Potential Use of Agave Genus in Neuroinflammation Management
Abstract
1. Introduction
2. Methodology
2.1. Search Planning
2.2. Study Selection
2.3. Data Extraction
3. Pharmacological Background of Agave Species
3.1. Antioxidant Activity
3.2. Anti-Inflammatory and Immunomodulatory Activities
3.3. Anti-Cancer and Anti-Bacterial Activities
3.4. Other Activities of Agave Genus
4. Natural Products from Agave Species
4.1. Phytosteroles
4.1.1. β-Sitosterol and Neuroinflammation
4.1.2. Agave β-Sitosterol
4.2. Flavonoids
4.2.1. Flavonoids and Neuroinflammation
4.2.2. Agave Flavonoids
4.3. Terpenes
4.3.1. Terpenes and Neuroinflammation
4.3.2. Agave Terpenes
4.4. Saponins
4.4.1. Saponins from Agave Species
4.4.2. Saponins and Neuroinflammation
5. Neuroinflammation
Neuroinflammatory Molecules
6. CNS Cells
6.1. Blood–Brain Barrier (BBB)
6.2. Microglia
6.3. Astrocytes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pérez, A.J.; Calle, J.M.S.; Simonet, A.M.; Guerra, J.O.; Stochmal, A.; Macías, F.A. Bioactive steroidal saponins from Agave offoyana flowers. Phytochemistry 2013, 95, 298–307. [Google Scholar] [CrossRef] [PubMed]
- García-Mendoza, A.; Galván, R. Riqueza de las familias Agavaceae y Nolinaceae en México. Bol. Soc. Bot. México. 1995, 56, 7–24. [Google Scholar] [CrossRef]
- López-Romero, J.C.; Ayala-Zavala, J.F.; Peña-Ramos, E.A.; Hernández, J.; González-Ríos, H. Antioxidant and antimicrobial activity of Agave angustifolia extract on overall quality and shelf life of pork patties stored under refrigeration. J. Food Sci. Technol. 2018, 55, 4413–4423. [Google Scholar] [CrossRef] [PubMed]
- Statista. Mexico: Distilled Agave-Based Beverages Production Value 2013–2020. 2022. Available online: https://www.statista.com/statistics/717349/distilled-Agave-based-beverage-production-value-in-mexico/ (accessed on 16 May 2022).
- Xiong, L.; Maki, M.; Guo, Z.; Mao, C.; Qin, W. Agave biomass is excellent for production of bioethanol and xylitol using Bacillus strain 65S3 and Pseudomonas strain CDS3. J. Biobased Mater. Bioenergy 2014, 8, 422–428. [Google Scholar] [CrossRef]
- Bermúdez-Bazán, M.; Castillo-Herrera, G.A.; Urias-Silvas, J.E.; Escobedo-Reyes, A.; Estarrón-Espinosa, M. Hunting bioactive molecules from de Agave genus: An update on extraction and biological potential. Molecules 2021, 26, 6789. [Google Scholar] [CrossRef]
- Wang, J.; Chio, C.; Chen, X.; Su, E.; Cao, F.; Jin, Y.; Qin, W. Efficient saccharification of Agave biomass using Aspergillus niger produced low-cost enzyme cocktail with hyperactive pectinase activity. Bioresour. Technol. 2018, 272, 26–33. [Google Scholar] [CrossRef]
- Blomberg, L. Tequila, Mezcal y Pulque: Lo Auténtico Mexicano, 1st ed.; Planeta Mexicana, S.A. de C.V. of México: Mexico City, Mexico, 2000; pp. 23–57. ISBN 9681332695. [Google Scholar]
- Monroy, C.; Castillo, P. Plantas Medicinales Utilizadas en el Estado de Morelos, 1st ed.; Universidad Autónoma del Estado de Morelos: Cuernavaca, Mexico, 2007; pp. 265–266. ISBN 968-878-277-7. [Google Scholar]
- Lozoya, X.; Lozoya, M. Flora Medicinal de Mexico. Volume 1: Plantas Indígenas; Instituto Mexicano del Seguro Social Mexico, D.F., Ed.; Instituto Mexicano del Seguro Social Mexico D.F.: Torreon, Coahuila, Mexico, 1982; pp. 32–48. [Google Scholar]
