Abstract
Nitric oxide (NO), as a signal molecule, is involved in the mediation of heavy-metal-stress-induced physiological responses in plants. In this study, we investigated the effect of NO on Camellia sinensis pollen tubes exposed to aluminum (Al) stress. Exogenous application of the NO donor decreased the pollen germination rate and pollen tube length and increased the malondialdehyde (MDA) content and antioxidant enzyme activities under Al stress. Simultaneously, the NO donor effectively increased NO content in pollen tube of C. sinensis under Al stress and could aggravate the damage of Al3+ to C. sinensis pollen tubes by promoting the uptake of Al3+. In addition, application of the NO-specific scavenger significantly alleviated stress damage in C. sinensis pollen tube under Al stress. Moreover, 18 CsALMT members from a key Al-transporting gene family were identified, which could be divided into four subclasses. Pearson correlation analysis showed the expression level of CsALMT8 showed significant positive correlation with the Al3+ concentration gradient and NO levels, but a significant negative correlation with pollen germination rate and pollen tube length. The expression level of CsALMT5 was negatively correlated with the Al3+ concentration gradient and NO level, and positively correlated with pollen germination rate and pollen tube length. The expression level of CsALMT17 showed a significant negative correlation with Al3+ concentration and NO content in pollen tubes, but significant positive correlation with pollen germination rate and pollen tube length. In conclusion, a complex signal network regulated by NO-mediated CsALMTs revealed that CsALMT8 was regulated by environmental Al3+ and NO to assist Al3+ entry into pollen tubes; CsALMT5 might be influenced by the Al3+ signal, stimulate malate efflux in vacuoles and chelate with Al3+ to detoxify Al in C. sinensis pollen tube.
1. Introduction
Aluminum (Al) is the most abundant metal in the Earth’s crust (comprising approximately 6.6% of the soil), and it is also an indispensable inorganic mineral in the soil []. Stable Al salt will become free Al ions (Al3+) that can be taken up by plants when the pH of the soil is lower than 5, which will affect plant growth []. As an acid-loving plant, Camellia sinensis (L.) O. Kuntze is better-suited to growing in acidic soil (pH 4.5–5.5), which has higher bioavailable Al in comparison to other plants. Thus, C. sinensis can absorb and accumulate more Al3+. In general, the root tip is the main part of plants responding to Al stress and generating a stress response []. On the one hand, aluminum stress can not only inhibit the absorption and metabolism of mineral ions such as Ca2+, Mg2+, K+, Fe2+, Cu2+, PO43- and water in plant root tip cells, but also inhibit the elongation and cell division of plant root tip cells []. It can also form Al-ATP complexes to inhibit the cytoplasmic membrane of plant roots by affecting the structure and function of calmodulin [], thereby disrupting the Ca2+ balance and Ca2+ concentration gradient in plant cells. On the other hand, Al3+ can not only damage the plasma membrane of plants, but also change the functional structure of the cytoskeleton by affecting the morphological changes in microtubules and microfilaments []. Pollen tube, as the male reproductive organ of higher plants, plays an important role in sexual reproduction. The pollen tube grows from the top of style and presents a polarized pattern, which is a typical polar top growth, which is very similar to the growth of plant root tips []. In addition, the polar growth of pollen tubes is closely related to the cytoplasmic Ca2+ concentration gradient homeostasis and the morphology of microtubules and filaments [].Therefore, pollen tubes provide a useful system for studying the mechanism of Al toxicity in plants. Many reports have shown that Al toxicity inhibits the growth of pollen tubes of tomato [], lily [], tea [] and apple [], but the mitigation measures for Al poisoning in pollen tubes have not been effectively explored.
Nitric oxide (NO) is involved in the regulation of plant growth and development, which is mainly reflected in seed development, root morphogenesis and stomatal movement [,]. Numerous studies in recent years have confirmed that NO is a crucial signal molecule for plants’ ability to deal with various challenges during growth [,]. In our previous studies, NO has been shown to play a role as a negative regulating factor in the response of C. sinensis pollen tubes to low-temperature stress []. In addition, some studies have demonstrated that applying an exogenous NO donor can significantly alleviate the Al stress on plants, such as alleviating the inhibition of Al stress on root length and reducing the accumulation of Al3+ in root tips []. NO has dual effects in relieving oxidative stress. On the one hand, NO reduces the accumulation of reactive oxygen species (ROS) by acting as an antioxidant and interacting with different ROS groups. On the other hand, NO can regulate the activity of antioxidant enzymes in plants and indirectly eliminate ROS accumulation induced by metal ions. Furthermore, NO can improve the capacity to alleviate Al stress by altering the polysaccharide components of rice cell walls [].
Exploring the mechanism of plant Al resistance from genetic traits is an important way to screen Al-resistant genotypes. Many achievements have been made in elucidating the molecular mechanism of Al tolerance in plants, and the functions of aluminum-activated malate transporter (ALMT), a kind of Al-tolerance-related organic acid transporter, have been confirmed [,]. TaALMT1 is structurally expressed in wheat root tips, and can be activated by Al3+ in acidic soil, releasing malate anion into the apoplast [], and then utilizing malate to chelate Al3+, thereby improving the Al tolerance of wheat []. The release of organic acids (malate, oxalate, citrate, etc.) can relieve Al toxicity by chelating and immobilizing Al3+ has been confirmed in rice, barley, buckwheat, etc. In addition, members of the ALMT gene family also show similar Al tolerance in Arabidopsis thaliana [], Brassica napus [], Glycine max [] and Medicago sativa []. To date, the ALMT gene family has been deeply studied in other plants, but rarely in C. sinensis. Pollen tube, as the male reproductive organ of higher plants, has a multinuclear single-cell structure, which is an ideal model to study the response of plants to various signals and stresses. In our pre-experiment, we found that ALMTs were also expressed in C. sinensis pollen tubes, and the expression levels of some CsALMTs in C. sinensis pollen tubes were higher than those in tea roots.
In this regard, we hypothesized that: (1) the Al tolerance of tea pollen tubes can be improved by increasing or decreasing NO levels in the environment; and (2) some CsALMTs can enhance the Al tolerance of C. sinensis pollen tubes through the mediation of NO signaling molecule tolerance. Thus, the present study aimed to explore the possible Al tolerance mechanism mediated by NO signals and CsALMTs, taking tea pollen tube as the research material for which the determination of physiological and biochemical indices, bioinformatics analysis and gene expression level analysis would be carried out.
2. Results
2.1. Pollen Germination and Pollen Tube Elongation
Exogenous Al3+ had a clear dose-dependent influence on the pollen germination rate and pollen tube length, both of which significantly decreased with an increase in the Al concentration gradient (p < 0.05) (Figure 1A,B and Figure S1). Moreover, the pollen germination rate and pollen tube length were considerably reduced by exogenous NO donor treatments compared to Al treatments, and the inhibitory effect of NO on pollen germination and growth increased with the increase in the Al concentration gradient (Figure 1A). Furthermore, Al + cPTIO treatments demonstrated a favorable impact on the pollen germination rate and pollen tube length when compared to Al treatments, and the alleviating effect of cPTIO on the exogenous Al3+-inhibited reduction in the pollen germination rate and pollen tube length of C. sinensis also increased with the increase in the Al concentration gradient (Figure 1A,B).
