Chemical Composition, Antioxidant Potentials, and Calcium Oxalate Anticrystallization Activity of Polyphenol and Saponin Fractions from Argania spinosa L. Press Cake
Abstract
1. Introduction
2. Results
2.1. GC–MS Analysis
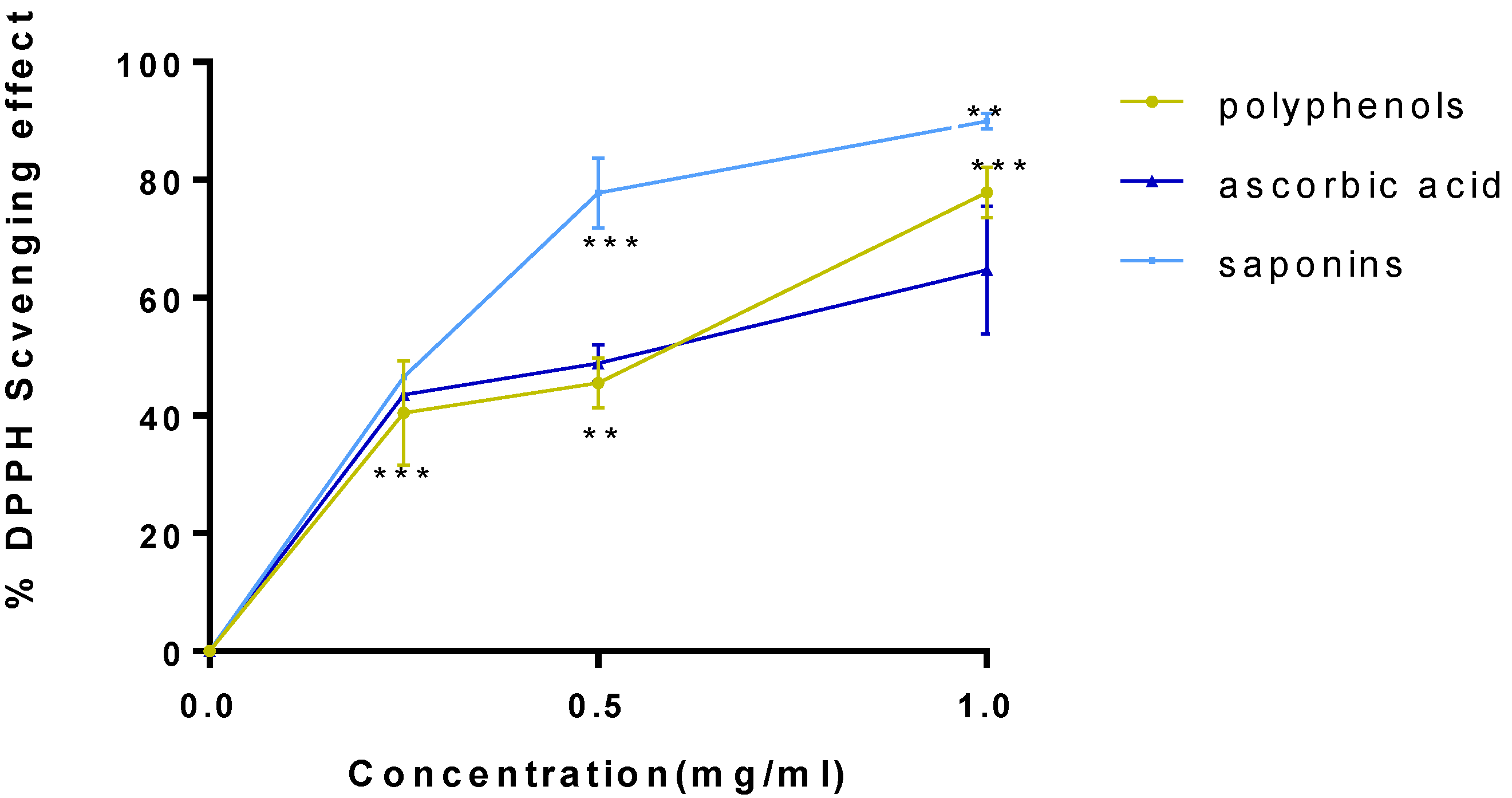
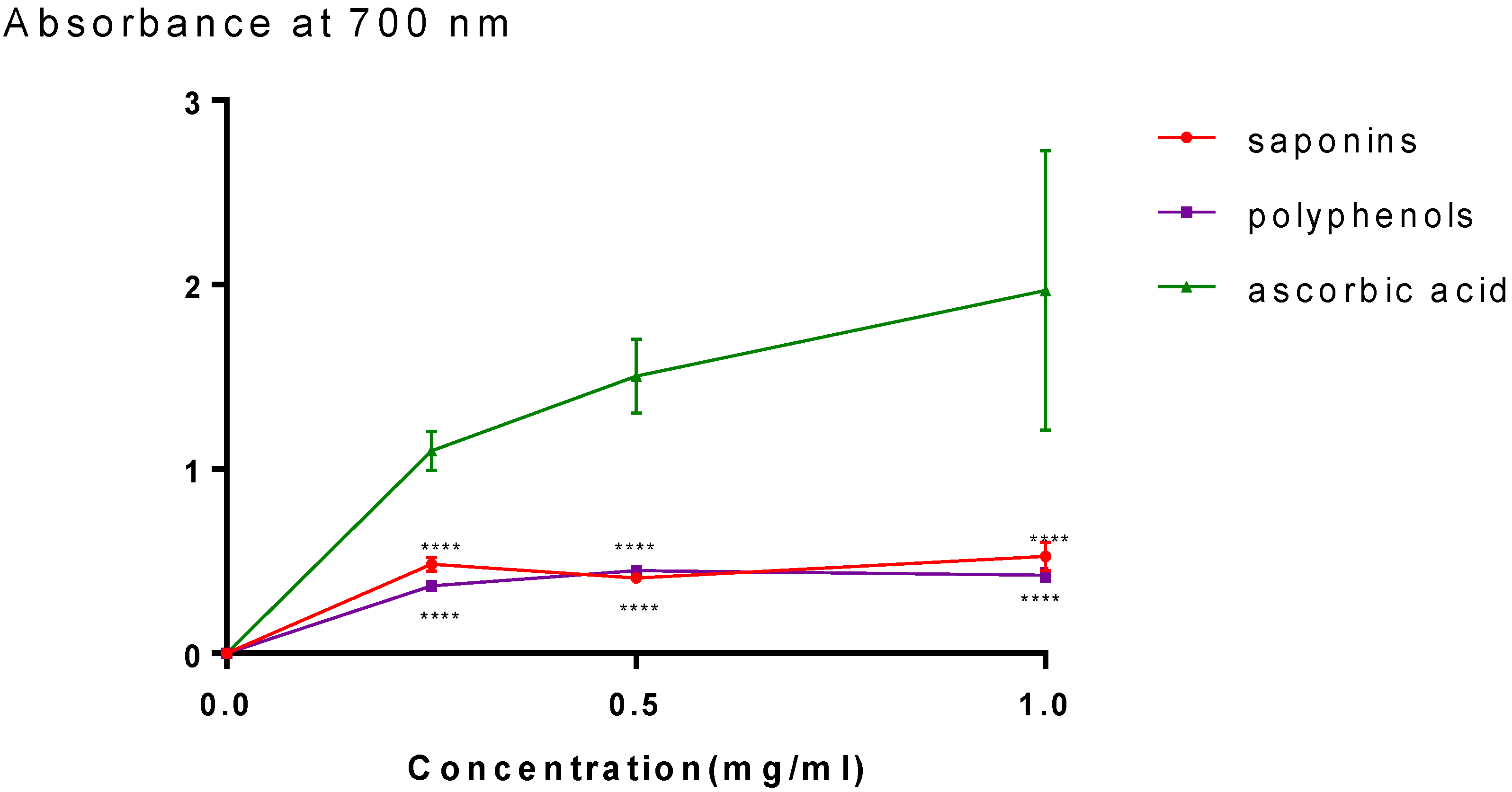
2.2. Antioxidant Activity
2.3. In Vitro Calcium Oxalate Crystallization Assay
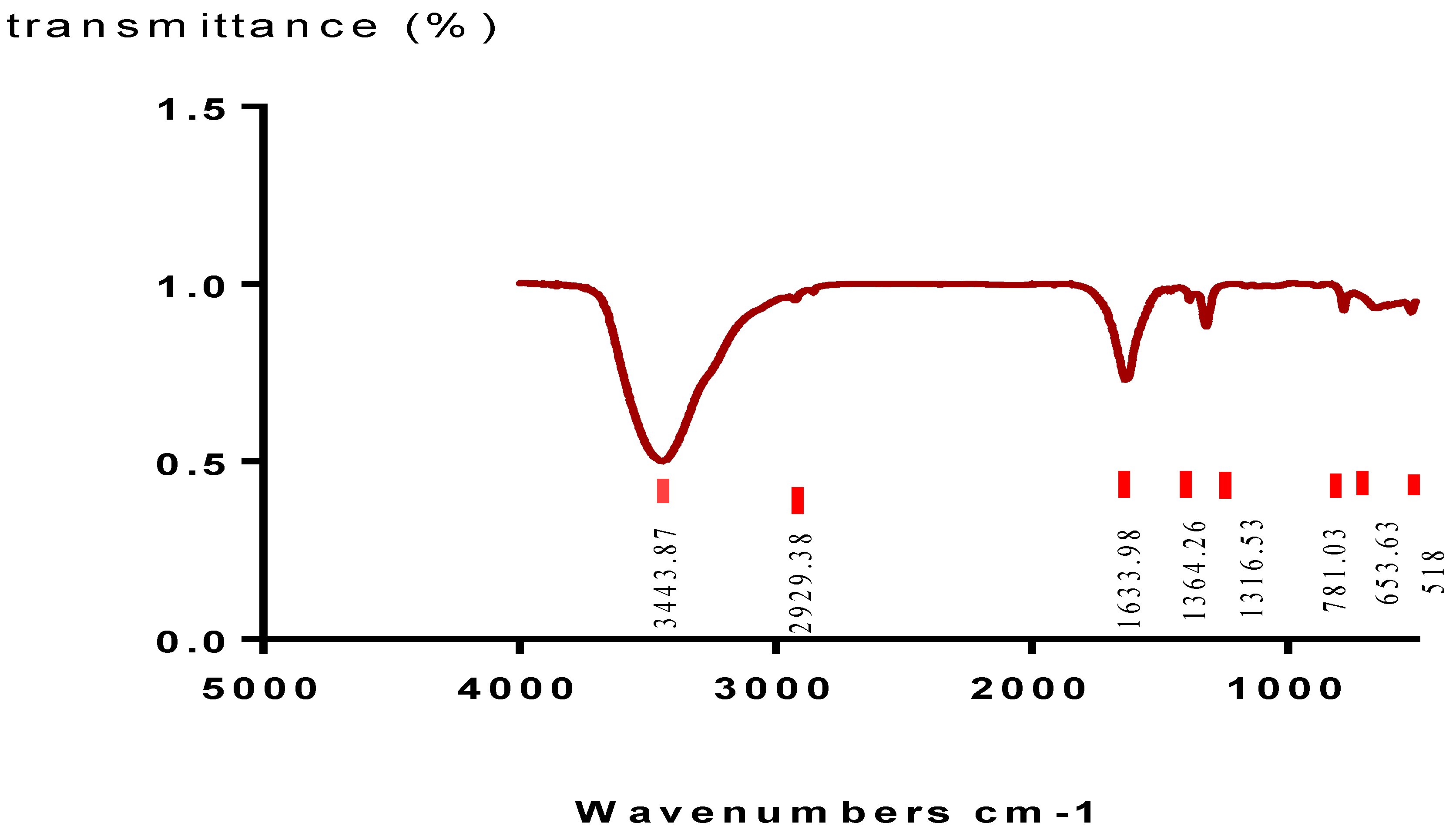
2.4. Characterization of Crystals by Fourier-Transform Infrared Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction Methods
4.2.1. Polyphenols Extraction Method
4.2.2. Saponin Extraction Method
4.3. GC–MS Analysis
4.4. Antioxidant Activity
4.4.1. DPPH Method
4.4.2. FRAP Method
4.5. In Vitro Calcium Oxalate Crystallization Assay
4.6. Characterization of Crystals by Fourier-Transform Infrared Spectroscopy
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrasekaran, S.; Veerasamy, V. Anti-Urolithiatic Activity of Whole Plant Aqueous and Ethanolic Extract of Coriandrum sativum L.—An in Vitro Approach. Malaya J. Biosci. 2018, 5, 28–36. [Google Scholar]
- Habbani, R.; Chaqrounea, A.; Sqalli Houssainib, T.; Arrayhanib, M.; El Ammaric, J.; Damib, F.; Chouhanib, B.A.; Habbani, A.L. Étude épidémiologique sur les calculs urinaires dans la région de Fès et sur le risque de récidive. Prog. Urol. 2016, 26, 287–294. [Google Scholar] [CrossRef]
- Castiglione, V.; Jouret, F.; Bruyère, O.; Dubois, B.; Thomas, A.; Waltregny, D.; Bekaert, A.-C.; Cavalier, É.; Gadisseur, R. Épidémiologie de la lithiase urinaire en Belgique sur base d’une classification morpho-constitutionnelle. Néphrol. Thér. 2015, 11, 42–49. [Google Scholar] [CrossRef]