- Ahumada-Santos, Y.P.; Montes-Avila, J.; Uribe-Beltrán, M.J.; Díaz-Camacho, S.P.; López-Angulo, G.; Vega-Aviña, R.; Delgado-Vargas, F. Chemical characterization, antioxidant and antibacterial activities of six Agave species from Sinaloa, Mexico. Ind. Crops Prod. 2013, 49, 143–149. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Rosas-Pérez, A.M.; Leal-Díaz, A.M.; Gutiérrez-Uribe, J.A. Variability in Saponin Content, Cancer Antiproliferative Activity and Physicochemical Properties of Concentrated Agave Sap. J. Food Sci. 2016, 81, H2069–H2075. [Google Scholar] [CrossRef]
- Monterrosas-Brisson, N.; Ocampo, M.L.; Jiménez-Ferrer, E.; Jiménez-Aparicio, A.R.; Zamilpa, A.; González-Cortazar, M.; Tortoriello, J.; Herrera-Ruiz, M. Anti-inflammatory activity of different Agave plants and the compound cantalasaponin-1. Molecules 2013, 18, 8136–8146. [Google Scholar] [CrossRef]
- Hernández-Valle, E.; Herrera-Ruiz, M.; Salgado, G.R.; Zamilpa, A.; Ocampo, M.L.; Aparicio, A.R.; Tortoriello, J.; Jiménez-Ferrer, E. Anti-inflammatory effect of 3-O-[(6’-O-Palmitoyl)-β-Dglucopyranosyl Sitosterol]), from Agave angustifolia on ear edema in mice. Molecules 2014, 19, 15624–15637. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Jiménez-Ferrer, E.; Tortoriello, J.; Zamilpa, A.; Alegría-Herrera, E.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Martínez-Duncker, I.; Monterrosas-Brisson, N. Anti-neuroinflammatory effect of Agaves and cantalasaponin-1 in a model of LPS-induced damage. Nat. Prod. Res. 2019, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- García Mendoza, A.J. Ficha técnica de Agave ornithobroma. In Revisión de las Agavaceae (sensu stricto), Crassulaceae y Liliaceae Incluidas en el PROY-NOM-059-ECOL-2000; Bases de datos SNIB-CONABIO. Proyecto No. W020; Jardín Botánico, Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2003; pp. 1–4. [Google Scholar]
- González-Elizondo, M.; Galván-Villanueva, R.; López-Enríquez, I.; Reséndiz-Rojas, L.; González-Elizondo, M.S. Agaves Magueyes, Lechuguillas y Notas del Estado de Durango y sus Alrededores, 1st ed.; CIIDIR Unidad Durango, Instituto Politécnico Nacional: Mexico City, Mexico, 2009; pp. 1–159. ISBN 978-970-95117-1-8. [Google Scholar]
- Argueta, V.A.; Cano, L.M.; Rodarte, M.E. Atlas de las Plantas de la Medicina Tradicional Mexicana, Volume I, II y III; Instituto Nacional Indigenista: Mexico City, Mexico, 1994; p. 1786. ISBN 9682961327. [Google Scholar]
- García-Regalado, G. Plantas Medicinales de Aguascalientes, 2nd ed.; Universidad Autónoma de Aguascalientes Mexico: Aguascalientes, Mexico, 2015; pp. 266–276. ISBN 978-607-8359-83-7. [Google Scholar]
- Pérez-Zavala, M.L.; Hernández-Arzaba, J.C.; Bideshi, D.K.; Barboza-Corona, J.E. Agave: A natural renewable resource with multiple applications. J Sci. Food Agric. 2020, 100, 5324–5333. [Google Scholar] [CrossRef]
- Rendón-Salcido, L.A.; Colunga-GarcíaMarín, P.; Barahona-Pérez, L.F.; Pimienta-Barrios, E.; Magdub-Méndez, A.; Larqué-Saavedra, A. Sugars and alcoholic by products from henequén (Agave fourcroydes) as influenced by plant age and climate. Rev. Fitotec. Mex. 2009, 32, 39–44. [Google Scholar]
- United States Department of Agriculture. Plants Database. Available online: https://plants.usda.gov/home/plantProfile?symbol=AGUT (accessed on 3 June 2022).
- Southwest Desert Flora. “Plants Profile: Agave Schottii Engelm. Schott’s Century Plant”. Available online: http://southwestdesertflora.com/WebsiteFolders/All_Species/Agavaceae/Agave%20schottii,%20Schott’s%20Century%20Plant.html (accessed on 4 May 2022).
