Pharmaceutical Potential of High-Altitude Plants for Fatigue-Related Disorders: A Review
Abstract
1. Introduction
2. Habitat and Adaptation
3. Environmental Stresses of Natural Plants at High Altitude
3.1. Low Oxygen Concentration
3.2. Ultraviolet Radiation
3.3. Extreme Climates
3.4. Other Factors
4. Pharmacological Effects on Treatment of Fatigue-Related Disorders
4.1. Neuroprotective Agent: Adjustment of the Level of the Central Neurotransmitters
4.2. Energy Supply and Metabolism: Maintainance of Energy Homeostasis
4.3. Removal of Accumulated Metabolites: Enhancement of Muscle and Organ Adaptation
4.4. Free Radical Scavenger: Antioxidant Activity
4.5. Inflammatory Response Inhibitor: Anti-Inflammatory Activity
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef] [PubMed]
- Khanum, F.; Bawa, A.S.; Singh, B. Rhodiola rosea: A versatile adaptogen. Compr. Rev. Food Sci. Food Saf. 2005, 4, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Muhamad, N.A.; Ismail, H.; Nasir, A.; Khalil, A.A.K.; Anwar, Y.; Khan, Z.; Ali, A.; Taha, R.M.; Al-Shara, B.; et al. Potential Nutraceutical Benefits of In Vivo Grown Saffron (Crocus sativus L.) As Analgesic, Anti-inflammatory, Anticoagulant, and Antidepressant in Mice. Plants 2020, 9, 1414. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Q.; Meng, Q.; Wang, L.; Xiong, W.; Zhang, L. Anti-fatigue activity of polysaccharide fractions from Lepidium meyenii Walp. (maca). Int. J. Biol. Macromol. 2017, 95, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Gao, T.; Wang, H.; Du, Y.; Li, J.; Li, C.; Wei, L.; Bi, H. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J. Ethnopharmacol. 2013, 150, 529–535. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hou, C.W.; Bernard, J.R.; Chen, C.C.; Hung, T.C.; Cheng, L.L.; Liao, Y.H.; Kuo, C.H. Rhodiola crenulata- and Cordyceps sinensis-Based Supplement Boosts Aerobic Exercise Performance after Short-Term High Altitude Training. High Alt. Med. Biol. 2014, 15, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Kong, D.X.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Jiang, J.G. Anti-fatigue Effects of Active Ingredients from Traditional Chinese Medicine: A Review. Curr. Med. Chem. 2019, 26, 1833–1848. [Google Scholar] [CrossRef]
- Ma, C.Y.; Hu, L.M.; Tao, G.J.; Lv, W.P.; Wang, H.X. An UPLC-MS-based metabolomics investigation on the anti-fatigue effect of salidroside in mice. J. Pharm. Biomed. Anal. 2015, 105, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, W.-C.; Wu, Z.-Y.; Fu, C.-X.; Hui, A.-L.; Gao, H.; Chen, P.-P.; Du, B.; Zhang, H.-W. Two macamide extracts relieve physical fatigue by attenuating muscle damage in mice. J. Sci. Food Agric. 2019, 99, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Geng, C.-A.; Yang, T.-H.; Yang, Y.-P.; Chen, J.-J. Phytochemical and Health-Beneficial Progress of Turnip (Brassica rapa). J. Food Sci. 2019, 84, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.-S.; Leyva-Gomez, G.; Del Prado-Audelo, M.L.; Cortes, H.; Singh, Y.D.; Panda, M.K.; Mishra, A.P.; Nigam, M.; Saklani, S.; et al. Cordyceps spp.: A Review on Its Immune-Stimulatory and Other Biological Potentials. Front. Pharmacol. 2021, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.C.; Li, S.; Peng, H. Anti-fatigue efficacy of crocin in mice via regulation of NRF-2/HO-1 pathway-mediated oxidative stress. Rev. Bras. Med. Esporte 2022, 28, 295–299. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The Roles of Environmental Factors in Regulation of Oxidative Stress in Plant. Biomed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef]