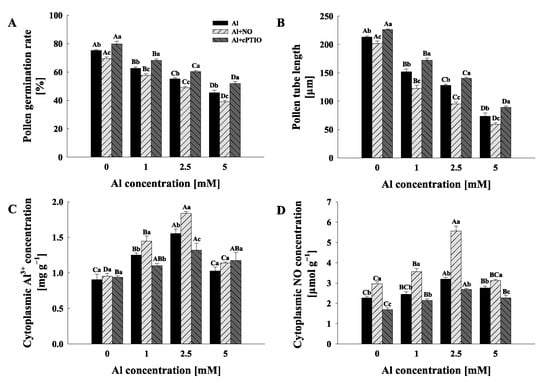
Figure 1.
The effects of different treatments on the pollen germination rate (A), pollen tube length, (B) cytoplasmic Al3+ concentration, (C) and cytoplasmic NO concentration (D) of Camellia sinensis. Al represents the treatment group treated with Al concentration gradient only, Al + NO represents the treatment group treated with Al3+ concentration gradient treatment and 25 μM DEA NONOate (NO donor), and Al + cPTIO represents the treatment group treated with Al3+ concentration gradient treatment and 200 μM carboxy PTIO potassium salt (NO scavenger). Different lowercase letters represent significant differences among different nitric oxide conditions under the same Al3+ concentration treatment, and different uppercase letters represent significant differences among different Al3+ concentration treatments under the same nitric oxide conditions (for pollen germination rate and pollen tube length, n = 10, and for cytoplasmic Al3+ and NO, n = 3, p < 0.05), as determined by the Duncan test.
2.2. Cytoplasmic Al3+ and NO
The results show that cytoplasmic Al3+ and NO concentrations were significantly increased under the treatment of exogenous Al3+, and cytoplasmic Al3+ and NO concentrations reached the highest value under 2.5 mM Al3+ treatment (Figure 1C,D). Additionally, the pollen tube Al3+ and NO concentrations of Al1 + NO and Al2.5 + NO were significantly higher than those of Al1 and Al2.5, respectively, while the cytoplasmic Al3+ and NO concentrations of Al1 + cPTIO and Al2.5 + cPTIO were significantly lower than those of Al1 and Al2.5, respectively (Figure 1C,D).
2.3. Physiochemical Responses
Malondialdehyde (MDA) contents in pollen tubes increased with the increasing Al concentration gradient (Figure 2A). It is worth noting that an exogenous addition the NO donor and NO scavenger increased and decreased MDA contents in pollen tubes, respectively. However, compared with Al0, Al0 + NO increased the MDA contents in pollen tubes by 21.98% but Al0 + cPTIO decreased the content by 16.80%, while there was no significant difference in MDA contents among Al5, Al5 + NO and Al5 + cPTIO (p > 0.05).
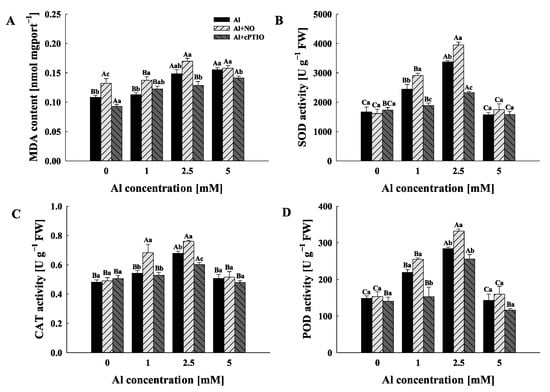
Figure 2.
The effects of different treatments on the malondialdehyde (MDA) contents (A), superoxide dismutase (SOD) (B), catalase (CAT) (C) and peroxidase (POD) (D) activities of Camellia sinensis pollen tubes. FW: fresh weight. Al represents the treatment group treated with Al concentration gradient only, Al + NO represents the treatment group treated with Al3+ concentration gradient treatment and 25 μM DEA NONOate (NO donor), and Al + cPTIO represents the treatment group treated with Al3+ concentration gradient treatment and 200 μM carboxy PTIO potassium salt (NO scavenger). Different lowercase letters represent significant differences among different nitric oxide conditions under the same Al3+ concentration treatment, and different uppercase letters represent significant differences among different Al3+ concentration treatments under the same nitric oxide condition (n = 3, p < 0.05), as determined by the Duncan test.
The activities of antioxidant enzymes in pollen tubes were significantly influenced by the Al concentration gradient and NO level (Figure 2B–D). The activities of all three antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD)) in Al, Al + NO and Al + cPTIO showed the same trend with the exogenous Al concentration gradient; that is, they increased until reaching the highest activity under the 2.5 mM Al3+ treatment and then decreased. In addition, the NO donor/NO scavenger could promote/inhibit the activities of SOD, CAT and POD in pollen tube only when the concentrations of Al3+ in the culture medium were 1 mM and 2.5 mM.
2.4. Bioinformatics Analysis for ALMT Gene Family in C. sinensis
2.4.1. Identification, Classification and Naming of ALMT Gene Family in C. sinensis
By comparing the genomic coding sequences of C. sinensis and the AtALMTs sequences using Local BLASTp and Bioedit, 18 potential ALMT amino acid sequences in C. sinensis were discovered. The ALMT conserved domain (PF11744) of the obtained sequences was verified again by online services SMART and Pfam. It was found that all 18 amino acid sequences contained the ALMT conserved domain, and finally, 18 ALMT amino acid sequences in C. sinensis were obtained.
In the present study, the phylogenetic tree was constructed by using amino acid sequences of CsALMTs, AtALMTs and OsALMTs (Figure S2). The result of the phylogenetic tree showed that ALMTs could be divided into four subclasses, among which subclass I includes 5 AtALMTs, 4 OsALMTs and 10 CsALMTs. Subclass II includes five AtALMTs, four OsALMTs and four CsALMTs. Subclass III includes four AtALMTs, one OsALMT and two CsALMTs. It is worth noting that only CsALMT17 and CsALMT18 are included in subclass IV (Figure S2). Previous studies have shown that AtALMT1 encodes Al-activated root malate efflux transporter and is related to Al tolerance []. Therefore, we speculate that CsALMTs in subclass I may be related to Al resistance.
