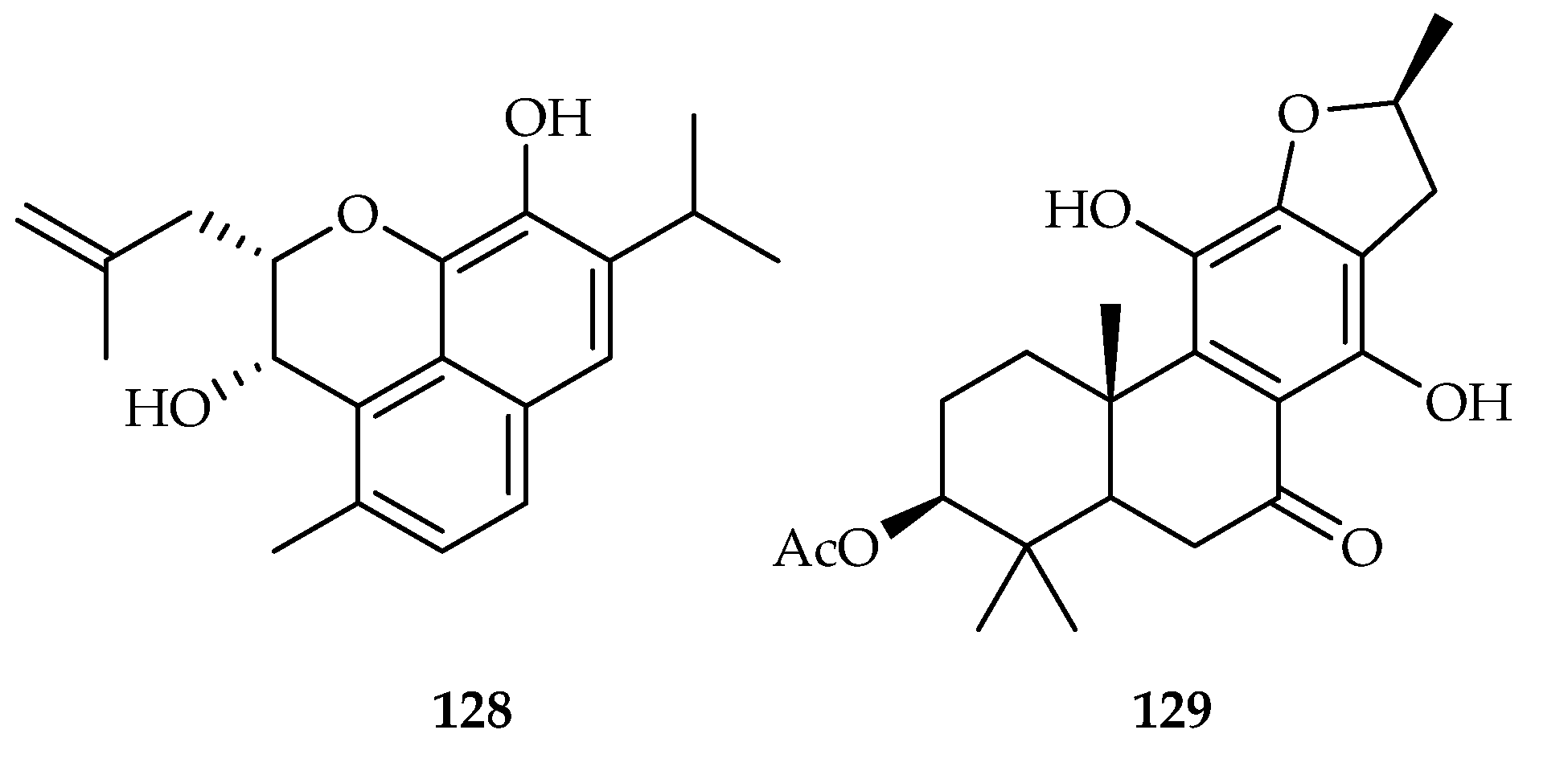
| 128 | 7.9 | MOLT-4 | DDP (2.7) | [80] | |
| 129 | 10.70 | A549 | - | [81] | |
| 130 | 5.89 | A549 | DDP (20.66) | [82] | |
| 131 | 6.94 | A549 | DDP (20.66) | [82] | |
| 132 | 16.6, 17.4 | KB, MCF7 | Paclitaxel (0.0031, 0.008) | [83] | |
| 133 | 13.71 | A549 | DDP (15.27) | [84] | |
| 134 | 10.91, 18.42 | HL-60, A549 | DDP (11.70, 15.27) | [84] | |
| 135 | 10.75 | HL-60 | ADR (0.019) | [85] | |
| 136 | 10.58 | HL-60 | ADR (0.019) | [85] | |
| 137 | 2.86, 5.03 | A549, HL-60 | ADR (0.852, 0.019) | [85] | |
| 138 | 1.00, 1.36 | A549, HL-60 | ADR (0.852, 0.019) | [85] | |
| 139 | 9.9 | HL-60 | ADR (0.02) | [86] | |
| 140 | 3.8, 2.8 | A549, HL-60 | ADR (0.85, 0.02) | [86] | |
| 141 | 9.0, 2.5 | A549, HL-60 | ADR (0.85, 0.02) | [86] | |
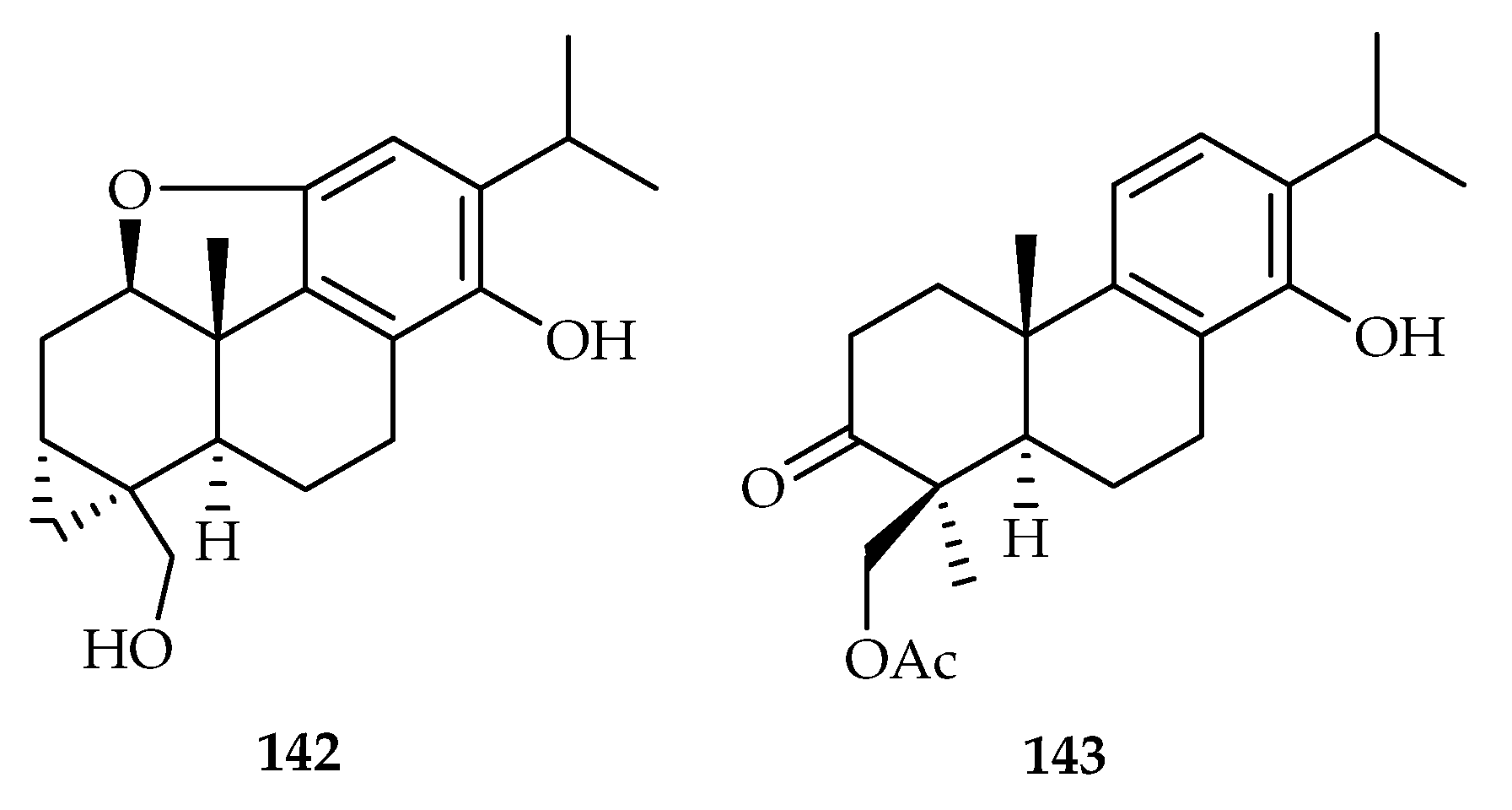
| 142 | 19.28 | SW1990 | Paclitaxel (92.16) | [87] | |
| 143 | 9.91 | SW1990 | Paclitaxel (92.16) | [87] | |
| 144 | 17.6 | MM-CSCs | Bortezomib (0.008) | [88] | |
| 145 | 2.63, 2.52, 4.84, 1.18, 3.23 | HL-60, SMMC-7721, A549, MCF-7, SW480 | DDP (1.81, 8.86, 11.68, 15.92, 8.86) | [89] | |
| 146 | 17.34 | MSTO-211H | Staurosporine (0.0011) | [90] | |
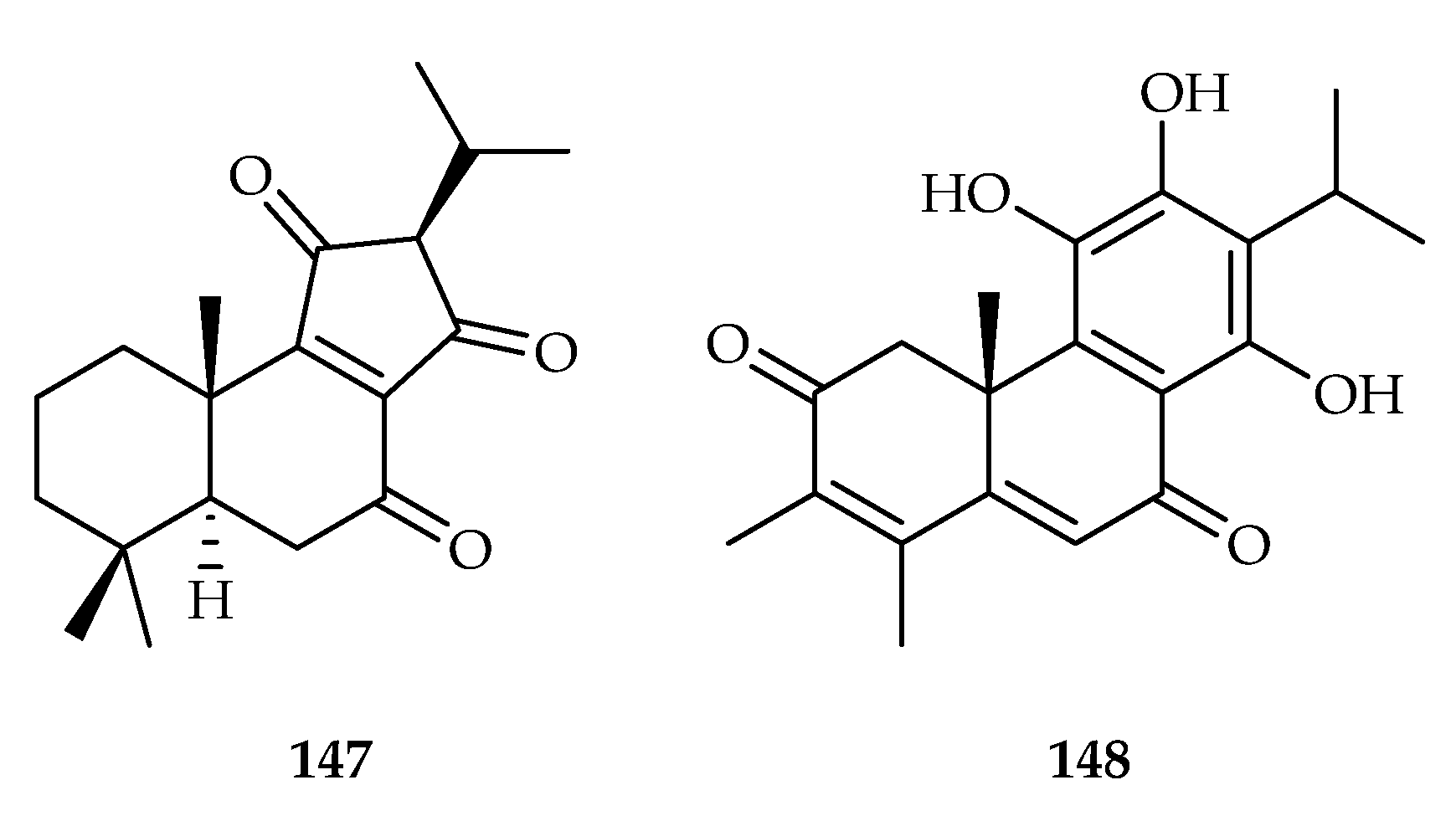
| 147 | 2.6 | L6 | Podophyllotoxin (0.02) | [91] | |
| 148 | 15.32, 8.36 | CCRF-CEM, CEM/ADR5000 | DOX (0.01, 66.83) | [92] | |
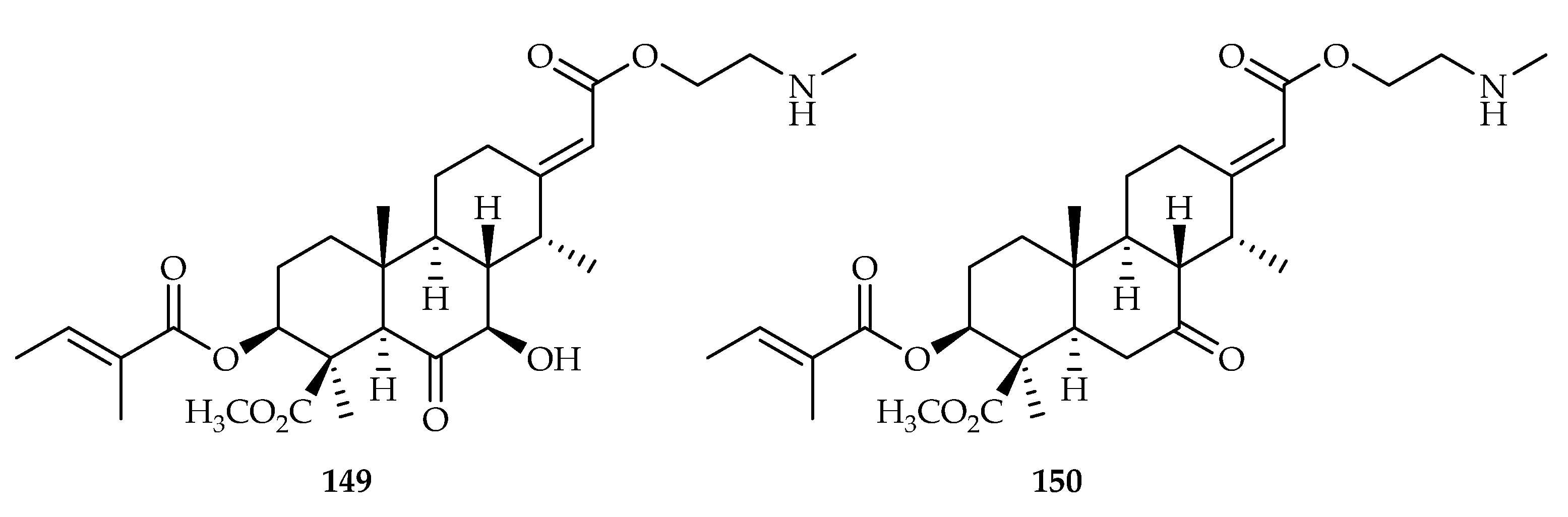
| Cassane | 149 | 1.4, 1.1, 1.2 | A549, NCI-H1975, NCI-H1299 | Camptothecin (0.7, 1.2, 0.8) | [94] |
| 150 | 0.5, 0.4, 0.9 | A549, NCI-H1975, NCI-H1299 | Camptothecin (0.7, 1.2, 0.8) | [94] | |
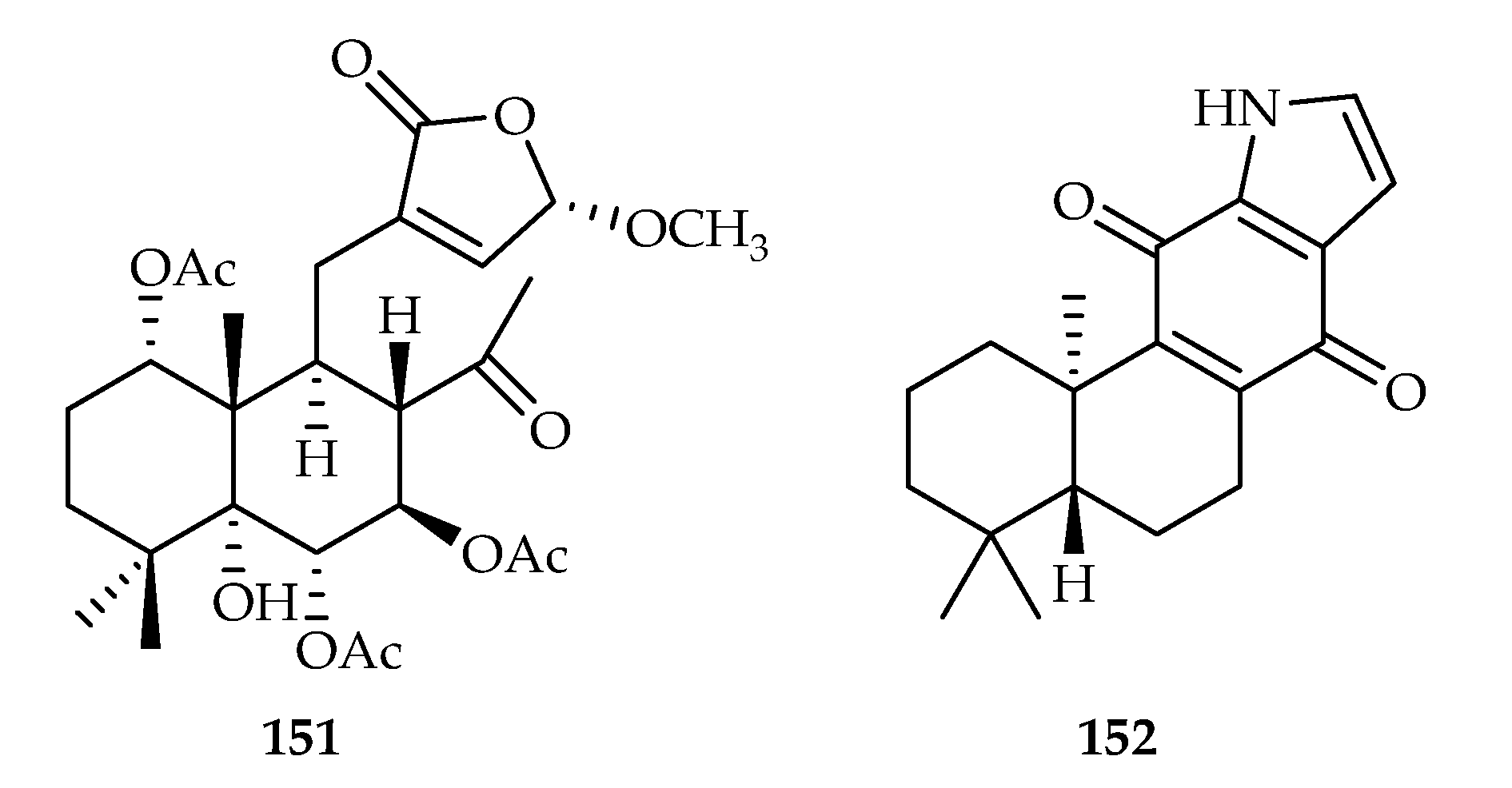
| 151 | 11.42 | HeLa | DDP (1.65) | [95] | |
| 152 | 3.2 | HEL | DOX (0.12) | [96] | |
| Dolabrane | 153 | 8.97, 8.97, 4.62, 17.11, 3.93 | MDA-MB-453, MDA-MB-231, SK-BR-3, ZR-75-1, MT-1 | DDP (4.37, 6.25, 8.42, 20.65, 7.90) | [99] |
| 154 | 18.06, 17.24, 8.07 | MDA-MB-453, SK-BR-3, MT-1 | DDP (4.37, 8.42, 7.90) | [99] | |
| 155 | 1.73, 8.12, 2.45, 12.03, 3.75, 1.97 | MD-MBA-453, MD-MBA-231, SK-BR-3, MCF-7, MT-1, ZR-75-1 | DDP (4.37, 3.73, 8.42, 3.21, 7.90, 20.65) | [100] | |
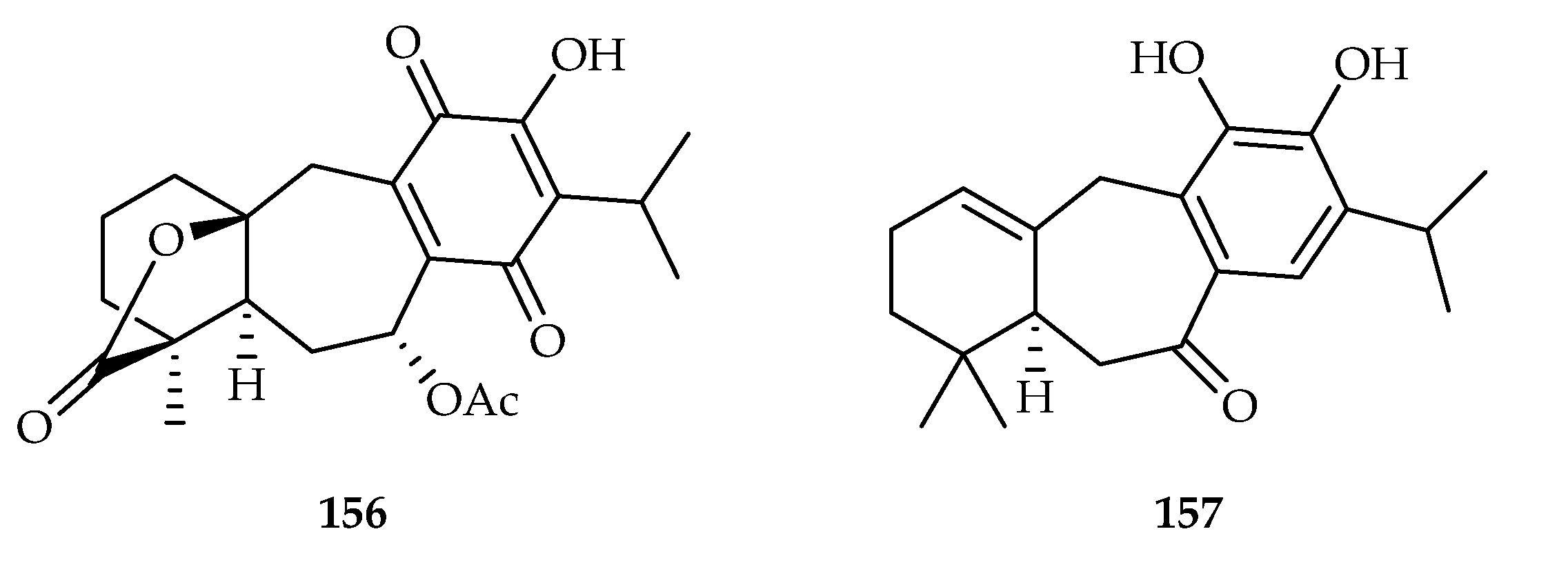
| Icetexane | 156 | 1.4, 0.82 | U-251, SKLU-1 | ADR (0.08, 0.05) | [101] |
| 157 | 17.70 | HL-60 | DDP (2.47) | [102] | |
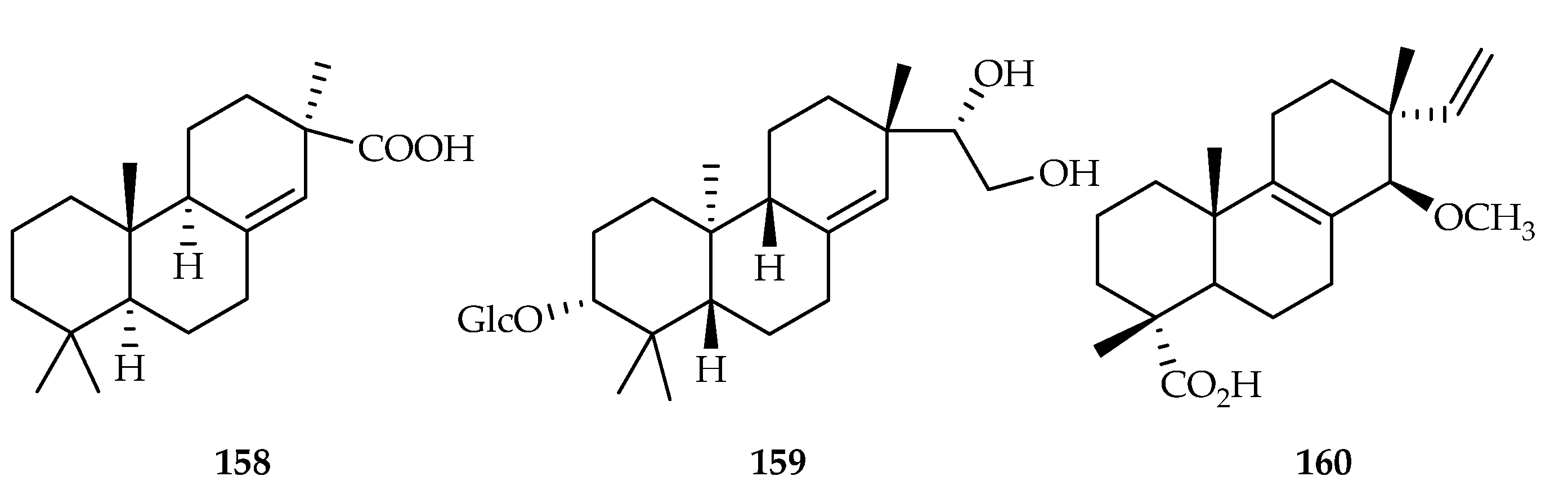
| Pimarane | 158 | 3.75 | MT-1 | DDP (7.90) | [99] |
| 159 | 0.27 | MB-MDA-231 | 2-morpholin-4-yl-8-phenylchromen-4-on (0.38) | [103] | |
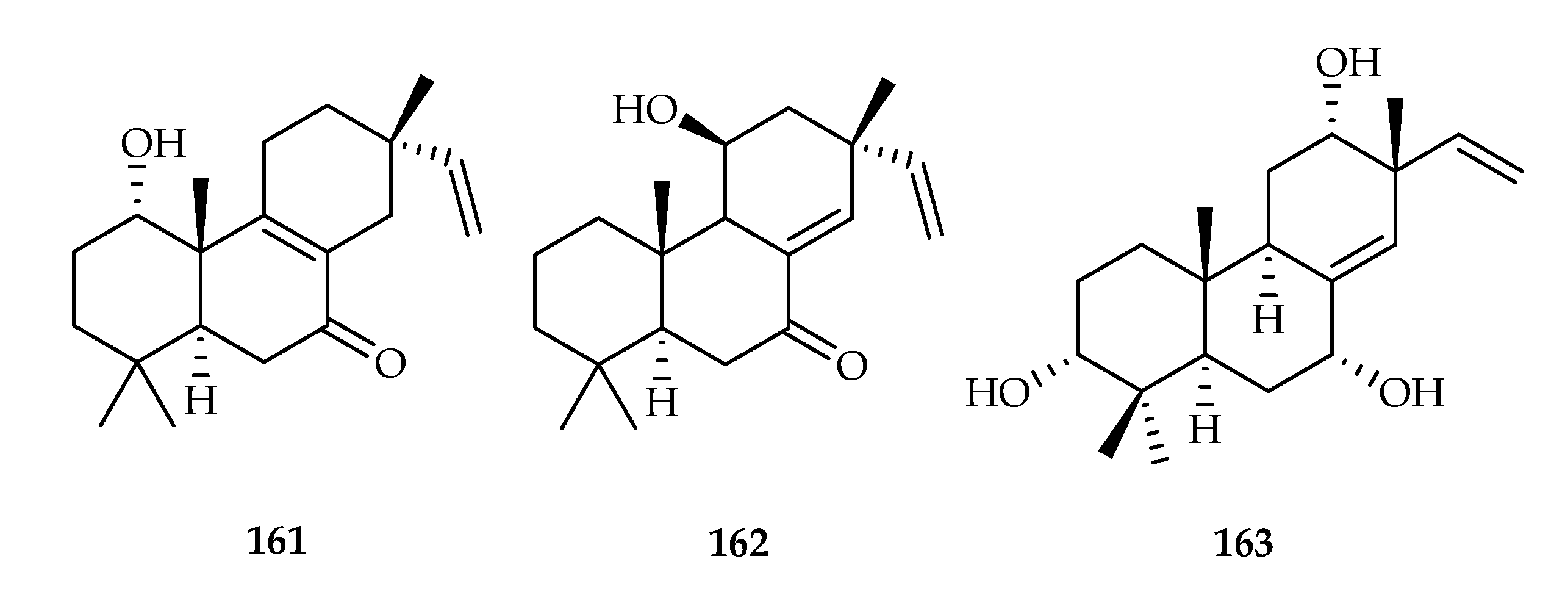
| 160 | 2.6 | A549 | - | [104] | |
| 161 | 0.35, 0.23, 1.42 | HepG2, HL60, HeLa | DDP (0.17, 0.21, 0.06) | [105] | |
| 162 | 4.29, 1.55, 1.27 | HepG2, HL60, HeLa | DDP (0.17, 0.21, 0.06) | [105] | |
| 163 | 14.327, 12.033 | MCF-7, A549 | DOX (15.7, 4.2) | [106] | |
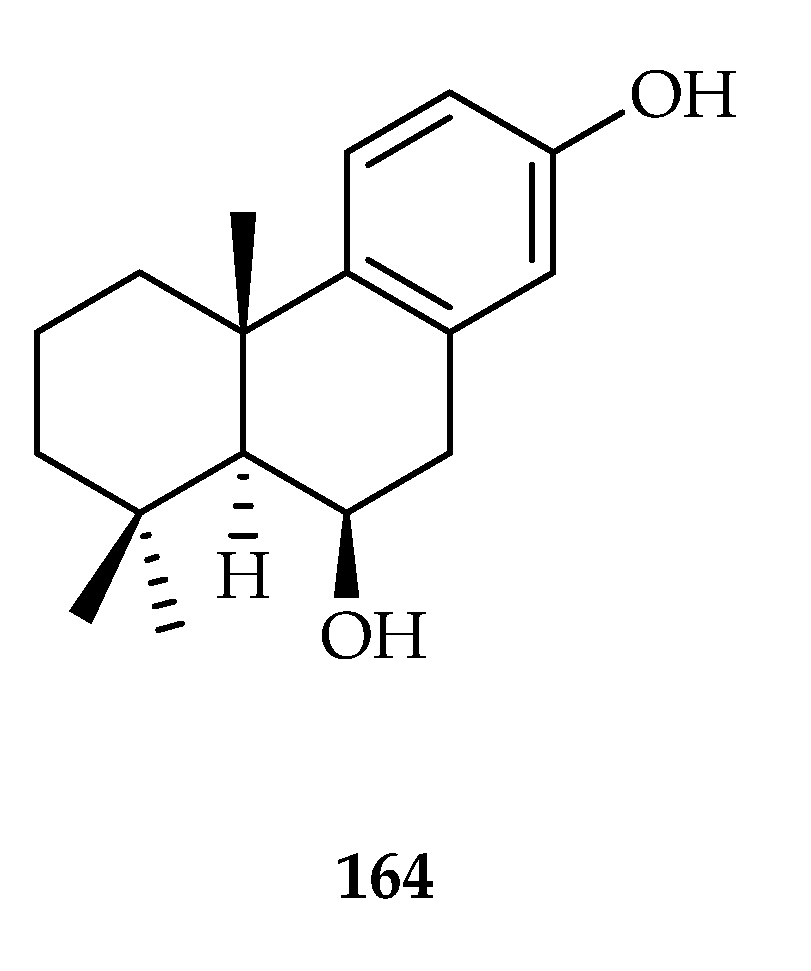
| Podocarpane | 164 | 13.2 | SW480 | DDP (12.8) | [107] |
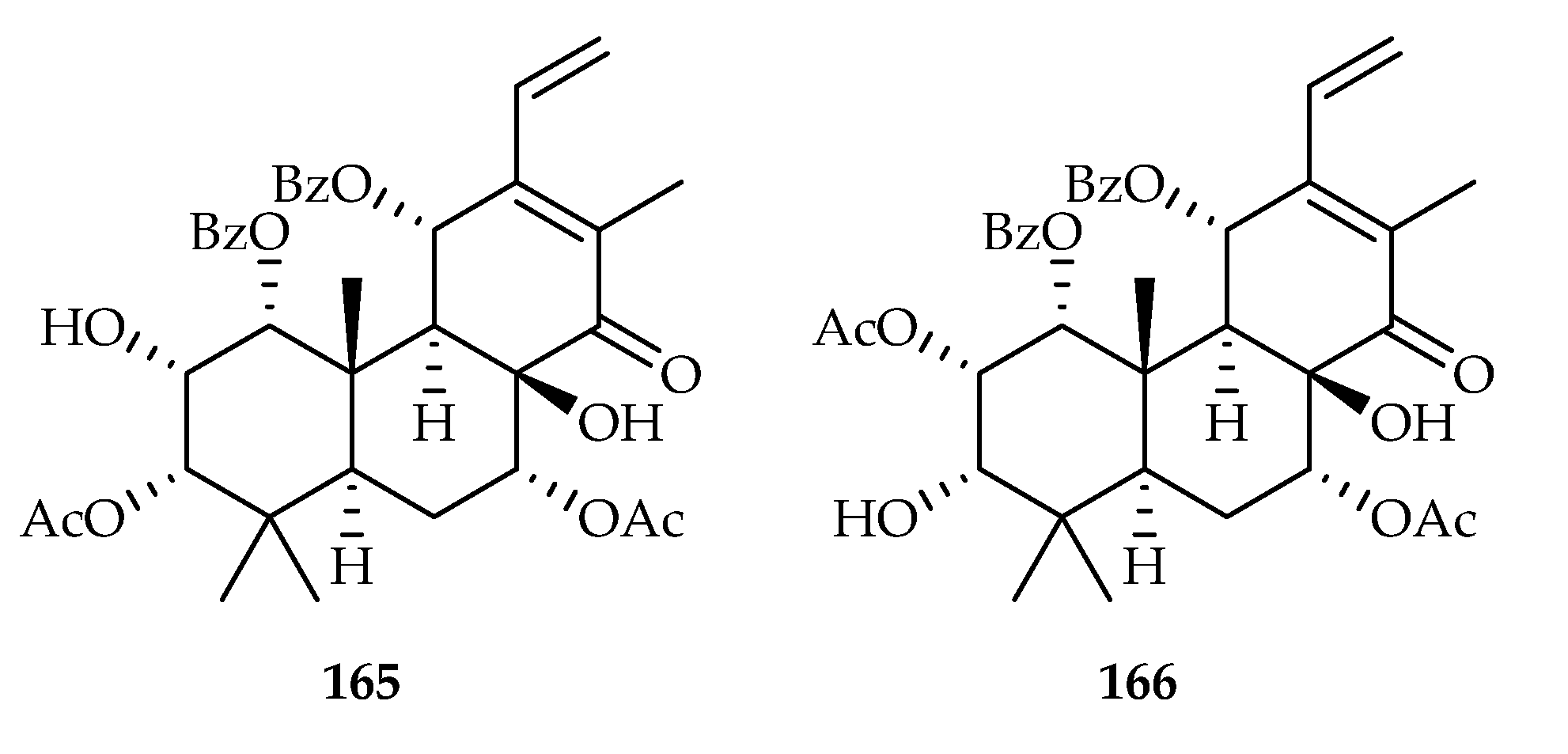
| Staminane | 165 | 12.31, 16.36, 5.62, 14.70, 15.24 | HL-60, A549, SMMC-7721, MCF-7, SW-480 | DDP (3.19, 23.25, 22.53, 19.56, 25.57) | [108] |
| 166 | 15.76, 19.15, 19.84 | HL-60, SMMC-7721, SW-480 | DDP (3.19, 22.53, 25.57) | [108] | |
| Atisane | 167 | 4.1, 4.0 | A549, HL-60 | ADR (0.85, 0.02) | [86] |
| Beyerane | 168 | 11.1 | SKOV3 | DDP (-) | [109] |
| Cephalotane | 169 | 4.5, 6.8 | A549, HL-60 | ADR (0.44, 0.075) | [111] |
| 170 | 2.4, 2.2 | A549, HL-60 | ADR (0.44, 0.075) | [111] | |
| 171 | 1.0, 9.8 | A549, HL-60 | ADR (0.44, 0.075) | [111] | |
| 172 | 0.77, 1.129 | HL60, A549 | DOX (0.031, 0.124) | [112] | |
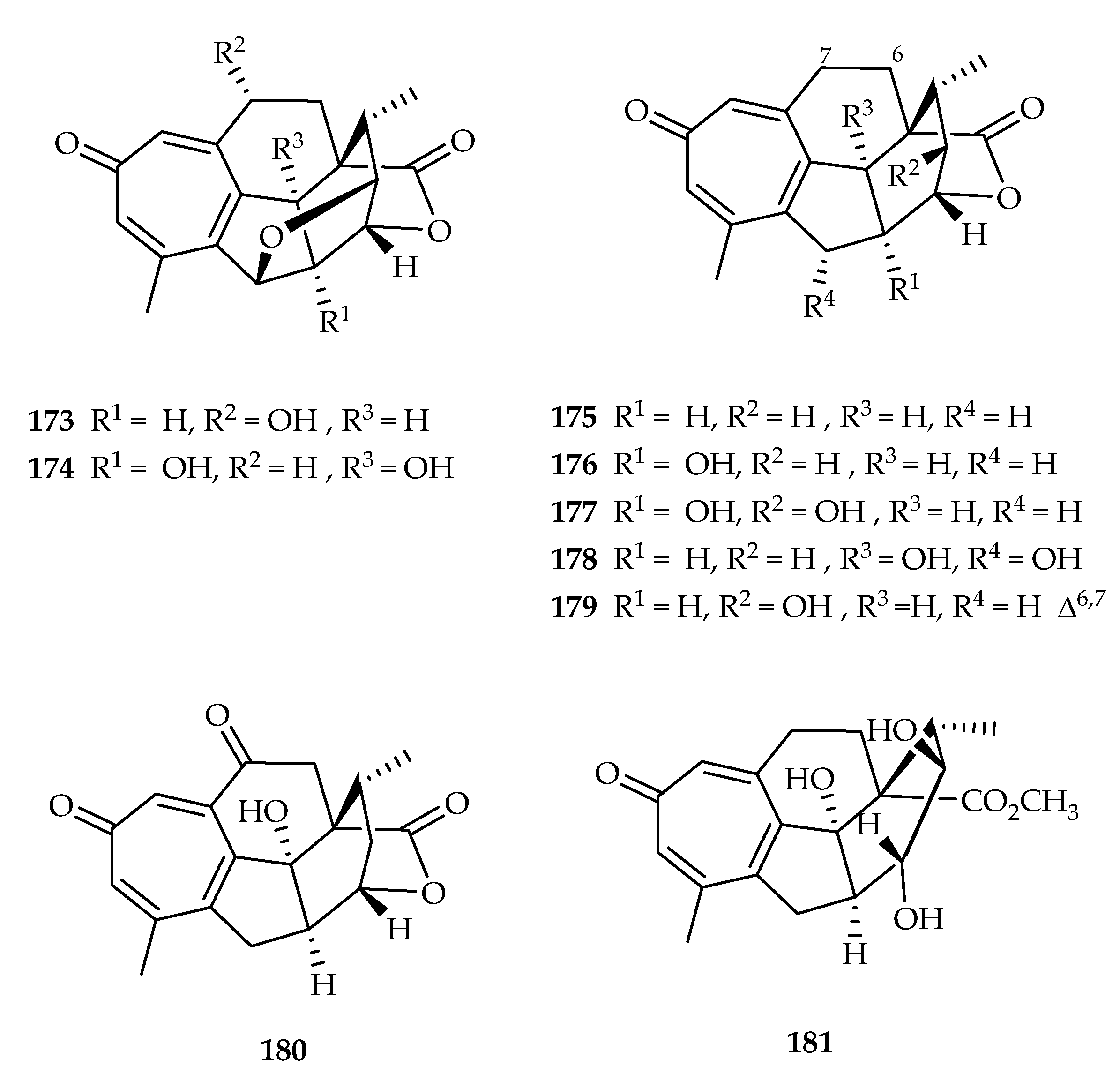
| 173 | 1.63, 1.72, 1.73 | A549, HeLa, SGC7901 | DDP (7.32, 8.42, 11.43) | [113] | |
| 174 | 4.64, 6.73, 3.84 | A549, HeLa, SGC7901 | DDP (7.32, 8.42, 11.43) | [113] | |
| 175 | 0.10, 0.13, 0.14 | A549, HeLa, SGC7901 | DDP (7.32, 8.42, 11.43) | [113] | |
| 176 | 0.31, 0.71, 0.35 | A549, HeLa, SGC7901 | DDP (7.32, 8.42, 11.43) | [113] | |
| 177 | 4.92, 6.85, 5.88 | A549, HeLa, SGC7901 | DDP (7.32, 8.42, 11.43) | [113] | |
| 178 | 16.5, 18.3 | A549, HeLa | DDP (7.32, 8.42) | [113] | |
| 179 | 19.7 | HeLa | DDP (8.42) | [113] | |
| 180 | 14.6, 15.3 | A549, HeLa | DDP (7.32, 8.42) | [113] | |
| 181 | 16.6 | HeLa | DDP (8.42) | [113] | |
| Kaurane | 182 | 13.3 | HMy2.CIR | - | [119] |
| 183 | 1.0, 1.5, 4.4, 2.9, 0.9 | HL-60, SMMC-7721, A549, MCF-7 SW-480 | DDP (3.0, 10.2, 16.0, 15.3, 9.3) | [120] | |
| 184 | 7.0, 4.7, 19.6, 11.0, 2.5 | HL-60, SMMC-7721, A549, MCF-7, SW-480 | DDP (3.0, 10.2, 16.0, 15.3, 9.3) | [120] | |
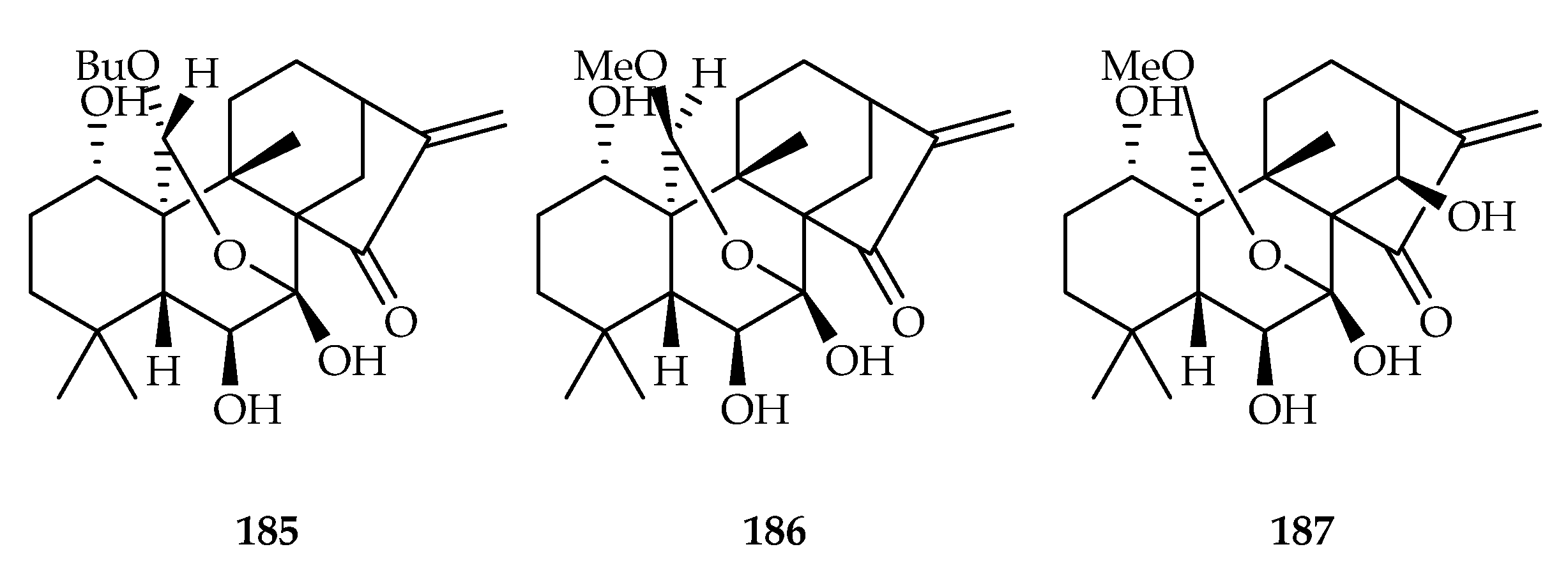
| 185 | 1.2, 5.3, 3.0, 2.9, 0.8 | HL-60, A549, SMMC-7721, MCF-7, SW-480 | DDP (2.1, 14.7, 5.7, 15.3, 9.2) | [121] | |
| 186 | 3.4, 8.6, 4.1, 2.1 | HL-60, SMMC-7721, MCF-7, SW-480 | DDP (2.1, 5.7, 15.3, 9.2) | [121] | |
| 187 | 1.0, 5.8, 3.2, 3.4, 1.9 | HL-60, A549, SMMC-7721, MCF-7, SW-480 | DDP (2.1, 14.7, 5.7, 15.3, 9.2) | [121] | |
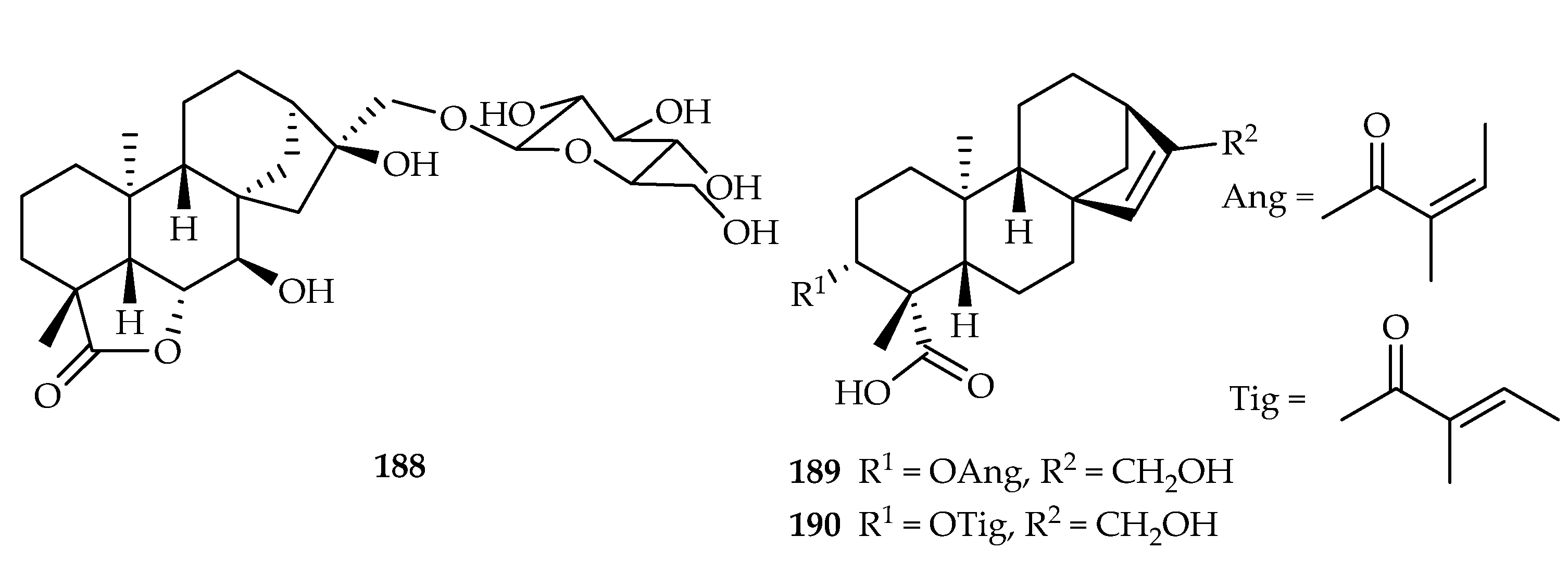
| 188 | 5.92, 15.31, 17.27 | SKOV3, SK-MEL-2, HCT15 | Etoposide (-) | [122] | |
| 189 | 16.94 | HepG2 | DDP (22.19) | [123] | |
| 190 | 12.38 | HepG2 | DDP (22.19) | [123] | |
| 191 | 8.7, 11.3 | SKOV3, MCF-7 | DDP (-) | [109] | |
| 192 | 8.1 | Hep-3B | 5-FU (9.36) | [124] | |
| 193 | 5.1, 9.8, 6.8, 2.9, 4.1, 1.9 | HeLa, A549, MDA-MB-231, SKOV3, Huh-7, HCT-116 | Paclitaxel (0.004, 0.005, 0.003, 0.002, 0.005, 0.006) | [55] | |
| 194 | 17.28, 11.56 | SMMC-7721, HepG2 | - | [125] | |
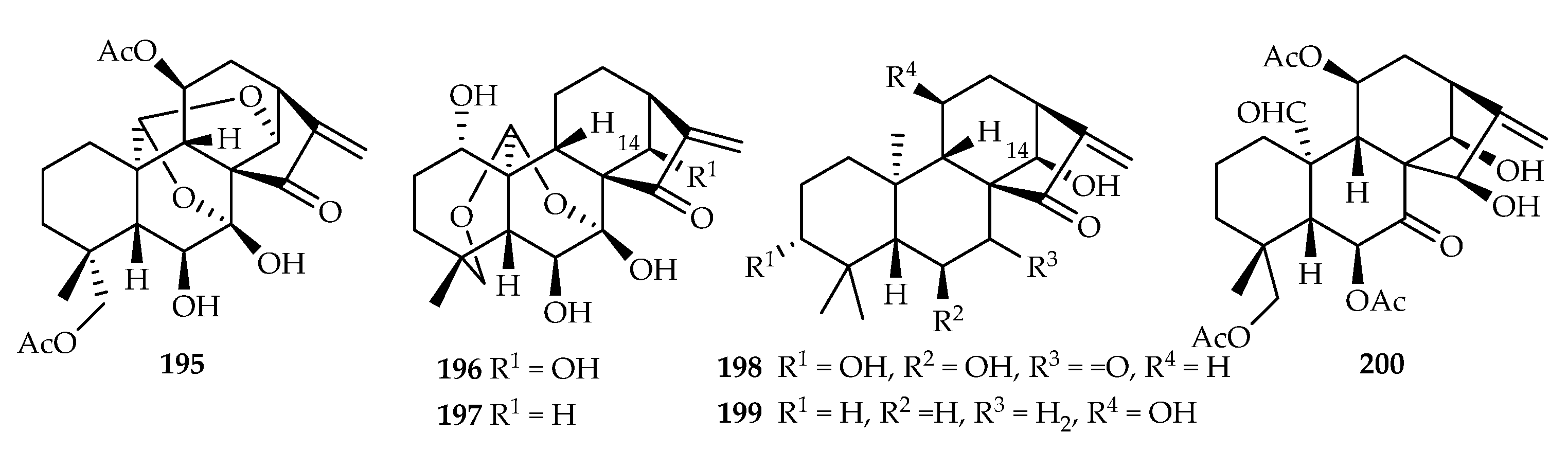
| 195 | 4.84, 1.89, 12.10, 13.54, 8.02 | HL-60, SMMC-7721, A549, MCF-7, SW-480 | DDP (2.06, 11.73, 9.09, 13.93, 7.79) | [126] | |
| 196 | 17.91 | SW-480 | DDP (7.79) | [126] | |
| 197 | 14.94, 14.06, 13.78, 13.95, 3.26 | HL-60, SMMC-7721, A549, MCF-7, SW-480 | DDP (2.06, 11.73, 9.09, 13.93, 7.79) | [126] | |
| 198 | 14.91 | SW-480 | DDP (7.79) | [126] | |
| 199 | 5.61, 2.20, 3.33, 4.45, 2.59 | HL-60, SMMC-7721, A549, MCF-7, SW-480 | DDP (2.06, 11.73, 9.09, 13.93, 7.79) | [126] | |
| 200 | 6.03, 2.58, 5.71, 3.96, 3.40 | HL-60, SMMC-7721, A549, MCF-7, SW-480 | DDP (2.06, 11.73, 9.09, 13.93, 7.79) | [126] | |
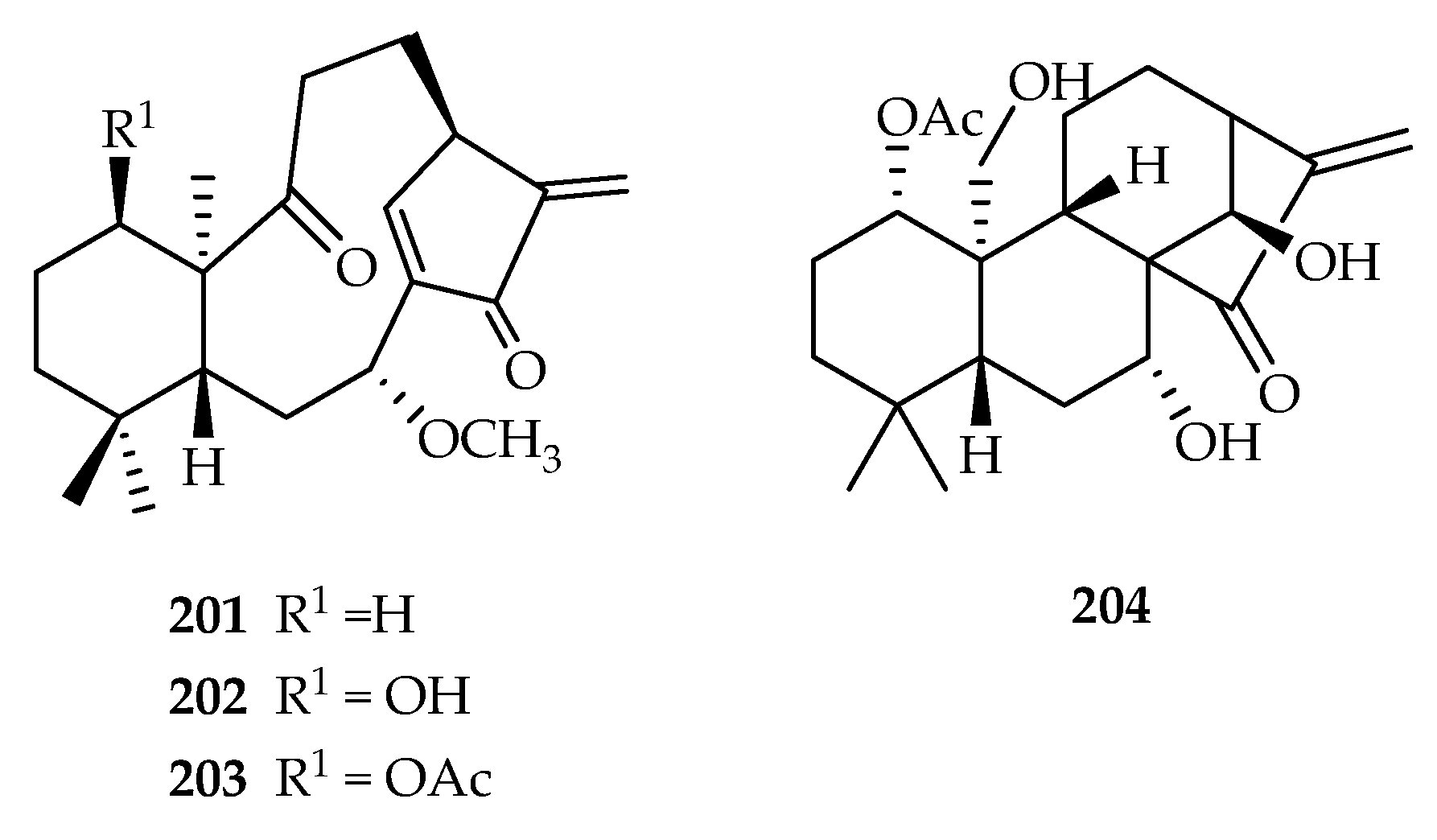
| 201 | 1.27, 5.74 | HL-60, A549 | ADR (0.06, 0.50) | [127] | |
| 202 | 0.47, 3.25 | HL-60, A549 | ADR (0.06, 0.50) | [127] | |
| 203 | 0.58 | HL-60 | ADR (0.06) | [127] | |
| 204 | 1.34, 1.07, 3.60, 2.35, 2.53 | HCT-116, HepG2, BGC-823, NCI-H1650, A-2780 | DDP (7.81, >10, 8.56, >10, 8.65) | [128] | |
| 205 | 19.5, 19.6 | SNU638, HCT116 | Etoposide (3.10, 7.94) | [129] | |
| 206 | 15.18, 7.22, 5.57, 1.99 | MDA-MB-231, SK-Hep-1 SNU638, HCT116 | Etoposide (28.56, 0.83, 3.10, 7.94) | [129] | |
| 207 | 1.99, 2.97, 1.11, 1.51 | A549, KB, MCF7, HCT116 | Paclitaxel (0.011, 0.0031, 0.0083, 0.0069) | [83] | |
| 208 | 17.36, 12.08, 12.47, 9.10, 2.66 | A549, Hep-3B, PC-3, HT29, U937 | CPT-11 (15.26, 23.21, 31.03, 15.11, 4.95) | [130] | |
| 209 | 7.39, 7.06, 4.19, 2.78, 1.97 | A549, Hep-3B, PC-3, HT29, U937 | CPT-11 (15.26, 23.21, 31.03, 15.11, 4.95) | [130] | |
| 210 | 17.12, 17.08, 16.49, 14.18, 6.73 | A549, Hep-3B, PC-3, HT29, U937 | CPT-11 (15.26, 23.21, 31.03, 15.11, 4.95) | [130] | |
| 211 | 19.39, 19.86, 15.96, 12.64, 8.25 | A549, Hep-3B, PC-3, HT29, U937 | CPT-11 (15.26, 23.21, 31.03, 15.11, 4.95) | [130] | |
| 212 | 3.56, 14.60, 3.50, 10.23, 2.96, 3.28, 2.07, 4.04 | HeLa, SK-OV-3, SK-HEP-1, Caco2, MDA-MB-231, PC-3, SW480, A549/Taxol | DDP (23.12, 26.98, 8.37, 6.53, 18.54, 16.76, 22.80, 10.22) | [131] | |
| 213 | 9.92, 13.37, 10.2, 16.12 | HeLa, SW480, A549, ACHN | CDDP (16.2, 29.93, NA, 20.36) | [132] | |
| 214 | 8.27, 10.37 | HeLa, SW480 | CDDP (16.2, 29.93) | [132] | |