- Shi, Y.; Su, Z.; Yang, H.; Wang, W.; Jin, G.; He, G.; Siddique, A.N.; Zhang, L.; Zhu, A.; Xue, R.; et al. Alternative splicing coupled to nonsense-mediated mRNA decay contributes to the high-altitude adaptation of maca (Lepidium meyenii). Gene 2019, 694, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Huarancca Reyes, T.; Esparza, E.; Crestani, G.; Limonchi, F.; Cruz, R.; Salinas, N.; Scartazza, A.; Guglielminetti, L.; Cosio, E. Physiological responses of maca (Lepidium meyenii Walp.) plants to UV radiation in its high-altitude mountain ecosystem. Sci. Rep. 2020, 10, 2654. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumari, M. Adaptive mechanisms of medicinal plants along altitude gradient: Contribution of proteomics. Biol. Plant. 2018, 62, 630–640. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants Metabolome Study: Emerging Tools and Techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bartolini, G.; Ganino, T.; Zelasco, S.; Lombardo, L.; Perri, E.; Durante, M.; Bernardi, R. Cold Stress, Freezing Adaptation, Varietal Susceptibility of Olea europaea L.: A Review. Plants 2022, 11, 1367. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Pluskal, T.; Li, F.-S.; Carballo, V.; Weng, J.-K. Complete Pathway Elucidation and Heterologous Reconstitution of Rhodiola Salidroside Biosynthesis. Mol. Plant 2018, 11, 205–217. [Google Scholar] [CrossRef]
- Peschel, W.; Kump, A.; Zomborszki, Z.P.; Pfosser, M.; Kainz, W.; Csupor, D. Phenylpropenoid content in high-altitude cultivated Rhodiola rosea L. provenances according to plant part, harvest season and age. Ind. Crops Prod. 2018, 111, 446–456. [Google Scholar] [CrossRef]
- Baba, S.A.; Ashraf, N. Functional characterization of flavonoid 3’-hydroxylase, CsF3’H, from Crocus sativus L: Insights into substrate specificity and role in abiotic stress. Arch. Biochem. Biophys. 2019, 667, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Reyes, T.H.; Scartazza, A.; Pompeiano, A.; Guglielminetti, L. Physiological responses of Lepidium meyenii plants to ultraviolet-B radiation challenge. BMC Plant Biol. 2019, 19, 186. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.L.; Xia, W.W.; Mu, J.Q.; Feng, Y.J.; Liu, R.N.; Yan, P.Y.; Wang, A.Y.; Lin, Z.P.; Guo, Y.; et al. De Novo Transcriptome Sequencing and the Hypothetical Cold Response Mode of Saussurea involucrata in Extreme Cold Environments. Int. J. Mol. Sci. 2017, 18, 1155. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhang, L.; Dong, G.Q.; Xu, Z.H.; Li, G.M.; Liu, N.; Wang, A.Y.; Zhu, J.B. A novel cold-regulated protein isolated from Saussurea involucrata confers cold and drought tolerance in transgenic tobacco (Nicotiana tabacum). Plant Sci. 2019, 289, 110246. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, D.; Guo, S.; Xiao, L.; Zhao, Z.; Zhao, Z.; Xing, X.; Tang, G.; Xu, L.; Fu, Z.; et al. QTL analysis and the development of closely linked markers for days to flowering in spring oilseed rape (Brassica napus L.). Mol. Breed. 2016, 36, 52. [Google Scholar] [CrossRef]