2.4.2. Basic Physicochemical Properties, Subcellular Localization and Protein Secondary Structure Prediction of CsALMTs
Based on fundamental physical and chemical features, it was determined that each of the 18 CsALMTs encoded a different number of amino acids, with CsALMT18 encoding the fewest (302 amino acids) and CsALMT5 encoding the most (793 amino acids) (Table S1). The molecular weights of 18 CsALMTs ranged from 32,991.54 to 88,719.08 Da, and the theoretical isoelectric points ranged from 5.66 to 8.98. Total average hydrophobicity ranged from −0.152 to 0.421, and protein instability coefficient ranged from 28.24 to 41.93. The analysis of the number of transmembrane proteins showed that CsALMTs contained three to six transmembrane proteins except CsALMT18, which contained no transmembrane domain. The subcellular localization of members of this gene family was predicted by SoftBerry ProtComp 9.0, and it was found that CsALMT1, CsALMT2 and CsALMT6-16 were all located on the plasma membrane, CsALMT3-5 was located on the endoplasmic reticulum, and CsALMT17 and CsALMT18 were located outside the cells.
The protein secondary structures of CsALMTs were predicted using the web program SOPMA (Table S2). The results show that the protein secondary structure of all the 18 CsALMTs contained an α helix, β turn, irregular curl and extended chain. Among them, the α helix (36.47–64.11%) was the main structure in CsALMTs, followed by irregular coiled structure and an extended chain. The β-turn accounts for the lowest number of the secondary structures in CsALMTs, ranging from 1.88% to 11.06%. Irregular curly structure and extended chains accounted for 20.33–30.45% and 8.82–29.18%, respectively.
2.4.3. Analysis of Gene Structure and Amino Acid Structure of CsALMTs
According to Figure S3, the majority of CsALMTs had six exons and were primarily found in subclasses I and II. The exon count for CsALMT5 was 11, whereas the exon count for CsALMT18 was only 4. Seventeen amino acid motifs of CsALMTs (Figure S4) were obtained using the MEME online service, and the E value and multilevel consensus sequence of each motif are shown in Table S3. CsALMTs contains at least 2 motifs and at most 15 motifs, among which 12 motifs (motifs 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 14) were commonly included in subclasses I-III. Motif 1 was present in each CsALMT, and only the CsALMTs in subclasses I and II included motif 17 and motif 12, respectively. However, CsALMTs in subclass II did not contain motif 13, and CsALMTs in subclass III did not contain motif 15.
2.5. Expression Analysis of CsALMTs in Different C. sinensis Tissues
To explore the expression differences in CsALMTs in different tissues of C. sinensis, RT-qPCR was used to analyze the expression patterns of CsALMTs in four different tissues (root, stem, leaf, flower and pollen tube) of C. sinensis. The results show that the expression of 18 CsALMTs had tissue specificity (Figure 3). All 18 CsALMTs were expressed in the root and stem, but CsALMT2, 7, 10, 13 and 15 were not expressed in leaves, CsALMT7 and 11 were not expressed in flowers, and CsALMT3, 6, 7, 11, 12, 13, 14 and 15 were not expressed in pollen tubes. Among the genes which had significantly higher expression levels in the stem than in the root (CsALMT3, 4, 6, 7, 8, 9, 11, 14, 15 and 17), CsALMT3, 6, 9 and 17 and CsALMT3, 8, 9, 14, 15 and 17 also had significantly higher expression levels in leaves and flowers than in the root, respectively. In addition, only CsALMT18 had a significantly higher expression level in pollen tubes than in the root.
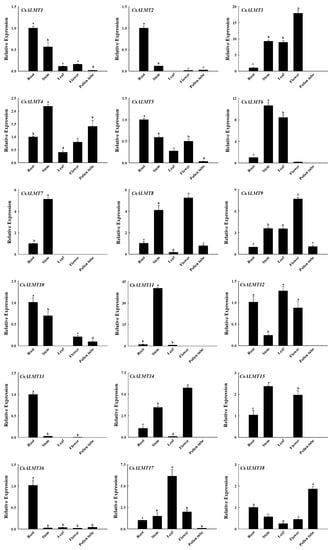
Figure 3.
The expression level of CsALMTs in different tissues of Camellia sinensis. Different lowercase letters represent significant differences among different tissues (n = 3, p < 0.05), as determined by the Duncan test.
2.6. Effect of Al3+ and NO on CsALMTs’ Expression in Pollen Tube
In order to explore the expression level of CsALMTs in C. sinensis pollen tubes under different treatments, RT-qPCR technology was also used to quantitatively analyze C. sinensis pollen tubes.
In Al treatment, Al + NO and Al + cPTIO, the expression level of CsALMT1 and CsALMT8 increased with the increased Al concentration gradient (Figure 4). Compared with Al treatment, cPTIO significantly increased the relative expression level of CsALMT1 (p < 0.05), but had no significant effect on CsALMT8; however, the relative expression levels of CsALMT8 in Al0 + NO and Al1 + NO were significantly higher than those in Al0 and Al1, respectively. In Al treatment and Al + NO, the concentration gradient of Al3+ had no significant effect on the expression level of CsALMT10. However, in Al + cPTIO, the expression level of CsALMT10 increased until the highest expression level under Al2.5 + cPTIO was reached and then decreased. Furthermore, both the NO donor and NO scavenger enhanced the expression level of CsALMT16 with 0 mM and 1 mM Al3+ in the culture medium.
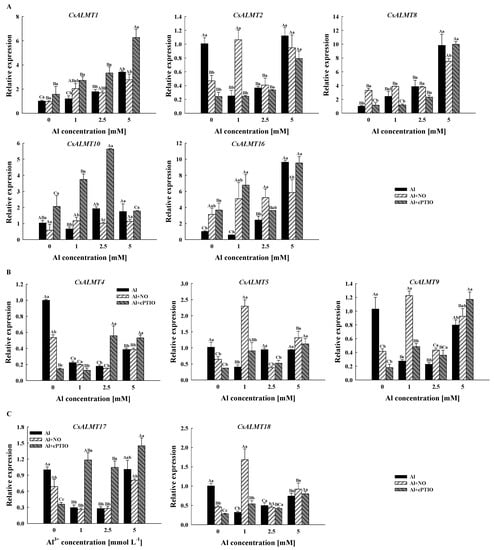
Figure 4.
The relative expression levels of CsALMTs belong to subclass I (A), subclass II (B) and subclass IV (C) in Camellia sinensis pollen tubes under different treatments. Al concentration represents the Al3+ concentration gradient in the culture medium, Al treatment represents the treatment group treated with Al concentration gradient only, Al + NO represents the treatment group treated with Al3+ concentration gradient treatment and 25 μM DEA NONOate (nitric oxide donor), Al + cPTIO represents the treatment group treated with Al3+ concentration gradient treatment and 200 μM carboxy PTIO potassium salt (nitric oxide scavenger). Different lowercase letters represent significant differences among different nitric oxide conditions under the same Al3+ concentration treatment, and different uppercase letters represent significant differences among different Al3+ concentration treatments under the same nitric oxide conditions (n = 3, p < 0.05), as determined by the Duncan test.