| 215 | 12.48, 14.20, 16.04, 14.65, 3.74 | HL-60, SMMC-7721, A-549, MCF-7, SW-480 | DDP (2.06, 11.73, 9.09, 13.93, 7.79) | [133] | |
| 216 | 5.53, 2.69, 6.23, 3.86, 3.17 | HL-60, SMMC-7721, A-549, MCF-7, SW-480 | DDP (2.06, 11.73, 9.09, 13.93, 7.79) | [133] | |
| 217 | 18.04, 12.28, 14.50, 15.95, 9.68 | HL-60, SMMC-7721, A-549, MCF-7, SW-480 | DDP (2.06, 11.73, 9.09, 13.93, 7.79) | [133] | |
| 218 | 3.77, 3.99, 6.80, 3.20, 1.23 | HL-60, SMMC-7721, A-549, MCF-7, SW-480 | DDP (2.06, 11.73, 9.09, 13.93, 7.79) | [133] | |
| 219 | 6.6, 2.3, 11.5 | MCF-7, A549, PC-3 | ADR (0.4, 0.1, 0.8) | [134] | |
| 220 | 15.81, 1.93 | A549, K562 | ADR (3.48, 3.49) | [135] | |
| 221 | 9.89, 0.59 | A549, K562 | ADR (3.48, 3.49) | [135] | |
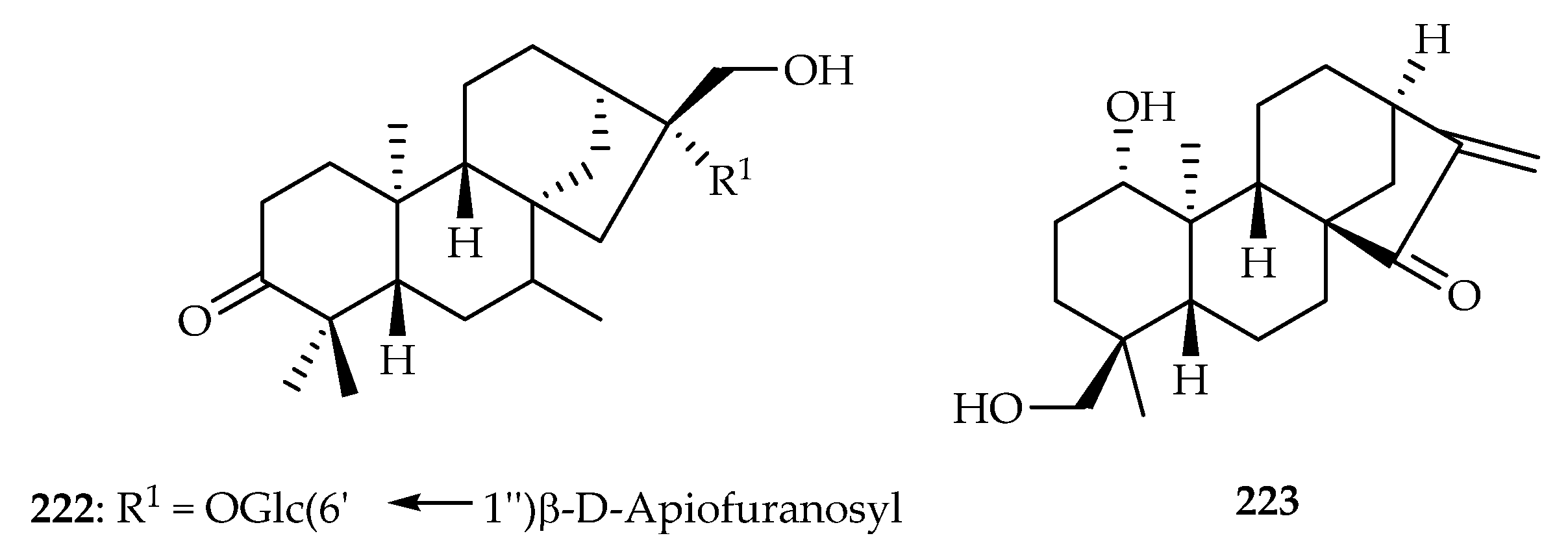
| 222 | 5.11 | A549 | Etoposide (36.5) | [136] | |
| 223 | 2.8, 3.4, 3.6, 4.0, 2.6 | HL-60, SMMC-7721, A549, MCF-7, SW480 | DDP (3.0, 10.2, 16.0, 15.3, 9.3) | [137] | |
| 224 | 3.9, 2.4, 4.2 | EC109, SHG-44, MCF-7 | Adenanthin (6.5, 4.8, 7.6) | [138] | |
| 225 | 15.83, 17.37, 19.47, 19.50 | HepG2, Caco2, U2OS, MDA-MB-231 | - | [139] | |
| 226 | 15.45, 10.05, 3.01, 3.38 | A549, HCT116, CCRF-CEM, HL-60 | DOX (0.20, 0.070, 0.014, 0.01) | [140] | |
| 227 | 11.60, 8.64, 2.77, 3.16 | A549, HCT116, CCRF-CEM, HL-60 | DOX (0.20, 0.070, 0.014, 0.01) | [140] | |
| 228 | 15.59 | EC109 | - | [141] | |
| 229 | 4.07 | HCT116 | - | [142] | |
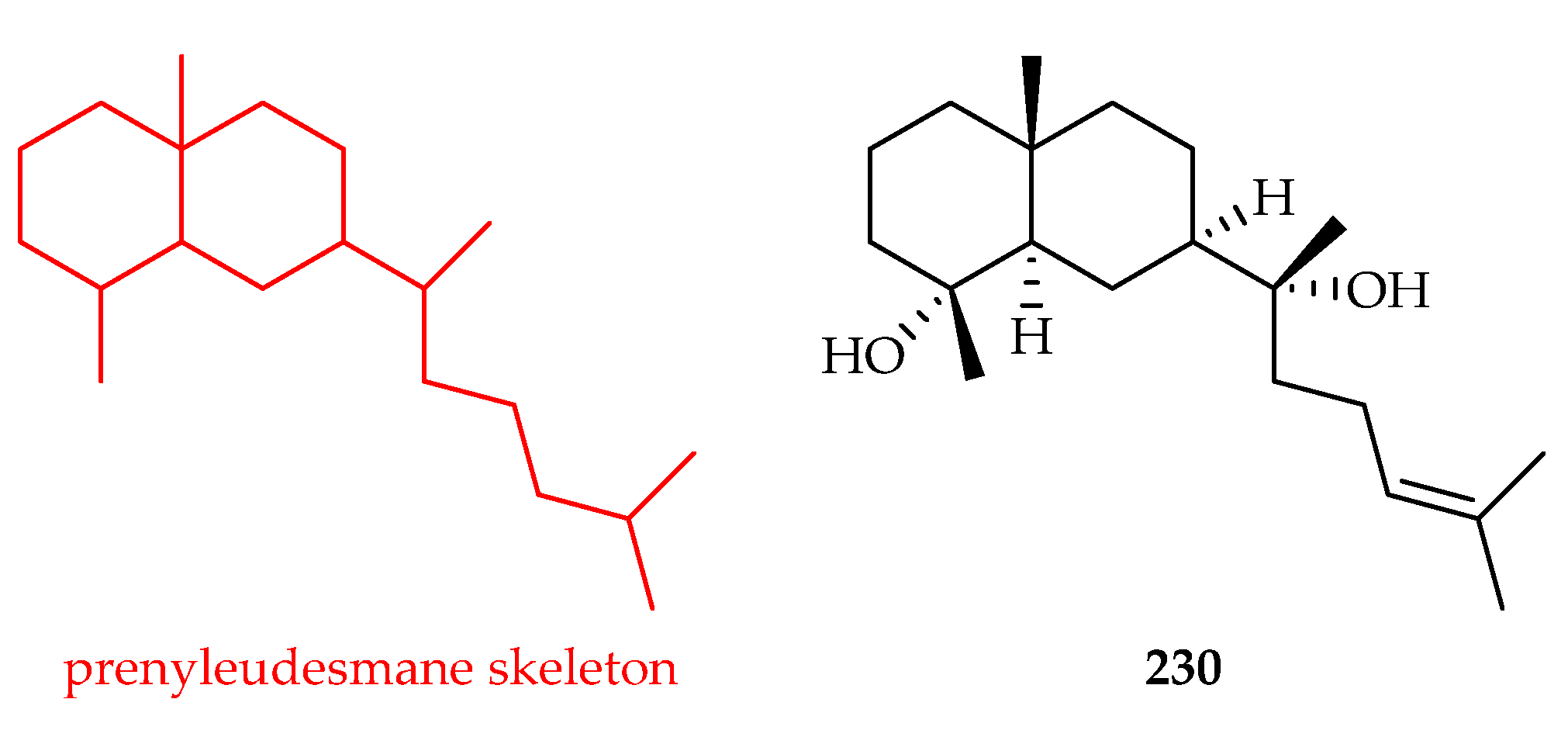
| Prenyleudesmane | 230 | 13.8 | HeLa | Etoposide (21.2) | [143] |
| Norditerpenoids and Dinorditerpenoids | 231 | 3.8, 6.4 | A-2780, HEY | Paclitaxel (0.16, 0.10) | [144] |
| 232 | <2.5 | A-2780, HEY | Paclitaxel (0.16, 0.10) | [144] | |
| 233 | 5.8 | A549 | DOX (0.090) | [145] | |
| 234 | 0.7 | U87-MG | DOX (0.0996) | [145] | |
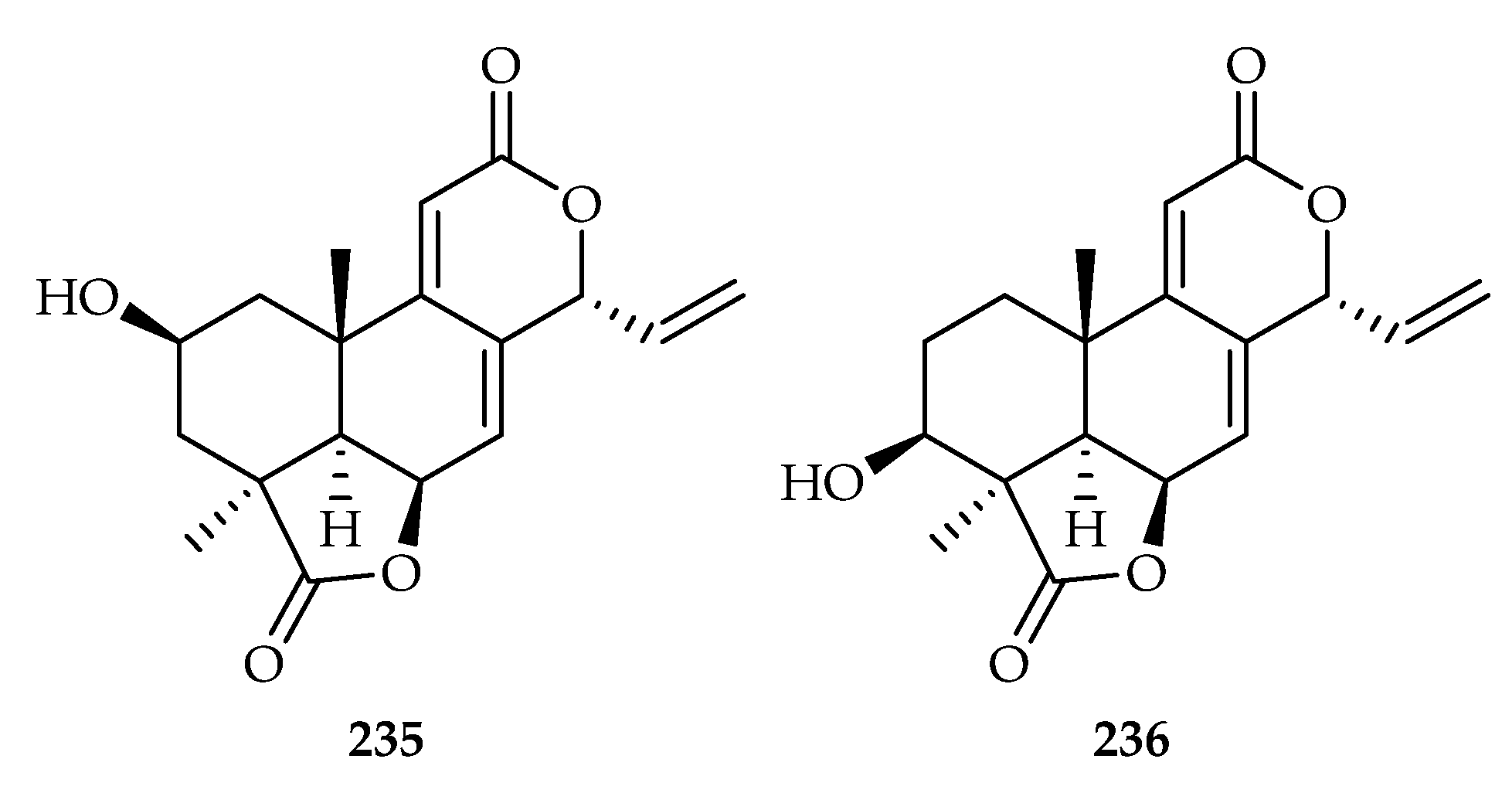
| 235 | 0.87, 0.38, 4.23, 19.17 | HeLa, AGS, MDA-MB-231, HepG2 | DDP (6.30, 20.98, 16.26, 24.07) | [146] | |
| 236 | 11.61, 0.88, 5.46, 5.56, 1.35 | HeLa, AGS, MDA-MB-231, HepG2 | DDP (6.30, 20.98, 16.26, 24.07, 18.27) | [146] | |
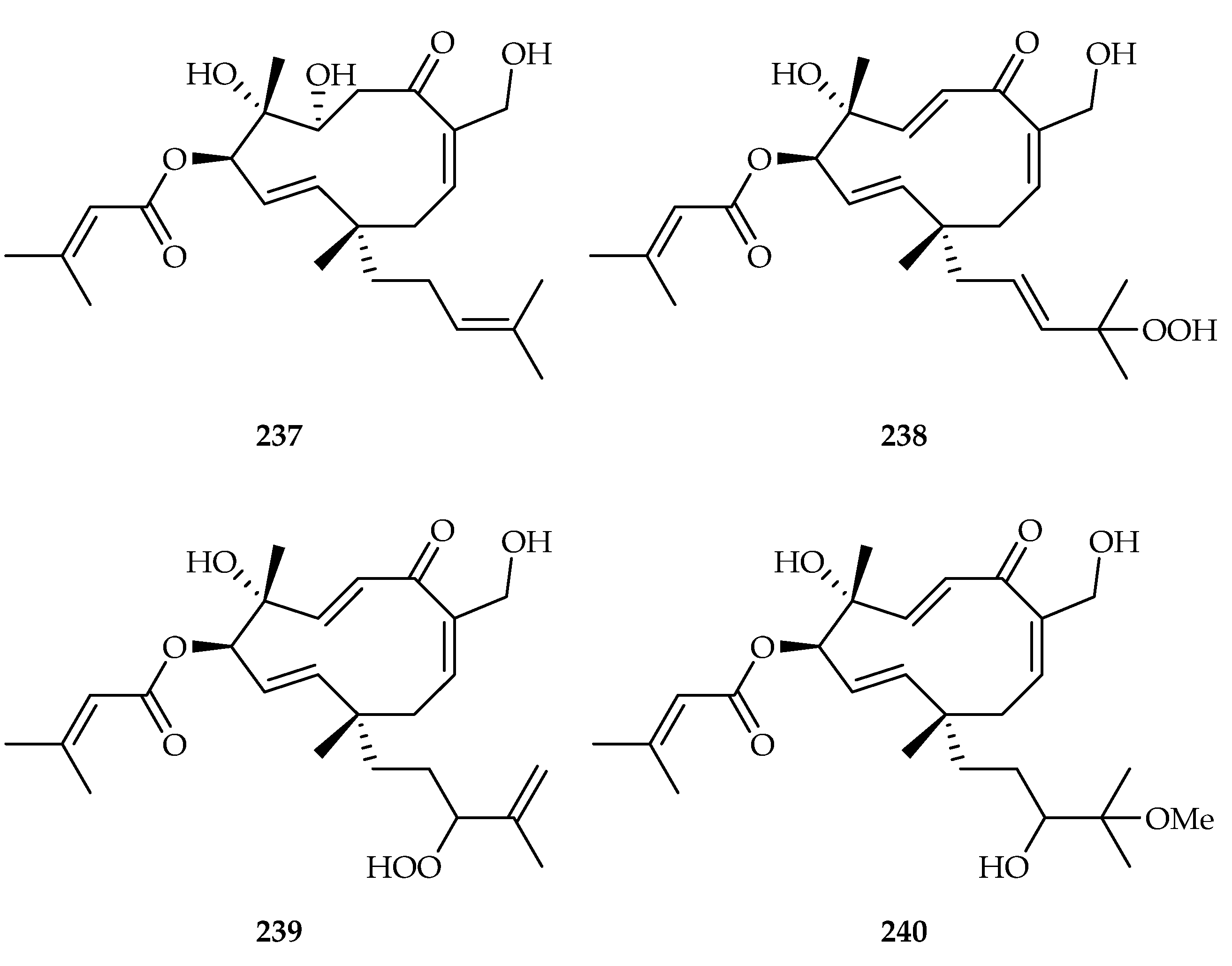
| Vibsane-type diterpenoid | 237 | 2.88, 7.31, 6.89, 5.88, 5.22 | HL60, A549, SMMC-7721, MCF7, SW480 | DDP (5.77, 19.68, 22.32, 21.17, 29.21) | [148] |
| 238 | 14.45, 18.47 | HL60, SMMC-7721 | DDP (5.77, 22.32) | [148] | |
| 239 | 2.56, 17.36, 3.42, 14.61, 5.34 | HL60, A549, SMMC-7721, MCF7, SW480 | DDP (5.77, 19.68, 22.32, 21.17, 29.21) | [148] | |
| 240 | 13.09, 16.51, 19.46, 15.98 | HL60, SMMC-7721, MCF7, SW480 | DDP (5.77, 22.32, 21.17, 29.21) | [148] | |
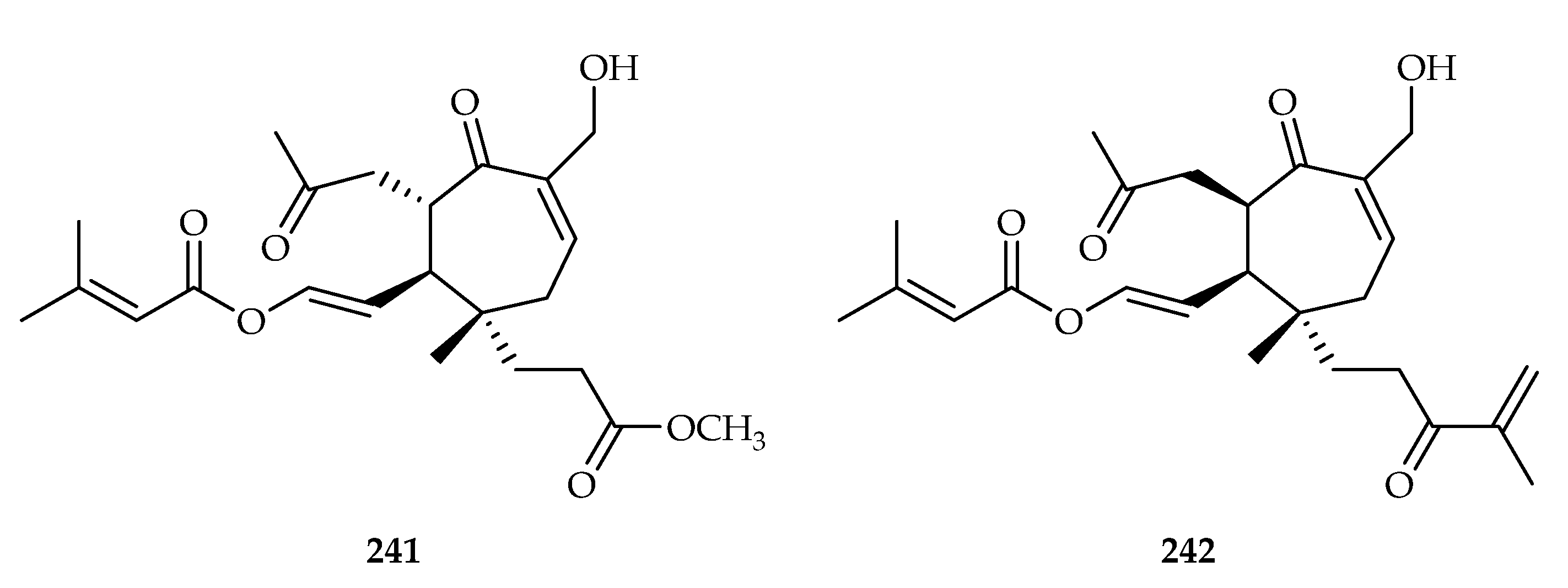
| 241 | 1.11, 13.62 | A549, HepG2 | Taxol (0.024), sorafenib (0.49) | [149] | |
| 242 | 19.75, 12.61 | A549, HepG2 | Taxol (0.024), sorafenib (0.49) | [149] | |
| Other skeletons | 243 | 2.6 | HL-60 | mitomycin C (0.19) | [150] |
| 244 | 18.0 | A549 | 5-FU (9.5) | [151] | |
| 245 | 1.24, 1.92 μM | HL-60, A549 | ADR (0.06, 0.50) | [152] | |
| 246 | 8.2, 5.4, 10.4 | PC-9, H1650, A549 | 5-FU (>10, >10, >10) | [153] | |
| 247 | 15.75 | HepG2 | DDP (16.28) | [154] | |
| 248 | 14.48 | T24 | Gemcitabine (6.50) | [43] | |
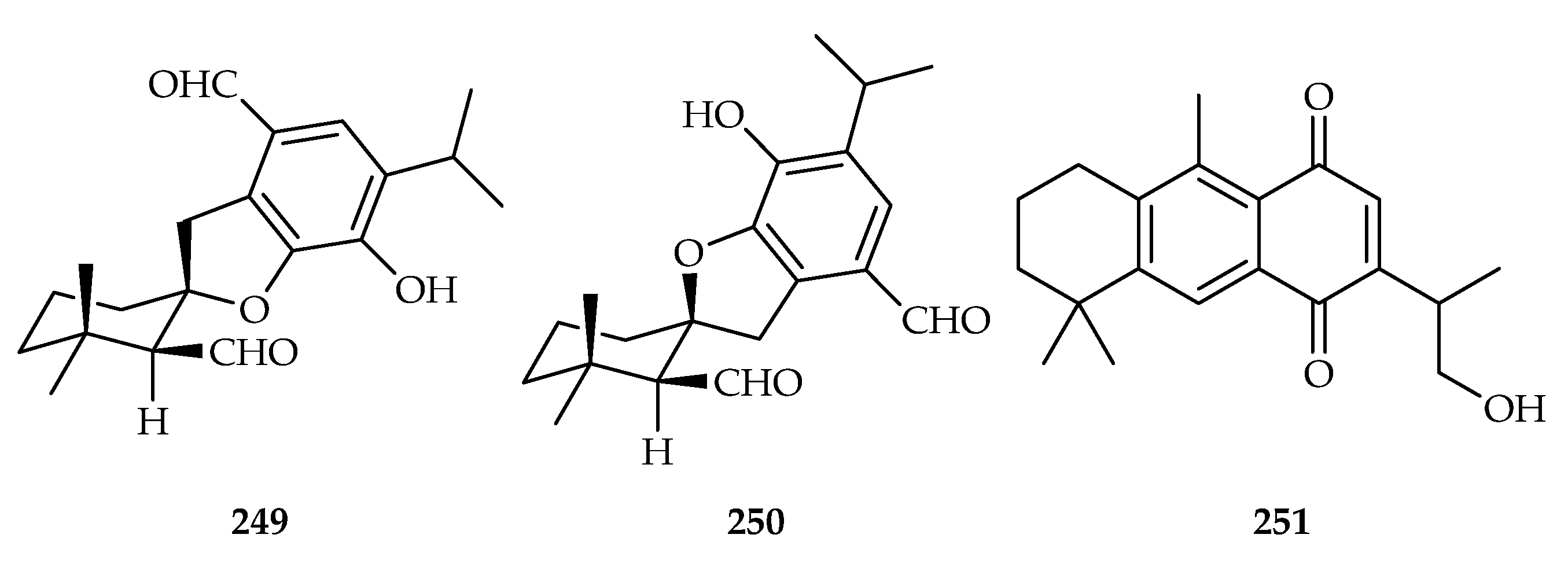
| 249 | 6.60, 7.13, 11.32, 11.50, 18.20 | A549, SMMC-7721, HL-60, MCF-7, SW480 | DDP (13.84, 7.82, 2.47, 13.46, 10.06) | [102] | |
| 250 | 18.00, 13.64, 18.42, 18.21, 21.65 | A549, SMMC-7721, HL-60, MCF-7, SW480 | DDP (13.84, 7.82, 2.47, 13.46, 10.06) | [102] | |
| 251 | 7.56, 10.34, 5.47, 5.48 | MCF-7, B16F10, PC-3, C26 | DOX (1.72, 0.68, 1.84, 0.25) | [155] |
| Organ | Cell Lines | Compound (Grouped by Cell Line Higlighted in Red) |
|---|---|---|
| Bladder | T24 | - |
| Blood | CCRF-CEM, CEM/ADR5000, HEL, HL-60, HMy2.CIR, Jurkat, K562, L5178Y, MM-CSCs, MOLT-4, MV4-11, Namalwa, NB4, p388D1, U937 | K562: 44, 45, 59, 220, 221; L5178Y: 86, 87; Namalwa: 117, 118; HL-60: 121, 122, 134, 183, 185, 187, 223, 237, 239; CEM/ADR5000: 148; 208, U937: 209 |
| Bones | MG-63, Saos-2, U2OS | Saos-2 and MG-63: 29 |
| Brain | SHG-44, U87, U87-TxR, U87-MG, U-251 | SHG-44: 224 |
| Breast | BT474, MCF-7, MDA-MB-231, MDA-MB-453, MM-231, MT-1, SK-BR-3, ZR-75-1 | MCF-7: 35, 47, 48, 49, 92, 98, 99, 116, 121, 122, 145, 163, 165, 183, 184, 185, 186, 187, 195, 199, 200, 216, 223, 224, 237, 239, 240, 249; MDA-MB-231: 47, 48, 49, 127, 159, 206, 212, 235, 236; MDA-MB-453: 155; MT-1: 153, 155, 158; SK-BR-3: 153, 155; ZR-75-1: 153, 155 |
| Cervix | HeLa | HeLa: 56, 57, 58, 59, 60, 61, 62, 116, 117, 173–177, 212, 213, 214, 230, 235 |
| Colon | C26, CaCo2, HCT-15, HCT-15/5-FU, HCT-116, HT29, LoVo, RKO, SW480, SW620 | SW620 and RKO: 7; HCT-116: 81, 204, 206; SW480: 120, 121, 122, 145, 165, 166, 183, 184, 185, 186, 187, 197, 199, 200, 212, 213, 214, 215, 216, 218, 223, 237, 239, 240; HT29: 208, 209, 210, 211 |
| Endometrium | ECC-1 | - |
| Epithelium | A375, B16F10, B16melanoma4A5, HMCB, KB, KB-VIN, MDA-MB-435, MM96L, SK-Mel2 | KB: 47, 48, 49; KB-VIN: 47, 48, 49, 50, 73, 74, 79; B16melanoma4A5: 83–85 |
| Esophage | EC, EC109, EC9706, KYSE-450, | EC109: 224 |
| Kidney | ACHN | ACHN: 1, 2, 213 |
| Liver | HepG2, HepG2/DOX, Hep3B, Huh-7, SK-Hep-1, SMMC-7721 | Hep3B: 9, 10, 11, 192, 208, 209, 210, 211; HepG2: 12, 59, 62, 189, 190, 204, 235, 236, 247;HepG2/DOX: 37, 38; SMMC-7721: 121, 122, 145, 165, 166, 183, 185, 187, 195, 199, 200, 216, 218, 223, 237, 238, 239, 240, 249; SK-Hep-1: 212 |
| Lung | 95-D, A549, A549/CDDP, A549/Taxol, Chago-K1, LUPF003, LUPF045, MSTO-211H, NCI-H1229, NCI-H1650, NCI-H1975, NCI-H460, PC-9, SKLU-1, XWLC-05 | A549: 47, 48, 57, 62, 65, 66, 82, 121, 122, 130, 131, 133, 145, 150, 165, 173–177, 183, 185, 187, 199, 200, 209, 216, 218, 222, 223, 237, 239, 249; LUPF045: 104, 105; NCI-H1975: 149, 150; A549/Taxol: 212; PC-9: 246; NCI-H1650: 204, 246 |
| Ovaries | A2780, HEY, OVPF008, OVPF038, SKOV3 | A2780: 204; SKOV3: 212 |
| Pancreas | PANC-1, SW1990 | SW1990: 142, 143 |
| Prostate | C4-2B, C4-2B/ENZR, DU145, PC3 | PC-3: 81, 208, 209, 210, 211, 212 |
| Stomach | ACP01, AGS, BGC-823, KATO-III, SGC7901, SNU638 | SGC7901: 173–177; BGC-823: 204, AGS: 235, 236 |
| Plant Family | Species | Compound |
|---|---|---|
| Acanthaceae | Hypoestes forsskaolii | 15 |
| Annonaceae | Annona squamosa | 194 |
| Araucariaceae | Araucaria bidwillii | 86–88 |
| Asteraceae | Sheareria nana | 65–68 |
| Siegesbeckia pubescens | 159 | |
| Tagetes minuta | 3,4 | |
| Wedelia prostrata | 189, 190 | |
| Caprifoliaceae | Lonicera macranthoides | 230 |
| Celastraceae | Euonymus oblongifolius | 160, 161 |
| Salacia cochinchinensis | 162 | |
| Tripterygium hypoglaucum | 142, 143 | |
| Tripterygium regelii | 100, 121 | |
| Compositae | Ligularia fischeri | 182 |
| Convolvulaceae | Pharbitis nil | 188 |
| Ebenaceae | Diospyros maritima | 222 |
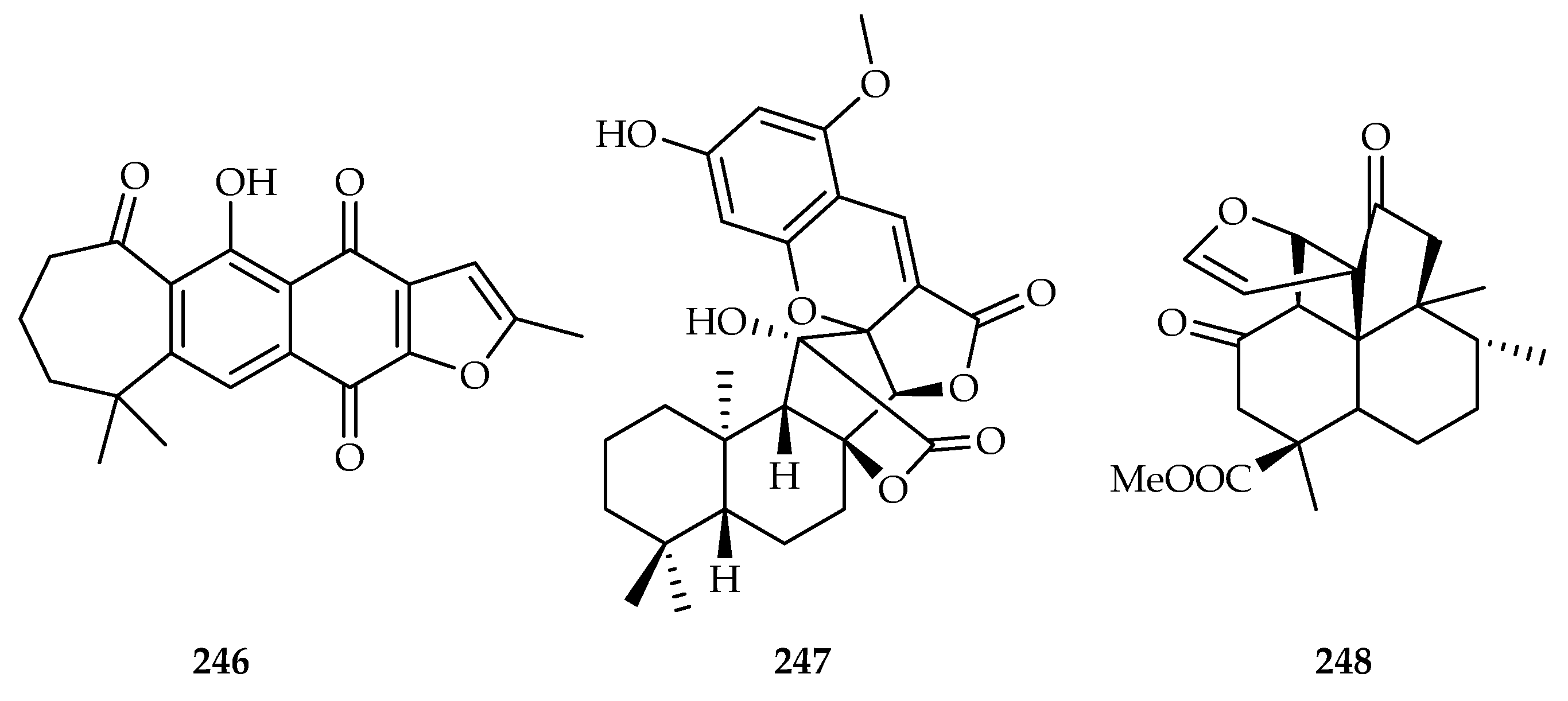
| Euphorbiaceae | Croton crassifolius | 53, 64, 82, 248 |
| Croton damayeshu | 39–43 | |
| Croton insularis | 5,6 | |
| Croton kongensis | 201–203, 245 | |
| Croton sublyratus | 95 | |
| Croton tiglium | 44, 45 | |
| Croton yanhuii | 213, 214 | |
| Ephorbia alatavica | 163 | |
| Euphorbia deflexa | 22, 24, 36 | |
| Euphorbia erythradenia | 19 | |
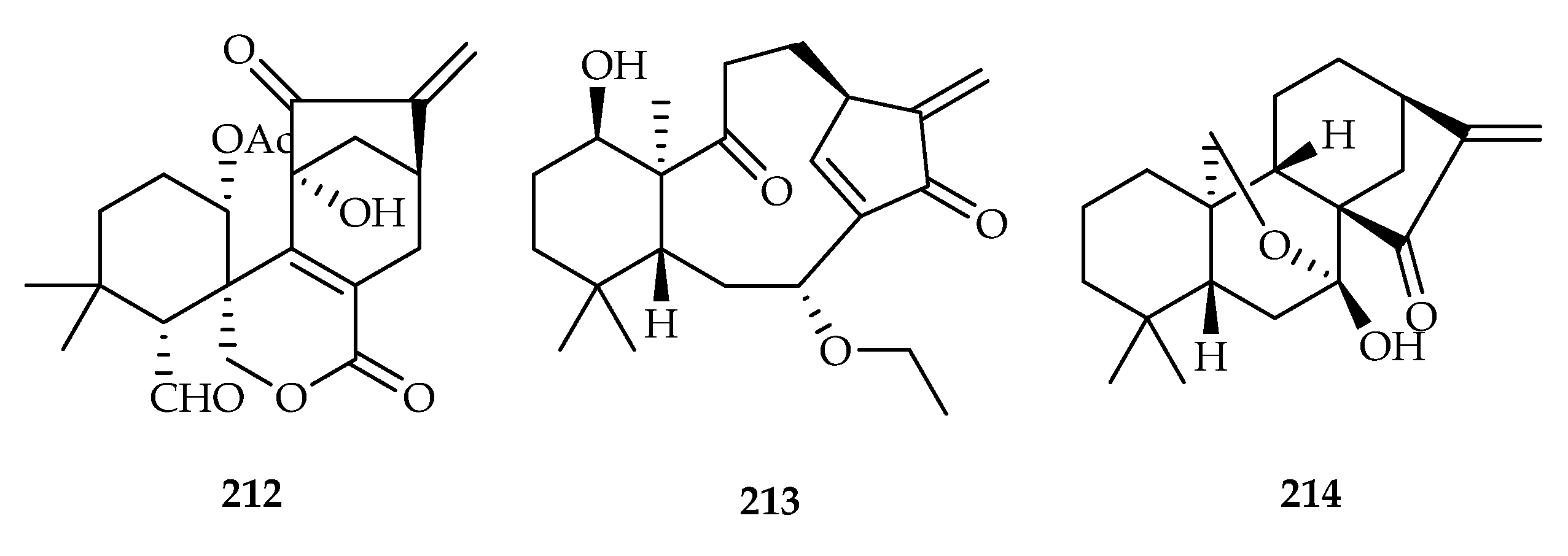
| Euphorbia fischeriana | 12, 13, 14, 32, 108–118, 152, 192, 247 | |
| Euphorbia helioscopia | 123, 124 | |
| Euphorbia kansuensis | 20 | |
| Euphorbia kansui | 16–18, 25–28 | |
| Euphorbia lathyrism | 35 | |
| Euphorbia neriifolia | 139–141, 167 | |
| Euphorbia nicaeensis | 23 | |
| Euphorbia pekinensis | 97 | |
| Euphorbia stracheyi | 21, 33, 34 | |
| Jatropha multifida | 30, 31, 37, 38 | |