- Roger, C.; Abid, N.; Dubourg, L.; Auvergnon, C.; Lemoine, S.; Machon, C. Composition of Urinary Calculi: Lessons from a French Epidemiologic Retrospective Study. Prog. Urol. 2020, 30, 339–345. [Google Scholar] [CrossRef] [PubMed]
- El oumari, F.E.; Bousta, D.; Grafov, A.; Sqalli Houssaini, T. Phytomolecules Investigated for the Prevention and Treatment of Urinary Stones. Mediterr. J. Chem. 2021, 11, 126. [Google Scholar] [CrossRef]
- Khare, P.; Mishra, V.K.; Arun, K.; Bais, N.; Singh, R. Study on In Vitro Anti-lithiatic Activity of Phyllanthus niruri Linn. Leaves by Homogenous Precipitation and Turbiditory Method. Int. J. Pharm. Pharm. Sci. 2014, 6, 124–127. [Google Scholar]
- Miller, N.L.; Lingeman, J.E. Management of Kidney Stones. Br. Med. J. 2007, 334, 468–472. [Google Scholar] [CrossRef]
- Khan, A.; Bashir, S.; Khan, S.R.; Gilani, A.H. Antiurolithic Activity of Origanum vulgare Is Mediated through Multiple Pathways. BMC Complement. Altern. Med. 2011, 11, 96. [Google Scholar] [CrossRef]
- Srisubat, A.; Potisat, S.; Lojanapiwat, B.; Setthawong, V.; Laopaiboon, M. Extracorporeal Shock Wave Lithotripsy (ESWL) versus Percutaneous Nephrolithotomy (PCNL) or Retrograde Intrarenal Surgery (RIRS) for Kidney Stones. Cochrane Database Syst. Rev. 2014, 11, CD007044. [Google Scholar] [CrossRef]
- Butterweck, V.; Khan, S. Herbal Medicines in the Management of Urolithiasis: Alternative or Complementary? Planta Med. 2009, 75, 1095–1103. [Google Scholar] [CrossRef]