The expression levels of CsALMT4, 5 and 9 in subclass II did not show an obvious trend along the Al concentration gradient (Figure 4). However, it should be noted that the expression levels of CsALMT4 and CsALMT9 in Al0 + NO and Al0 + cPTIO were significantly lower than those of Al0. Similar to CsALMT4 and CsALMT9, the expression levels of CsALMT17 and CsALMT18 were significantly lower than those of the control under normal cytoplasmic Al3+ concentration after the addition of the NO donor and NO scavenger. Moreover, the expression levels of CsALMT17 in Al + cPTIO were significantly higher than those of the other two treatment groups after the addition of Al3+. However, for CsALMT18, only the expression level in Al1 + NO was significantly higher than that in Al1 and Al1 + cPTIO.
2.7. Two-Way ANOVA Analysis and Pearson Correlation Coefficients
In order to further clarify the effects of the Al concentration gradient and NO level on physiological and biochemical indices and the CsALMTs expression level in C. sinensis pollen tube, two-way ANOVA was performed (Table 1). One the one hand, the Al concentration gradients and NO levels were highly significantly (p < 0.001), and influenced all eight physiological and biochemical indices. Aside from the cytoplasmic NO concentration, the other seven indices were affected by the Al concentration gradient to a higher degree than the NO level. Furthermore, the combined effects of the Al concentration gradient and NO level on the pollen germination rate and POD activity were not significant. On the other hand, the relative expression levels of CsALMT1, 2, 4, 5, 8, 9, 10, 16, 17 and 18 were all significantly (p < 0.05) affected by Al concentration gradient and NO level. Among them, CsALMT1, 2, 4, 5, 8, 9 and 16 were more influenced by the Al concentration gradient than NO level, while CsALMT10, 17 and 18 were more influenced by the NO level.

Table 1.
Two-way ANOVA test F value of pollen germination rates (PGRs), pollen tube lengths (PTLs), cytoplasmic Al3+ (CAl) and NO (CNO)concentrations, malondialdehyde (MDA) contents, antioxidant enzyme activities and relative expression level of CsALMTs for C. sinensis cv ‘Longjing43’ affected by Al concentration gradient and NO level in the culture medium.
The Pearson correlation analysis was carried out to further explore the role of CsALMTs in NO involved in the process of the C. sinensis pollen tube response to Al stress (Figure 5). The data for the 5 mM Al3+ treatment were eliminated due to the NO level, which had only a weak effect on the indicators of tea pollen tubes under the 5 mM Al3+ treatment. Three levels of NO were assigned: Al + cPTIO treatment = 0, Al treatment = 1 and Al + NO = 2. The expression levels of CsALMT1, CsALMT8 and CsALMT10 in subclass I showed a significant positive correlation with the Al concentration gradient. Among these three CsALMTs in subclass I, CsALMT8 also showed a significant positive correlation with the NO level and cytoplasmic concentration of Al3+ and NO, but a significant negative correlation with the pollen germination rate and pollen tube length. CsALMT1, 10 and 17 showed a significant negative correlation with the NO level, and CsALMT5 and CsALMT17 showed a significant negative correlation with cytoplasmic Al3+ concentration (Figure 5).
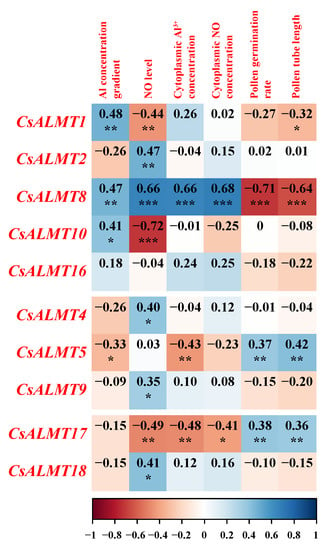
Figure 5.
The modified (the data under 5 mM Al3+ treatment were removed) Pearson correlation coefficients between Al3+ concentration gradient and NO level in the culture medium, cytoplasmic Al3+ and NO concentration, pollen germination rates, pollen tube lengths and CsALMTs expression levels. Significant differences are reported as * (0.01 < p < 0.05), ** (0.001 < p < 0.01) and *** (p < 0.001), as determined by the Duncan test.
3. Discussion
Depending on the timing and level of NO production in response to stress, plants may create NO endogenously and use it to respond in particular ways [,]. When NO concentrations rise under stressful circumstances, it is thought that it can potentially enhance cytotoxicity in addition to acting as a signal molecule [,]. In the present study, exogenous Al3+ significantly inhibited the pollen germination and the pollen tube elongation of C. sinensis in a dose-dependent manner (Figure 1). This result is consistent with the results reported for Al-stressed pollen tubes of apple []. In addition, the exogenous NO donor could inhibit pollen tube growth and enhance the inhibition of Al3+ for the pollen germination rate and pollen tube length, while exogenous cPTIO alleviated this inhibition. On the other hand, exogenous NO donors exacerbated the oxidative damage to Al3+ in pollen tubes, while exogenous NO scavengers lessened the oxidative damage to Al3+ in pollen tubes, as shown by MDA content (Figure 2A). These results, similar to those reported for alfalfa [], wheat [] and rice [], suggest that the high NO level conferred Al sensitivity on pollen tube polar growth in C. sinensis. Moreover, there was no significant difference in cytoplasmic Al3+ concentration, SOD, CAT and POD activities among Al0, Al0 + NO and Al0 + cPTIO (Figure 1 and Figure 2), indicating that the NO level had a weak effect on the absorption of Al3+ by pollen tubes without exogenous Al addition. Furthermore, the encouragement of DEA NONOate and the inhibition of cPTIO on pollen tube absorption of Al3+ steadily increased with the increase in the Al concentration gradient. This suggests that the NO level in the plant growth environment could regulate the absorption of Al3+ by plants, and had a positive correlation, which further indicated that NO could participate in the response of C. sinensis pollen tube extension to Al stress as a negative regulator. Previous research had demonstrated that NO generation and an increase in NOS activity occurred in response to biotic or abiotic stress in plants. For example, according to Wang et al. [], ultrasonography can cause Taxus cells to begin accumulating H2O2 and triggering programmed death, which can be facilitated by sodium nitroprusside (an NO donor), but partially blocked by an NO scavenger (cPTIO) and NOS scavenger (L-NNA). The significant positive correlation between NO concentration and cytoplasmic Al3+ concentration in pollen tubes indicates that the C. sinensis pollen tube is accompanied by NO production under Al stress. On the other hand, the activities of SOD, POD and CAT in pollen tube were significantly increased by the exogenous NO donor, while the activities of these antioxidant enzymes were decreased when cPTIO was added; however, when the Al concentration gradient was increased up to 5 mM, the cytoplasmic Al3+ concentration, SOD, CAT and POD activities decreased significantly in the comparison to when the gradient was 2.5 mM, and there was no significant difference among Al5, Al5 + NO or Al5 + cPTIO (Figure 1 and Figure 2). This seems to imply that (i) excessive NO could activate the antioxidant system of C. sinensis pollen tube, but that it could also cause oxidative stress; (ii) high concentrations of exogenous Al3+ severely inhibit the growth and development of C. sinensis pollen tube, even destroying the antioxidant system, and mask the influence of NO level.
