The Anatomical Differences and Physiological Responses of Sunburned Satsuma Mandarin (Citrus unshiu Marc.) Fruits
Abstract
1. Introduction
2. Results
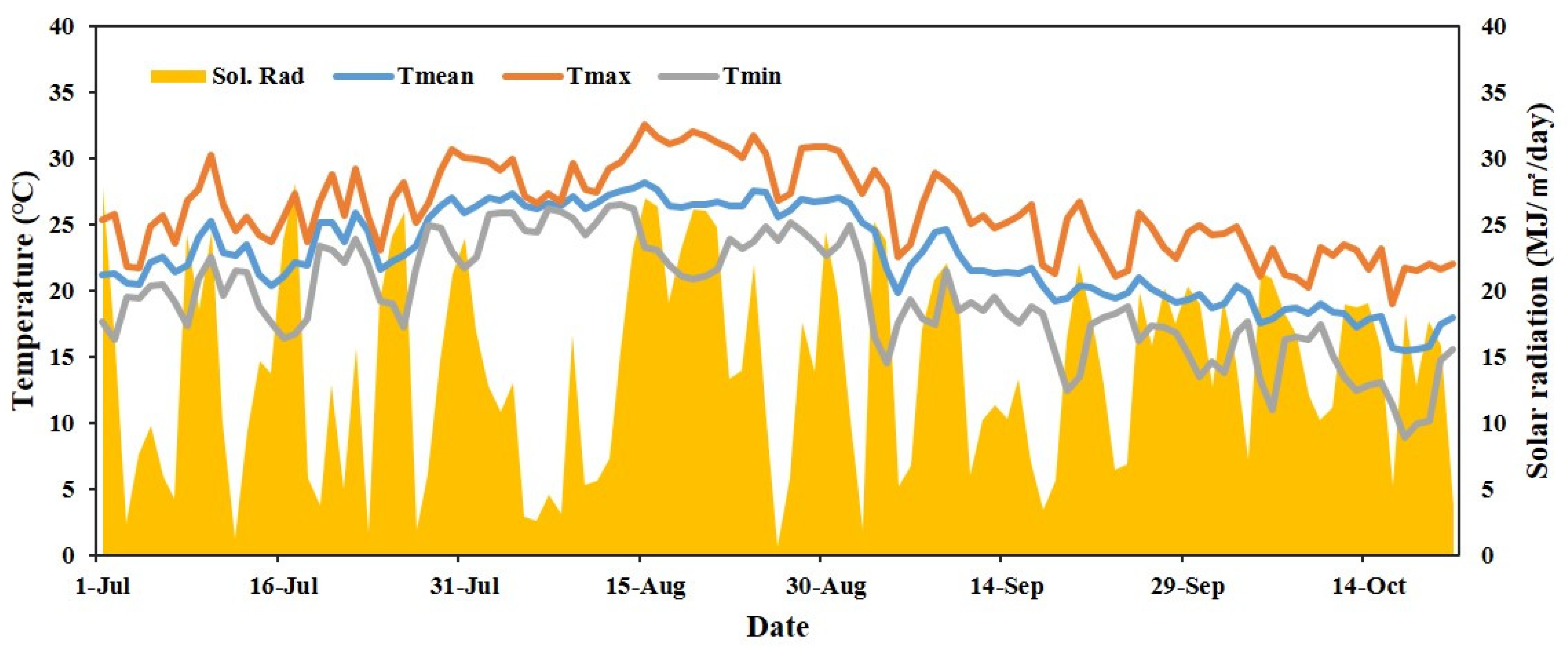
2.1. Experimental Conditions
2.2. Histological Observations according to the Sunburn Damage Severity
2.3. Physiological Analysis
2.3.1. Pigment Analysis
2.3.2. Flavonoid Analysis
2.3.3. Malondialdehyde (MDA) Analysis
2.3.4. Total Phenolic Content (TPC), Ferric Reducing Antioxidant Power (FRAP), and Antioxidant Activities
2.3.5. Analysis of Fruit Quality and Free Sugar and Organic Acid Contents
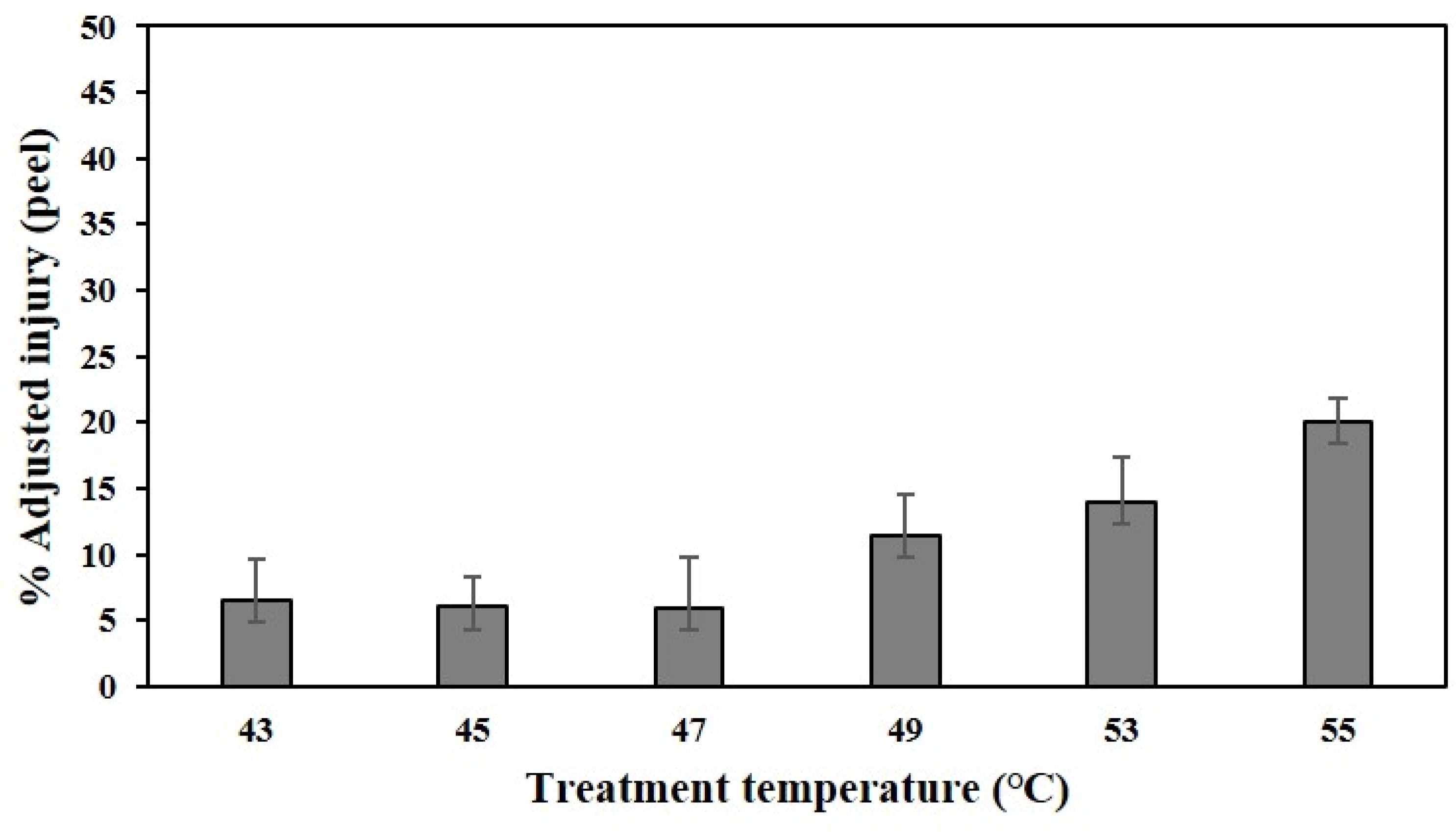
2.3.6. Heat Tolerance and Evaluation of Cell Membrane Damage
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Artificial Sunburn Induction
4.2. Anatomical Analysis Using Light Microscopy and SEM
4.3. Analysis of Pigment Contents
4.4. Lipid Peroxidation Analysis Using the MDA Assay
4.5. High-Performance Liquid Chromatography (HPLC) Analysis of Flavonoid Content and Antioxidant Activity
4.6. Analysis of Fruit Properties
4.7. HPLC Analysis of Sugar and Organic Acid
4.8. Electrolyte Leakage Analyses