- Cortés, A.J.; Sánchez-Mendoza, E.; Zamilpa, A.; González-Cortazar, M.; Herrera-Ruiz, M.; Almanza-Pérez, J.C.; Terán-Cabanillas, E.; Renaud, C.; Domínguez-Ramírez, L.; Montiel-Arcos, E.; et al. Steroidal saponin from Agave marmorata Roezl modulates inflammatory response by inhibiting NF-κB and AP-1. Nat. Prod. Res. 2020, 36, 1–6. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Gutiérrez-Mora, A.; García-Lara, S. Micropropagation of Agave salmiana: Means to production of antioxidant and bioactive principles. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Ben-Hamissa, A.M.; Seffen, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Converti, A. Phenolics extraction from Agave americana (L.) leaves using high-temperature, high-pressure reactor. Food Bioprod. Process. 2012, 90, 17–21. [Google Scholar] [CrossRef]
- López, M.; Mancilla, N.; Mendoza, G. Molecular structures of fructans from Agave tequilana Weber var azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef] [PubMed]
- Arreola-Vargas, J.; Ojeda-Castillo, V.; Snell-Castro, R.; Corona-González, R.I.; Alatriste-Mondragón, F.; Méndez-Acosta, H.O. Methane production from acid hydrolysates of Agave tequilana bagasse: Evaluation of hydrolysis conditions and methane yield. Bioresour. Technol. 2015, 181, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Gutiérez-Nava, Z.J.; Jiménez-Aparicio, A.R.; Herrera-Ruiz, M.L.; Jiménez-Ferrer, E. Immunomodulatory effect of Agave tequilana evaluated on an autoimmunity like-SLE model induced in Balb/c mice with pristane. Molecules 2017, 22, 848. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; Gutiérrez-Nava, Z.J.; Trejo-Moreno, C.; Zamilpa, A.; González-Cortazar, M.; Jiménez-Aparicio, A.; Jiménez-Ferrer, E. Agave tequilana counteracts chronic hypertension and associated vascular damage. J. Med. Food. 2021, 25, 443–455. [Google Scholar] [CrossRef]
- García, M.D.; Quílez, A.M.; Sáenz, M.T.; Martínez-Domínguez, M.E.; de la Puerta, R. Anti-inflammatory activity of Agave intermixta Trel. and Cissus sicyoides L., species used in the Caribbean traditional medicine. J. Ethnopharmacol. 2000, 71, 395–400. [Google Scholar] [CrossRef]
- Peana, A.T.; Moretti, M.D.; Manconi, V.; Desole, G.; Pippia, P. Anti-inflammatory activity of aqueous extracts and steroidal sapogenins of Agave americana. Planta Med. 1997, 63, 199–202. [Google Scholar] [CrossRef]
- Yokosuka, A.; Mimaki, Y.; Kuroda, M.; Sashida, Y. A new steroidal saponin from the leaves of Agave americana. Planta Med. 2000, 66, 393–396. [Google Scholar] [CrossRef]
- Jin, J.M.; Liu, X.K.; Yang, C.R. A new C-27 steroidal saponins from fermented leaves of Agave Americana. Zhongguo Zhong Yao Za Zhi 2002, 27, 431–434. [Google Scholar]
- Jin, J.M.; Zhang, Y.J.; Yang, C.R. Four new steroid constituents from the waste residue of fibre separation from Agave americana leaves. Chem. Pharm. Bull. 2004, 52, 654–658. [Google Scholar] [CrossRef]
- Nasri, S.; Ben-Salem, H. Effect of oral administration of Agave americana or Quillaja saponaria extracts on digestion and growth of Barbarine female lamb. Livest. Sci. 2012, 147, 59–65. [Google Scholar] [CrossRef]
- Quintans, J.S.; Barreto, R.S.; de Luca, W., Jr.; Villarreal, C.F.; Kaneto, C.M.; Soares, M.B.; Branco, A.; Almeida, J.R.; Taranto, A.G.; Antoniolli, A.R.; et al. Evidence for the involvement of spinal cord-inhibitory and cytokines-modulatory mechanisms in the anti-hyperalgesic effect of hecogenin acetate, a steroidal sapogenin-acetylated, in mice. Molecules 2014, 19, 8303–8316. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.K.; Varma, S.K.; Kumar, R. Anti-inflammatory effect of an extract of Agave americana on experimental animals. Pharmacogn. Res. 2018, 10, 104–108. [Google Scholar]
- Tinto, W.F.; Simmons-Boyce, J.L.; McLean, S.; Reynolds, W.F. Constituents of Agave americana and Agave barbadensis. Fitoterapia 2005, 76, 594–597. [Google Scholar] [CrossRef]
- Simmons-Boyce, J.L.; Tinto, W.F.; McLean, S.; Reynolds, W.F. Saponins from Furcraea selloa var. marginata. Fitoterapia 2004, 75, 634–638. [Google Scholar] [CrossRef]