- Gürocak, S.; Küpeli, B. Consumption of Historical and Current Phytotherapeutic Agents for Urolithiasis: A Critical Review. J. Urol. 2006, 176, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Atta-ur-Rahman. Trends in Ethnopharmacology. J. Ethnopharmacol. 2005, 100, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Boğa, R. Polyphenols Analysed by UHPLC-ESI-MS/MS and Antioxidant Activities of Molasses, Acorn and Leaves of Oak (Quercus robur subsp. pedunculiflora). Prog. Nutr. 2018, 20, 167–175. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Doğru, M.; Kiliç, Ö. Phenolic Content, Antioxidant Potentials of Saponaria prostrata Endemic Plant. Int. J. Life Sci. Biotechnol. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Youn, S.H.; Kwon, J.H.; Yin, J.; Tam, L.T.; Ahn, H.S.; Myung, S.C.; Lee, M.W. Anti-Inflammatory and Anti-Urolithiasis Effects of Polyphenolic Compounds from Quercus gilva Blume. Molecules 2017, 22, 1121. [Google Scholar] [CrossRef]
- Grases, F.; Prieto, R.M.; Fernandez-Cabot, R.A.; Costa-Bauzá, A.; Tur, F.; Torres, J.J. Effects of Polyphenols from Grape Seeds on Renal Lithiasis. Oxid. Med. Cell. Longev. 2015, 2015, 813737. [Google Scholar] [CrossRef]
- Patel, P.K.; Patel, M.A.; Vyas, B.A.; Shah, D.R.; Gandhi, T.R. Antiurolithiatic Activity of Saponin Rich Fraction from the Fruits of Solanum xanthocarpum Schrad. & Wendl. (Solanaceae) against Ethylene Glycol Induced Urolithiasis in Rats. J. Ethnopharmacol. 2012, 144, 160–170. [Google Scholar] [CrossRef]
- Fouada, A.; Yamina, S.; Nait, M.A.; Mohammed, B.; Abdlekrim, R. In Vitro and in Vivo Antilithiasic Effect of Saponin Rich Fraction Isolated from Herniaria Hirsuta. Orgão Soc. Bras. Lat.-Am. Nefrol. 2006, 28, 199–203. [Google Scholar]
- El oumari, F.E.; Bousta, D.; Imtara, H.; Lahrichi, A.; Elhabbani, R.; El mouhri, G.; Al kamaly, O.; Saleh, A.; Parvez, M.K.; Grafov, A.; et al. Chemical Composition and Anti-Urolithiatic Activity of Extracts from Argania spinosa (L.) Skeels Press-Cake and Acacia senegal (L.) Willd. Molecules 2022, 27, 3973. [Google Scholar] [CrossRef]