| Jatropha podagrica | 29 | |
| Trigonostemon howii | 122 | |
| Fabaceae | Caesalpinia sappan | 151 |
| Copaifera trapezifolia | 94 | |
| Erythrophleum fordii | 149, 150 | |
| Flacourtiaceae | Casearia graveolens | 63 |
| Cesearia grewiifolia | 51 | |
| Casearia kurzii | 54–62 | |
| Labiateae | Ajuga decumbens | 70 |
| Isodon oresbius | 212 | |
| Isodon pharicus | 215–218 | |
| Perovskia atriplicifolia | 101, 102 | |
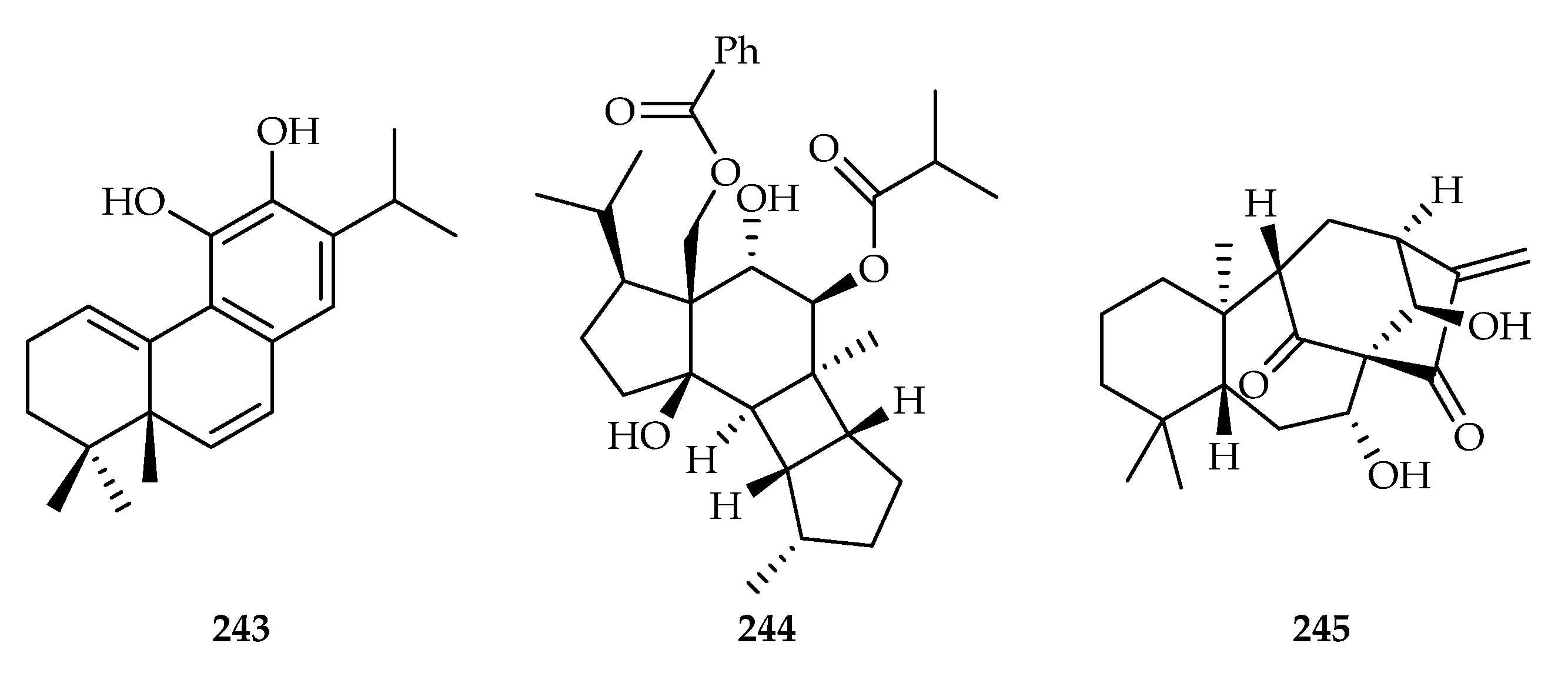
| Prunella vulgaris | 244 | |
| Salvia multicaulis | 148 | |
| Lamiaceae | Ajuga ovalifolia | 129 |
| Clerodendranthus spicatus | 165, 166 | |
| Clerodendrum bracteatum | 133, 134 | |
| Isodon excisoides | 204 | |
| Isodon forrestii | 119, 120, 225 | |
| Isodon interruptus | 132, 207 | |
| Isodon pharicus | 195–200 | |
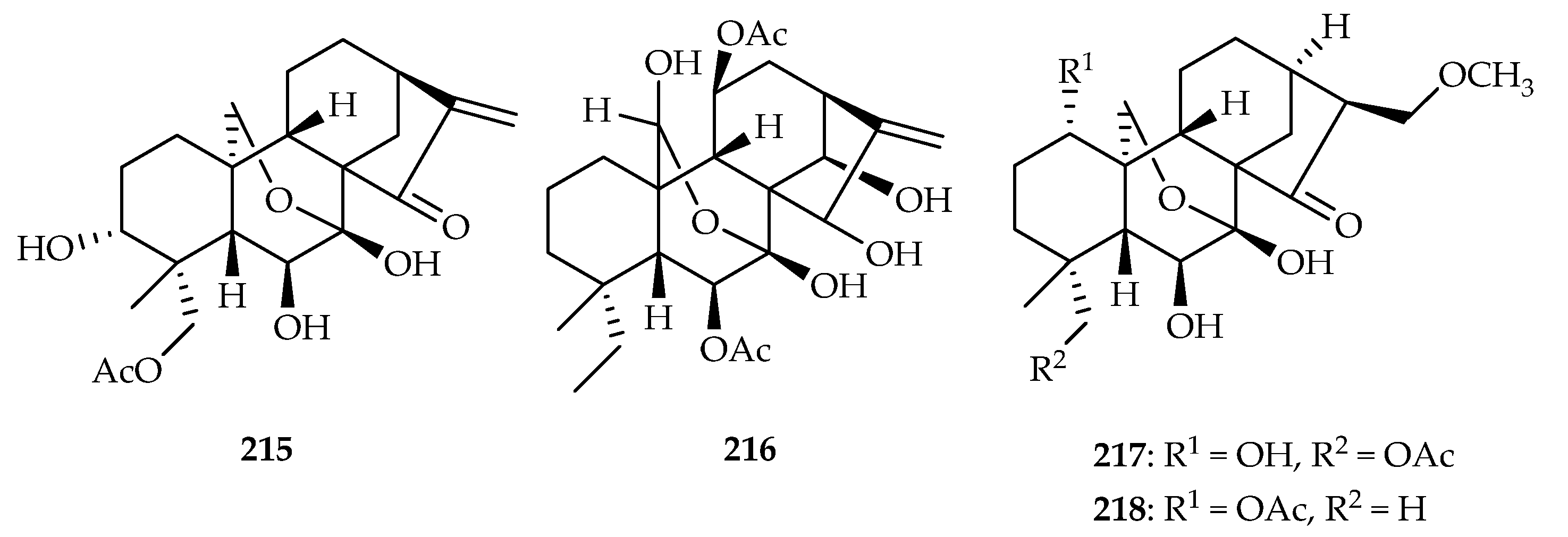
| Isodon rubescens | 185–187, 220, 221, 214 | |
| Isodon scoparius | 183, 184, 223 | |
| Isodon serra | 103 | |
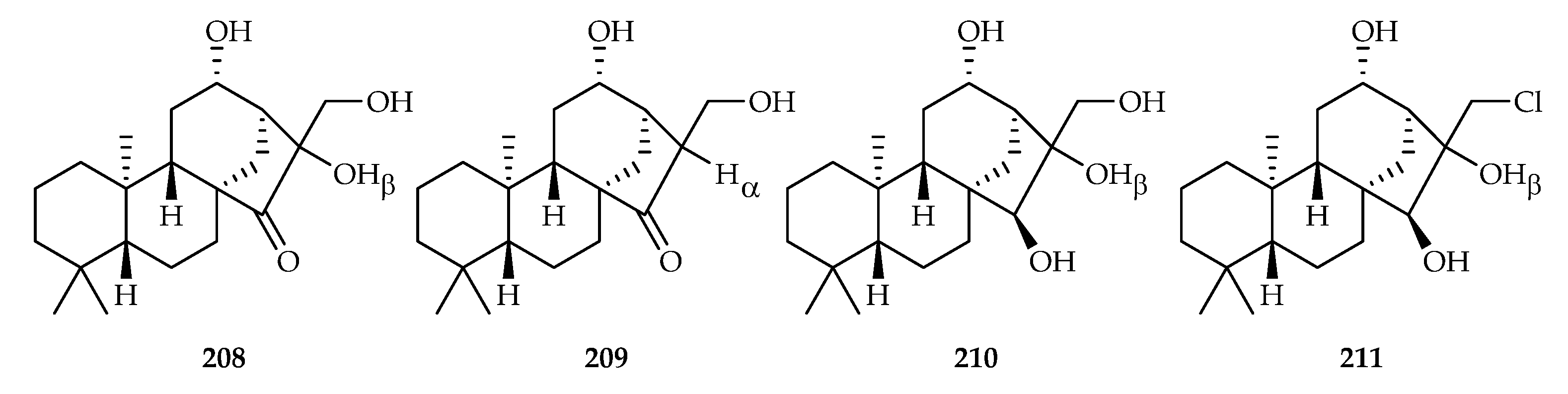
| Mesona procumbens | 208–211 | |
| Phlomoides betonicoides | 92 | |
| Plectranthus scutellarioides | 125, 144 | |
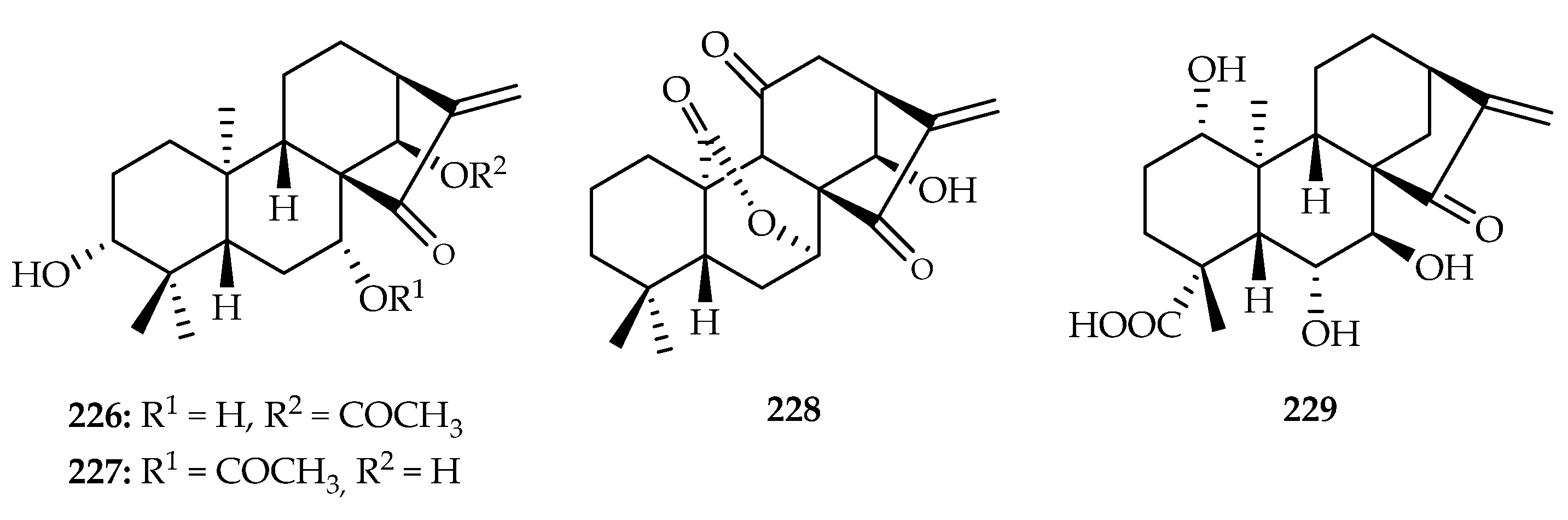
| Rabdosia japonica | 226, 227 | |
| Rabdosia rubescens | 224, 228 | |
| Salvia ballotiflora | 156 | |
| Salvia ceratophylla | 128 | |
| Salvia deserta | 130, 131, 157, 249, 250 | |
| Salvia leriifolia | 147 | |
| Salvia plebeia | 106, 107 | |
| Salvia prattii | 243 | |
| Salvia tebesana | 251 | |
| Salvia yunnanensis | 145 | |
| Scutellaria barbata | 52, 69 | |
| Vitex cofassus | 50 | |
| Lycopodiaceae | Lycopodium complanatum | 146 |
| Meliaceae | Aphanamixis polystachya | 1, 2 |
| Menispermaceae | Tinospora cordifolia | 81 |
| Tinospora crispa | 71, 72 | |
| Orchidaceae | Pholidota cantonensis | 89 |
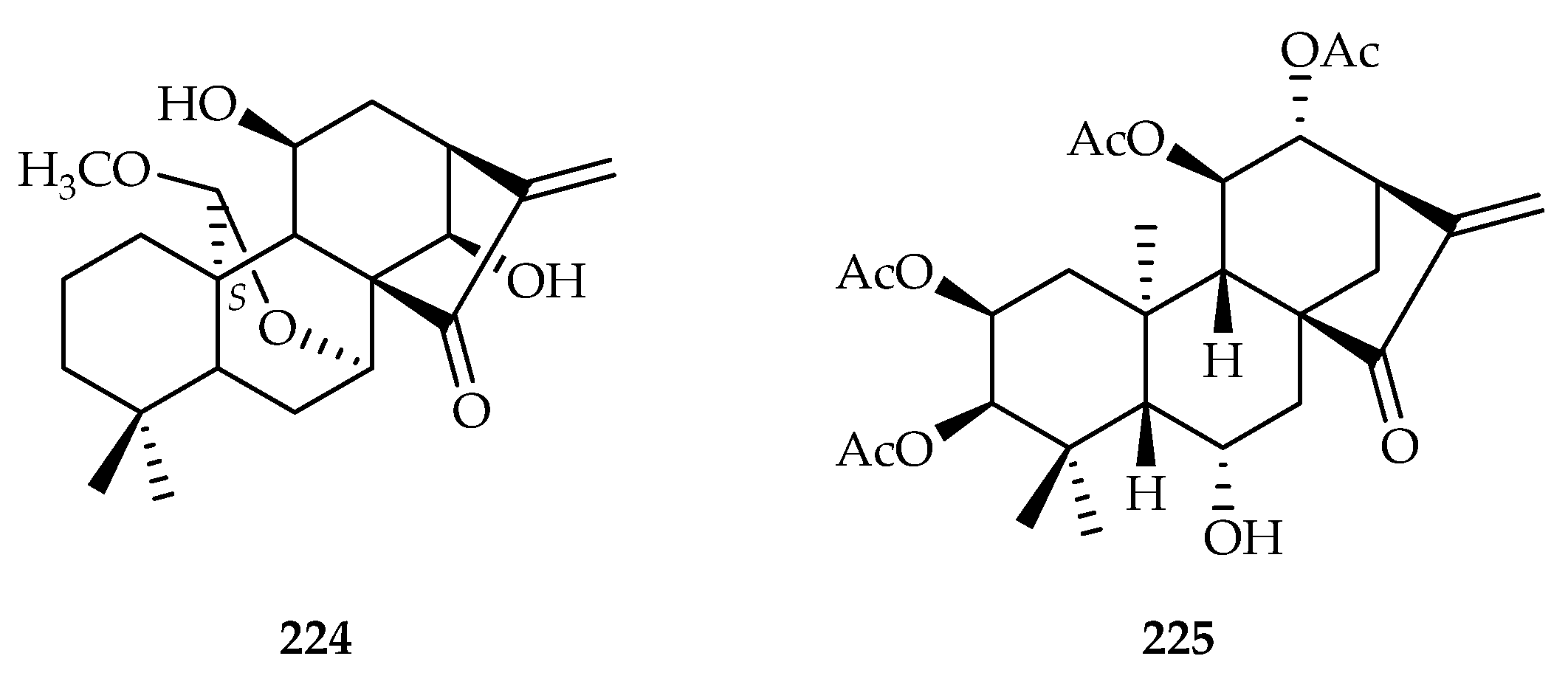
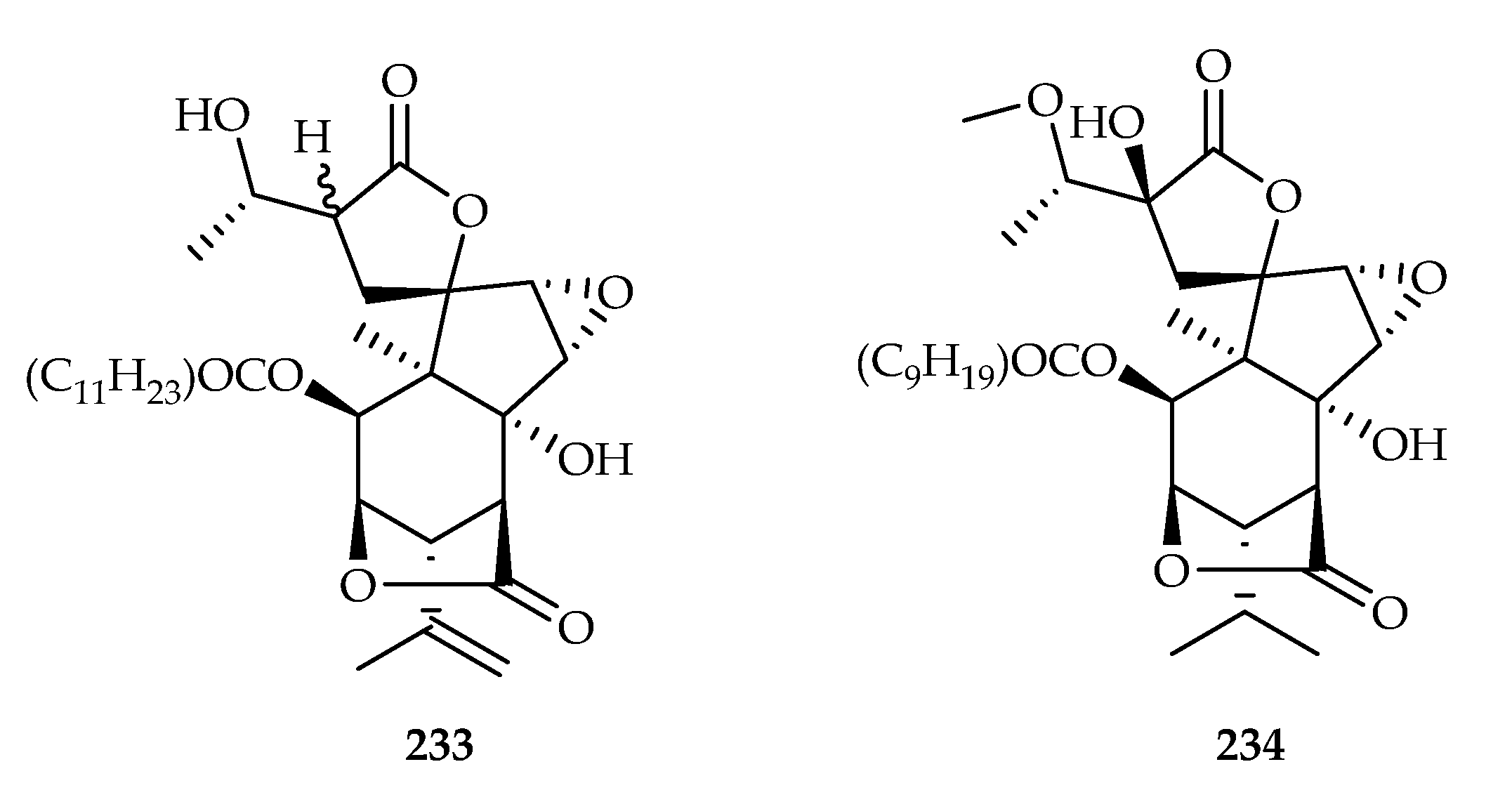
| Picrodendraceae | Austrobuxus carunculatus | 233, 234 |
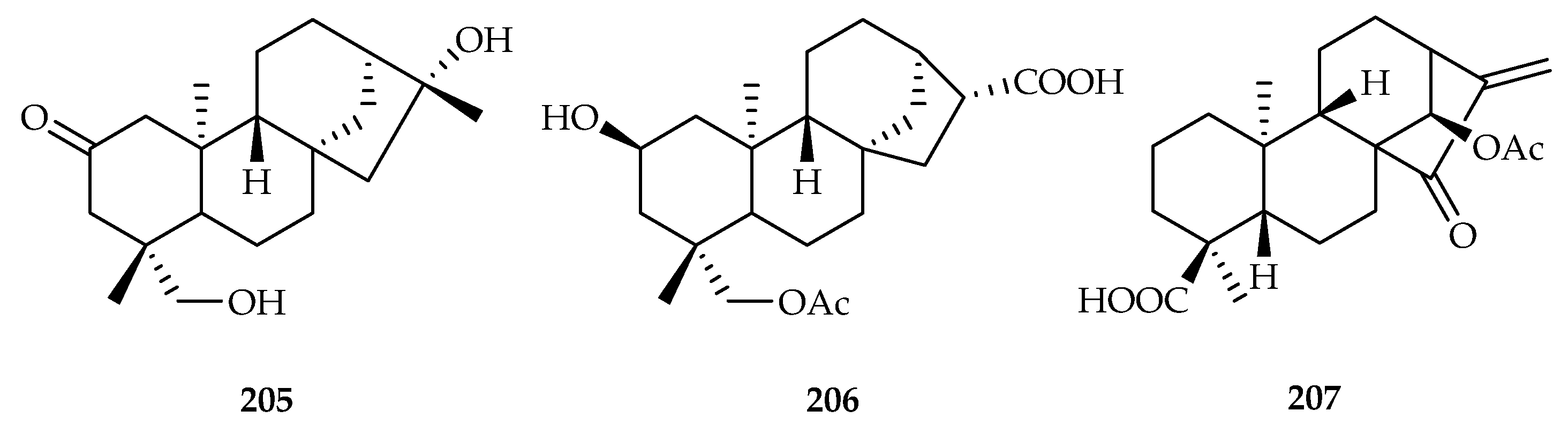
| Poaceae | Zea mays | 205, 206 |
| Podocarpaceae | Podocarpus macrophyllus | 235, 236 |
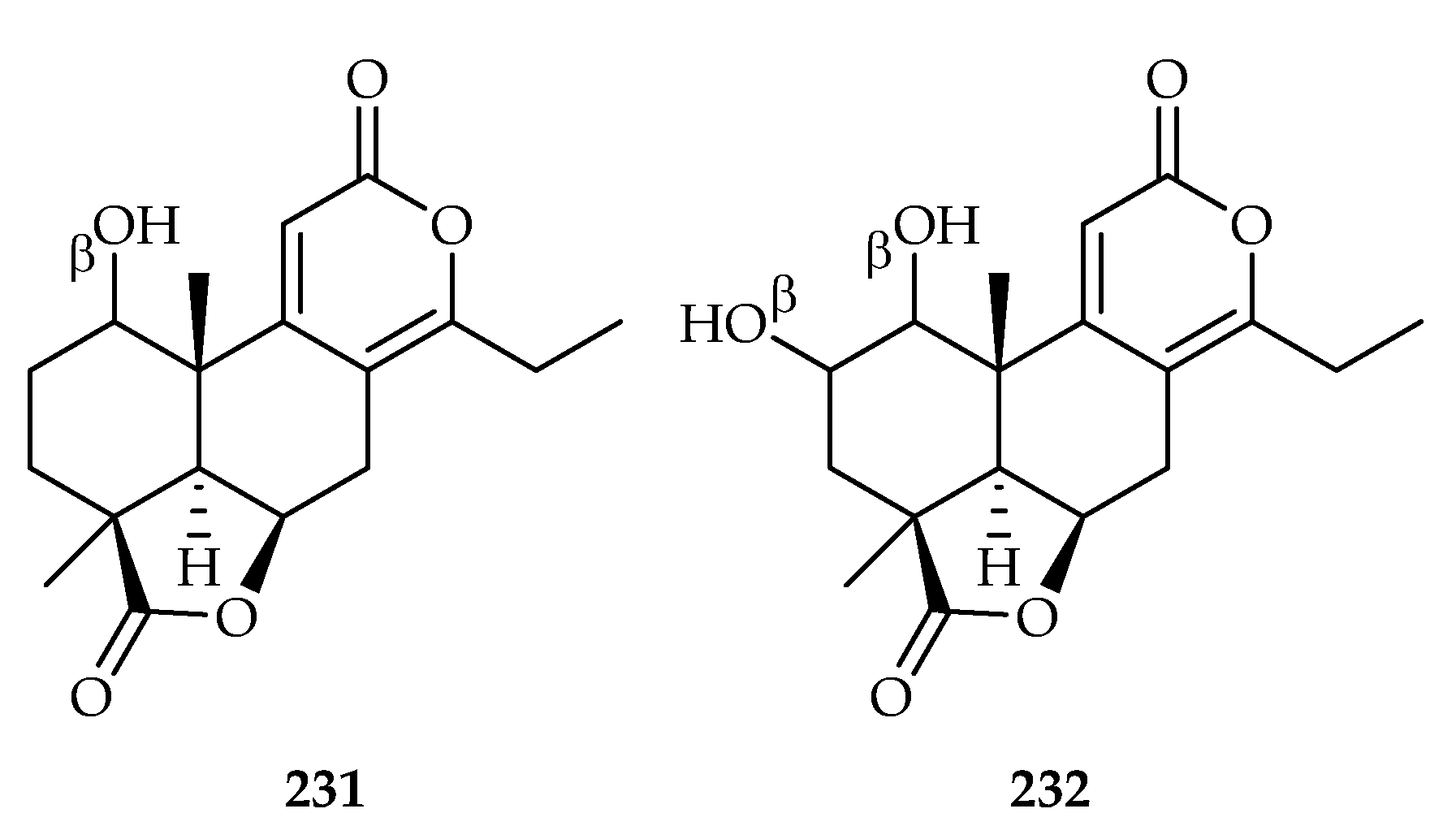
| Podocarpus nagi | 231, 232 | |
| Pteridaceae | Pteris semipinnata | 229 |
| Rhizophoraceae | Ceriops tagal | 153–155, 158 |
| Ranunculaceae | Anemone hupehensis | 164 |
| Salicaceae | Laetia corymbulosa | 47–49, 73–80 |
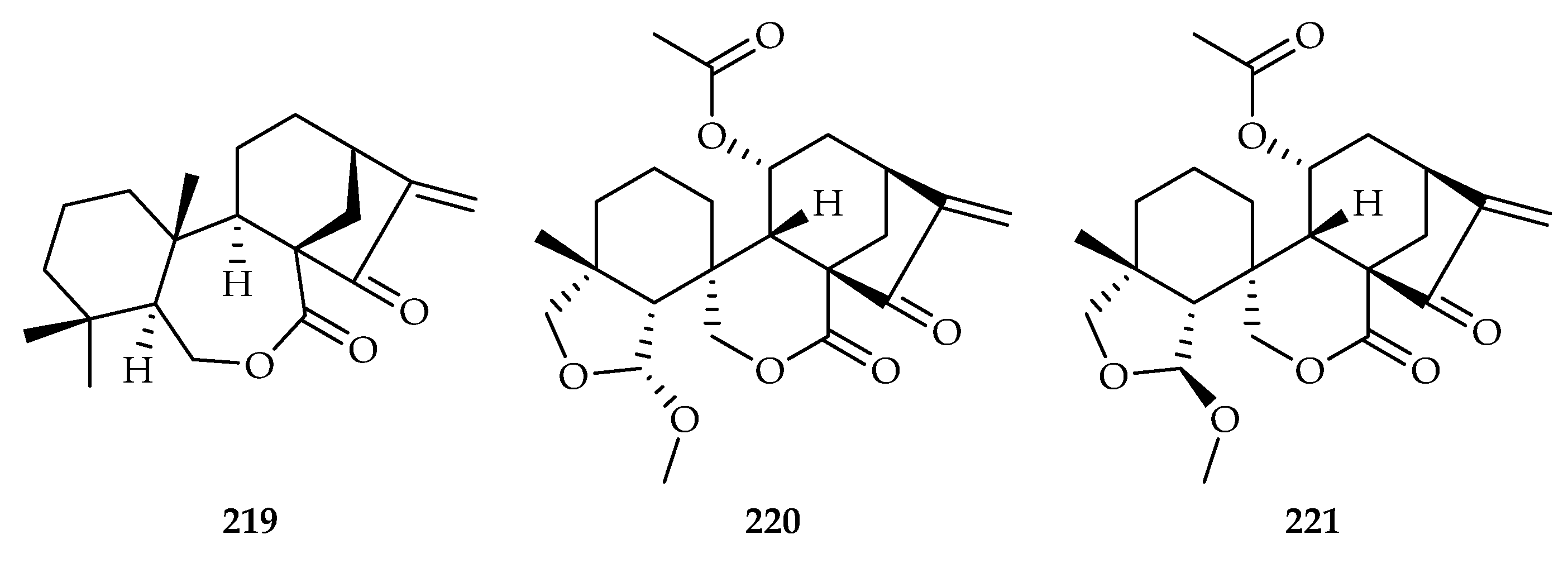
| Scapaniaceae | Scapania carinthiaca | 219 |
| Scrophulariaceae | Scoparia dulcis | 98, 99 |
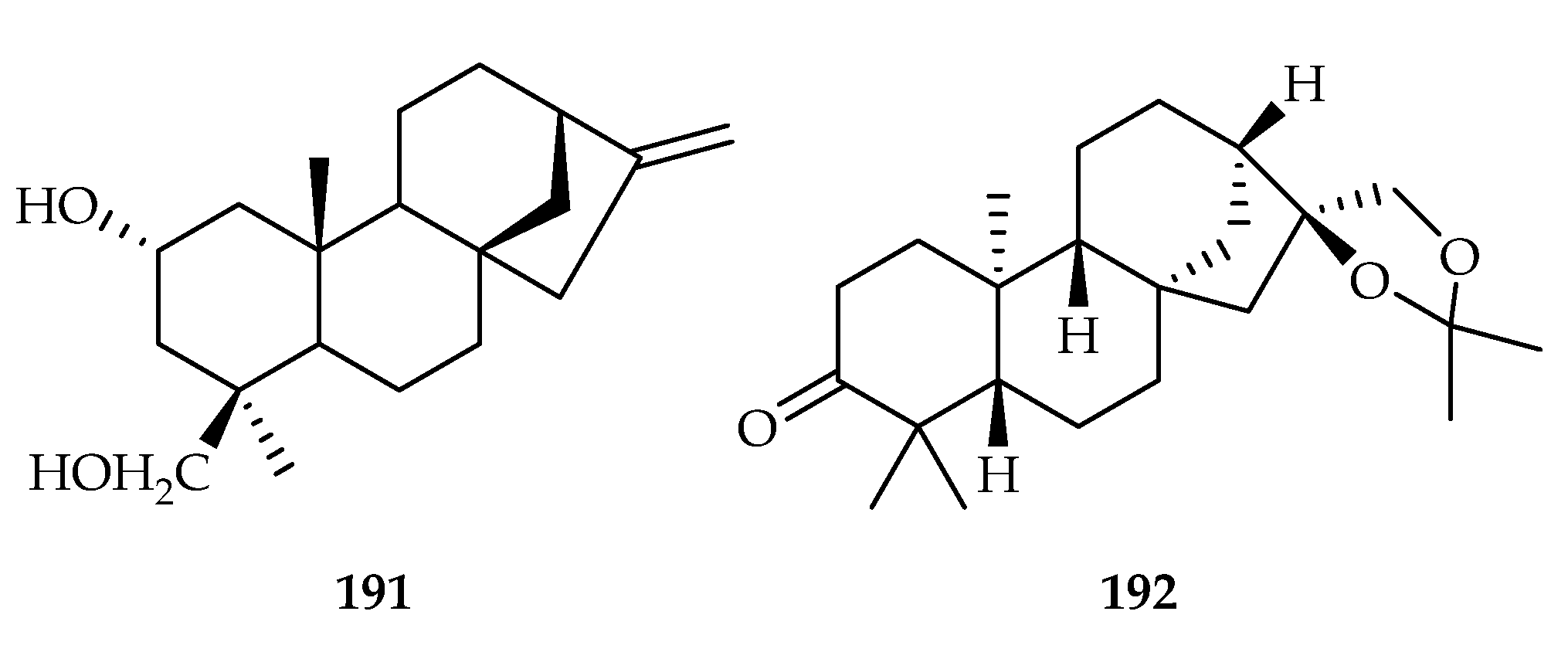
| Sinoptiridaceae | Aleuritopteris argentea | 168, 191 |
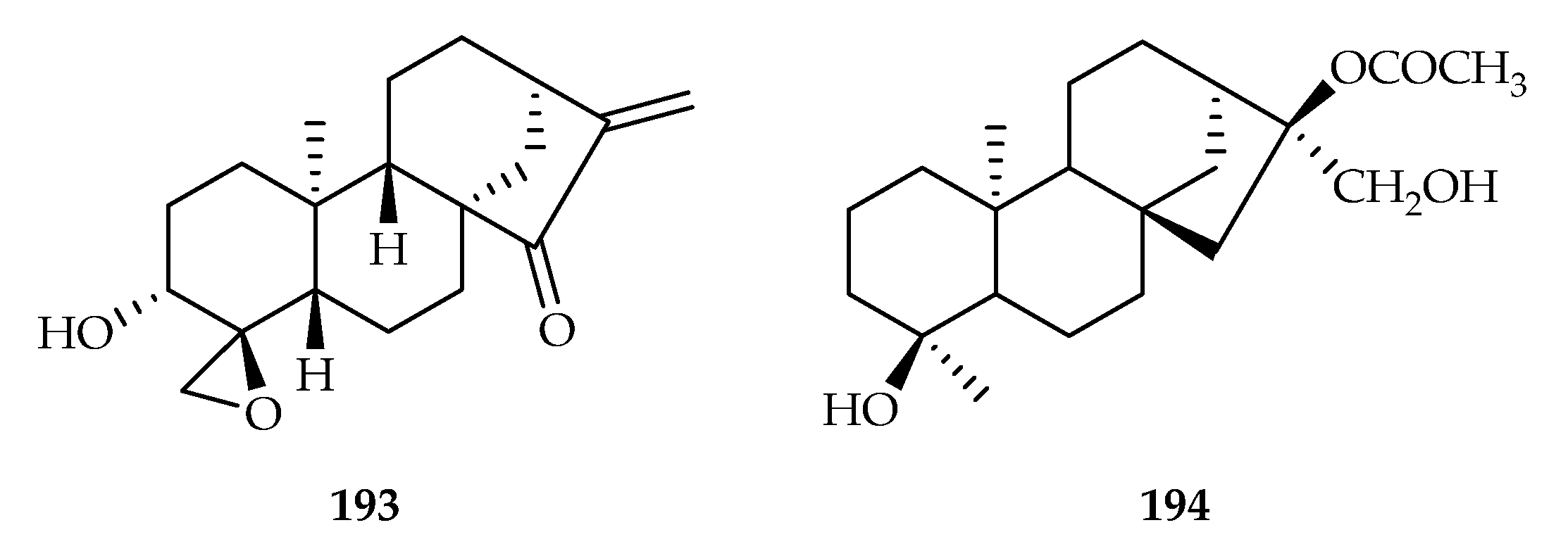
| Taxaceae | Amentotaxus argotaenia | 90–91, 193 |
| Cephalotaxus fortunei | 169–171, 172, 173–181 | |
| Cephalotaxus lanceolata | 173–181 | |
| Taxus wallichiana | 46 | |
| Taxodiaceae | Cunninghamia lanceolata | 126 |
| Metasequoia glyptostroboides | 127 | |
| Thymelaeaceae | Daphne genkwa | 7, 8–11 |
| Verbenaceae | Caryopteris aureoglandulosa | 246 |
| Caryopteris nepetaefolia | 104, 105 | |
| Clerodendrum chinense | 135–138 | |
| Viburnaceae | Viburnum odoratissimum | 237–242 |
| Zingiberaceae | Alpinia galanga | 83–85 |
| Hedychium forrestii | 93 | |
| Roscoea purpurea | 96 |
6. Methodology
7. Conclusions
8. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 95-D | lung giant-cell carcinoma |
| A2780 | human ovarian cancer |
| A375 | human melanoma cancer |
| A549 | human non-small-cell lung carcinoma |
| A549/CDDP | cisplatin resistant human adenocarcinomic alveolar basal epithelial |
| A549/Taxol | taxol resistant lung cancer |
| ACHN | human kidney cancer cells |
| ACP01 | gastric cancer |
| AGS | gastric cancer |
| B16F10 | melanoma |
| B16 melanoma 4A5 | theophylline-stimulated murine melanoma |
| BGC-823 | human gastric cancer |
| BT474 | breast ductal carcinoma |
| C26 | colon cancer |
| C4-2B | human prostate cancer |
| C4-2B/ENZR | enzalutamide-resistant C4-2B cell line |
| CaCo2 | human colorectal carcinoma |
| CCRF-CEM | drug-sensitive leukemia |
| CEM/ADR5000 | multidrug resistant P-glycoprotein-overexpressing subline |
| Chago-K1 | undifferentiated lung carcinoma |
| DU145 | human prostate cancer |
| EC | esophageal cancer |
| EC109 | human esophageal cancer |
| EC9706 | human esophageal cancer |
| ECC-1 | endometrial carcinoma |
| HCT-15 | human colon cancer |
| HCT-15/5-FU | fluorouracil resistant human colorectal adenocarcinoma |
| HCT-116 | colon cancer |
| HEL | human erythroleukemia |
| HeLa | human cervical carcinoma |
| HepG2 | human hepatocellular liver carcinoma |
| HepG2/DOX | doxorubicin-resistant human hepatocellular liver carcinoma |
| Hep3B | human liver cancer |
| HEY | human ovarian cancer |
| HL-60 | human erythroleukemia (promyelocytic leukemia) |
| HMy2.CIR | human B lymphoblast |
| HT29 | human colon cancer |
| HMCB | human melanoma cancer |
| Huh-7 | human hepatoma |
| Jurkat | T-cell lymphoma |
| K562 | human erythromyeloblastoid leukemia |
| KATO-III | gastric carcinoma |
| KB | human epithelial carcinoma |
| KB-VIN | P-gp-overexpressing MDR subline of KB |
| KYSE-450 | human esophageal cancer |
| L5178Y | mouse lymphoma |
| L6 | rat myoblast |
| LoVo | human colon cancer |
| LUPF003 | non-small-cell lung |
| LUPF045 | nonsmall-cell lung |
| MCF-7 | breast cancer |
| MDA-MB-231 | human breast carcinoma (triple-negative breast cancer) |
| MDA-MB-435 | melanoma |
| MDA-MB-453 | breast cancer |
| MG-63 | human osteosarcoma |
| MM-231 | breast cancer |
| MM96L | melanoma |
| MM-CSCs | human multiple myeloma cancer stem |
| MOLT-4 | human lymphoblastic leukemia |
| MSTO-211H | human lung cancer |
| MT-1 | human contaminated breast cancer |
| MV4-11 | lymphoblast from human biphenotypic B myelomonocytic leukemia |
| Namalwa | Burkitt lymphoma |
| NB4 | human acute promyelocytic leukemia cell |
| NCI-H1229 | lung large cell carcinoma |
| NCI-H1650 | human lung adenocarcinoma bronchioalveolar carcinoma |
| NCI-H1975 | human lung adenocarcinoma cells |
| NCI-H460 | non-small cell lung carcinoma |
| OVPF008 | ovarian cancer |
| OVPF038 | ovarian cancer |
| p388D1 | mouse leukemia |
| PANC-1 | human pancreatic |
| PC3 | human prostate cancer |
| PC-9 | lung cancer |
| RKO | human colon cancer |
| Saos-2 | human osteosarcoma |
| SGC7901 | human gastric cancer |
| SHG-44 | human glioblastoma |
| SK-BR-3 | human breast cancer |
| SK-Hep-1 | liver cancer cell lines |
| SKLU-1 | human lung adenocarcinoma |
| SK-Mel2 | human melanoma |
| SKOV3 | human ovarian cancer |
| SMMC-7721 | hepatoma cancer |
| SNU638 | stomach cancer cell lines |
| SW1990 | human pancreatic cancer |
| SW480 | human colon cancer |
| SW620 | human colon cancer |
| T24 | human urinary bladder cancer |
| U2OS | human osteosarcoma |
| U87 | glioblastoma |
| U87-TxR | resistant glioblastoma |
| U87-MG | glioblastoma |
| U-251 | human glioblastoma |
| U937 | human monoblastic leukemia |
| ZR-75-1 | epithelial from the mammary gland |
| XWLC-05 | lung adenocarcinoma |
References
- Alves, A.L.V.; da Silva, L.S.; Faleiros, C.A.; Silva, V.A.O.; Reis, R.M. The Role of Ingenane Diterpenes in Cancer Therapy: From Bioactive Secondary Compounds to Small Molecules. Nat. Prod. Commun. 2022, 17, 1–30. [Google Scholar] [CrossRef]
- De Sousa, I.P.; Sousa Teixeira, M.V.; Jacometti Cardoso Furtado, N.A. An Overview of Biotransformation and Toxicity of Diterpenes. Molecules 2018, 23, 1387. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, P.; Zhu, M.; Wang, Y.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the genus Euphorbia: Structure and biological activity (2013–2019). Phytochemistry 2021, 190, 112846. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Wu, X.-Z.; Fang, F.-H.; Huang, W.-J.; Shi, Y.-Y.; Pan, H.-Q.; Ning, L.; Yuan, C.-S. Two novel nornemoralisin-type diterpenoids from Aphanamixis polystachya (Wall.) R. Parker. Fitoterapia 2020, 140, 104431. [Google Scholar] [CrossRef]