- Basak, S.; Wang, G.; Sun, X.; Yang, Y. Variations in Genome Size of Turnip Landraces from Two High-altitude Environments. J. Am. Soc. Hortic. Sci. 2018, 143, 136–143. [Google Scholar] [CrossRef]
- Swastika, P.; Chang-An, G.; Tong-Hua, Y.; Yong-Ping, Y.; Ji-Jun, C. Comparative study of the glucosinolate profiles in turnip from four agroclimatic zones of China and neighboring countries. J. Food Meas. Charact. 2019, 13, 2798–2811. [Google Scholar] [CrossRef]
- Pham, T.A.; Hwang, S.Y. High temperatures reduce nutrients and defense compounds against generalist Spodoptera litura F. in Rorippa dubia. Arthropod-Plant Interact. 2020, 14, 333–344. [Google Scholar] [CrossRef]
- Cordeiro, L.M.S.; Rabelo, P.C.R.; Moraes, M.M.; Teixeira-Coelho, F.; Coimbra, C.C.; Wanner, S.P.; Soares, D.D. Physical exercise-induced fatigue: The role of serotonergic and dopaminergic systems. Braz. J. Med. Biol. Res. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Watson, P. Amino acids and the brain: Do they play a role in “Central fatigue”? Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S37–S46. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, M.; Bhlke, M.; Kelley, C.; Maher, T.; Pino-Figueroa, A. Inhibition of Fatty Acid Amide Hydrolase (FAAH) by Macamides. Mol. Neurobiol. 2019, 56, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.; Kim, J.; Oh, D.-R.; Kim, Y.; Choi, E.-J.; Lee, H.; Jung, M.-A.; Lee, S.-Y.; Jeong, C.; Lee, M.; et al. Multifunctional antistress effects of standardized aqueous extracts from Hippophae rhamnoides L. Anim. Cells Syst. 2016, 20, 369–383. [Google Scholar] [CrossRef]
- Hou, D.; Gu, F.; Liang, Z.; Helland, T.; Fu, W.; Cai, L. Sea buckthorn (Hippophae rhamnoides L.) oil protects against chronic stress-induced inhibitory function of natural killer cells in rats. Int. J. Immunopathol. Pharmacol. 2016, 29, 76–83. [Google Scholar] [CrossRef]
- Fan, J.-L.; Kayser, B. Fatigue and Exhaustion in Hypoxia: The Role of Cerebral Oxygenation. High Alt. Med. Biol. 2016, 17, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Y.; Jahan, I.; Wu, S.; Clark, B.C.; Wiseman, J.S. Extracts of maca (Lepidium meyenii) root induce increased glucose uptake by inhibiting mitochondrial function in an adipocyte cell line. J. Herb. Med. 2019, 17–18, 100282. [Google Scholar] [CrossRef]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y.; et al. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 12902. [Google Scholar] [CrossRef]
- Dinel, A.-L.; Guinobert, I.; Lucas, C.; Blondeau, C.; Bardot, V.; Ripoche, I.; Berthomier, L.; Pallet, V.; Laye, S.; Joffre, C. Reduction of acute mild stress corticosterone response and changes in stress-responsive gene expression in male Balb/c mice after repeated administration of a Rhodiola rosea L. root extract. Food Sci. Nutr. 2019, 7, 3827–3841. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.T.; Kuo, T.Y.; Liou, S.Y.; Chien, C.T. Chronic Rhodiola rosea Extract Supplementation Enforces Exhaustive Swimming Tolerance. Am. J. Chin. Med. 2009, 37, 557–572. [Google Scholar] [CrossRef]