- Mechqoq, H.; El Yaagoubi, M.; El Hamdaoui, A.; Momchilova, S.; Guedes da Silva Almeida, J.R.; Msanda, F.; El Aouad, N. Ethnobotany, Phytochemistry and Biological Properties of Argan Tree (Argania spinosa (L.) Skeels) (Sapotaceae)—A Review. J. Ethnopharmacol. 2021, 281, 114528. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Secondary Metabolites from Argania spinosa (L.) Skeels. Phytochem. Rev. 2002, 1, 345–354. [Google Scholar] [CrossRef]
- Bejaoui, M.; Taarji, N.; Saito, M.; Nakajima, M.; Isoda, H. Argan (Argania spinosa) Press Cake Extract Enhances Cell Proliferation and Prevents Oxidative Stress and Inflammation of Human Dermal Papilla Cells. J. Dermatol. Sci. 2021, 103, 33–40. [Google Scholar] [CrossRef] [PubMed]
- El Monfalouti, H.; Guillaume, D.; Denhez, C.; Charrouf, Z. Therapeutic Potential of Argan Oil: A Review. J. Pharm. Pharmacol. 2010, 62, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Kharbach, M.; Heyden, Y.V.; Yu, H.; Bouklouze, A.; Cherrah, Y.; Alaoui, K. In Vitro & In Vivo Anti-Hyperglycemic Potential of Saponins Cake and Argan Oil from Argania spinosa. Foods 2021, 10, 1078. [Google Scholar] [CrossRef]
- Ouyang, J.-M.; Zheng, H.; Deng, S.-P. Simultaneous Formation of Calcium Oxalate (Mono-, Di-, and Trihydrate) Induced by Potassium Tartrate in Gelatinous System. J. Cryst. Growth 2006, 293, 118–123. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Hess, B.; Meinhardt, U.; Zipperle, L.; Giovanoli, R.; Jaeger, P. Simultaneous Measurements of Calcium Oxalate Crystal Nucleation and Aggregation: Impact of Various Modifiers. Urol. Res. 1995, 23, 231–238. [Google Scholar] [CrossRef]