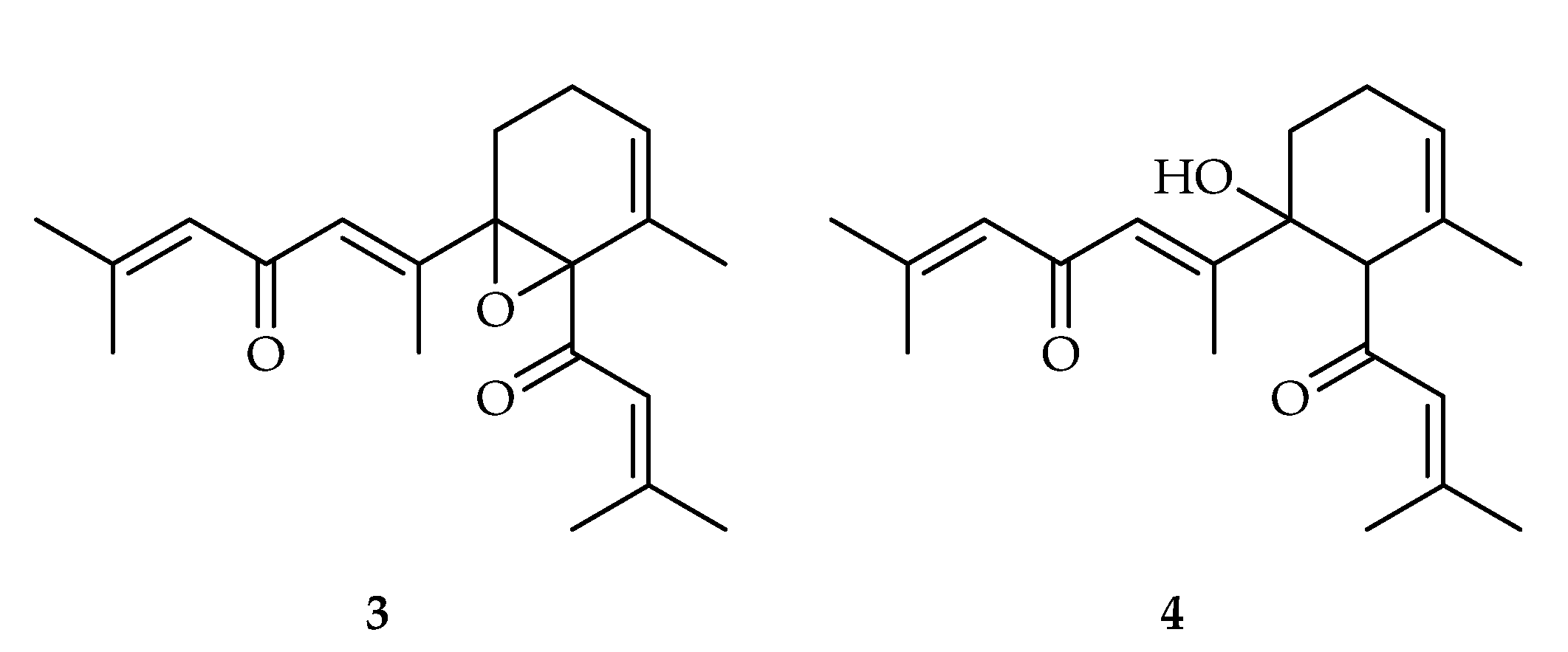
- Ibrahim, S.R.M.; Mohamed, G.A.A. Tagetones A and B, New Cytotoxic Monocyclic Diterpenoids from Flowers of Tagetes minuta. Chin. J. Nat. Med. 2017, 15, 546–549. [Google Scholar] [CrossRef]
- Xu, Z.H.; Sun, J.; Xu, R.S.; Qin, G.W. Casbane diterpenoids from Euphorbia ebracteolata. Phytochemistry 1998, 49, 149–151. [Google Scholar] [CrossRef]
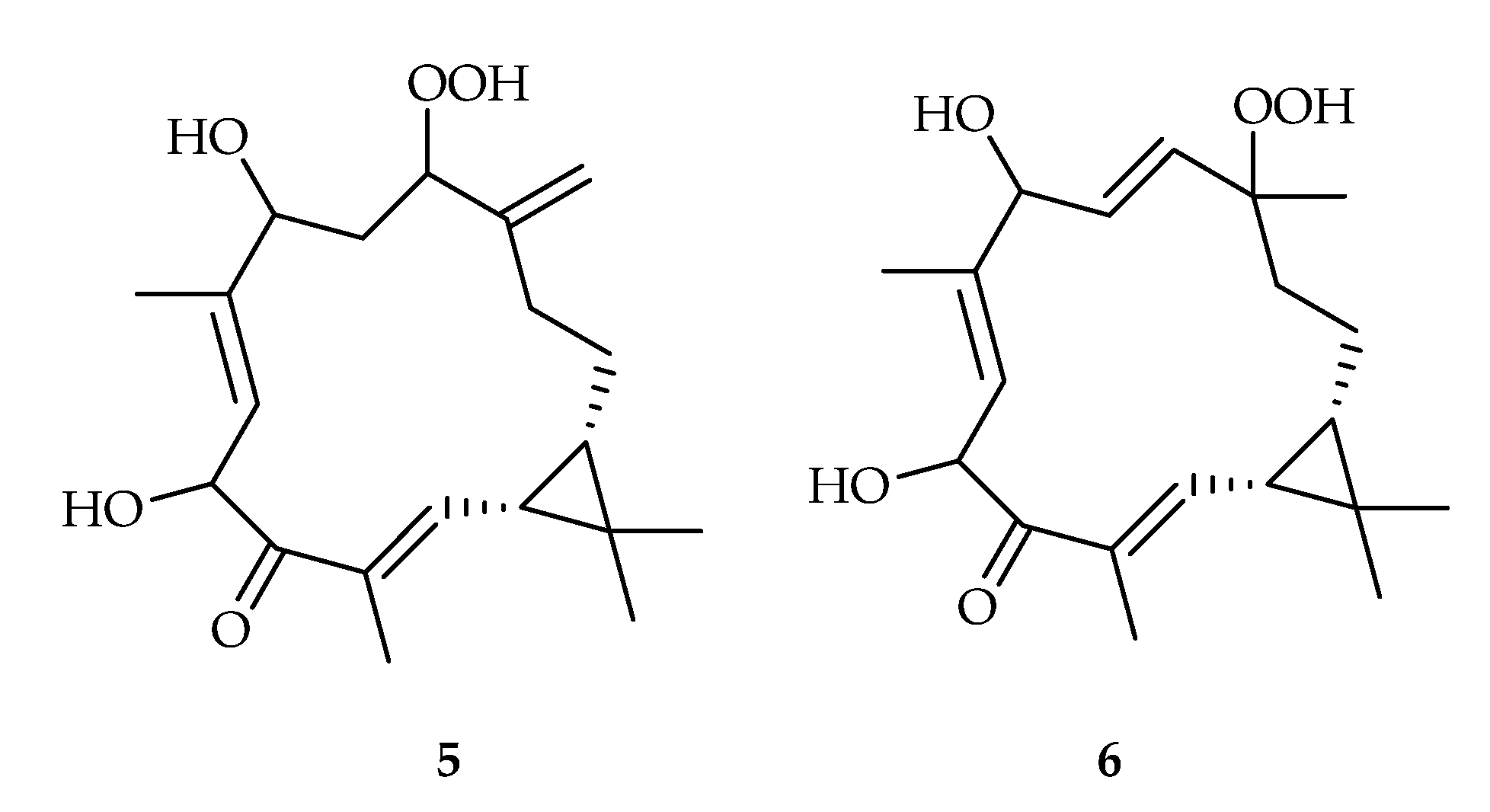
- Maslovskaya, L.A.; Savchenko, A.I.; Gordon, V.A.; Reddell, P.W.; Pierce, C.J.; Parsons, P.G.; Williams, C.M. The first casbane hydroperoxides EBC-304 and EBC-320 from the Australian rainforest. Chem. Eur. J. 2017, 23, 537–540. [Google Scholar] [CrossRef]
- Jin, Y.-X.; Shi, L.-L.; Zhang, D.-P.; Wei, H.-Y.; Si, Y.; Ma, G.-X.; Zhang, J. A Review on Daphnane-Type Diterpenoids and Their Bioactive Studies. Molecules 2019, 24, 1842. [Google Scholar] [CrossRef]
- Pan, R.-R.; Zhang, C.-Y.; Li, Y.; Zhang, B.-B.; Zhao, L.; Ye, Y.; Song, Y.-N.; Zhang, M.; Tie, H.-Y.; Zhang, H.; et al. Daphnane Diterpenoids from Daphne genkwa Inhibit PI3K/Akt/mTOR Signaling and Induce Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells. J. Nat. Prod. 2020, 83, 1238–1248. [Google Scholar] [CrossRef]
- Mi, S.-H.; Zhao, P.; Li, Q.; Zhang, H.; Guo, R.; Liu, Y.-Y.; Lin, B.; Yao, G.-D.; Song, S.-J.; Huang, X.-X. Guided isolation of daphnane-type diterpenes from Daphne genkwa by molecular network strategies. Phytochemistry 2022, 198, 113144. [Google Scholar] [CrossRef]
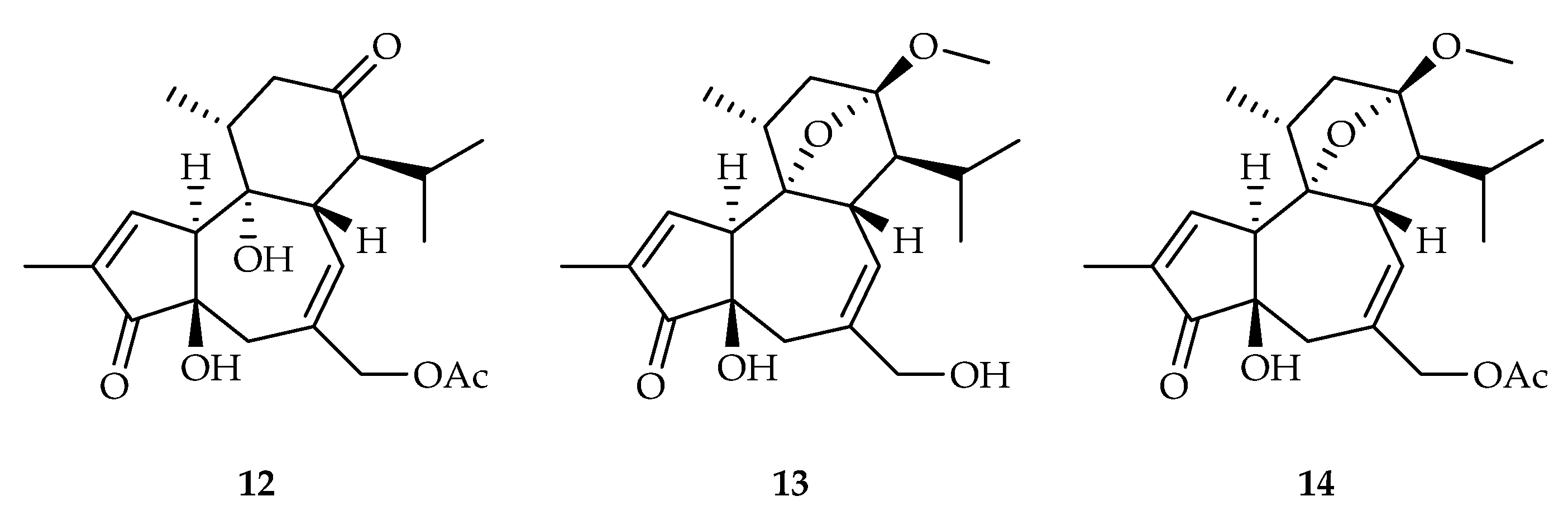
- Du, K.; Yang, X.; Li, J.; Meng, D. Antiproliferative diterpenoids and acetophenone glycoside from the roots of Euphorbia fischeriana. Phytochemistry 2020, 177, 112437. [Google Scholar] [CrossRef]
- Xie, R.; Xia, G.; Zhu, J.; Lin, P.; Fan, X.; Zi, J. Daphnane-type diterpenoids from Euphorbia fischeriana Steud and their cytotoxic activities. Fitoterapia 2021, 149, 104810. [Google Scholar] [CrossRef]
- D’Ambola, M.; Fiengo, L.; Chini, M.G.; Cotugno, R.; Bader, A.; Bifulco, G.; Braca, A.; de Tommasi, N.; Piaz, F.D. Fusicoccane Diterpenes from Hypoestes forsskaolii as Heat Shock Protein 90 (Hsp90) Modulators. J. Nat. Prod. 2019, 82, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G. Ingenane Diterpenoids. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 102. [Google Scholar] [CrossRef]
- Meng, X.-H.; Wang, K.; Chai, T.; Guo, Z.-Y.; Zhao, M.; Yang, J.-L. Ingenane and Jatrophane Diterpenoids from Euphorbia Kansui and Their Antiproliferative Effects. Phytochemistry 2020, 172, 112257. [Google Scholar] [CrossRef]
- Fallahian, F.; Ghanadian, M.; Aghaei, M.; Zarei, S.M. Induction of G2/M phase arrest and apoptosis by a new tetrahydroingenol diterpenoid from Euphorbia erythradenia Bioss. in melanoma cancer cells. Biomed. Pharmacother. 2017, 86, 334–342. [Google Scholar] [CrossRef]
- Yan, X.-L.; Sang, J.; Chen, S.-X.; Li, W.; Tang, G.-H.; Gan, L.-S.; Yin, S. Euphorkanlide A, a Highly Modified Ingenane Diterpenoid with a C 24 Appendage from Euphorbia kansuensis. Org. Lett. 2019, 21, 4128–4131. [Google Scholar] [CrossRef]
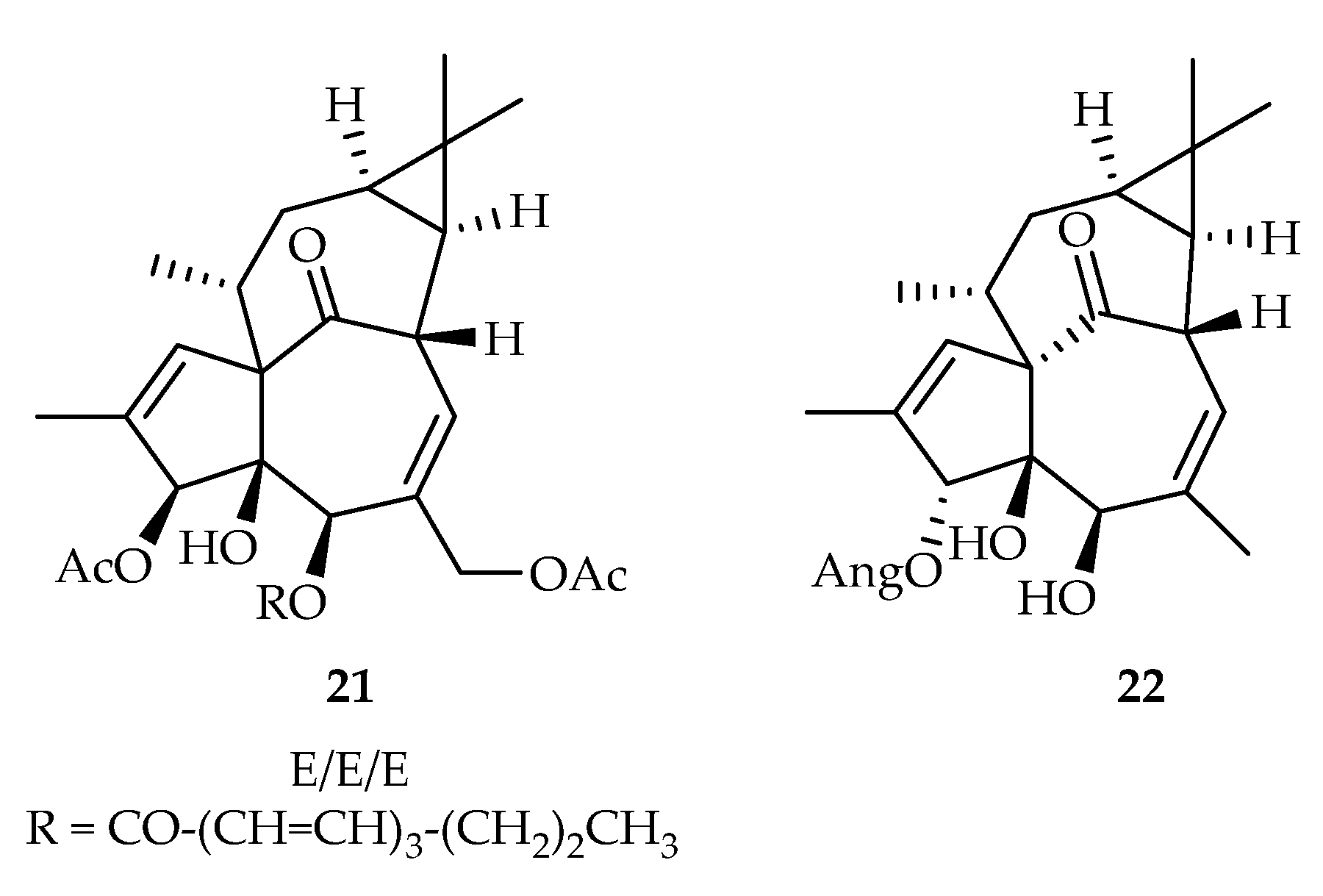
- Ye, Y.; Liu, G.-H.; Dawa, D.; Ding, L.-S.; Cao, Z.-X.; Zhou, Y. Cytotoxic diterpenoids from the roots of Euphorbia stracheyi. Phytochem. Lett. 2020, 36, 183–187. [Google Scholar] [CrossRef]
- Grafakou, M.-E.; Barda, C.; Heilmann, J.; Skaltsa, H. Macrocyclic Diterpenoid Constituents of Euphorbia deflexa, an Endemic Spurge from Greece. J. Nat. Prod. 2021, 84, 2893–2903. [Google Scholar] [CrossRef]
- Krstic, G.B.; Kostic, A.; Jadranin, M.B.; Pesic, M.; Novakovic, M.M.; Aljancic, I.S.; Vajs, V.V. Two new jatrophane diterpenes from the roots of Euphorbia nicaeensis. J. Serb. Chem. Soc. 2021, 86, 1219–1228. [Google Scholar] [CrossRef]
- Yuan, H.-T.; Li, Q.-F.; Tian, T.; Zhang, C.-Y.; Huang, Z.-Q.; Fan, C.-X.; Mei, K.; Zhou, J.; Zhai, X.-X.; Li, S.-B.; et al. Lathyrane diterpenoids from Jatropha podagrica and their antitumor activities in human osteosarcoma cells. Nat. Prod. Res. 2021, 35, 5089–5095. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Zhang, Y.; Li, S.; Ahmed, A.; Tang, G.-H.; Yin, S. Cytotoxic Macrocyclic Diterpenoids from Jatropha multifida. Bioorg. Chem. 2018, 80, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, J.; Yang, C.; Yan, X.; Yin, Z. Cytotoxic lathyrane diterpenoids from roots of Euphorbia fischeriana. Records Nat. Prod. 2020, 14, 286–291. [Google Scholar] [CrossRef]
- Wang, J.-X.; Wang, Q.; Zhen, Y.-Q.; Zhao, S.-M.; Gao, F.; Zhou, X.-L. Cytotoxic Lathyrane-Type Diterpenes from Seeds of Euphorbia lathyris. Chem. Pharm. Bull. 2018, 66, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, M.; Ghanadian, M.; Ali, Z.; Khan, I.A. Jatrophane and rearranged jatrophane-type diterpenes: Biogenesis, structure, isolation, biological activity and SARs (1984–2019). Phytochem. Rev. 2020, 19, 265–336. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, X.-Y.; Liu, L.-P.; Qin, G.-W.; Kang, T.-G. Tigliane Diterpenoids from the Euphorbiaceae and Thymelaeaceae Families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar] [CrossRef]
- Cui, J.-J.; Ji, K.-L.; Liu, H.-C.; Zhou, B.; Liu, Q.-F.; Xu, C.-H.; Ding, J.; Zhao, J.-X.; Yue, J.-M. Cytotoxic Tigliane Diterpenoids from Croton damayeshu. J. Nat. Prod. 2019, 82, 1550–1557. [Google Scholar] [CrossRef]
- Wang, J.; Qin, L.; Zhao, B.; Cai, L.; Zhong, Z.; Liu, Y.; Zhou, X. Crotonols A and B, Two Rare Tigliane Diterpenoid Derivatives against K562 Cells from Croton tiglium. Org. Biomol. Chem. 2019, 17, 195–202. [Google Scholar] [CrossRef]
- Dang, P.H.; Nguyen, H.X.; Duong, T.T.T.; Tran, T.K.T.; Nguyen, P.T.; Vu, T.K.T.; Vuong, H.C.; Phan, N.H.T.; Nguyen, M.T.T.; Nguyen, N.T.; et al. α-Glucosidase Inhibitory and Cytotoxic Taxane Diterpenoids from the Stem Bark of Taxus wallichiana. J. Nat. Prod. 2017, 80, 1087–1095. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschkeb, S.L.; Lee, K.-H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef]
- Suzuki, A.; Saito, Y.; Fukuyoshi, S.; Goto, M.; Miyake, K.; Newman, D.J.; O’Keefe, B.R.; Lee, K.-H.; Nakagawa-Goto, K. Corymbulosins D–H, 2-Hydroxy- and 2-Oxo-Clerodane Diterpenes from the Bark of Laetia Corymbulosa. J. Nat. Prod. 2017, 80, 1065–1072. [Google Scholar] [CrossRef]
- Rasyid, F.A.; Fukuyoshi, S.; Ando, H.; Miyake, K.; Atsumi, T.; Fujie, T.; Saito, Y.; Goto, M.; Shinya, T.; Mikage, M.; et al. A Novel Clerodane Diterpene from Vitex Cofassus. Chem. Pharm. Bull. 2017, 65, 116–120. [Google Scholar] [CrossRef]
- Nuanyai, T.; Chailap, B.; Buakeaw, A.; Puthong, S. Cytotoxicity of clerodane diterpenoids from fresh ripe fruits of Casearia grewiifolia. Songklanakarin J. Sci. Techn. 2017, 39, 517–521. [Google Scholar]
- Yuan, Q.-Q.; Song, W.-B.; Wang, W.-Q.; Xuan, L.-J. Scubatines A-F, new cytotoxic neo-clerodane diterpenoids from Scutellaria barbata D. Don. Fitoterapia 2017, 119, 40–44. [Google Scholar] [CrossRef]
- Tian, J.-L.; Yao, G.-D.; Wang, Y.-X.; Gao, P.-Y.; Wang, D.; Li, L.-Z.; Lin, B.; Huang, X.-X.; Song, S.-J. Cytotoxic clerodane diterpenoids from Croton crassifolius. Bioorg. Med. Chem. Lett. 2017, 27, 1237–1242. [Google Scholar] [CrossRef]