- Dun, Y.; Liu, S.; Zhang, W.; Xie, M.; Qiu, L. Exercise Combined with Rhodiola sacra Supplementation Improves Exercise Capacity and Ameliorates Exhaustive Exercise-Induced Muscle Damage through Enhancement of Mitochondrial Quality Control. Oxidative Med. Cell. Longev. 2017, 2017, 8024857. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Yuan, D.; Zheng, J.; Wu, X.C.; Wang, J.T.; Liu, X.; He, Y.M.; Zhang, C.C.; Liu, C.Q.; Wang, T.; et al. Chikusetsu saponin IVa attenuates isoprenaline-induced myocardial fibrosis in mice through activation autophagy mediated by AMPK/mTOR/ULK1 signaling. Phytomedicine 2019, 58, 152764. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.J.; Fu, T.T.; Kelly, D.P.; Vega, R.B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res 2018, 28, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Jung, S.K.; Chang, Y.H. Rheological properties of a neutral polysaccharide extracted from maca (Lepidium meyenii Walp.) roots with prebiotic and anti-inflammatory activities. Int. J. Biol. Macromol. 2020, 152, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-Y.; Yu, C.Y.; Chen, Y.-W.; Huang, Y.-T.; Chen, C.-T.; Wu, H.-F.; Chen, Y.-L.S. Rutin, a Flavonoid and Principal Component of Saussurea Involucrata, Attenuates Physical Fatigue in a Forced Swimming Mouse Model. Int. J. Med. Sci. 2014, 11, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, L.B.; Zhou, N.; Liu, C.; Cui, J.H.; Wu, R.X.; Jing, J.; Zhang, S.Y.; Chen, H.; Wang, S.W. Salidroside, a scavenger of ROS, enhances the radioprotective effect of Ex-RAD (R) via a p53-dependent apoptotic pathway. Oncol. Rep. 2017, 38, 3094–3102. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Saggu, S.; Kumar, R. Effect of seabuckthorn leaf extracts on circulating energy fuels, lipid peroxidation and antioxidant parameters in rats during exposure to cold, hypoxia and restraint (C-H-R) stress and post stress recovery. Phytomedicine 2008, 15, 437–446. [Google Scholar] [CrossRef]
- Jia, J.M.; Wu, C.F. Antifatigue activity of tissue culture extracts of Saussurea involucrata. Pharm. Biol. 2008, 46, 433–436. [Google Scholar] [CrossRef]
- Chu, B.; Chen, C.; Li, J.; Chen, X.; Li, Y.; Tang, W.; Jin, L.; Zhang, Y. Effects of Tibetan turnip (Brassica rapa L.) on promoting hypoxia-tolerance in healthy humans. J. Ethnopharmacol. 2017, 195, 246–254. [Google Scholar] [CrossRef]
- Qin, Y.; Wenwen, J.; Xueyuan, L.; Pengfei, D.; Yanxiao, A.; Mengying, W.; Wenjing, D.; Longjiang, Y. Effects of macamides on endurance capacity and anti-fatigue property in prolonged swimming mice. Pharm. Biol. 2016, 54, 827–834. [Google Scholar] [CrossRef]
- Noakes, T.D.; Gibson, A.S. Logical limitations to the "catastrophe" models of fatigue during exercise in humans. Br. J. Sports Med. 2004, 38, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nkubana, A.; Qian, H. A comparative study of antioxidant activity between black tea from Rwandan highlands with green and oolong teas from China. Int. J. Food Saf. Nutr. Public Health 2008, 1, 159–166. [Google Scholar] [CrossRef]
- Tang, W.; Jin, L.; Xie, U.; Huan, J.; Chu, B.; Dai, X.; Wang, R.; Zhang, Y. Purification, structural characterization and anti-fatigue effect in vivo of Tibetan Turnip (Brassica rapa L.) polysaccharide. J. Chin. Inst. Food Sci. Technol. 2018, 18, 22–31. [Google Scholar] [CrossRef]