- Allsopp, P.; Possemiers, S.; Campbell, D.; Oyarzábal, I.S.; Gill, C.; Rowland, I. An exploratory study into the putative prebiotic activity of fructans isolated from Agave angustifolia and the associated anticancer activity. Anaerobe 2013, 22, 38–44. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Koyano, T.; Kowithayakorn, T.; Sakai, S.; Kawahara, N.; Goda, Y.; Yamaguchi, N.; Ishibashi, M. New chlorogenin hexasaccharide isolated from Agave fourcroydes with cytotoxic and cell cycle inhibitory activities. Bioorg. Med. Chem. 2004, 12, 3841–3845. [Google Scholar] [CrossRef]
- Yokosuka, A.; Jitsuno, M.; Yui, S.; Yamazaki, M.; Mimaki, Y. Steroidal glycosides from Agave utahensis and their cytotoxic activity. J. Nat. Prod. 2009, 72, 1399–1404. [Google Scholar] [CrossRef]
- Yokosuka, A.; Mimaki, Y. Steroidal saponins from the whole plants of Agave utahensis and their cytotoxic activity. Phytochemistry 2009, 70, 807–815. [Google Scholar] [CrossRef]
- Fujino, T.; Yokosuka, A.; Higurashi, H.; Yokokawa, R.; Sakurai, R.; Harashima, W.; Miki, Y.; Fujiwara, Y.; Mimaki, Y.; Hayakawa, M. AU-1 from Agavaceae plants causes transient increase in p21/Cip1 expression in renal adenocarcinoma ACHN cells in an miR-34-dependent manner. J. Nat. Med. 2016, 71, 36–43. [Google Scholar] [CrossRef]
- Bianchi, E.; Cole, J.R. Antitumor Agents from Agave schottii (Amaryllidaceae). J. Pharm. Sci. 1969, 58, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Escobosa, A.R.; Gomez-Ojeda, A.; Wrobel, K.; Alcazar-Magana, A.; Wrobel, K. Methylglyoxal is associated with bacteriostatic activity of high fructose Agave syrups. Food Chem. 2014, 165, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Anajwala, C.C.; Patel, R.M.; Dakhara, S.L.; Jariwala, J.K. In vitro cytotoxicity study of Agave americana, Strychnos nuxvomica and Areca catechu extracts using mcf-7 cell line. J. Adv. Pharm. Technol. Res. 2010, 1, 245–252. [Google Scholar] [PubMed]
- Padilla-Camberos, E.; Barragán-Álvarez, C.P.; Díaz-Martínez, N.E.; Rathod, V.; Flores-Fernández, J.M. Effects of Agave fructans (Agave tequilana Weber var. azul) on Body Fat and Serum Lipids in Obesity. Plant Foods Hum. Nutr. 2018, 73, 34–39. [Google Scholar] [CrossRef]
- Mwale, M.; Masika, P.J. In vivo anthelmintic efficacy of Aloe ferox, Agave sisalana, and Gunnera perpensa in village chickens naturally infected with Heterakis gallinarum. Trop. Anim. Health Prod. 2015, 47, 131–138. [Google Scholar] [CrossRef]
- Santos-Cerqueira, G.; dos Santos-e Silva, G.; Rios-Vasconcelos, E.; Fragoso-de Freitas, A.P.; Arcanjo-Moura, B.; Silveira-Macedo, D.; Lopes-Souto, A.; Barbosa-Filho, J.M.; deAlmeida-Leal, L.K.; de Castro-Brito, G.A.; et al. Effects of hecogenine and its possible mechanism of action on experimental models of gastric ulcer in mice. Eur. J. Pharmacol. 2012, 683, 260–269. [Google Scholar] [CrossRef]
- BritoCortez-Lima, N.; DantasCavalcante-Abreu, R.N.; Barbosa-Filho, J.M.; Leal, L.K.A.M.; Ribeiro-Honório-Júnior, J.E.; Camelo-Chaves, E.M.; Magalhaes-de Brito, E.; Martins-Dias, L.; Miranda-Sousa, A.; Mendes-Vasconcelos, S.M. P.2.d.013. Evaluation of the action mechanism of hecogenin’s antidepressant effect from Agave sisalana Perrine in mice. Eur. Neuropsychopharmacol. 2012, 22, S275. [Google Scholar] [CrossRef]
- Durán, A.G.; Benito, J.; Macías, F.A.; Simonet, A.M. Agave steroidal saponins as potential bioherbicides. Agronomy 2021, 11, 2404. [Google Scholar] [CrossRef]
- Maazoun, A.M.; Hamdi, S.H.; Belhadj, F.; Jemâa, J.M.B.; Messaoud, C.; Marzouki, M.N. Phytochemical profile and insecticidal activity of Agave americana leaf extract towards Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Environ. Sci. Poll. Res. Int. 2019, 26, 19468–19480. [Google Scholar] [CrossRef]
- Yang, C.R.; Zhang, Y.; Jacob, M.R.; Khan, S.I.; Zhang, Y.J.; Li, X.C. Antifungal Activity of C-27 Steroidal Saponins. Antimicrob. Agents Chemother. 2006, 50, 1710–1714. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. Β-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. BioMed Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, J.C.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Peña-Ramos, E.A.; González-Ríos, H. Biological activities of Agave by-products and their possible applications in food and pharmaceuticals. J. Sci. Food Agric. 2017, 98, 2461–2474. [Google Scholar] [CrossRef] [PubMed]