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabinowitch, H.D.; Kedar, N.; Budowski, P. Induction of sunscald damage in tomatoes under natural and controlled conditions. Sci. Hortic. 1974, 2, 265–272. [Google Scholar] [CrossRef]
- Parchomchuk, P.; Meheriuk, M. Orchard cooling with pulsed over- tree irrigation to prevent solar injury and improve fruit quality of ‘Jonagold’ apples. Hortscience 1996, 3, 802–804. [Google Scholar] [CrossRef]
- Schrader, L.E.; Zhang, J.; Duplaga, W.K. Two types of sunburn in apple caused by high fruit surface (peel) temperature. Plant Health Prog. 2001, 2, 3. [Google Scholar] [CrossRef]
- Tarara, J.M.; Spayd, S.D. Tackling ‘sunburn’ in red wine grapes through temperature and sunlight exposure. Good Fruit Grow. 2005, 56, 40–41. [Google Scholar]
- Racsko, J.; Schrader, L.E. Sunburn of apple fruit: Historical background, recent advances and future perspectives. Crit. Rev. Plant Sci. 2012, 31, 455–504. [Google Scholar] [CrossRef]
- IPCC. Climate Change: The Physical science basis. In Contribution of Working Groups I to the sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Korea Meteorological Administration. Korean Climate Change Assessment Report. Available online: http://www.climate.go.kr/home/cc_data/2020/Korean_Climate_Change_Assessment_Report_2020_1_summary.pdf (accessed on 1 July 2020).