- Choi, E.H.; Kang, J.I.; Cho, J.Y.; Lee, S.H.; Kim, T.S.; Yeo, I.H.; Chun, H.S. Supplementation of standardized lipid-soluble extract from maca (Lepidium meyenii) increases swimming endurance capacity in rats. J. Funct. Foods 2012, 4, 568–573. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Wu, J.; Ushida, Y.; Suganuma, H.; et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J. Nutr. Biochem. 2017, 39, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.W.; Fang, Q.Q.; Ma, W.J.; Zhang, Q.Y.; Qiu, J.Y.; Gu, X.S.; Yang, H.L.; Sun, H.L. Skeletal Muscle Atrophy Was Alleviated by Salidroside Through Suppressing Oxidative Stress and Inflammation During Denervation. Front. Pharmacol. 2019, 10, 997. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Lee, D.S.; Son, C.G. Oxidative Stress is a Convincing Contributor to Idiopathic Chronic Fatigue. Sci. Rep. 2018, 8, 12890. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jin, W.; Dong, X.; Ao, M.; Liu, H.; Yu, L. Safety evaluation and protective effects of ethanolic extract from maca (Lepidium meyenii Walp.) against corticosterone and H2O2 induced neurotoxicity. Regul. Toxicol. Pharmacol. 2020, 111, 104570. [Google Scholar] [CrossRef] [PubMed]
- King-Himmelreich, T.S.; Möser, C.V.; Wolters, M.C.; Schmetzer, J.; Schreiber, Y.; Ferreirós, N.; Russe, O.Q.; Geisslinger, G.; Niederberger, E. AMPK contributes to aerobic exercise-induced antinociception downstream of endocannabinoids. Neuropharmacology 2017, 124, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, R.; Hua, H.; Cheng, Y.; Guo, Y.; Qian, H.; Du, P. The macamide relieves fatigue by acting as inhibitor of inflammatory response in exercising mice: From central to peripheral. Eur. J. Pharmacol. 2022, 917, 174758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Han, X.; Li, R.; Zhao, W.; Bai, B.; Yan, C.; Dong, X. Anti-atherosclerosis of oligomeric proanthocyanidins from Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function. Phytomedicine 2018, 51, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Suryakumar, G.; Prasad, R.; Ganju, L.; Singh, S.B. Enhanced hypoxic tolerance by Seabuckthorn is due to upregulation of HIF-1 alpha and attenuation of ER stress. J. Appl. Biomed. 2016, 14, 71–83. [Google Scholar] [CrossRef]
- Yang, D.-W.; Kang, O.-H.; Lee, Y.-S.; Han, S.-H.; Lee, S.-W.; Cha, S.-W.; Seo, Y.-S.; Mun, S.-H.; Gong, R.; Shin, D.-W.; et al. Anti-inflammatory effect of salidroside on phorbol-12-myristate-13-acetate plus A23187-mediated inflammation in HMC-1 cells. Int. J. Mol. Med. 2016, 38, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Kausar, F.; Kim, K.H.; Farooqi, H.M.U.; Farooqi, M.A.; Kaleem, M.; Waqar, R.; Khalil, A.A.K.; Khuda, F.; Rahim, C.S.A.; Hyun, K.; et al. Evaluation of Antimicrobial and Anticancer Activities of Selected Medicinal Plants of Himalayas, Pakistan. Plants 2022, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Jung, Y.S.; You, D.M.; Lee, S.H.; Lee, G.; Kwon, K.-B.; Kim, D.-O. Neuroprotective effects of ethanolic extract from dry Rhodiola rosea L. rhizomes. Food Sci. Biotechnol. 2021, 30, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Ivanova Stojcheva, E.; Quintela, J.C. The Effectiveness of Rhodiola rosea L. Preparations in Alleviating Various Aspects of Life-Stress Symptoms and Stress-Induced Conditions-Encouraging Clinical Evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Kang, H. Rhodiola rosea Extract Improves the Antioxidant Capacity of High-intensity Treadmill Mice. Genom. Appl. Biol. 2020, 39, 955–960. [Google Scholar]