- Morreeuw, Z.P.; Escobedo-Fregoso, C.; Ríos-González, L.J.; Castillo-Quiroz, D.; Reyes, A.G. Transcriptome-based metabolic profiling of flavonoids in Agave lechuguilla waste biomass. Plant Sci. 2021, 305, 110748. [Google Scholar] [CrossRef]
- Shegute, T.; Wasihun, Y. Antibacterial Activity and Phytochemical Components of Leaf Extracts of Agave americana. J. Ex. Pharmacol. 2020, 12, 447–454. [Google Scholar] [CrossRef]
- Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; Hernández-Vargas, V.; Ortega-Chávez, M.; Orea-Lara, G.; Leon, A.C.; Avila-Reyes, J.A.; Muniz-Martinez, R. Profiling of phenolic compounds of somatic and reproductive tissues of Agave durangensis Gentry (Agavaceae). Am. J. Appl. Sci. 2009, 6, 1076–1085. [Google Scholar]
- Mulati, A.; Zhang, X.; Zhao, T.; Ren, B.; Wang, L.; Liu, X.; Lan, Y.; Liu, X. Isorhamnetin attenuates high-fat and high-fructose diet induced cognitive impairments and neuroinflammation by mediating MAPK and NFκB signalling pathways. Food Funct. 2021, 12, 9261–9272. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jin, C.Y.; Kim, C.H.; Yoo, Y.H.; Choi, S.H.; Kim, G.Y.; Yoon, H.M.; Park, H.T.; Choi, Y.H. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-κB, blocking the TLR4 pathway and reducing ROS generation. Int. J Mol. Med. 2019, 43, 682–692. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, H.; Jiang, Z.; Li, X.; Jiao, K.; Jia, X.; Hou, Y.; Li, N. Natural potential neuroinflammatory inhibitors from Alhagi sparsifolia Shap. Bioorg. Med. Chem. Lett. 2017, 27, 973–978. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein-Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Kim, J.; Wie, M.B.; Ahn, M.; Tanaka, A.; Matsuda, H.; Shin, T. Benefits of hesperidin in central nervous system disorders: A review. Anat. Cell Biol. 2019, 52, 369–377. [Google Scholar] [CrossRef]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Haghmorad, D.; Mahmoudi, M.B.; Salehipour, Z.; Jalayer, Z.; Momtazi-Brojeni, A.A.; Rastin, M.; Kokhaei, P.; Mahmoudi, M. Hesperidin ameliorates immunological outcome and reduces neuroinflammation in the mouse model of multiple sclerosis. J Neuroimmunol. 2017, 302, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.O.; Çelik, H.; Caglayan, C.; Kandemir, F.M.; Gür, C.; Bayav, İ.; Genç, A.; Kandemir, Ö. Neuromodulatory effects of hesperidin against sodium fluoride-induced neurotoxicity in rats: Involvement of neuroinflammation, endoplasmic reticulum stress, apoptosis and autophagy. Neurotoxicology 2022, 90, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Zhu, L.L.; Ju, K.F.; Liu, J.L.; Li, K.P. Anti-inflammatory effect of delphinidin on intramedullary spinal pressure in a spinal cord injury rat model. Exp. Ther. Med. 2017, 14, 5583–5588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharmacol. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Protective Effects of Quercetin on Anxiety-Like Symptoms and Neuroinflammation Induced by Lipopolysaccharide in Rats. Evid. Based Complement Alternat. Med. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.D.S.; de Almeida, M.M.A.; Bispo-da Silva, A.; Dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; da Silva, J.S.; Souza, D.O.; Costa, M.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated With Alzheimer’s Disease. Front Aging Neurosci. 2020, 12, 119–133. [Google Scholar] [CrossRef]
- Patel, M.; Singh, S. Apigenin Attenuates Functional and Structural Alterations via Targeting NF-kB/Nrf2 Signaling Pathway in LPS-Induced Parkinsonism in Experimental Rats: Apigenin Attenuates LPS-Induced Parkinsonism in Experimental Rats. Neurotox. Res. 2022, 40, 941–960. [Google Scholar] [CrossRef]
- Tang, Y.; Xiong, R.; Wu, A.G.; Yu, C.L.; Zhao, Y.; Qiu, W.Q.; Wang, X.L.; Teng, J.F.; Liu, J.; Chen, H.X.; et al. Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation. Int. J Mol. Sci. 2018, 19, 2109. [Google Scholar] [CrossRef] [PubMed]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2018, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Qin, Q.; Wang, Y.; Xu, J.; He, X. Pronounced anti-neuroinflammatory jasmonates and terpenes isolated from lychee seeds. Fitoterapia 2021, 152, 104924. [Google Scholar] [CrossRef] [PubMed]