Gene expression analysis is a very important part of gene function research. The expression analysis of the ALMT family in different tissues of rice has been studied. OsALMT1, OsALMT7 and OsALMT9, which belong to subclass I, are expressed in the root of rice []. In this study, CsALMT1, CsALMT2, CsALMT8, CsALMT10 and CsALMT16, which belonged to subclass I, were expressed both in the root and pollen tube (Figure 3), and the expression levels of all except CsALMT2 showed an up-regulated pattern along the Al concentration gradient in Al treatment (Figure 4A). This was consistent with the expression of HbALMT1, HbALMT2 and HbALMT15, which were involved in Al detoxification [], suggesting that the CsALMTs in subclass I might play a role in alleviating Al stress. However, results of Pearson correlation analysis show that CsALMT2 and CsALMT10 only had a significant correlation with the NO level (Figure 5); therefore, we further ruled out the Al detoxification effect of CsALMT2 and CsALMT10 in the C. sinensis pollen tube. In addition, the expression levels of CsALMT1 and CsALMT8 under Al5 + cPTIO were significantly higher than those under other Al + cPTIO treatments, so we speculate that CsALMT1 and CsALMT8 might have synergistic effects under the induction of exogenous cPTIO to jointly alleviate Al stress. In addition, CsALMT8 also showed a significant positive correlation with the NO level, cytoplasmic Al3+ concentration and cytoplasmic NO concentration, but a significant negative correlation with the pollen germination rate and pollen tube length, suggesting that CsALMT8 participated in the response of C. sinensis pollen tube to Al stress as a negative regulator.
On the other hand, except for CsALMTs in subclass II, the expression levels of CsALMT5 in subclass II and CsALMT17 in subclass IV had a significant positive correlation with the pollen germination rate and pollen tube length (Figure 5). CsALMT5 was also positively correlated with the concentration gradient of Al and the concentration of Al3+ in the cytoplasm. In the research of AtALMTs in subclass II, AtALMT6 is aimed at the vacuole membrane of guard cells, which not only serves as the inflow and outflow channel of malate, but also controls the gas exchange; AtALMT9 is a vacuole chlorine channel activated by malate, which plays an important role in the response of plant cells to the stomatal opening signaling pathway and contributes to the transportation of malate through the vacuole membrane []. Thus, we speculate that CsALMT5 might alleviate the Al stress of the C. sinensis pollen tube by mediating the efflux of malate and then chelating or fixing extracellular Al3+. As for CsALMT17, it is not phylogenetically close to Al tolerance ALMT genes with a low response to the Al concentration gradient (Figure S2 and Figure 5). Unexpectedly, CsALMT17 showed a significant negative correlation with cytoplasmic Al3+ concentration. Since CsALMT17 is subcellularly localized outside the cell, we speculate that it may be a secreted protein, which is synthesized by ribosomes on the surface of the endoplasmic reticulum and transported outside the cell. We speculate that Al3+ entering pollen tube cells could slow down the synthesis of CsALMT17 protein by inhibiting the expression of CsALMT17. In addition, the expression level of CsALMT17 was increased by exogenous cPTIO and negatively correlated with the NO level and NO concentration in the cytoplasm, indicating that CsALMT17 was negatively regulated by the cytoplasmic concentration of both Al3+ and NO. However, due to the lack of research on ALMT with extracellular subcellular localization, the relationship between this negative regulatory relationship and the promotion effect of CsALMT17 on pollen germination and pollen tube growth are still unclear. Therefore, the mechanism of NO-mediated CsALMT17 alleviating C. sinensis pollen tube Al toxicity still needs further study.
According to the results of this study, based on the comprehensive study of Al toxicity and tolerance in plants, the fundamental signal regulation network mainly relies on the NO and CsALMTs regulated, and their crosstalk is shown in Figure 6. On the one hand, CsALMT8 localized on the plasma membrane can sense Al3+ outside pollen tubes and promote the uptake of Al3+, and this process is positively regulated by NO signaling molecules. After Al3+ enters the pollen tube, it accelerates the production of ROS, such as superoxide anions (·O2-), and then leads to programmed cell death through lipid peroxidation. These ROS are gradually converted into H2O2 and H2O under the action of SOD, POD and CAT. On the other hand, CsALMT5 is negatively regulated both by the extracellular Al concentration gradient signal and cytoplasmic Al3+ concentration, which may hinder malate efflux from vacuoles, further weaken the chelation and fixation of Al3+ by extracellular malate and finally inhibit the growth of C. sinensis pollen tube. CsALMT17 encodes an extracellular protein that is synthesized and secreted by cells and its expression level was negatively regulated by NO signaling molecules and cytoplasmic Al3+, and can promote the growth of C. sinensis pollen tube. In addition, Al3+ readily reacts with the negatively charged plasma membrane and ultimately disrupts the uptake of mineral ions, such as Ca2+ []. Additionally, it then affects the Ca2+ concentration gradient, which acts as an important factor regulating the polar growth of C. sinensis pollen tubes.
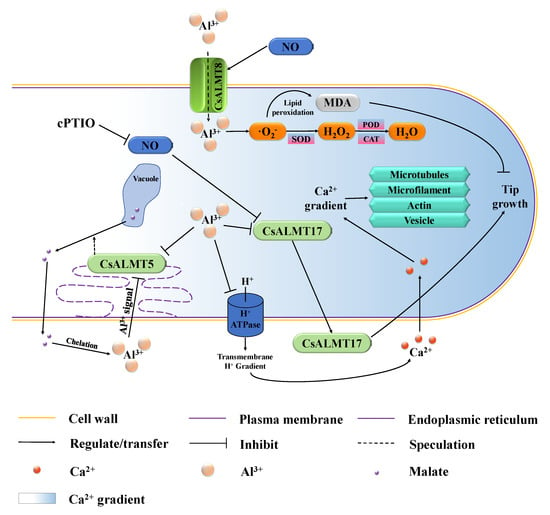
Figure 6.
Potential hypothesis model that nitric oxide (NO) participates in exogenous Al3+ inhibition of C. sinensis pollen tube growth by regulating CsALMTs.
In summary, reducing or eliminating NO in the environment can be an effective way to enhance the Al resistance of C. sinensis; however, the regulatory effect of NO seems to be extremely weak in the case of a high Al3+ concentration in the environment. In addition, CsALMTs also played different roles under Al stress: CsALMT8 was regulated by environmental Al3+ and NO to assist Al3+ entry into pollen tubes, while the up-regulated expression of CsALMT5 could prevent Al3+ from entering pollen tubes by promoting malate efflux. This study also provides a new idea for using exogenous regulation of the NO level as a means to reduce Al content in tea.