- Driouch, A.; Djelloul, A.; Kaid-Omar, Z.; Semmoud, A.; Rais, A.; Addou, A. Optimized Experimental Design for the Inhibition of Calcium Oxalate Using a Turbidimetrical Model. Asia-Pac. J. Chem. Eng. 2008, 3, 425–431. [Google Scholar] [CrossRef]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in Vitro Antioxidant Activity of Fennel (Foeniculum vulgare) Seed Extracts. LWT Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Raman, S.; Ganeshan, A.K.G.; Chen, C.; Jin, C.; Li, S.-H.; Chen, H.-J.; Gui, Z. In Vitro and In Vivo Antioxidant Activity of Flavonoid Extracted from Mulberry Fruit (Morus alba L.). Pharm. Mag. 2016, 12, 128. [Google Scholar] [CrossRef]
- Bao, Y.; Qu, Y.; Li, J.; Li, Y.; Ren, X.; Maffucci, K.; Li, R.; Wang, Z.; Zeng, R. In Vitro and In Vivo Antioxidant Activities of the Flowers and Leaves from Paeonia rockii and Identification of Their Antioxidant Constituents by UHPLC-ESI-HRMSn via Pre-Column DPPH Reaction. Molecules 2018, 23, 392. [Google Scholar] [CrossRef] [PubMed]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A Comprehensive Review on Biological Activities of P-Hydroxy Benzoic Acid and Its Derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Juurlink, B.H.; Azouz, H.J.; Aldalati, A.M.; AlTinawi, B.M.; Ganguly, P. Hydroxybenzoic Acid Isomers and the Cardiovascular System. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef]