- Peña-Alvarez, A.; Díaz, L.; Medina, A.; Labastida, C.; Capella, S.; Vera, L.E. Characterization of three Agave species by gas chromatography and solid-phase microextraction–gas chromatography–mass spectrometry. J Chromatogr. A 2004, 1027, 131–136. [Google Scholar] [CrossRef]
- Savage, G.P. Saponins. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L., Finglas, P.M., Eds.; Academic Press: London, UK, 2003; pp. 5095–5097. [Google Scholar]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef]
- Jones, H.D.; Furbeck, G.N.; Colorado, N. Isolation and study of the saponin content of the juice and leaf of the Agave plant, Maguey, manso fino. J. Am. Pharm. Assoc. 1932, 21, 787–793. [Google Scholar]
- Marker, R.E.; Wagner, R.B.; Ulshafer, P.R.; Wittbecker, E.L.; Goldsmith, D.P.J.; Ruo, C.H. Isolation and structures of thirteen new steroidal sapogenins. New sources for known sapogenins. J. Am. Chem. Soc. 1943, 65, 1199–1209. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, B.; Gutiérrez-Uribe, J.A.; Guajardo-Flores, D.; Santos-Zea, L. Microencapsulation of steroidal saponins from Agave sap concentrate using different carriers in spray drying. Food sci. Technol. Int. 2021, 10820132211049949, Advance online publication. [Google Scholar] [CrossRef]
- Simonet, A.M.; Durán, A.G.; Pérez, A.J.; Macías, F.A. Features in the NMR spectra of the aglycones of Agave spp. saponins. HMBC method for aglycone identification (HMAI). Phytochem. Anal. 2021, 32, 38–61. [Google Scholar] [CrossRef]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of Agave: Chemistry and bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Araújo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; Araújo, A.; Almeida, J.; Thangaraj, P.; Júnior, L.; Quintans, J. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: A review of pre-clinical research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Xu, X.; Lin, J.; Cui, X.; Xu, R. Neuroprotection by Saponins. Phytother. Res. 2014, 29, 187–200. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Higashi, M.; Kobayashi, H. Effects of ginsenosides on maze performance and brain choline acetyltransferase activity in scopolamine treated young rats and aged rats. Eur. J. Pharmacol. 1997, 329, 37–41. [Google Scholar] [CrossRef]
- Jin, S.H.; Park, J.K.; Nam, K.Y.; Park, S.N.; Jung, N.P. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine induced learning disability and spatial working memory in mice. J. Ethnopharmacol. 1999, 66, 123–129. [Google Scholar] [CrossRef]
- Benishin, C.G.; Lee, R.; Wang, L.C.; Liu, H.J. Effects of ginsenoside Rbl on centralcholinergic metabolism. Pharmacology 1991, 42, 223–229. [Google Scholar] [CrossRef]
- Wu, C.F.; Bi, X.L.; Yang, J.Y.; Zhan, J.Y.; Dong, Y.X.; Wang, J.H.; Wang, J.M.; Zhang, R.; Li, X. Differential effects of ginsenosides on NO and TNF–alpha production by LPS-activated N9 microglia. Int. J. Immunopharmacol. 2007, 7, 313–320. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, Z.; Zhou, X.; He, W.; Chen, C.; Luo, P.; Liu, D.D.; Ai, Q.D.; Gong, H.F.; Wang, Z.Z.; et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol. Sin. 2018, 40, 13–25. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Liu, X.; Zhang, C.; Shang, W.; Xue, J.; Chen, R.; Xing, Y.; Song, D.; Xu, R. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur. J. Pharmacol. 2019, 856, 172418. [Google Scholar] [CrossRef]
- Lee, K.W.; Jung, S.Y.; Choi, S.M.; Yang, E.J. Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement. Altern. Med. 2012, 12, 196–203. [Google Scholar] [CrossRef]
- Cai, M.; Yang, E.J. Ginsenoside Re Attenuates Neuroinflammation in a Symptomatic ALS Animal Model. Am. J. Chin. Med. 2016, 44, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Wang, J.; Zhao, S.; Zhang, K.; Wang, J.; Zhang, W.; Wu, C.; Yang, J. Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4mediated TAK1IKKNF-κB, MAPKs and Akt signaling pathways. Neuropharmacology 2014, 79, 642–656. [Google Scholar] [CrossRef]