- Zhao, W.; Zhang, W.; Liu, L.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Fractionation, characterization and anti-fatigue activity of polysaccharides from Brassica rapa L. Process Biochem. 2021, 106, 163–175. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, H.; Hua, H.; Liu, C.; Cheng, Y.; Guo, Y.; Du, P.; Qian, H. Anti-fatigue activity of Brassica rapa L. extract and correlation among biochemical changes in forced swimming mice. Food Biosci. 2022, 47, 101633. [Google Scholar] [CrossRef]
- Akbari-Fakhrabadi, M.; Najafi, M.; Mortazavian, S.; Rasouli, M.; Memari, A.-H.; Shidfar, F. Effect of saffron (Crocus sativus L.) and endurance training on mitochondrial biogenesis, endurance capacity, inflammation, antioxidant, and metabolic biomarkers in Wistar rats. J. Food Biochem. 2019, 43, e12946. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Bandieri, E.; Arletti, R. Lepidium meyenii Walp. improves sexual behaviour in male rats independently from its action on spontaneous locomotor activity. J. Ethnopharmacol. 2001, 75, 225–229. [Google Scholar] [CrossRef]
- Hongkang, Z.; Wenqian, X.; Ning, W.; Wenhao, J.; Yuliang, C.; Yahui, G.; Weirong, Y.; Bin, H.; Peng, D.; He, Q. Anti-fatigue effect of Lepidium meyenii Walp. (Maca) on preventing mitochondria-mediated muscle damage and oxidative stress in vivo and vitro. Food Funct. 2021, 12, 3132–3141. [Google Scholar] [CrossRef]
- Roesch, D.; Krumbein, A.; Muegge, C.; Kroh, L.W. Structural investigations of flavonol glycosides from sea buckthorn (Hippophae rhamnoides) pomace by NMR spectroscopy and HPLC-ESI-MSn. J. Agric. Food Chem. 2004, 52, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Xiangqun, G.; Ohlander, M.; Jeppsson, N.; Bjork, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophaerhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Gong, G.; Huang, J.; Yang, Y.; Qi, B.; Han, G.; Zheng, Y.; He, H.; Chan, K.; Tsim, K.W.K.; Dong, T.T.X. Saussureae Involucratae Herba (Snow lotus): Review of Chemical Compositions and Pharmacological Properties. Front. Pharmacol. 2020, 10, 1549. [Google Scholar] [CrossRef]
- Yi, T.; Zhao, Z.-Z.; Yu, Z.-L.; Chen, H.-B. Comparison of the anti-inflammatory and anti-nociceptive effects of three medicinal plants known as "Snow lotus" herb in traditional Uighur and Tibetan medicines. J. Ethnopharmacol. 2010, 128, 405–411. [Google Scholar] [CrossRef]
- Zheng, R.L.; Liu, G.S.; Xing, G.X.; Jia, Z.J.; Du, M.; Tan, L.Q. Free-radical scavenging and antifatigue activities of saussurea-involucrata polysaccharides. Acta Pharmacol. Sin. 1993, 14, S47–S49. [Google Scholar]
- Lee, J.-C.; Kao, J.-Y.; Kuo, D.-H.; Liao, C.-F.; Huang, C.-H.; Fan, L.-L.; Way, T.-D. Antifatigue and Antioxidant Activity of Alcoholic Extract from Saussurea involucrata. J. Tradit. Complement. Med. 2011, 1, 64–68. [Google Scholar] [CrossRef]