- Freeman, T.L.; Thiele, G.M.; Klassen, L.W.; Klassen, B.T.; Mailliard, M.E. N-(Methylamino)Isobutyric Acid Inhibits Proliferation of CFSC-2C Hepatic Stellate Cells. Biochem. Pharmacol. 2004, 68, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Persky, A.M.; Berry, N.S.; Pollack, G.M.; Brouwer, K.L.R. Modelling the Cardiovascular Effects of Ephedrine. Br. J. Clin. Pharmacol. 2004, 57, 552–562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kommera, H.; Kaluđerović, G.N.; Bette, M.; Kalbitz, J.; Fuchs, P.; Fulda, S.; Mier, W.; Paschke, R. In Vitro Anticancer Studies of α- and β-d-Glucopyranose Betulin Anomers. Chem. Biol. Interact. 2010, 185, 128–136. [Google Scholar] [CrossRef]
- Carrillo, C.; del Cavia, M.M.; Alonso-Torre, S. Papel del Ácido Oleico en el Sistema Inmune; Mecanismos de Acción; Revisión Científica. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef]
- Leland, D.L.; Kotick, M.P. Analgesic Narcotic Antagonists. 4.0 7-Met Hyl-N-(Cycloalkylmet Hyl)-3-Hydroxymorp Hinan-6-Ones and -Isomorphinan-6-Ones. J. Med. Chem. 1980, 23, 1427–1431. [Google Scholar] [CrossRef]
- Guillaume, D.; Charrouf, Z. Saponines et métabolites secondaires de l’arganier (Argania spinosa). Cah. Agric. 2005, 14, 509–516. [Google Scholar]
- Souhila, M.; Mustapha, K. Etude de l’extraction des composés phénoliques de différentes parties de la fleur d’artichaut (Cynara scolymus L.). Nat. Technol. 2013, 6, 35–40. [Google Scholar]
- Majinda, R.R.T. Extraction and Isolation of Saponins. In Natural Products Isolation; Methods in Molecular Biology; Sarker, S.D., Nahar, L., Eds.; Humana Press: Totowa, HJ, USA, 2012; Volume 864, pp. 415–426. ISBN 978-1-61779-623-4. [Google Scholar]
- Kabran, G.R.; Mamyrbekova-Bekro, J.A.; Pirat, J.-L.; Bekro, Y.-A.; Sommerer, N.; Verbaere, A.; Meudec, E. Identification de composés Phénoliques Extraits de Deux Plantes de la Pharmacopée Ivoirienne. J. Soc. Ouest-Afr. Chim. 2014, 38, 57–63. [Google Scholar]