- Park, Y.; Kim, M.; Yun, S.K.; Kim, S.S.; Joa, J. A simple model for predicting sunburn on Satsuma mandarin fruit. Sci. Hortic. 2022, 292, 110658. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Marin, F.R.; Ortuño, A.; Del Río, J.A. Uses and properties of citrus flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4514. [Google Scholar] [CrossRef]
- Bocco, A.; Cuvelier, M.; Richard, H.; Berset, C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and oranges juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef]
- Laganá, V.; Giuffrè, A.M.; De Bruno, A.; Poiana, M. Formulation of biscuits fortified with a flour obtained from bergamot by-products (Citrus bergamia, Risso). Foods 2022, 11, 1137. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, K.J.; Yun, S.H.; Choi, Y.H. Bioactive compounds and antioxidant capacity of domestic citrus cultivar ‘Haryejosaeng’. Korean J. Food Preserv. 2019, 26, 681–689. [Google Scholar] [CrossRef]
- Jeju Special Self-Governing Province Citrus Marketing & Shipping Association. Citrus Annual Distribution Processing Analysis. Available online: http://www.citrus.go.kr (accessed on 20 October 2020).
- Minessy, F.A.; Nasr, T.A.A.; El-Shurafa, M.Y. Citrus fruit temperature in relation to sunburn. In Proceedings of the Conference on Tropical and Subtropical Fruits, London, UK, 15–19 September 1969; pp. 245–252. [Google Scholar]
- Wan, J.F.; Li, J.; Chen, J.Z. Changes in antioxidant metabolism in the fruit pericarps of citrus during sunburn development. Acta Hortic. Sin. 2012, 39, 2009. [Google Scholar] [CrossRef]
- Kang, S.-B.; Moon, Y.-E.; Yankg, K.-R.; Joa, J.-H.; Lee, H.J. Effect of the harvest season on the yield and growth of unripe fruit and biennial flowering of ‘Miyagawa’ satsuma mandarin in open field cultivation. Korean J. Environ. Agric. 2019, 38, 314–320. [Google Scholar] [CrossRef]
- Tasi, M.-S.; Lee, T.-C.; Chang, P.-T. Comparison of paper bags, calcium carbonate, and shade nets for sunscald protection in ‘Murcott’ Tangor fruit. HortTechnology 2013, 23, 659–667. [Google Scholar]
- Lee, T.-C.; Zhong, P.-J.; Chang, P.-T. The effects of preharvest shading and postharvest storage temperatures on the quality of ‘Ponkan’ (Citrus reticulate Blanco) mandarin fruits. Sci. Hortic. 2015, 188, 57–65. [Google Scholar] [CrossRef]
- Ennab, H.A.; El-Sayed, S.A.; Abo El-Enin, M.M.S. Effect of kaolin applications on fruit sunburn, yield and fruit quality of balady mandarin (Citrus reticulate, Blanco). Menoufia J. Plant Prod. 2017, 2, 129–138. [Google Scholar] [CrossRef]
- Tajvar, Y.; Ghazvini, R.F.; Hamidoghli, Y.; Sajedi, R.H. Antioxidant changes of Thomson navel orange (Citrus sinensis) on three rootstocks under low temperature stress. Hortic. Environ. Biotechnol. 2011, 52, 576–580. [Google Scholar] [CrossRef]
- Kim, M.; Yun, S.K.; Kim, S.S.; Park, Y.; Joa, J.; Han, S. Influence of freezing temperatures on metabolite composition and antioxidant activity in Shiranuhi mandarin. Sci. Horti. 2021, 288, 110397. [Google Scholar] [CrossRef]
- Wang, L.-J.; Loescher, W.; Duan, W.; Li, W.-D.; Yang, S.-H.; Li, S.-H. Heat acclimation induced acquired heat tolerance and cross adaptation in different grape cultivars: Relationships to photosynthetic energy partitioning. Funct. Plant Biol. 2009, 36, 516–526. [Google Scholar] [CrossRef]
- Araújo, M.; Santos, C.; Dias, M.C. Can Young olive plants overcome heat shock? In Theory and Practice of Climate Adaptation; Alves, F., Filho, W.L., Azeiteiro, U., Eds.; Springer: Cham, Switzerland, 2018; pp. 193–203. [Google Scholar] [CrossRef]
- Yasuhiko, K.; Tomoka, S.; Mari, N. Studies on the mechanism of sunscald occurrence and its mitigation in citrus fruit. Bull. Yamaguchi Agric. Fore. Gene Tec. Ctr. 2020, 11, 61–73. [Google Scholar]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Zappia, C.; Capocasale, M. Physico-chemical stability of blood orange juice during frozen storage. Int. J. Food Prop. 2017, 20, 1930–1943. [Google Scholar] [CrossRef]