- Xin-xia, C.; Zhong-ming, L.U.; Gen-yong, S.H.I.; De-zhou, X.U. Study on the mitigation role of physical fatigue of Cordyceps sinensis mycelium. Chin. J. Biochem. Pharm. 2009, 30, 321–323. [Google Scholar]
- Wang, K.; Xu, C.; Li, Z. Study on effect and mechanism of Cordyceps sinensis on fatigue in mice induced by exercise. J. Harbin Med. Coll. 2003, 37, 311–314. [Google Scholar]
- Guo, P.; Li, Y.S.; Xu, J.; Guo, Y.Q.; Jin, D.Q.; Gao, J.; Hou, W.B.; Zhang, T.J. neo-Clerodane diterpenes from Ajuga ciliata Bunge and their neuroprotective activities. Fitoterapia 2011, 82, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.J.; Yang, X.Y.; Liu, W.P.; An, L.J.; Zhang, X.K.; Tuerhong, M.; Du, Q.; Wang, C.Y.; Abudukeremu, M.; Xu, J.; et al. Anti-inflammatory neo-Clerodane Diterpenoids from Ajuga pantantha. J. Nat. Prod. 2020, 83, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Liu, L.; Wang, Y.; Yan, G.; Zhang, Y. Long-term systemic toxicity of shikonin derivatives in Wistar rats. Pharm. Biol. 2014, 52, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiong, Z. Experimental study on the determination of gromwell extract and its influence on the metabolism of free radical in skeletal muscle and anti-fatigue effect. Chin. J. Pharm. Anal. 2016, 36, 444–450. [Google Scholar]
- Cui-fang, L.I.; Fang, W.; Hao, M.A.; Yue-hua, Y.U.; Yong-jun, W. Study on Antioxidant Activity of Arnebia euchroma (Royle) Johnst Hairy Roots. Xinjiang Agric. Sci. 2010, 47, 291–295. [Google Scholar]
- Guerrero, E.; Abad, A.; Montenegro, G.; del Olmo, E.; Luis Lopez-Perez, J.; San Feliciano, A. Analgesic and anti-inflammatory activity of podophyllotoxin derivatives. Pharm. Biol. 2013, 51, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, C.; Li, X.; Gao, Q.; Huang, L.; Xiao, P.; Gao, W. The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 214, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Q.; Li, Y.-Q.; Yu, L.-P.; Li, X.; Mu, J.-K.; Shang, J.; Gu, W.; Li, J.-P.; Yu, J.; Yang, X.-X. Muscle Fatigue-Alleviating Effects of a Prescription Composed of Polygonati Rhizoma and Notoginseng Radix et Rhizoma. Biomed Res. Int. 2020, 2020, 3963045. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Hu, Z.P.; Du, G.Y.; Zhang, J.Z.; Dong, Q.J.; Fu, F.H.; Tian, J.W. Antidepressant-like effects of the extract from Cimicifuga foetida L. J. Ethnopharmacol. 2012, 144, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Huang, R.; Li, N.Z.; Xu, H.Y.; Yang, J.H. Triterpenes from Stachyurus himalaicus var. himalaicus Hook. f. et Thoms. ex Benth. Molecules 2010, 15, 2096–2102. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lu, H.; Huang, R.; Wang, Y.-S. Flavonoids from leaves and twigs of Stachyurus himalaicus var. himalaicus. Chem. Nat. Compd. 2011, 47, 112–113. [Google Scholar] [CrossRef]
- Chen, X.-L.; Peng, X.-R.; Gong, X.-Y.; Liu, Y.-Q.; Qing, Z.; Ren, X.-X.; Su, R.; Fang, L.-M.; Qiu, M.-H.; Dong, K. Flavonoid glycosides from the nectar of Camellia reticulata Lindl. Nat. Prod. Res. 2022, 36, 1827–1833. [Google Scholar] [CrossRef]
- Li, J.-B.; Hashimoto, F.; Shimizu, K.; Sakata, Y. Anthocyanins from red flowers of Camellia reticulata Lindl. Biosci. Biotechnol. Biochem. 2007, 71, 2833–2836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, J.; Kan, H.; Liu, Y. Free radicals scavenging effects of polyphenols in Camellia reticulata Lindl. seed oil. China Oils Fats 2011, 36, 54–57. [Google Scholar]