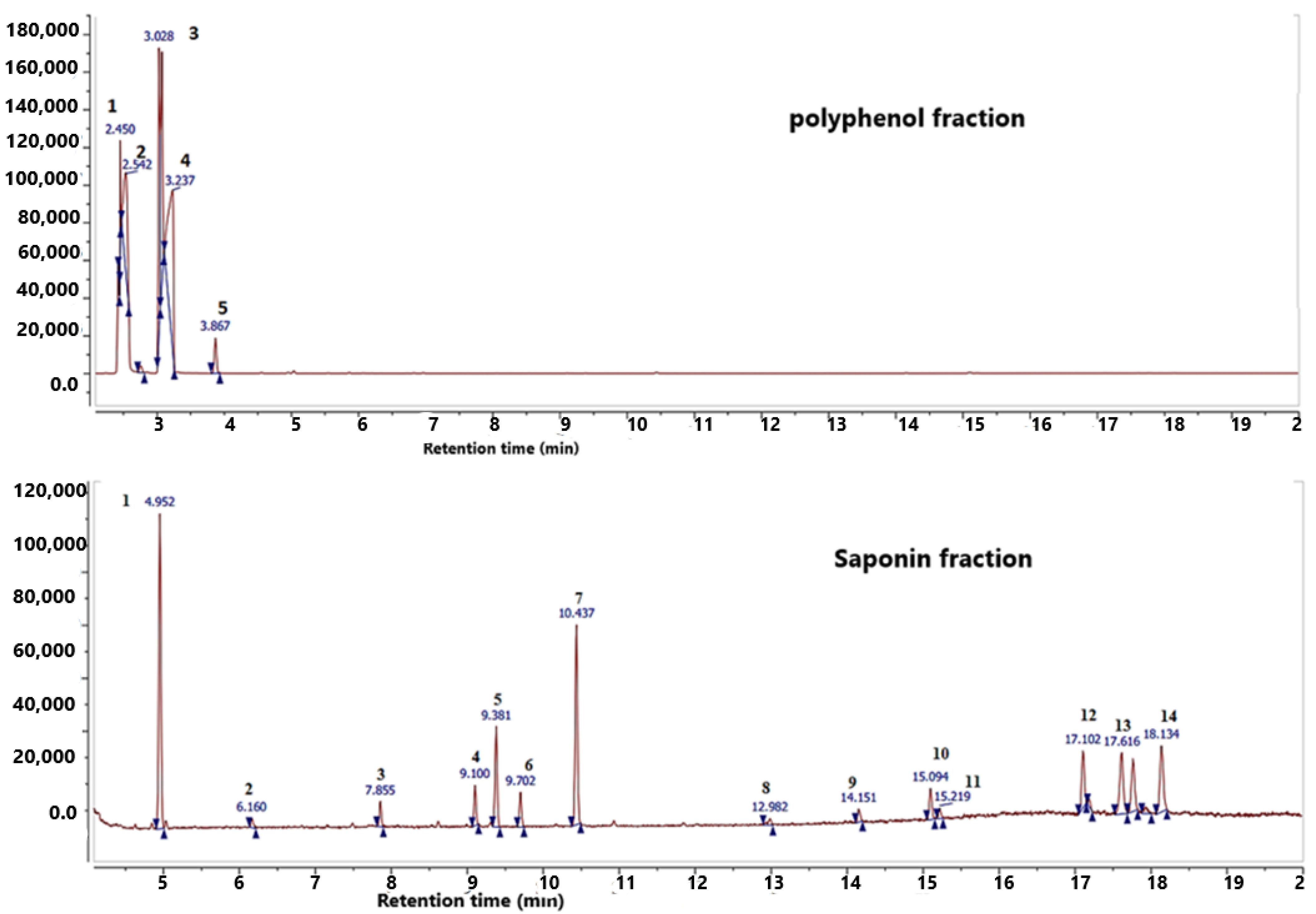



| No. | Retention Time (Min) | Compounds | Chemical Formula |
|---|---|---|---|
| 1 | 2.43 | Isocyanic acid/carbimide hydrogen isocyanate | HNCO |
| 2 | 2.75 | Propanoic acid,2-methyl-[cas] (isobutyric acid) | C4H8O2 |
| 3 | 3.03 | p-Hydroxybenzoic acid | C7H6O3 |
| 4 | 3.23 | Catechol | C6H6O2 |
| 5 | 3.87 | Ephedrine | C10H15NO |
| No. | Retention Time (Min) | Compounds | Chemical Formula |
|---|---|---|---|
| 1 | 4.96 | Silanamine, N,1,1,1-tetramethyl-N-(trimethylsilyl) | SiH5N |
| 2 | 7.85 | 3-Methyl-6-phenyl-imidazole [2,1-b] oxazole | C13H10N2O2S |
| 3 | 9.1 | 3,7-Dioxa-2,8disilanonane,2,2,8,8-tetramethyl-5-(trimethylsilyl)oxypropoxy)silane) | C9H24O2Si2 |
| 4 | 9.38 | N-methyl-n-phenyl-n’-(3-methoxyphenyl)-urea | C15H16N2O |
| 5 | 9.7 | Morphinan-6-one, 3-methoxy-17-methyl | C18H21NO3 |
| 6 | 10.43 | Imidazole,1,4-dimethyl-2-phenyl | C23H20N2 |
| 7 | 14.15 | Quinidine | C20H24N2O2 |
| 8 | 15.09 | Oleic acid TMS | C18H34O2 |
| 9 | 15.21 | Tetramethoxyflavone | C19H18O6 |
| 10 | 17.1 | Beta-D-galactofuranose,1,2,3,5,6-pentakis-o-[trimethylsilyl] | C21H52O6Si5 |
| 11 | 17.61 | D-xylofuranose,1,2,3,5 tetrakis-o-[trimethylsilyl] | C17H42O5Si4 |
| 12 | 18.13 | Glucofuranoside, methyl-tetrakis-o-trimethylsilyl | C19H46O6Si4 |
| 13 | 17.78 | D-ribopyranose,1,2,3,5 tetrakis-o-[trimethylsilyl] | C17H42O5Si4 |
| Concentration g/L | % Inhibition | R | CV | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P.F | S.F | Cit.P | P.F | S.F | Cit.P | P.F | S.F | Cit.P | |
| 0.25 | 79.76 ± 5.4 | 78.87 ± 4.03 | 75.47 ± 6.76 | 0.96 | 0.97 | 0.99 | 6.77 | 4.46 | 8.96 |