- Zhang, L.-T.; Wang, X.-L.; Wang, T.; Zhang, J.-S.; Huang, Z.-Q.; Shen, T.; Lou, H.-X.; Ren, D.-M.; Wang, X.-N. Dolabellane and Clerodane Diterpenoids from the Twigs and Leaves of Casearia kurzii. J. Nat. Prod. 2020, 83, 2817–2830. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Zhang, Q.; Zhang, X.; Xie, C.; Tuerhong, M.; Zhang, J.; Jin, D.-Q.; Lee, D.; Xu, J.; et al. Cytotoxic clerodane diterpenoids from the leaves of Casearia kurzii. Bioorg. Chem. 2019, 85, 558–567. [Google Scholar] [CrossRef]
- Shuo, Y.; Zhang, C.; Yang, X.; ·Liu, F.; Zhang, Q.; Li, A.; Ma, J.; Lee, D.; Ohizumi, Y.; Guo, Y. Clerodane diterpenoids from Casearia kurzii and their cytotoxic activities. J. Nat. Med. 2019, 73, 826–833. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Q.; Yang, X.; Li, Y.; Zhang, X.; Li, Y.; Du, Q.; Jin, D.-Q.; Cui, J.; Lall, N.; et al. Diterpenoids from the leaves of Casearia kurzii showing cytotoxic activities. Bioorg. Chem. 2020, 98, 103741. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Q.; Yang, X.; Xi, Y.; Zhang, X.; Wang, H.; Zhang, J.; Tuerhong, M.; Jin, D.-Q.; Lee, D.; et al. Cytotoxic diterpenoids as potential anticancer agents from the twigs of Casearia kurzii. Bioorg. Chem. 2019, 89, 102995. [Google Scholar] [CrossRef]
- Liu, F.; Ma, J.; Shi, Z.; Zhang, Q.; Wang, H.; Li, D.; Song, Z.; Wang, C.; Jin, J.; Xu, J.; et al. Clerodane Diterpenoids Isolated from the Leaves of Casearia graveolens. J. Nat. Prod. 2020, 83, 36–44. [Google Scholar] [CrossRef]
- Qiu, M.; Jin, J.; Zhou, L.; Zhou, W.; Liu, Y.; Tan, Q.; Cao, D.; Zhao, Z. Diterpenoids from Croton crassifolius include a novel skeleton possibly generated via an intramolecular [2+2]-photocycloaddition reaction. Phytochemistry 2018, 145, 103–110. [Google Scholar] [CrossRef]
- Tang, Z.; Shen, J.; Zhang, F.; Liang, J.; Xia, Z. Sulfated Neo-Clerodane Diterpenoids and Triterpenoid Saponins from Sheareria nana S. Moore. Fitoterapia 2018, 124, 12–16. [Google Scholar] [CrossRef]
- Tang, Z.; Xia, Z. A New Diterpenoid Against Endometrial Cancer from Sheareria nana. Chem. Nat. Comp. 2021, 57, 691–694. [Google Scholar] [CrossRef]
- Yang, G.-C.; Hu, J.-H.; Li, B.-L.; Liu, H.; Wang, J.-Y.; Sun, L.-X. Six New Neo-Clerodane Diterpenoids from Aerial Parts of Scutellaria barbata and Their Cytotoxic Activities. Planta Med. 2018, 84, 1292–1299. [Google Scholar] [CrossRef]
- Chen, H.; Tang, B.-Q.; Chen, L.; Liang, J.-Y.; Sun, J.-B. Neo-Clerodane Diterpenes and Phytoecdysteroids from Ajuga decumbens Thunb. and Evaluation of Their Effects on Cytotoxic, Superoxide Anion Generation and Elastase Release In Vitro. Fitoterapia 2018, 129, 7–12. [Google Scholar] [CrossRef]
- Noman, M.A.A.; Hossain, T.; Ahsan, M.; Jamshidi, S.; Hasan, C.M.; Rahman, K.M. Crispenes F and G, Cis-Clerodane Furanoditerpenoids from Tinospora crispa, Inhibit STAT3 Dimerization. J. Nat. Prod. 2018, 81, 236–242. [Google Scholar] [CrossRef]
- Aimaiti, S.; Suzuki, A.; Saito, Y.; Fukuyoshi, S.; Goto, M.; Miyake, K.; Newman, D.J.; O’Keefe, B.R.; Lee, K.-H.; Nakagawa-Goto, K. Corymbulosins I–W, Cytotoxic Clerodane Diterpenes from the Bark of Laetia corymbulosa. J. Org. Chem. 2018, 83, 951–963. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, A.; Sharma, P.R.; Qayum, A.; Singh, S.K.; Dutt, P.; Paul, S.; Gupta, V.; Verma, M.K.; Satti, N.K.; et al. A New Clerodane Furano Diterpene Glycoside from Tinospora cordifolia Triggers Autophagy and Apoptosis in HCT-116 Colon Cancer Cells. J. Ethnopharmacol. 2018, 211, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-Q.; Tang, S.; Song, W.-B.; Wang, W.-Q.; Huang, M.; Xuan, L.-J. Crassins A-H, diterpenoids from the roots of Croton crassifolius. J. Nat. Prod. 2017, 80, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Hashimoto, Y.; Chaipech, S.; Muraoka, O.; Morikawa, T. Labdane-Type Diterpenes, Galangalditerpenes A–C, with Melanogenesis Inhibitory Activity from the Fruit of Alpinia galanga. Molecules 2017, 22, 2279. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Talaat, A.N.; Labib, R.M.; Mándi, A.; Kurtán, T.; Müller, W.E.G.; Singab, A.; Proksch, P. Cytotoxic Labdane Diterpenes and Bisflavonoid Atropisomers from Leaves of Araucaria bidwillii. Tetrahedron 2017, 73, 3048–3055. [Google Scholar] [CrossRef]
- Li, B.; Ali, Z.; Chan, M.; Li, J.; Wang, M.; Abe, N.; Wu, C.-R.; Khan, I.A.; Wang, W.; Li, S.-X. Chemical constituents of Pholidota cantonensis. Phytochemistry 2017, 137, 132–138. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.-R.; Chen, S.-X.; Wang, W.-X.; Zou, Y.; Nuryyeva, S.; Houk, K.N.; Xiong, J.; Hu, J.-F. Amentotaxins C-V, Structurally Diverse Diterpenoids from the Leaves and Twigs of the Vulnerable Conifer Amentotaxus argotaenia and Their Cytotoxic Effects. J. Nat. Prod. 2020, 83, 2129–2144. [Google Scholar] [CrossRef]
- Geng, H.; Liu, Y.-C.; Li, D.-S.; Xiao, C.-J.; Liu, Y.; Li, X.-N.; Li, S.-H. Unusual glycosidic labdane diterpenoids with cytotoxicity from the root of Phlomoides betonicoides. Phytochemistry 2020, 173, 112325. [Google Scholar] [CrossRef]
- Zhao, Q.; Gao, J.-J.; Qin, X.-J.; Hao, X.-J.; He, H.-P.; Liu, H.-Y. Hedychins A and B, 6,7-Dinorlabdane Diterpenoids with a Peroxide Bridge from Hedychium forrestii. Org. Lett. 2018, 20, 704–707. [Google Scholar] [CrossRef]
- Carneiro, L.J.; Tasso, T.O.; Santos, M.F.C.; Goulart, M.O.; dos Santos, R.A.; Bastos, J.K.; da Silva, J.J.M.; Crotti, A.E.M.; Parreira, R.L.T.; Orenha, R.P.; et al. Copaifera multijuga, Copaifera pubiflora and Copaifera trapezifolia oleoresins: Chemical characterization and in vitro cytotoxic potential against tumoral cell lines. J. Braz. Chem. Soc. 2020, 31, 1679–1689. [Google Scholar] [CrossRef]
- Qi, J.-J.; Zhou, J.-S.; Zhang, Y.; Fan, Y.-Y.; Zhou, B.; Liu, H.-C.; Zhao, J.-X.; Yue, J.-M. Sublyratins A-O, Labdane-Type Diterpenoids from Croton sublyratus. J. Nat. Prod. 2021, 84, 2971–2980. [Google Scholar] [CrossRef]
- Singamaneni, V.; Lone, B.; Singh, J.; Kumar, P.; Gairola, S.; Singh, S.; Gupta, P. Coronarin K and L: Two novel labdane diterpenes from Roscoea purpurea: An ayurvedic crude drug. Front. Chem. 2021, 9, 642073. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Zeng, X.-T.; Xu, D.-Q.; Yue, S.-J.; Fu, R.-J.; Yang, X.; Liu, Z.-X.; Tang, Y.-P. Pimarane, abietane, and labdane diterpenoids from Euphorbia pekinensis Rupr. and their anti-tumor Activities. Phytochemistry 2022, 197, 113113. [Google Scholar] [CrossRef]
- Li, Y.-P.; Wu, D.-X.; Ye, T.; Zhang, H. Cytotoxic diterpenoids from the aerial parts of Scoparia dulcis. Phytochem. Lett. 2022, 49, 21–26. [Google Scholar] [CrossRef]
- Fan, D.; Zhou, S.; Zheng, Z.; Zhu, G.-Y.; Yao, X.; Yang, M.-R.; Jiang, Z.-H.; Bai, L.-P. New Abietane and Kaurane Type Diterpenoids from the Stems of Tripterygium Regelii. Int. J. Mol. Sci. 2017, 18, 147. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, J.; Zhu, L.-Y.; Zhang, J.-R.; Jing, Y.-X.; Zhao, J.-W.; Huang, X.-Z.; Li, G.-P.; Jiang, Z.-Y.; Xue, D.-Y. Four New Diterpene Glucosides from Perovskia Atriplicifolia. Chem. Biodivers. 2017, 14, e1700071. [Google Scholar] [CrossRef]
- Wan, J.; Jiang, H.-Y.; Tang, J.-W.; Li, X.-R.; Du, X.; Li, Y.; Sun, H.-D.; Pu, J.-X. Ent-abietanoids isolated from Isodon serra. Molecules 2017, 22, 309. [Google Scholar] [CrossRef]
- Zhang, C.-G.; Chou, G.-X.; Mao, X.-D.; Yang, Q.-S.; Zhou, J.-L. Nepetaefolins A-J, Cytotoxic Chinane and Abietane Diterpenoids from Caryopteris nepetaefolia. J. Nat. Prod. 2017, 80, 1742–1749. [Google Scholar] [CrossRef]
- Zhang, C.-G.; Jin, M.-R.; Chou, G.-X.; Yang, Q.-S. Plebeins A-F, sesquiterpenoids and diterpenoids from Salvia plebeian. Phytochem. Lett. 2017, 19, 254–258. [Google Scholar] [CrossRef]