- Su, X.D.; Jang, H.J.; Wang, C.Y.; Lee, S.W.; Rho, M.C.; Kim, Y.H.; Yang, S.Y. Anti-inflammatory Potential of Saponins from Aster tataricus via NF-κBMAPK. J. Nat. Prod. 2019, 82, 1139–1148. [Google Scholar]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548–552. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Shubhakaran, K.P.; Chin, J.H. The global burden of neurologic diseases. Neurology. 2015, 84, 758. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R. Molecular Basis of Etiological Implications in Alzheimer’s Disease: Focus on Neuroinflammation. Mol. Neurobiol. 2013, 48, 412–428. [Google Scholar] [CrossRef]
- Niranjan, R.; Nagarajan, R.; Hanif, K.; Nath, C.; Shukla, R. LPS induces mediators of neuroinflammation, cell proliferation, and GFAP expression in human astrocytoma cells U373MG: The anti-inflammatory and anti-proliferative effect of guggulipid. Neurol. Sci. 2014, 35, 409–414. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2016, 127, 624–633. [Google Scholar] [CrossRef]
- Woodroofe, M.N. Cytokine production in the central nervous system. Neurology 1995, 45, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Na, K.S.; Myint, A.M.; Leonard, B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016, 64, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Pitossi, F.; del Rey, A.; Kabiersch, A.; Besedovsky, H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J. Neurosci. Res. 1997, 48, 287–298. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Koper, O.M.; Kamińska, J.; Sawicki, K.; Kemona, H. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv. Clin. Exp. Med. 2018, 27, 849–856. [Google Scholar] [CrossRef]
- Miguel-Álvarez, M.; Santos-Lozano, A.; Sanchis-Gomar, F.; Fiuza-Luces, C.; Pareja-Galeano, H.; Garatachea, N.; Lucia, A. Non-Steroidal Anti-Inflammatory Drugs as a Treatment for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Treatment Effect. Drugs Aging 2015, 32, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.F.; Wan, C.Q.; Cai, M.; Zhou, N.K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Zhang, X.; Gu, X.; Mao, Y.; Peng, B. The origin and repopulation of microglia. Dev. Neurobiol. 2022, 82, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Matsumoto, J.; Dohgu, S.; Takata, F.; Machida, T.; Bölükbaşi-Hatip, F.F.; Hatip-Al-Khatib, I.; Yamauchi, A.; Kataoka, Y. TNF-α-sensitive brain pericytes activate microglia by releasing IL-6 through cooperation between IκB-NFκB and JAK-STAT3 pathways. Brain Res. 2018, 1692, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.; Giannopoulos, P.; Chu, J.; Praticò, D. Modulation of lipopolysaccharide-induced memory insult γ-secretase, and neuroinflammation in triple transgenic mice by 5-lipoxygenase. Neurobiol. Aging 2014, 35, 1024–1031. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Taipa, R.; Ferreira, V.; Brochado, P.; Robinson, A.; Reis, I.; Marques, F.; Mann, D.M.; Melo-Pires, M.; Sousa, N. Inflammatory pathology markers (activated microglia and reactive astrocytes) in early and late onset Alzheimer disease: A post-mortem study. Neuropathol. Appl. Neurobiol. 2018, 44, 298–313. [Google Scholar] [CrossRef]
- Villarejo-Galende, A.; González-Sánchez, M.; Blanco-Palmero, V.A.; Llamas-Velasco, S.; Benito-León, J. Non-steroidal Anti-inflammatory Drugs as Candidates for the Prevention or Treatment of Alzheimer’s Disease: Do they Still Have a Role? Curr. Alzheimer Res. 2020, 17, 1013–1022. [Google Scholar] [CrossRef]
- Granger, K.T.; Barnett, J.H. Postoperative cognitive dysfunction: An acute approach for the development of novel treatments for neuroinflammation. Drug Discov. 2021, 26, 1111–1114. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
| Scientific Name | Common Name (s) | Uses | Distribution in Mexico | References |
|---|---|---|---|---|
| A. offoyana Jacobi., 1864 | No data found | No data found | No data found | [1] |
| A. angustifolia Haw | “Maguey espadín” “Maguey de monte” “Bacanora” Caribbean Agave | Urticaria Dysentery pain of wounds relieve rheumatic pain inflammation. Mezcal production | Oaxaca Durango | [9] |