- Yatoo, M.I.; Dimri, U.; Gopalakrishnan, A.; Karthik, K.; Gopi, M.; Khandia, R.; Saminathan, M.; Saxena, A.; Alagawany, M.; Farag, M.R.; et al. Beneficial health applications and medicinal values of pedicularis plants: A review. Biomed. Pharmacother. 2017, 95, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, Q.; Wang, J.; Tang, H.; Li, C. Purification and Identification of Chemical Constituents of Pedicularis longiflora Rudolph, var. Tubiformis (Klotz). Tsoong. Chin. Pharm. J. 2017, 52, 2146–2150. [Google Scholar]
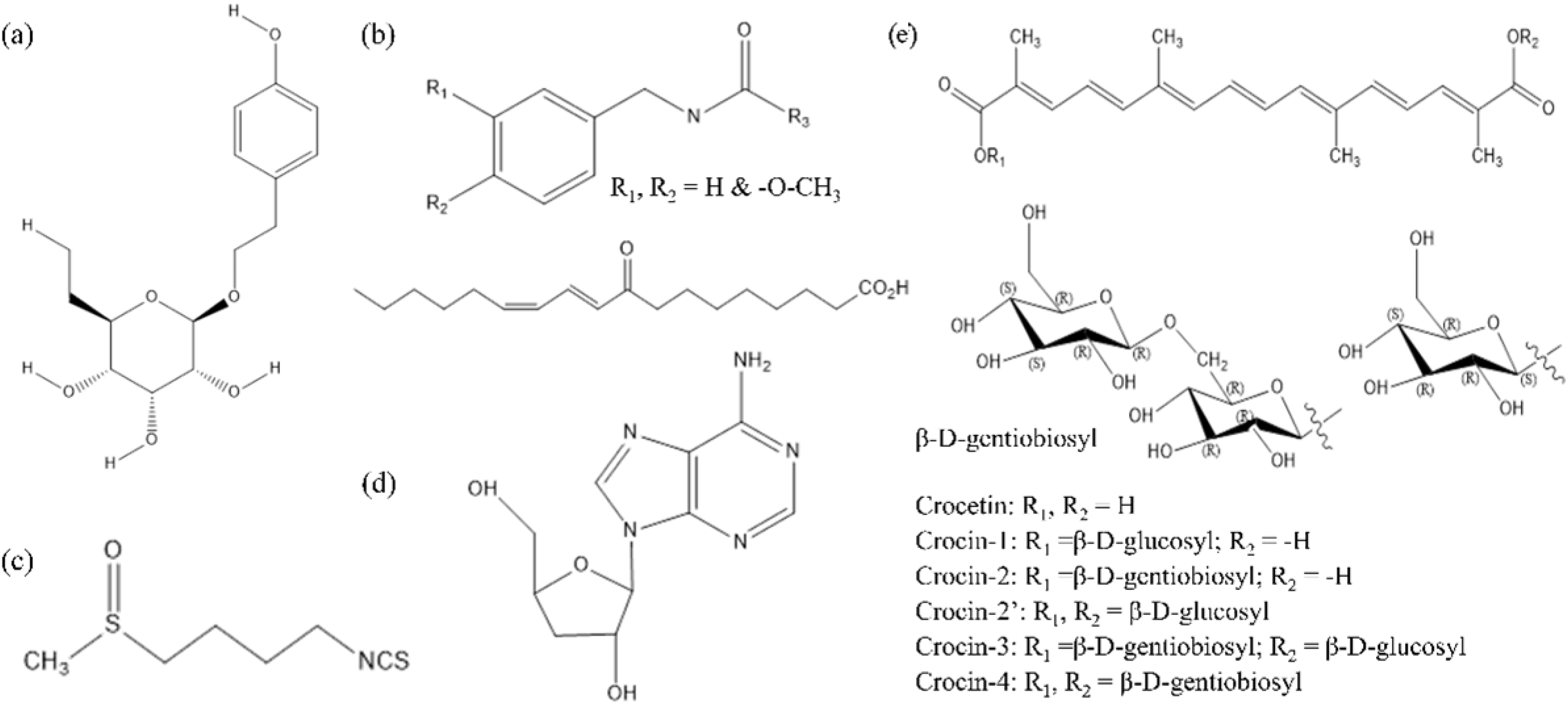
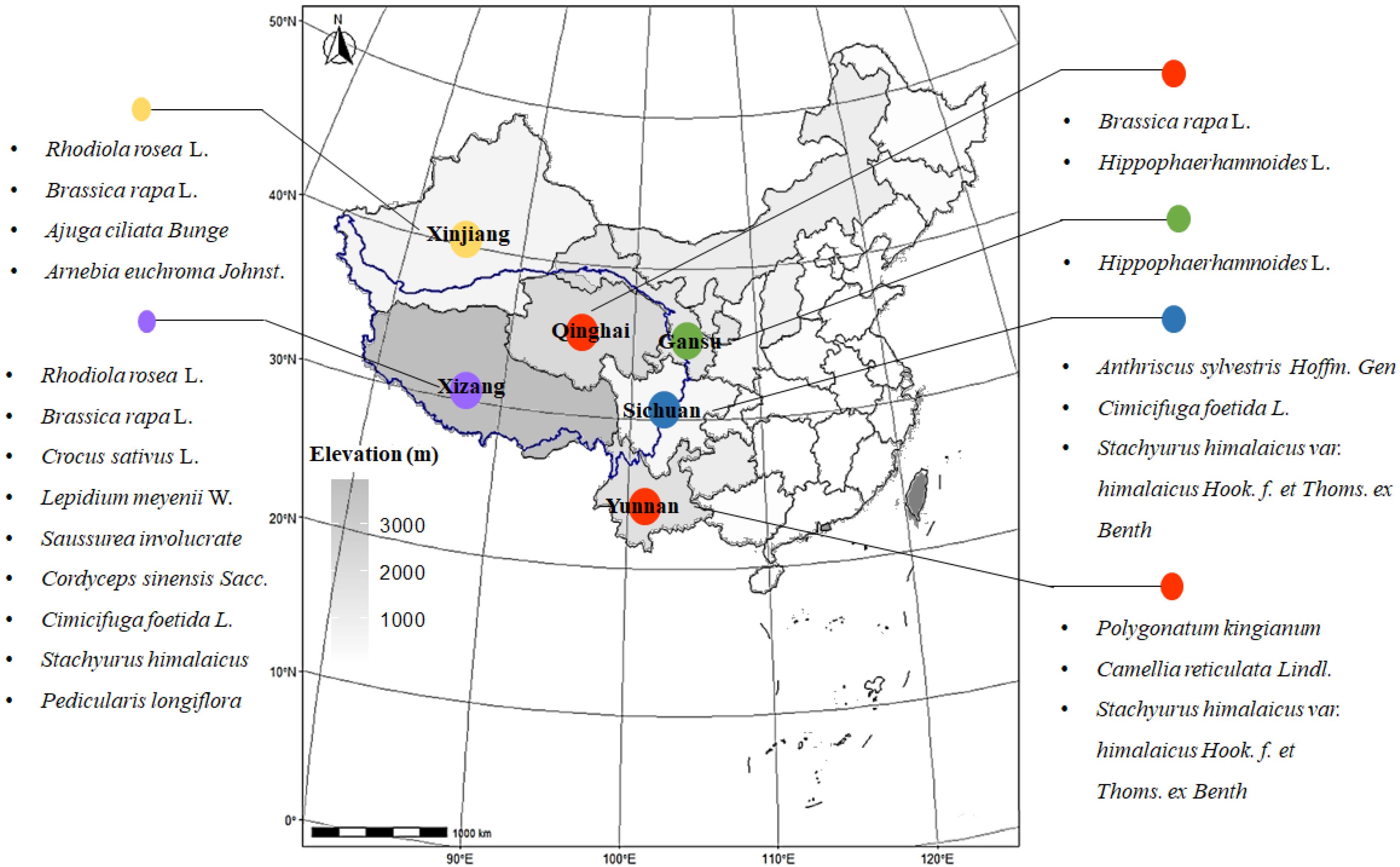
| No. | Latin Name | Family | Elevation/m | Distributions | Used Part | Main Active Ingredients |
|---|---|---|---|---|---|---|
| 1 | Rhodiola rosea L. | Crassulaceae | 2800– | Tibet, Xinjiang | Root, rhizome | Flavonoids, salidroside |
| 2 | Brassica rapa L. | Brassica | 3500- | Tibet, Xinjiang, Qinghai | Root | Polysaccharide, isothiocyanates |
| 3 | Crocus sativus L. | Iridaceae | 5000- | Tibet | Filament | Flavonoids, crocin |
| 4 | Lepidium meyenii W. | Brassicaceae | 3800- | Tibet | Root | Polysaccharide, alkaloids (macamides) |
| 5 | Hippophaerhamnoides L. | Elaeagnaceae | 800– | Qinghai, Gansu | Fruit | Flavonoids |
| 6 | Saussurea involucrata Sch.-Bip. | Compositae | 4300- | Tibet | Flower | Flavonoids |
| 7 | Cordyceps sinensis Sacc. | Clavicipitaceae | 5000- | Tibet | Complex | Polysaccharide, cordycepin |
| 8 | Ajuga ciliata Bunge | Labiatae | 2500– | Xinjiang | Whole grass | Flavonoids, triterpenes |
| 9 | Arnebia euchroma Johnst. | Boraginaceae | 2500– | Xinjiang | Root | Polysaccharide |
| 10 | Anthriscus sylvestris Hoffm. Gen | Umbelliferae | 4500- | Liaoning, Sichuan | Root | Lactones |
| 11 | Polygonatum kingianum | Liliaceae | 700– | Yunnan | Root | Polysaccharide, flavonoids, triterpenes |
| 12 | Cimicifuga foetida L. | Ranunculaceae | 1700– | Tibet, Liaoning, Sichuan | Root | Triterpenes |
| 13 | Stachyurus himalaicus var. himalaicus Hook. f. et Thoms. ex Benth | Stachyuraceae | 1500– | Tibet, Yunnan, Sichuan | Stem pith | Polyphenols, triterpenes |
| 14 | Camellia reticulata Lindl. | Theaceae | 2200– | Yunnan | Flower, leaves | Polyphenols |
| 15 | Pedicularis longiflora var. tubiformis | Pedicularis | 2700– | Tibet | Whole grass | Flavonoids, boschnaloside |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Liu, C.; Qian, H. Pharmaceutical Potential of High-Altitude Plants for Fatigue-Related Disorders: A Review. Plants 2022, 11, 2004. https://doi.org/10.3390/plants11152004
Zhu H, Liu C, Qian H. Pharmaceutical Potential of High-Altitude Plants for Fatigue-Related Disorders: A Review. Plants. 2022; 11(15):2004. https://doi.org/10.3390/plants11152004
Chicago/Turabian StyleZhu, Hongkang, Chang Liu, and He Qian. 2022. "Pharmaceutical Potential of High-Altitude Plants for Fatigue-Related Disorders: A Review" Plants 11, no. 15: 2004. https://doi.org/10.3390/plants11152004
APA StyleZhu, H., Liu, C., & Qian, H. (2022). Pharmaceutical Potential of High-Altitude Plants for Fatigue-Related Disorders: A Review. Plants, 11(15), 2004. https://doi.org/10.3390/plants11152004