- Yan, X.-L.; Zhang, J.-S.; Huang, J.-L.; Zhang, Y.; Chen, J.-Q.; Tang, G.-H.; Yin, S. Euphonoids A–G, Cytotoxic Diterpenoids from Euphorbia fischeriana. Phytochemistry 2019, 166, 112064. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Wang, X.-X.; Shi, Y.-X.; Zhang, N.; Ma, B.-Z.; Zhang, W.-K.; Xu, J.-K. Ent-Abietane Diterpenoids and Their Probable Biogenetic Precursors from the Roots of Euphorbia fischeriana. RSC Adv. 2017, 7, 55859–55865. [Google Scholar] [CrossRef]
- Li, M.; He, F.; Zhou, Y.; Wang, M.; Tao, P.; Tu, Q.; Lv, G.; Chen, X. Three New Ent-Abietane Diterpenoids from the Roots of Euphorbia fischeriana and Their Cytotoxicity in Human Tumor Cell Lines. Arch. Pharm. Res. 2019, 42, 512–518. [Google Scholar] [CrossRef]
- Wei, J.-C.; Gao, Y.-N.; Wang, D.-D.; Zhang, X.-Y.; Fan, S.-P.; Bao, T.-R.-G.; Gao, X.-X.; Hu, G.-S.; Wang, A.-H.; Jia, J.-M. Discovery of Highly Oxidized Abietane Diterpenoids from the Roots of Euphorbia fischeriana with Anti-tumor Activities. Chin. J. Chem. 2021, 39, 2973–2982. [Google Scholar] [CrossRef]
- Meng, J.; Li, B.-T.; Sheng, G.; Zhang, A.-L.; Li, X.-C.; Tian, J.-M. Cytotoxic Diterpenoids from Euphorbia fischeriana. Chem. Biodivers. 2021, 18, e2000919. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Q.; Hu, K.; Li, X.-N.; Sun, H.-D.; Puno, P.-T. Isoforrethins A–D, Four ent-Abietane Diterpenoids from Isodon forrestii var. forrestii. Fitoterapia 2019, 134, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-H.; Liu, Y.-P.; He, M.; Zhao, J.-H.; Wang, T.-T.; Feng, X.-Y.; Yue, H.; An, R.-B.; Fu, Y.-H. A New Abietane Diterpenoid from the Roots of Tripterygium regelii. Nat. Prod. Res. 2018, 32, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Wen, Q.; Hu, S.; Ma, Y.-L.; Jiang, Z.-H.; Tang, J.-Y.; Fu, Y.-H. Structurally Diverse Diterpenoids from Trigonostemon howii. Nat. Prod. Res. 2019, 33, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-P.; Jiang, K.; Zhang, P.; Shen, K.-K.; Qu, S.-J.; Yu, X.-P.; Tan, C.-H. Highly Oxygenated and Structurally Diverse Diterpenoids from Euphorbia helioscopia. Phytochemistry 2018, 145, 93–102. [Google Scholar] [CrossRef]
- Ito, T.; Rakainsa, S.K.; Nisa, K.; Morita, H. Three New Abietane-Type Diterpenoids from the Leaves of Indonesian Plectranthus scutellarioides. Fitoterapia 2018, 127, 146–150. [Google Scholar] [CrossRef]
- Yu, J.-H.; Yu, Z.-P.; Wu, D.-X.; Yan, X.; Wang, Y.-Y.; Zhang, H. Cuceolatins A–D: New Bioactive Diterpenoids from the Leaves of Cunninghamia lanceolata. Chem. Biodivers. 2019, 16, e1900317. [Google Scholar] [CrossRef]
- Tua, W.-C.; Qi, Y.-Y.; Ding, L.-F.; Yang, H.; Liu, J.-X.; Peng, L.-Y.; Song, L.-D.; Gong, X.; Wu, X.-D.; Zhao, Q.-S. Diterpenoids and sesquiterpenoids from the stem bark of Metasequoia glyptostroboides. Phytochemistry 2019, 161, 86–96. [Google Scholar] [CrossRef]
- Hadavand, H.; Mirzaei, O.; Firuzi, J.N. Chandran, B. Schneider, A. Reza Jassbi. Two antiproliferative seco-4,5-abietane diterpenoids from roots of Salvia ceratophylla L. Phytochem. Lett. 2019, 29, 129–133. [Google Scholar] [CrossRef]
- Liu, D.-M.; Cao, Z.-X.; Yan, H.-L.; Li, W.; Yang, F.; Zhao, W.-J.; Diao, Q.-C.; Tan, Y.-Z. A new abietane diterpenoid from Ajuga ovalifolia var. calantha induces human lung epithelial A549 cell apoptosis by inhibiting SHP2. Fitoterapia 2020, 141, 104484. [Google Scholar] [CrossRef]
- Zheng, X.; Kadir, A.; Zheng, G.; Jin, P.; Qin, D.; Maiwulanjiang, M.; Aisa, H.A.; Yao, G. Antiproliferative abietane quinone diterpenoids from the roots of Salvia deserta. Bioorg. Chem. 2020, 104, 104261. [Google Scholar] [CrossRef]
- Li, Q.-J.; Zhao, C.-L.; Ku, C.F.; Zhu, Y.; Zhu, X.-J.; Zhang, J.-J.; Deyrup, S.T.; Pan, L.-T.; Zhang, H.-J. Two new bioactive diterpenes identified from Isodon interruptus. Bioorg. Chem. 2020, 95, 103512. [Google Scholar] [CrossRef]
- Li, P.; Li, L.; Zhu, Q.; Xu, M. Abietane Diterpenoids Isolated from Clerodendrum bracteatum and Their Antioxidant and Cytotoxic Activities. Molecules 2021, 26, 4870. [Google Scholar] [CrossRef]
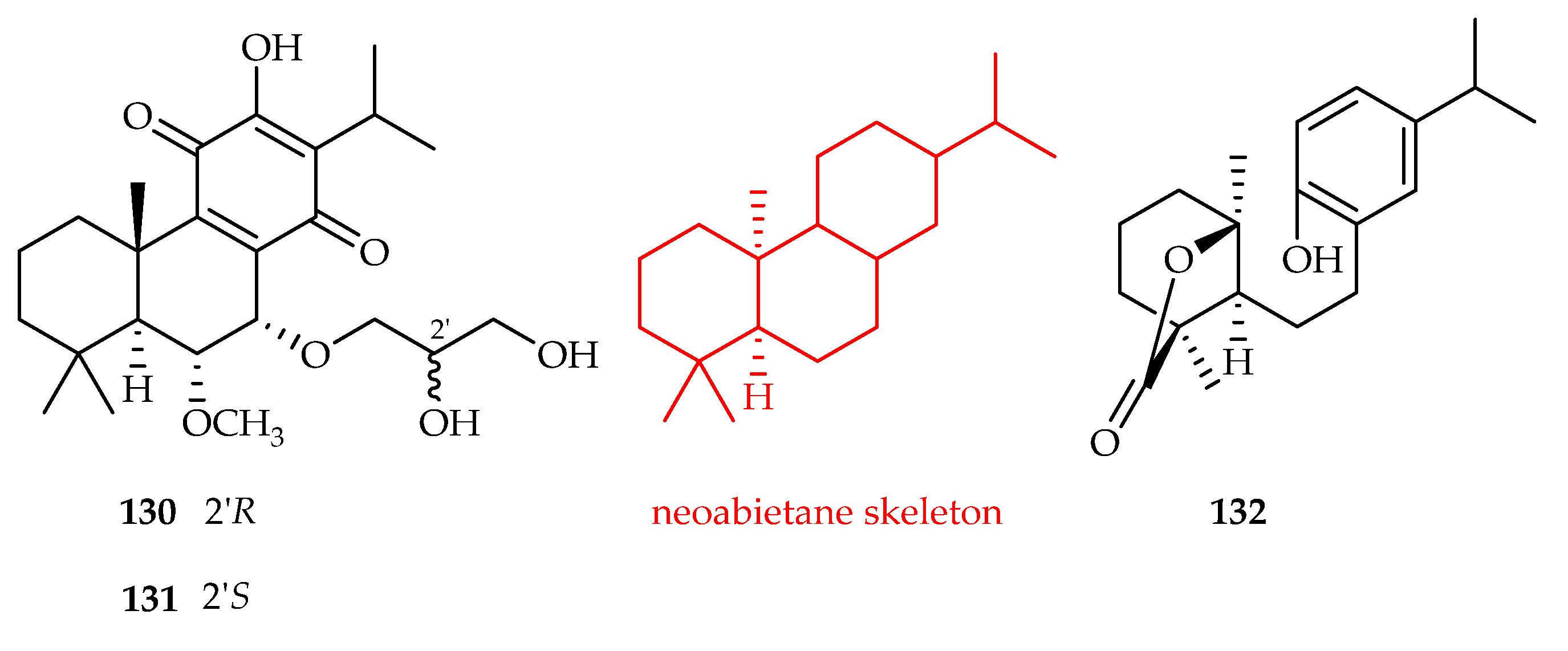
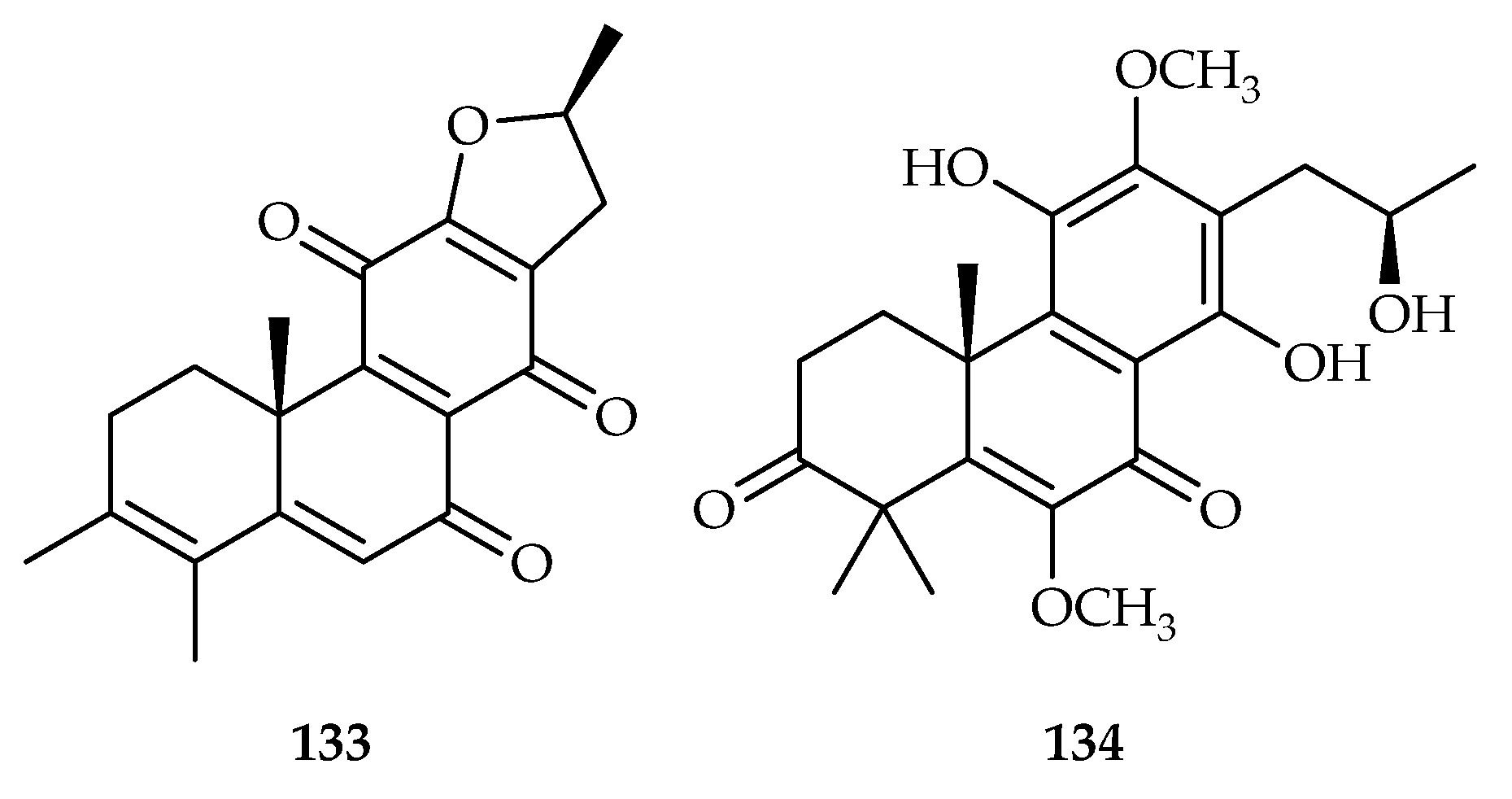
- Qi, J.; Zhang, Y.; Liu, Q.; Liu, H.; Fan, Y.; Yue, J. Clerodenoids A-F: C-ring Aromatized and/or Rearranged Abietane Diterpenoids from Clerodendrum chinense var. simplex. Chin. J. Chem. 2021, 39, 1891–1897. [Google Scholar] [CrossRef]
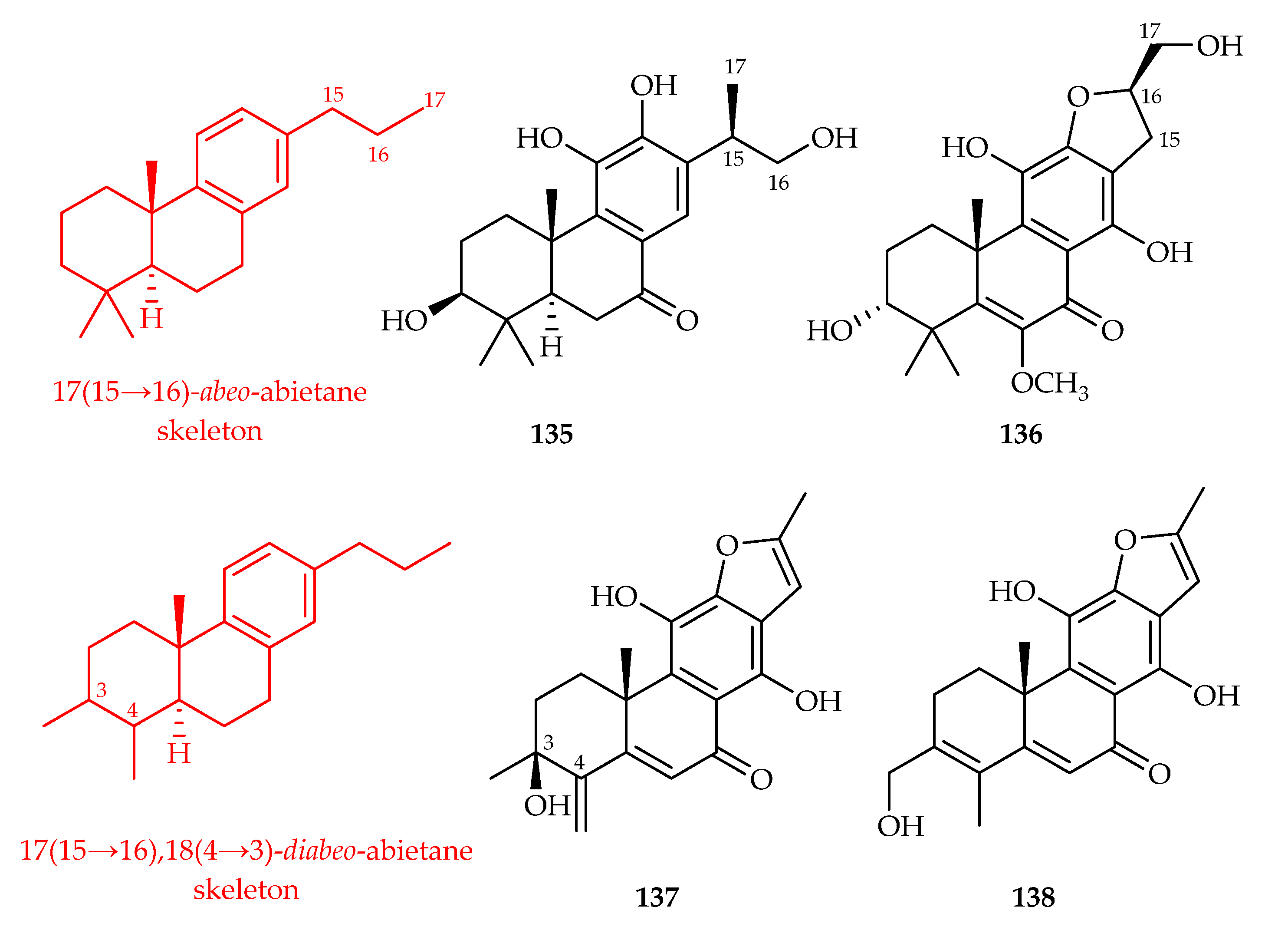
- Gao, Y.; Zhou, J.-S.; Liu, H.-C.; Zhang, Y.; Yin, W.-H.; Liu, Q.-F.; Wang, G.-W.; Zhao, J.-X.; Yue, J.-M. Phorneroids A-M, diverse types of diterpenoids from Euphorbia neriifolia. Phytochemistry 2022, 198, 113142. [Google Scholar] [CrossRef]
- Chen, X.-L.; Geng, Y.-J.; Li, F.; Hu, W.-Y.; Zhang, R.-P. Cytotoxic terpenoids from Tripterygium hypoglaucum against human pancreatic cancer cells SW1990 by increasing the expression of Bax protein. J. Ethnopharm. 2022, 289, 115010. [Google Scholar] [CrossRef]
- Cretton, S.; Saraux, N.; Monteillier, A.; Righi, D.; Marcourt, L.; Genta-Jouve, G.; Wolfender, J.-L.; Cuendet, M.; Christen, P. Anti-Inflammatory and Antiproliferative Diterpenoids from Plectranthus scutellarioides. Phytochemistry 2018, 154, 39–46. [Google Scholar] [CrossRef]
- Xia, F.; Zhang, D.-W.; Wu, C.-Y.; Geng, H.-C.; Xu, W.-D.; Zhang, Y.; Yang, X.-W.; Qin, H.-B.; Xu, G. Isolation, Structural Elucidation, and Synthetic Study of Salviyunnanone A, an Abietane Derived Diterpenoid with a 7/5/6/3 Ring System from Salvia yunnanensis. Org. Chem. Front. 2018, 5, 1262–1266. [Google Scholar] [CrossRef]
- Weng, Y.; Yu, X.; Li, J.; Dong, Q.; Li, F.; Cheng, F.; Zhang, Y.; Yao, C.; Zou, Z.; Zhou, W.; et al. Abietane Diterpenoids from Lycopodium complanatum. Fitoterapia 2018, 128, 135–141. [Google Scholar] [CrossRef]
- Farimani, M.M.; Khodaei, B.; Moradi, H.; Aliabadi, A.; Ebrahimi, S.N.; De Mieri, M.; Kaiser, M.; Hamburger, M. Phytochemical Study of Salvia leriifolia Roots: Rearranged Abietane Diterpenoids with Antiprotozoal Activity. J. Nat. Prod. 2018, 81, 1384–1390. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Hamed, A.R.; El-Halawany, A.M.; Hussien, T.A.; Abdelfatah, S.; Ohta, S.; Paré, P.W.; Abdel-Sattar, E.; Efferth, T. Cytotoxicity of Abietane Diterpenoids from Salvia multicaulis towards Multidrug-Resistant Cancer Cells. Fitoterapia 2018, 130, 54–60. [Google Scholar] [CrossRef]
- Jing, W.; Zhang, X.X.; Zhou, H.; Wang, Y.; Yang, M.; Long, L.; Gao, H. Naturally occurring cassane diterpenoids (CAs) of Caesalpinia: A systematic review of its biosynthesis, chemistry and pharmacology. Fitoterapia 2019, 134, 226–249. [Google Scholar] [CrossRef]
- Ha, M.T.; Tran, M.H.; Phuong, T.T.; Kim, J.A.; Woo, M.H.; Choi, J.S.; Lee, S.; Lee, J.H.; Lee, H.K.; Min, B.S. Cytotoxic and Apoptosis-Inducing Activities against Human Lung Cancer Cell Lines of Cassaine Diterpenoids from the Bark of Erythrophleum fordii. Bioorg. Med. Chem. Lett. 2017, 27, 2946–2952. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, R.; Yang, L.; Liang, H. Two new cassane-type diterpenoids from the seeds of Caesalpinia sappan. Nat. Prod. Res. 2022, 36, 2078–2084. [Google Scholar] [CrossRef]
- Chen, B.-L.; Zhu, Q.-F.; Zhang, X.; Lin, Y.; Long, Q.-D.; Liu, W.-L.; Yan, X.-L. An unusual indole-diterpenoid with C-17 norcassane skeleton from Euphorbia fischeriana induces HEL cell cycle arrest and apoptosis. Fitoterapia 2022, 159, 105195. [Google Scholar] [CrossRef]
- Peng, Y.; Ni, S.-J.; Li, J.; Li, M.-Y. Three New Dolabrane Diterpenes from the Chinese Mangrove Plant of Ceriops tagal. Phytochem. Lett. 2017, 21, 38–41. [Google Scholar] [CrossRef]