| 0.5 | 82.83 ± 4.32 | 83.49 ± 3.73 | 97.28 ± 0.29 | 0.97 | 0.97 | 0.97 | 5.21 | 5.11 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El oumari, F.E.; Mammate, N.; Imtara, H.; Lahrichi, A.; Elhabbani, R.; El mouhri, G.; Alqahtani, A.S.; Noman, O.M.; Ibrahim, M.N.; Grafov, A.; et al. Chemical Composition, Antioxidant Potentials, and Calcium Oxalate Anticrystallization Activity of Polyphenol and Saponin Fractions from Argania spinosa L. Press Cake. Plants 2022, 11, 1852. https://doi.org/10.3390/plants11141852
El oumari FE, Mammate N, Imtara H, Lahrichi A, Elhabbani R, El mouhri G, Alqahtani AS, Noman OM, Ibrahim MN, Grafov A, et al. Chemical Composition, Antioxidant Potentials, and Calcium Oxalate Anticrystallization Activity of Polyphenol and Saponin Fractions from Argania spinosa L. Press Cake. Plants. 2022; 11(14):1852. https://doi.org/10.3390/plants11141852
Chicago/Turabian StyleEl oumari, Fatima Ezzahra, Naima Mammate, Hamada Imtara, Anissa Lahrichi, Radouane Elhabbani, Ghita El mouhri, Ali S. Alqahtani, Omar M. Noman, Mansour N. Ibrahim, Andriy Grafov, and et al. 2022. "Chemical Composition, Antioxidant Potentials, and Calcium Oxalate Anticrystallization Activity of Polyphenol and Saponin Fractions from Argania spinosa L. Press Cake" Plants 11, no. 14: 1852. https://doi.org/10.3390/plants11141852
APA StyleEl oumari, F. E., Mammate, N., Imtara, H., Lahrichi, A., Elhabbani, R., El mouhri, G., Alqahtani, A. S., Noman, O. M., Ibrahim, M. N., Grafov, A., Bousta, D., & Sqalli Houssaini, T. (2022). Chemical Composition, Antioxidant Potentials, and Calcium Oxalate Anticrystallization Activity of Polyphenol and Saponin Fractions from Argania spinosa L. Press Cake. Plants, 11(14), 1852. https://doi.org/10.3390/plants11141852