4. Materials and Methods
4.1. Pollen Source and Culture
The mature pollen from 5-year-old C. sinensis cv. ‘Longjing43′ was collected on the day before blooms in November 2020. The C. sinensis plantation was located at Sun Yat-sen Tea Factory in Jiangsu Province, China (32°2′57′’ N, 118°50′35′’ E). The detailed information of in vitro pollen culture condition was mainly based on the previous publication with slight modifications []. Briefly, the pre-incubation of pollen was abandoned and directly treated in the dark at 25 °C for 60 min. For normal NO level treatments, the pollen was only treated with four Al concentration gradients (0 mM, 1 mM, 2.5 mM and 5 mM Al2(SO4)3·18H2O), and was marked as Al0, Al1, Al2.5 and Al5, respectively. For high-NO-level treatments, the pollen was treated with 25 µM diethylamine nonoate (DEA NONOate, an NO donor) and the above four Al concentration gradientsand was marked as Al + NO (Al0 + NO, Al1 + NO, Al2.5 + NO and Al5 + NO, respectively. For low-NO-level treatments, the pollen was treated with 200 µM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, an NO scavenger) and the above four Al concentration gradients, and was marked as Al + cPTIO (Al0 + cPTIO, Al1 + cPTIO, Al2.5 + cPTIO and Al5 + cPTIO, respectively. Three biological replicates were performed for each treatment. A portion of the culture solution containing pollen tubes was sucked after the pollen incubation process was complete in order to measure the pollen germination rate and pollen tube length. The remaining pollen tubes were filtered to remove ungerminated pollen grains and culture material using a nylon sieve with a hole size of 0.74 m, frozen in liquid nitrogen and then kept at −80 °C.
4.2. Observation of Pollen Germination Rate and Pollen Tube Elongation
To measure the mean pollen germination rate and pollen tube length, approximately 50 pollen tubes were detected in each of the three replicates after different treatments using a fluorescence microscope (DM6B, Leica, Wetzlar, Germany) and Image J (version 1.8.0, National Institutes of Health, Bethesda, MD, USA).
4.3. Measurement of Cytoplasmic Al3+ and NO
The cytoplasmic Al3+ concentration in the pollen tubes was measured as described by Havlin and Soltanpour with slight modifications []. Briefly, oven-dried and ground 100 mg pollen tube samples were digested with 5 mL of HNO3 and analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES, iCAP 7400, Thermo Fisher, Waltham, MA, USA). The cytoplasmic NO concentration in pollen tubes were determined according to the operating instructions provided in the NO determination kit (Beyotime Biotechnology, Shanghai, China, Cat No. S0023).
4.4. Measurement of Cytoplasmic MDA and Antioxidant Enzyme Activity
The cytoplasmic MDA in pollen tubes was analyzed spectrophotometrically as described by Lei et al. [] with slight modifications. In brief, 0.100 g of fresh sample was homogenized in 1 mL of 10% trichloracetic acid (TCA). Five hundred microliters of the supernatant was mixed with 500 μL of 10% TCA containing 0.5% thiobarbituric acid (TBA), and the mixture was heated in boiling water for 15 min. After rapid cooling, it was then centrifuged at 4 °C for 10 min at 10,000 rpm. The absorbance values were determined spectrophotometrically at 450, 532 and 600 nm by a multidetection microplate reader (CYTATION3, BioTek, Winooski, VT, USA).
For the determination of antioxidant enzyme activities in pollen tubes, 0.1 g of fresh sample was ground with 2 mL of 0.05 mM phosphate buffer (pH 7.8) in the ice bath. After grinding, the homogenate was centrifuged at 4 °C for 30 min at 10,000 rpm, and the supernatant was kept at 4 °C to be tested for enzyme activity.
Superoxide dismutase (SOD) activity was determined by measuring the inhibition of the photochemical reduction on pyrogallol utilizing the pyrogallol autoxidation method by spectrophotometry at 325 nm []. In brief, two test tubes were taken; one was added with 2.98 mL 50 mM Tris-HCl and 0.02 mL 10 mM HCl, and the other contained 2.98 mL 50 mM Tris-HCl, 0.2 mL 10 mM HCl and 50 mM pyrogallol mixture. The absorbance changes (ΔOD) were determined within 4 min by spectrophotometry at 325 nm. The volume of pyrogallol was fixed in the mixture to obtain an OD value at 0.07. In the determination of enzyme activity, 0.01 mL of HCl was replaced with 0.01 mL of enzyme solution, and ΔOD was determined spectrophotometrically at 325 nm for 4 min and recorded every 30 s. The SOD activity was calculated using the following formula:
where ΔOD represents the average change value of OD in the sample tube per minute for 4 min. V represents the total volume of sample extract. V′ represents the total volume of reaction solution. V represents the volume of the sample extract contained in the reaction solution. M represents the fresh weight of the sample used in the sample extract.
Catalase (CAT) activity was determined by decomposition of H2O2 and was measured spectrophotometrically by assessing the decrease in absorbance at 240 nm []. In brief, 3 mL of phosphate buffers, 0.3 mL of enzyme solution, 2 mL of distilled water and 0.6 mL of 0.1 M H2O2 were added to the test tube and immediately shaken. Then, the ΔOD was quickly determined using the colorimetric method at 260 nm. The enzyme solution was replaced with phosphate buffer as the control. ΔOD was determined spectrophotometrically at 325 nm for 4 min and recorded every 30 s. The CAT activity was calculated using the following formula:
where ΔOD represents the average change value of OD in the sample tube per minute over 4 min. V represents the total volume of sample extract. v represents the volume of the sample extract contained in the reaction solution. m represents the fresh weight of the sample used in the sample extract.
Peroxidase (POD) activity was determined by the degree of oxidation of guaiacol by the spectrophotometer at 470 nm []. In brief, 2 mL of phosphate buffers, 1 mL of enzyme solution, 1 mL of 0.05 M guaiacol, and 1 mL of 2% H2O2 were added to the test tube and immediately shaken. ΔOD was determined spectrophotometrically at 470 nm for 4 min and recorded every 30 s. The POD activity was calculated using the following formula:
where ΔOD represents the average change value of OD in the sample tube per minute over 4 min. V represents the total volume of sample extract. v represents the volume of the sample extract contained in the reaction solution. m represents the fresh weight of the sample used in the sample extract.
All the indices of samples, as well as the control experiments were tested with independent replicates.
4.5. Identification of ALMT Family Genes in C. sinensis
The amino acid sequence of C. sinensis genomic coding sequences (http://tpia.teaplant.org/index.html) (accessed on 23 January 2021) was compared with the reported ALMT gene family of A. thaliana (https://www.arabidopsis.org/) (accessed on 23 January 2021) by using Local BLASTp and Bioedit (v8.1.0, Manchester, UK). Furthermore, Pfam protein analysis online services (http://pfam.xfam.org/) (accessed on 23 January 2021) and SMART online services (http://smart.embl-heidelberg.de/) (accessed on 23 January 2021) were used to verify the candidate ALMT genes of C. sinensis, and finally the members of CsALMTs family were identified.