- Ni, S.-J.; Li, J.; Li, M.-Y. Two New Dolabrane Diterpenes from the Chinese Mangrove Ceriops tagal. Chem. Biodivers. 2018, 15, e1700563. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Shen, L.; Wu, J. Four New Diterpenes from the Mangrove Ceriops tagal and Structure Revision of Four Dolabranes with a 4,18-Epoxy Group. Fitoterapia 2018, 124, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Yang, Y.; Liu, J.-J.; Shen, L.; Shi, Z.; Wu, J. Tagalide A and Tagalol A, Naturally Occurring 5/6/6/6- and 5/6/6-Fused Cyclic Dolabrane-Type Diterpenes: A New Insight into the Anti-Breast Cancer Activity of the Dolabrane Scaffold. Org. Chem. Front. 2018, 5, 1176–1183. [Google Scholar] [CrossRef]
- Esquivel, B.; Bustos-Brito, C.; Sánchez-Castellanos, M.; Nieto-Camacho, A.; Ramírez-Apan, T.; Joseph-Nathan, P.; Quijano, L. Structure, Absolute Configuration, and Antiproliferative Activity of Abietane and Icetexane Diterpenoids from Salvia ballotiflora. Molecules 2017, 22, 1690. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Kadir, A.; Zheng, X.; Jin, P.; Liu, J.; Maiwulanjiang, M.; Yao, G.; Aisa, H.A. Spirodesertols A and B, two highly modified spirocyclic diterpenoids with an unprecedented 6-isopropyl-3H-spiro[benzofuran-2,1’-cyclohexane] motif from Salvia deserta. Org. Chem. Front. 2020, 7, 3137–3145. [Google Scholar] [CrossRef]
- Wang, J.; Xie, K.; Duan, H.; Wang, Y.; Ma, H.; Fu, H. Isolation and Characterization of Diterpene Glycosides from Siegesbeckia pubescens. Bioorg. Med. Chem. Lett. 2017, 27, 1815–1819. [Google Scholar] [CrossRef]
- Li, F.; Ma, J.; Li, C.-J.; Yang, J.-Z.; Zhang, D.; Chen, X.-G.; Zhang, D.-M. Bioactive Isopimarane Diterpenoids from the Stems of Euonymus oblongifolius. Phytochemistry 2017, 135, 144–150. [Google Scholar] [CrossRef]
- Jing, Y.-X.; You, H.-M.; Zhao, J.-W.; Wang, W.; Jiang, Y.-T.; Zuo, A.-X.; Fan, J.-T.; Zhang, S.-Y.; Jiang, Z.-Y. Bioactive constituents from Salacia cochinchinensis. J. Asian Nat. Prod. Res. 2020, 22, 738–745. [Google Scholar] [CrossRef]
- Rozimamat, R.; Hu, R.; Aisa, H.A. New Isopimarane Diterpenes and Nortriterpene with Cytotoxic Activity from Ephorbia alatavica Boiss. Fitoterapia 2018, 127, 328–333. [Google Scholar] [CrossRef]
- Yu, X.; Duan, K.-T.; Wang, Z.-X.; Chen, H.-P.; Gan, X.-Q.; Huang, R.; Li, Z.-H.; Feng, T.; Liu, J.-K. Anemhupehins A–C, Podocarpane Diterpenoids from Anemone hupehensis. Nat. Prod. Bioprospect. 2018, 8, 31–35. [Google Scholar] [CrossRef]
- Luo, Y.; Li, X.-Z.; Xiang, B.; Luo, Q.; Liu, J.-W.; Yan, Y.-M.; Sun, Q.; Cheng, Y.-X. Cytotoxic and renoprotective diterpenoids from Clerodendranthus spicatus. Fitoterapia 2018, 125, 135–140. [Google Scholar] [CrossRef]
- Li, J.-C.; Yuan, X.-R.; Liu, Y.-L.; Li, Y.; Cui, N.-N.; Li, L.-L.; Ha, J. Two new diterpenoids from Aleuritopteris argentea. Phytochem. Lett. 2017, 20, 22–25. [Google Scholar] [CrossRef]
- Jiang, C.; Xue, J.; Yuan, Y.; Li, Y.; Zhao, C.; Jing, Q.; Zhang, X.; Yang, M.; Han, T.; Bai, J.; et al. Progress in structure, synthesis and biological activity of natural cephalotane diterpenoids. Phytochemistry 2021, 192, 112939. [Google Scholar] [CrossRef]
- Ge, Z.-P.; Liu, H.-C.; Wang, G.-C.; Liu, Q.-F.; Xu, C.-H.; Ding, J.; Fan, Y.-Y.; Yue, J.-M. 17-nor-Cephalotane-Type Diterpenoids from Cephalotaxus fortunei. J. Nat. Prod. 2019, 82, 1565–1575. [Google Scholar] [CrossRef]
- Zhao, J.-X.; Fan, Y.-Y.; Xu, J.-B.; Gan, L.-S.; Xu, C.-H.; Ding, J.; Yue, J.-M. Diterpenoids and Lignans from Cephalotaxus fortunei. J. Nat. Prod. 2017, 80, 356–362. [Google Scholar] [CrossRef]
- Ni, L.; Zhong, X.-H.; Chen, X.-J.; Zhang, B.-J.; Bao, M.-F.; Cai, X.-H. Bioactive Norditerpenoids from Cephalotaxus fortunei var. alpina and C. lanceolata. Phytochemistry 2018, 151, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Guercia, E.; Berti, F.; Navarini, L.; Demitri, N.; Forzato, C. Isolation and characterization of major diterpenes from C. canephora roasted coffee oil. Tetrahedron Asymmetry 2016, 27, 649–656. [Google Scholar] [CrossRef]
- Guercia, E.; Forzato, C.; Navarini, L.; Berti, F. Interaction of coffee compounds with serum albumins. Part II: Diterpenes. Food Chem. 2016, 199, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Navarini, L.; Guercia, E.; Oreski, A.; Gasparini, A.; Scoltock, J.; Forzato, C. Interaction of the Coffee Diterpenes Cafestol and 16-O-Methyl-Cafestol Palmitates with Serum Albumins. Int. J. Mol. Sci. 2020, 21, 1823. [Google Scholar] [CrossRef]
- Finotello, C.; Forzato, C.; Gasparini, A.; Mammi, S.; Navarini, L.; Schievano, E. NMR quantification of 16-O-methylcafestol and kahweol in Coffea canephora var. robusta beans from different geographical origins. Food Control 2017, 75, 62–69. [Google Scholar] [CrossRef]
- Sarwar, S.; Xia, Y.-X.; Liang, Z.-M.; Tsang, S.W.; Zhang, H.-J. Mechanistic Pathways and Molecular Targets of Plant-Derived Anticancer ent-Kaurane Diterpenes. Biomolecules 2020, 10, 144. [Google Scholar] [CrossRef]
- Gobu, F.-R.; Chen, J.-J.; Zeng, J.; Wei, W.-J.; Wang, W.-F.; Lin, C.-J.; Gao, K. Isolation, structure elucidition, and immunosuppressive activity of diterpenoids from Ligularia fischeri. J. Nat. Prod. 2017, 80, 2263–2268. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Wang, W.-G.; Tang, J.-W.; Liu, M.; Li, X.-R.; Hu, K.; Du, X.; Li, X.-N.; Zhang, H.-B.; Pu, J.-X.; et al. Structurally Diverse Diterpenoids from Isodon scoparius and Their Bioactivity. J. Nat. Prod. 2017, 80, 2026–2036. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Jiang, H.-Y.; Liu, M.; Hu, K.; Wang, W.-G.; Du, X.; Li, X.-N.; Pu, J.-X.; Sun, H.-D. Bioactive ent-kaurane diterpenoids from Isodon rubescens. Phytochemistry 2017, 143, 199–207. [Google Scholar] [CrossRef]
- Woo, K.W.; Park, K.J.; Choi, S.Z.; Son, M.W.; Kim, K.H.; Lee, K.R. A New Ent-Kaurane Diterpene Glycoside from Seeds of Pharbitis nil. Chem. Nat. Compd. 2017, 53, 468–471. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Yang, L.; Chen, N.; Jiang, L.; Jiang, S.; Li, G.; Li, Y.; Wang, G. Three New Ent-Kaurane Diterpenes from the Herbs of Wedelia prostrata. J. Nat. Med. 2017, 71, 305–309. [Google Scholar] [CrossRef]
- Shi, Q.; Sun, Y.-W.; Meng, D. Phytochemical and cytotoxic studies on the roots of Euphorbia fischeriana. Bioorg. Med. Chem. Lett. 2017, 27, 266–270. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Ma, C.-Y.; Wang, M.-L.; Lu, J.-H.; Hu, P.; Chen, J.-W.; Li, X.; Chen, Y. Five new ent-kaurane diterpenes from Annona squamosa L. pericarps. Nat. Prod. Res. 2020, 34, 2243–2247. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Xu, H.-C.; Hu, K.; Liu, M.; Li, X.-N.; Li, X.-R.; Du, X.; Zhang, Y.-H.; Puno, P.-T.; Sun, H.-D. Structurally Diverse Diterpenoids from Isodon pharicus. Org. Chem. Front. 2018, 5, 2379–2389. [Google Scholar] [CrossRef]
- Shi, S.-Q.; Fan, Y.-Y.; Xu, C.-H.; Ding, J.; Wang, G.-W.; Yue, J.-M. Cytotoxic 8,9- Seco -Ent-Kaurane Diterpenoids from Croton kongensis. J. Asian Nat. Prod. Res. 2018, 20, 920–927. [Google Scholar] [CrossRef]
- Dai, L.-P.; Zhang, L.-X.; Liu, Y.-L.; Wu, H.; Liu, R.-X.; Zhao, M.; Tian, S.-S.; Jiang, X.; Chen, S.-Q. Isolation and purification of diterpenoids from the aerial parts of Isodon excisoides target-guided by UPLCLTQ-Orbitrap-MS. Nat. Prod.Res. 2018, 32, 2424–2430. [Google Scholar] [CrossRef]
- Wang, A.; Fan, Y.; Ouyang, Q.; Fan, C.; Lin, B.; Liu, J.; Xu, Y. Antiproliferative ent-kaurane diterpenoids isolated from the roots of Zea mays L. Fitoterapia 2019, 134, 44–49. [Google Scholar] [CrossRef]