| A. rzedowskiana P. Carrillo, Vega & R. Delgad. | No data found | No data found | Nayarit/Sinaloa/Jalisco | [21] |
| A. ornithobroma Gentry, 1982 | “Maguey Pajarito”, “Amole”, “Lechuguilla”. | Fiber for the manufacture of backpacks and mats | Nayarit/Sinaloa | [21] |
| A. salmiana Otto ex Salm-Dyck | “Maguey pinto”, En Michoacán: “Akamha” (purépecha *) “Maguey cimarrón” “Maguey pulquero” “Pulque Agave” | Inflammation, gastritis, diabetes, wounds, blows and cough | Tlaxcala, Hidalgo, Querétaro, San Luis Potosí y Zacatecas. | [22,23] |
| A. barbadensis Trel | No data found | No data found | No data found | [21] |
| A. impressa Gentry | “Maguey masparillo” “Lechugilla” | Ornamental | Sinaloa Durango Nayarit | [22] |
| A. marmorata Roezl | Maguey de caballo Pitzomel | Cough Asthma internal bumps Ornamental. | Puebla Oaxaca | [22] |
| A. intermixta Trel | No data found | It is used as a decoction of the plant to treat cancer problems | No data found | [22] |
| A. schidigera Lem. | “Lechugilla mansa” Thread-leaf Agave | To relieve gastric problems in children | Sonora y Chihuahua a Michoacán, Jalisco, Aguascalientes, San Luis Potosí | [22,24] |
| A. americana L., 1753 | “Maguey pinto”, “Mezcal”, American Agave | Gastric ulcers, eye inflammation, diuretic, wounds, diarrhea. Ornamental | Tropical America, widely distributed in Mexican territory | [24] |
| A. tequilana F.A.C. Weber | “Maguey azul” Blue Agave | Tequila production | Jalisco, Michoacán, Guanajuato y Nayarit. Tamaulipas | [25] |
| A. fourcroydes LEM. | “Henequen” | It is a natural fiber used to make hammocks and mats. It is fermented to produce alcohol (similar to mezcal) | Yucatán | [26] |
| A. utahensis Engelm | Utah Agave | Nursery Stock Product | No data found | [27] |
| A. schotti Engelm | “Maguey” Arizona shin-dagger “Amol” (Náhuatl) ** | No data found | Chihuahua, Sonora, and Baja California. | [28] |
| Natural Compound Name | Number | Chemical Structure |
|---|---|---|
| smilagenin-3-O-[β-D-glucopyranosyl (1→2)-β-D-galactopyranoside] | 1 | 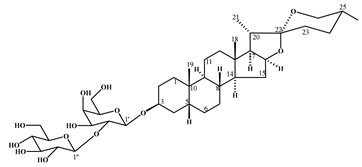 |
| 3-O-[(6’-O-palmitoyl)-β-D-glucopyranosyl sitosterol | 2 | 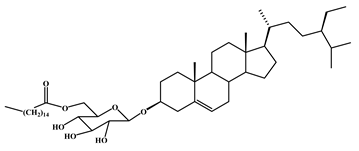 |
| Agavesaponin E | 3 |  |
| Agavasaponin H | 4 | 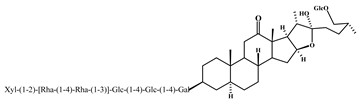 |
| Hecogenin | 5 |  |
| Tigogenin | 6 | 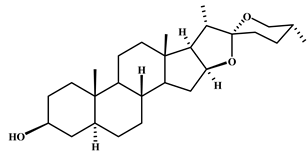 |
| β-sitosterol | 7 | 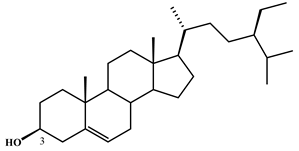 |
| Isorhamnetin | 8 |  |
| Hesperidin | 9 | 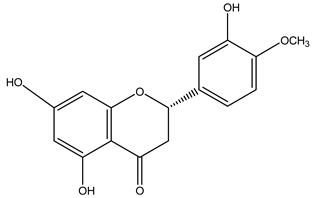 |
| Delphinidin | 10 | 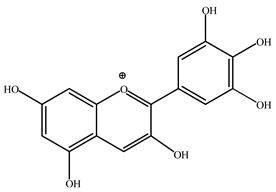 |
| Quercetin | 11 | 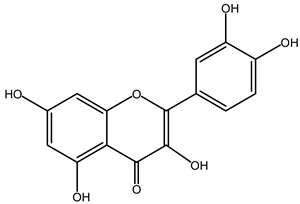 |
| Apigenin | 12 | 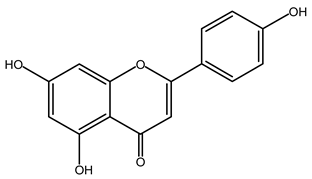 |
| Catechin | 13 |  |
| Ginsenoside Rg1 | 14 |  |
| Ginsenoside Re | 15 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Ruiz, M.; Jiménez-Ferrer, E.; González-Cortazar, M.; Zamilpa, A.; Cardoso-Taketa, A.; Arenas-Ocampo, M.L.; Jiménez-Aparicio, A.R.; Monterrosas-Brisson, N. Potential Use of Agave Genus in Neuroinflammation Management. Plants 2022, 11, 2208. https://doi.org/10.3390/plants11172208
Herrera-Ruiz M, Jiménez-Ferrer E, González-Cortazar M, Zamilpa A, Cardoso-Taketa A, Arenas-Ocampo ML, Jiménez-Aparicio AR, Monterrosas-Brisson N. Potential Use of Agave Genus in Neuroinflammation Management. Plants. 2022; 11(17):2208. https://doi.org/10.3390/plants11172208
Chicago/Turabian StyleHerrera-Ruiz, Maribel, Enrique Jiménez-Ferrer, Manasés González-Cortazar, Alejandro Zamilpa, Alexandre Cardoso-Taketa, Martha Lucía Arenas-Ocampo, Antonio Ruperto Jiménez-Aparicio, and Nayeli Monterrosas-Brisson. 2022. "Potential Use of Agave Genus in Neuroinflammation Management" Plants 11, no. 17: 2208. https://doi.org/10.3390/plants11172208
APA StyleHerrera-Ruiz, M., Jiménez-Ferrer, E., González-Cortazar, M., Zamilpa, A., Cardoso-Taketa, A., Arenas-Ocampo, M. L., Jiménez-Aparicio, A. R., & Monterrosas-Brisson, N. (2022). Potential Use of Agave Genus in Neuroinflammation Management. Plants, 11(17), 2208. https://doi.org/10.3390/plants11172208