4.6. Bioinformatics Analysis for CsALMTs
ClustalW was used to compare the domains of CsALMTs, AtALMTs and OsALMTs, and MEGA 7.0 was used to construct the phylogenetic tree by using the neighbor-joining method and related parameters (Poisson model, pairwise deletion and 1000 bootstrap replications) []. According to the evolutionary relationship and referring to the classification of AtALMTs sequences, all identified CsALMTs were classified and named. The standardized naming rules were adopted in the present study. Briefly, each of the ALMT gene sequences was named using a prefix, ‘Cs’ (Camellia sinensis), and distinguished by additional numbers, such as CsALMT1, CsALMT2 and soon on.
The basic physical and chemical properties, such as amino acid number and average hydrophobicity of CsALMTs protein sequence in C. sinensis, were predicted by using the ExPASy-ProtParam online services (https://web.expasy.org/protparam/) (accessed on 23 January 2021). Meanwhile, SoftBerry ProtComp 9.0 (http://linux1.softberry.com/berry.phtml) (accessed on 23 January 2021) and TMHMM Server.2.0 (http://www.cbs.dtu.dk/services/TMHMM) (accessed on 23 January 2021) were used to complete subcellular localization of CsALMT proteins and predict their transmembrane domains. SOPMA online services (https://npsa-prabi.ibcp.fr/cgibin/npsa_automat.pl?Page=npsa_sopma.html) (accessed on 23 January 2021) were used to predict the secondary structure of CsALMT proteins.
According to the conserved structure of CsALMTs and the annotation file of the C. sinensis genome (http://www.plantkingdomgdb.com/tea_tree/data/gff3/) (accessed on 23 January 2021), the schematic diagram of CsALMTs gene structure was constructed by using The TBtools online service (https://github.com/CJ-Chen/TBtools) (accessed on 23 January 2021). The MEME online service (http://meme-suite.org/tools/meme) (accessed on 23 January 2021) was used to identify the conserved motif (E-Value < 20) of the CsALMT amino acid sequence, and TBtools was used to construct the structural map of CsALMTs.
4.7. Total RNA Extraction and RT-qPCR Analysis
Total RNA was extracted from pollen tubes as described in item 4.1 of three independent biological replicates for each treatment and different C. sinensis tissues (root, stem, leaf and flower) of 5-year-old C. sinensis cv. ‘Longjing43’ without treatment using EASYspin Plus Complex Plant RNA Kit (Aidlab, Beijing, China, Cat No. RN53) following the manufacturer’s protocol. The quality and integrity of total RNA was checked with reference to the method of Wang et al. []. For RT-qPCR analysis of CsALMTs, HiScript® III RT SuperMix for qPCR with gDNA wiper (Vazyme, Nanjing, China, Cat No. R323-01) was used to synthesize the first-strand cDNA. RT-qPCR analysis was conducted using the ChanQ® SYBR qPCR Master Mix (Vazyme, Nanjing, China, Cat No. Q311-02) with the specific primer pairs shown in Table S4. β-Actin served as a reference gene [].
All the RT-qPCR tests were performed on the Bio-Rad BFX96 fluorescence (Bio-Rad C1000 TouchTM Thermal Cycler, Hercules, CA, USA). Each sample was run in three technical triplicates with three biological replicates. The specificity was confirmed by the melting-curve analysis of the amplified products at the end of the RT-qPCR test. The expression levels of CsALMTs were normalized to the β-actin based on the 2-∆∆Ct method [].
4.8. Statistical Analysis
Data analysis and correlation analysis were performed using SPSS software (SPSS Inc. version 22.0, Chicago, IL, USA, 2013) with Duncan’s test. The data diagrams were drawn with SigmaPlot software (SigmaPlot, version 12.5, Systat Software Inc., San Jose, CA, USA) and R software (R, version 4.1.0, Auckland, New Zealand, 2021).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11172233/s1, Figure S1: The phenotypes of Camellia sinensis pollen tubes under different treatments; Figure S2: Phylogenetic tree of CsALMTs; Figure S3: Gene structure of CsALMTs; Figure S4: Motif analysis of CsALMTs. Table S1: Bioinformatics analysis of ALMT genes in Camellia sinensis; Table S2: Second structure prediction of proteins encoded by ALMT genes in Camellia sinensis; Table S3: Multilevel consensus motifs observed in CsALMTs; Table S4: Primer sequences information for ALMT genes.
Author Contributions
Conceptualization, X.X., Z.T. and Y.W.; methodology, X.X. and Y.W.; validation, A.X., Z.W., X.L. and L.D.; formal analysis, X.X.; investigation, X.X. and Z.T.; resources, Y.W.; data curation, X.X. and Z.T.; writing—original draft preparation, X.X.; writing—review and editing, X.X., Y.Y., J.Y. and Y.W.; visualization, X.X.; supervision, Y.W.; project administration, Y.W.; funding acquisition, Y.Y., J.Y. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31770733, 31972458), the earmarked fund for Jiangsu Agricultural Industry Technology System (JATS [2022]458), Changzhou Science and Technology Support Program (CE20212002) and the Pilot Project of Collaborative Extension Plan of Major Agricultural Technologies in Jiangsu Province (2020-SJ-047-02-1).
Institutional Review Board Statement
Not applicable. The study did not involve humans or animals.
Informed Consent Statement
Not applicable. The study did not involve humans or animals.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its information files).
Acknowledgments
We thank Yuehua Ma (Central Laboratory of College of Horticulture, Nanjing Agricultural University) for assistance in using ICP-OES (iCAP 7400, Thermo Fisher, USA), the multidetection microplate reader (CYTATION3, BioTek, USA) and quantitative real-time PCR (Bio-rad CFX96, USA).
Conflicts of Interest
The authors declare that there are no competing interests.