- Huang, H.-T.; Liaw, C.-C.; Lin, Y.-C.; Liao, G.-Y.; Chao, C.-H.; Chiou, C.-T.; Kuo, Y.-H.; Lee, K.-T. New Diterpenoids from Mesona procumbens with Antiproliferative Activities Modulate Cell Cycle Arrest and Apoptosis in Human Leukemia Cancer Cells. Pharmaceuticals 2021, 14, 1108. [Google Scholar] [CrossRef]
- Qiu, C.-L.; Ye, Z.-N.; Yan, B.-C.; Hu, K.; Yang, J.; Yang, X.-Z.; Li, H.-M.; Li, X.-N.; Sun, H.-D.; Puno, P.-T. Structurally diverse diterpenoids from Isodon oresbius and their bioactivity. Bioorg. Chem. 2022, 124, 105811. [Google Scholar] [CrossRef]
- Li, Y.; Hou, B.; Wang, M.; Wang, R.; Chen, X.; Liu, X.; Fei, D.; Zhang, Z.; Li, E. Diterpenoids and C nor-isoprenoid identified from the leaves and twigs of Croton yanhuii activating apoptosis and pyroptosis. Front. Chem. 2022, 10, 861278. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Liu, M.; Wang, W.-G.; Li, X.-N.; Hu, K.; Li, X.-R.; Du, X.; Zhang, Y.-H.; Puno, P.-T.; Sun, H.-D. 7α,20-Epoxy-Ent-Kaurane Diterpenoids from the Aerial Parts of Isodon pharicus. J. Nat. Prod. 2018, 81, 106–116. [Google Scholar] [CrossRef]
- Qiao, Y.; Zheng, H.; Li, L.; Zhang, J.; Li, Y.; Li, S.; Zhu, R.; Zhou, J.; Zhao, S.; Jiang, Y.; et al. Terpenoids with Vasorelaxant Effects from the Chinese Liverwort Scapania carinthiaca. Bioorg. Med. Chem. 2018, 26, 4320–4328. [Google Scholar] [CrossRef]
- Luo, G.-Y.; Deng, R.; Zhang, J.-J.; Ye, J.-H.; Pan, L.-T. Two Cytotoxic 6,7-Seco -Spiro-Lacton-Ent-Kauranoids from Isodon Rubescens. J. Asian Nat. Prod. Res. 2018, 20, 227–233. [Google Scholar] [CrossRef]
- Kawakami, S.; Nishida, S.; Nobe, A.; Inagaki, M.; Nishimura, M.; Matsunami, K.; Otsuka, H.; Aramoto, M.; Hyodo, T.; Yamaguchi, K. Eight Ent-Kaurane Diterpenoid Glycosides Named Diosmariosides A–H from the Leaves of Diospyros maritima and Their Cytotoxic Activity. Chem. Pharm. Bull. 2018, 66, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Li, X.-N.; Sun, H.-D.; Zhang, H.-B.; Puno, P.-T. Scopariusols L-T, Nine New Ent-Kaurane Diterpenoids Isolated from Isodon scoparius. Chin. J. Nat. Med. 2018, 16, 456–464. [Google Scholar] [CrossRef]
- Shi, X.-J.; Ding, L.; Zhou, W.; Ji, Y.; Wang, J.; Wang, H.; Ma, Y.; Jiang, G.; Tang, K.; Ke, Y.; et al. Pro-Apoptotic Effects of JDA-202, a Novel Natural Diterpenoid, on Esophageal Cancer Through Targeting Peroxiredoxin I. Antioxid. Redox Signal. 2017, 27, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, W.; Chen, J.; Cai, X.; Yang, J.; Yang, Y.; Yan, H.; Cheng, X.; Ye, J.; Lu, W.; et al. The Natural Diterpenoid Isoforretin A Inhibits Thioredoxin-1 and Triggers Potent ROS-Mediated Antitumor Effects. Cancer Res. 2017, 77, 926–936. [Google Scholar] [CrossRef]
- Liu, H.-C.; Xiang, Z.-B.; Wang, Q.; Li, B.-Y.; Jin, Y.-S.; Chen, H.-S. Monomeric and Dimeric Ent-Kauranoid-Type Diterpenoids from Rabdosia japonica and Their Cytotoxicity and Anti-HBV Activities. Fitoterapia 2017, 118, 94–100. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Y.-Q.; Han, B.-K.; Li, X.-R.; Shi, X.-J.; Yin, F.; Wang, J.-W.; Zhao, P.-R.; Ke, Y.; Liu, H.-M. Gene Expression Profiling and Pathway Network Analysis of Anti-Tumor Activity by Jaridon 6 in Esophageal Cancer. Eur. J. Pharmacol. 2017, 815, 478–486. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, X.; Liao, H.; Zeng, X.; Zhang, S.; Lu, X.; He, X.; Zhang, X.; Ye, W.; Wu, H.; et al. Pteisolic Acid G, a Novel Ent-kaurane Diterpenoid, Inhibits Viability and Induces Apoptosis in Human Colorectal Carcinoma Cells. Oncol. Lett. 2017, 14, 5540–5548. [Google Scholar] [CrossRef][Green Version]
- Lyu, H.; Liu, W.; Bai, B.; Shan, Y.; Paetz, C.; Feng, X.; Chen, Y. Prenyleudesmanes and A Hexanorlanostane from the Roots of Lonicera macranthoides. Molecules 2019, 24, 4276. [Google Scholar] [CrossRef]
- Feng, Z.-L.; Zhang, L.-L.; Zheng, Y.-D.; Liu, Q.-Y.; Liu, J.-X.; Feng, L.; Huang, L.; Zhang, Q.-W.; Lu, J.-J.; Lin, L.-G. Norditerpenoids and Dinorditerpenoids from the Seeds of Podocarpus nagi as Cytotoxic Agents and Autophagy Inducers. J. Nat. Prod. 2017, 80, 2110–2117. [Google Scholar] [CrossRef]
- Olivon, F.; Retailleau, P.; Desrat, S.; Touboul, D.; Roussi, F.; Apel, C.; Litaudon, M. Isolation of Picrotoxanes from Austrobuxus carunculatus Using Taxonomy-Based Molecular Networking. J. Nat. Prod. 2020, 83, 3069–3079. [Google Scholar] [CrossRef]
- Qi, Y.-Y.; Su, J.; Zhang, Z.-J.; Li, L.-W.; Fan, M.; Zhu, Y.; Wu, X.-D.; Zhao, Q.-S. Two New Anti-Proliferative C18-Norditerpenes from the Roots of Podocarpus macrophyllus. Chem. Biodivers. 2018, 15, e1800043. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Z.-P.; Yuan, Z.-F.; Zhao, Q.-S. Vibsane-Type Diterpenoids: Structures, Derivatives, Bioactivities, and Synthesis. Chem. Biodiversity 2022, 19, e202100861. [Google Scholar] [CrossRef]
- Zhu, Q.-F.; Qi, Y.-Y.; Zhang, Z.-J.; Fan, M.; Bi, R.; Su, J.; Wu, X.-D.; Shao, L.-D.; Zhao, Q.-S. Vibsane-Type Diterpenoids from Viburnum odoratissimum and Their Cytotoxic and HSP90 Inhibitory Activities. Chem. Biodivers. 2018, 15, 1800049. [Google Scholar] [CrossRef]
- Li, S.-F.; Yu, X.-Q.; Li, Y.-L.; Bai, M.; Lin, B.; Yao, G.-D.; Song, S.-J. Vibsane-type diterpenoids from Viburnum odoratissimum and their cytotoxic activities. Bioorg. Chem. 2021, 106, 104498. [Google Scholar] [CrossRef]
- Kawabe, H.; Suzuki, R.; Hirota, H.; Matsuzaki, K.; Gong, X.; Ohsaki, A. A New Diterpenoid with a Rearranged Skeleton from Salvia prattii. Nat. Prod. Comm. 2017, 12, 1934578X1701200807. [Google Scholar] [CrossRef]
- Lou, H.-Y.; Jin, L.; Huang, T.; Wang, D.-P.; Liang, G.-Y.; Pan, W.-D. Vulgarisins B-D, three novel diterpenoids with a rare skeleton isolated from Prunella vulgaris Linn. Tetrahedron Lett. 2017, 58, 401–404. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Shi, S.-Q.; Deng, G.-Z.; Liu, H.-C.; Xu, C.-H.; Ding, J.; Wang, G.-W.; Yue, J.-M. Crokonoids A-C, A Highly Rearranged and Dual-Bridged Spiro Diterpenoid and Two Other Diterpenoids from Croton kongensis. Org. Lett. 2020, 22, 929–933. [Google Scholar] [CrossRef]
- Mao, X.-D.; Zhang, C.-G.; Chen, T.; Zhao, S.-M.; Chou, G.-X. Cytotoxic Diterpenoids from Caryopteris aureoglandulosa. J. Nat. Prod. 2020, 83, 2093–2101. [Google Scholar] [CrossRef]
- He, J.; Xu, J.-K.; Zhang, J.; Bai, H.-J.; Ma, B.-Z.; Cheng, Y.-C.; Zhang, W.-K. Fischeriana A, a Meroterpenoid with an Unusual 6/6/5/5/5/6/6 Heptacyclic Carbon Skeleton from the Roots of Euphorbia fischeriana. Org. Biomol. Chem. 2019, 17, 2721–2724. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Emami, S.A.; Tayarani-Najaran, Z.; Iranshahi, M.; Shakeri, A.; Hohmann, J.; Asili, J. Cytotoxic Diterpene Quinones from Salvia tebesana Bunge. Fitoterapia 2018, 128, 97–101. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Chapter Six-Validation of in-Vitro Bioassay Methods: Application in Herbal Drug Research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. ISBN 1871-5125. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forzato, C.; Nitti, P. New Diterpenes with Potential Antitumoral Activity Isolated from Plants in the Years 2017–2022. Plants 2022, 11, 2240. https://doi.org/10.3390/plants11172240
Forzato C, Nitti P. New Diterpenes with Potential Antitumoral Activity Isolated from Plants in the Years 2017–2022. Plants. 2022; 11(17):2240. https://doi.org/10.3390/plants11172240
Chicago/Turabian StyleForzato, Cristina, and Patrizia Nitti. 2022. "New Diterpenes with Potential Antitumoral Activity Isolated from Plants in the Years 2017–2022" Plants 11, no. 17: 2240. https://doi.org/10.3390/plants11172240
APA StyleForzato, C., & Nitti, P. (2022). New Diterpenes with Potential Antitumoral Activity Isolated from Plants in the Years 2017–2022. Plants, 11(17), 2240. https://doi.org/10.3390/plants11172240