References
- Dai, B.; Chen, C.; Liu, Y.; Liu, L.; Qaseem, M.F.; Wang, J.; Li, H.; Wu, A.-M. Physiological, biochemical, and transcriptomic responses of neolamarckia cadamba to aluminum stress. Int. J. Mol. Sci. 2020, 21, 9624. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ai, H.; Wei, M.; Qin, C.; Feng, Y.; Ran, S.; Wei, Z.; Niu, H.; Zhu, Q.; Zhu, H.; et al. Distribution and phytotoxicity of soil labile aluminum fractions and aluminum species in soil water extracts and their effects on tall fescue. Ecotoxicol. Environ. Saf. 2018, 163, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Blamey, F.P.C.; Wheeler, D.M.; Christie, R.A.; Edmeades, D.C. Variation in aluminum tolerance among and within lotus lines. J. Plant Nutr. 1990, 13, 745–755. [Google Scholar] [CrossRef]
- Blancaflor, E.B.; Jones, D.L.; Gilroy, S. Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol. 1998, 118, 159–172. [Google Scholar] [CrossRef]
- Hao, H.; Chen, T.; Fan, L.; Li, R.; Wang, X. 2, 6-dichlorobenzonitrile causes multiple effects on pollen tube growth beyond altering cellulose synthesis in Pinus bungeana Zucc. PLOS ONE 2013, 8, e76660. [Google Scholar] [CrossRef]
- Wang, W.D.; Sheng, X.Y.; Shu, Z.F.; Li, D.Q.; Pan, J.T.; Ye, X.L.; Chang, P.P.; Li, X.H.; Wang, Y.H. Combined cytological and transcriptomic analysis reveals a nitric oxide signaling pathway involved in cold-inhibited Camellia sinensis pollen tube growth. Front. Plant Sci 2016, 7, 456. [Google Scholar] [CrossRef]
- Searcy, K.B.; Mulcahy, D.L. Comparison of the response to aluminum toxicity in gametophyte and sporophyte of four tomato (Lycopersicon esculentum Mill.) cultivars. Theor. Appl. Genet. 1990, 80, 289–295. [Google Scholar] [CrossRef]
- Sawidis, T.; Reiss, H.D. Effects of heavy metals on pollen tube growth and ultrastructure. Protoplasma 1995, 185, 113–122. [Google Scholar] [CrossRef]
- Konishi, S.; Ferguson, I.B.; Putterill, J. Effect of acidic polypeptides on aluminium toxicity in tube growth of pollen from tea (Camellia sinensis L.). Plant Sci. 1988, 56, 55–59. [Google Scholar] [CrossRef]
- Fang, K.; Xie, P.; Zhang, Q.; Xing, Y.; Cao, Q.; Qin, L. Aluminum toxicity-induced pollen tube growth inhibition in apple (Malus domestica) is mediated by interrupting calcium dynamics and modification of cell wall components. Environ. Exp. Bot. 2020, 171, 103928. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 2000, 210, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, W.B.; Xu, L.L. Effects of nitric oxide on the germination of wheat seeds and its reactive oxygen species metabolisms under osmotic stress. Acta Bot. Sinica 2003, 45, 901–905. [Google Scholar]
- Tian, X.; Lei, Y. Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol. Plant. 2006, 50, 775–778. [Google Scholar] [CrossRef]
- Shao, R.; Wang, K.; Shangguan, Z. Cytokinin-induced photosynthetic adaptability of Zea mays L. to drought stress associated with nitric oxide signal: Probed by ESR spectroscopy and fast OJIP fluorescence rise. J. Plant Physiol. 2010, 167, 472–479. [Google Scholar] [CrossRef]
- Wang, H.H.; Huang, J.J.; Bi, Y.R. Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci. 2010, 179, 281–288. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wang, H.H.; Wang, X.M.; Bi, Y.R. Nitric oxide enhances aluminum tolerance by affecting cell wall polysaccharides in rice roots. Plant Cell Rep. 2011, 30, 1701–1711. [Google Scholar] [CrossRef]
- Bose, J.; Babourina, O.; Rengel, Z. Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 2011, 62, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. PlJ 2004, 37, 645–653. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, M.; Delhaize, E.; Ryan, R.P. Altered expression of a Malate-Permeable anion channel, OsALMT4, disrupts mineral nutrition. Plant Physiol. 2017, 175, 1745–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoekenga, O.A.; Maron, L.G.; Piñeros, M.A.; Cançado, G.M.A.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef] [PubMed]
- Ligaba, A.; Katsuhara, M.; Ryan, P.R.; Shibasaka, M.; Matsumoto, H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006, 142, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Piñeros, M.A.; Tian, J.; Yao, Z.; Sun, L.; Liu, J.; Shaff, J.; Coluccio, A.; Kochian, L.V.; Liao, H. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 2013, 161, 1347–1361. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, K.H.; Wang, P.; Yi, J.; Li, K.Z.; Yu, Y.X.; Chen, L.M. Overexpression of MsALMT1, from the aluminum-sensitive medicago sativa, enhances malate exudation and aluminum resistance in Tobacco. Plant Mol. Biol. Report. 2013, 31, 769–774. [Google Scholar] [CrossRef]
- Xu, L.; Qiao, X.; Zhang, M.; Zhang, S. Genome-Wide analysis of aluminum-activated malate transporter family genes in six rosaceae species, and expression analysis and functional characterization on malate accumulation in Chinese white pear. Plant Sci. 2018, 274, 451–465. [Google Scholar] [CrossRef]
- Puyaubert, J.; Baudouin, E. New clues for a cold case: Nitric oxide response to low temperature. Plant Cell Environ. 2014, 37, 2623–2630. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Yu, Y.; Liu, W.; Lu, L.; Jin, C.; Lin, X. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J. Integr. Plant Biol. 2015, 57, 550–561. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef]
- Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 2012, 166, 136–143. [Google Scholar] [CrossRef]
- Chen, M.; Cui, W.; Zhu, K.; Xie, Y.; Zhang, C.; Shen, W. Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J. Hazard. Mater. 2014, 267, 40–47. [Google Scholar] [CrossRef]
- Sun, C.; Lu, L.; Yu, Y.; Liu, L.; Hu, Y.; Ye, Y.; Jin, C.; Lin, X. Decreasing methylation of pectin caused by nitric oxide leads to higher aluminium binding in cell walls and greater aluminium sensitivity of wheat roots. J. Exp. Bot. 2016, 67, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, X.Y.; Chen, L.Q.; Yang, J.L.; Zheng, S.J. Nitric oxide exacerbates Al-induced inhibition of root elongation in rice bean by affecting cell wall and plasma membrane properties. Phytochemistry 2012, 76, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Zheng, L.P.; Wu, J.Y.; Tan, R.X. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide 2006, 15, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; An, F.; Wang, L.; Guo, D.; Xie, G.; Liu, Z. Genome-wide identification of Aluminum-Activated Malate Transporter (ALMT) gene family in rubber trees (Hevea brasiliensis) highlights their involvement in aluminum detoxification. Forests 2020, 11, 142. [Google Scholar] [CrossRef]
- Zhang, W.H.; Rengel, Z. Aluminium induces an increase in cytoplasmic calcium in intact wheat root apical cells. Funct. Plant Biol. 1999, 26, 401–409. [Google Scholar] [CrossRef]
- Havlin, J.L.; Soltanpour, P.N. A nitric acid plant tissue digest method for use with inductively coupled plasma spectrometry. Commun. Soil Sci. Plant Anal. 1980, 11, 969–980. [Google Scholar] [CrossRef]
- Lei, Y.; Korpelainen, H.; Li, C. Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere 2007, 68, 686–694. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Qin, G.; Wang, H.; Zhu, Y.; Zhang, K.; Yang, H. Growth, accumulation, and antioxidative responses of two Salix genotypes exposed to cadmium and lead in hydroponic culture. Environ. Sci. Pollu. Res. 2019, 26, 19770–19784. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Song, Y.; Cui, H.; Shi, Y.; Xue, J.; Ji, C.; Zhang, C.; Yuan, L.; Li, R. Genome-wide identification and functional characterization of the Camelina sativa WRKY gene family in response to abiotic stress. BMC Genom. 2020, 21, 786. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhu, X.; Shen, J.; Xing, H.; Zou, Z.; Ma, Y.; Wang, Y.; Fang, W. Genome-wide identification, characterization and expression analysis of the amino acid permease gene family in tea plants (Camellia sinensis). Genomics 2020, 112, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).