- Giuffrè, A.M. Bergamot (Citrus bergamia, Risso): The effects of cultivar and harvest date on functional properties of juice and cloudy juice. Antioxidants 2019, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Sayyari, M.; Ghanbari, F.; Fatahi, S.; Bavandpour, F. Chilling tolerance improving of watermelon seedling by salicylic acid seed and foliar application. Not. Sci. Biol. 2013, 5, 67–73. [Google Scholar] [CrossRef][Green Version]
- Petracek, P.D. Peel morphology and fruit blemishes. In Citrus Flowering and Fruiting Short Course; CREC: Lake Alfred, FL, USA, 1997; pp. 18–118. [Google Scholar]
- Wang, J.; Hao, H.; Liu, R.; Ma, Q.; Xu, J.; Chen, F.; Cheng, Y.; Deng, X. Comparative analysis of surface wax in mature fruits between satsuma mandarin (Citrus unshiu) and ‘Newhall’ navel orange (Citrus sinensis) from the perspective of crystal morphology, chemical composition and key gene expression. Food Chem. 2014, 153, 177–185. [Google Scholar] [CrossRef]
- Bain, J.M. Morphological, anatomical, and physiological changes in the developing fruit of the Valencia orange, Citrus sinesis (L.) Osbeck. Aust. J. Bot. 1958, 6, 1–24. [Google Scholar] [CrossRef]
- Spiegel-Roy, P.; Goldschmidt, E.E. Biology of Citrus Cambridge; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Schrader, L.E.; Kahn, C.B.; Felicetti, D.A.; Sun, J.; Xu, J.; Zhang, J. Effects of high temperature and high solar irradiance on sunburn, quality, and skin pigments of apple fruit. In Proceedings of the X International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems, Bologna, Italy, 28 August–2 September 2008; pp. 1025–1039. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.-Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplast to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef]
- Chen, L.-S.; Li, P.; Cheng, L. Effects of high temperature coupled with high light on the balance between photooxidation and photoprotection in the sun-exposed peel of apple. Planta 2008, 228, 745–756. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Changes in pigment concentrations associated with the degree of sunburn browning of ‘Fuji’ apple. J. Am. Soc. Hort. Sci. 2008, 133, 27–34. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Changes in pigment concentrations associated with sunburn browning of five apple cultivars. I. Chlorophylls and carotenoids. Plant Sci. 2009, 176, 78–83. [Google Scholar] [CrossRef]
- Sdiri, S.; Bermejo, A.; Aleza, P.; Navarro, P.; Salvador, A. Phenolic composition, organic acids, sugars, vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Food Res. Int. 2012, 49, 462–468. [Google Scholar] [CrossRef]
- Kim, M.; Yun, S.K.; Kim, S.S.; Park, Y.; Joa, J.; Han, S.; Shin, K.; Song, K.J. Response of citrus to freezing tolerance differs depending on genotypes and growing conditions. Hortic. Environ. Biotechnol. 2021, 62, 181–189. [Google Scholar] [CrossRef]
- Song, E.-Y.; Choi, Y.-H.; Kang, K.-H.; Koh, J.-S. Free sugar, organic acid, hesperidin, naringin and inorganic elements changes of Cheju citrus fruits according to harvest. Korean J. Food Sci. Technol. 1998, 4, 306–312. [Google Scholar]
- Yamazaki, Y.; Suh, D.-Y.; Sitthithaworn, W.; Ishiguro, K.; Kobayashi, Y.; Shibuya, M.; Ebizuka, Y.; Sankawa, U. Diverse chalcone synthase superfamily enzymes from the most primitive vascular plant, Psilotum nudum. Planta 2001, 214, 75–84. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, T. The significance of the study about the biological effects of solar ultraviolet radiation using the exposed facility on the international space station. Biol. Sci. Space 2004, 18, 255–260. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Roussos, P.A. Phytochemicals and antioxidant capacity of orange (Citrus sinensis (L.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Sci. Hortic. 2011, 129, 253–258. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf Senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Photooxidative sunburn of apples: Characterization of a third type of apple sunburn. Int. J. Fruit Sci. 2008, 8, 160–172. [Google Scholar] [CrossRef]
- Clément, C.; Burrus, M.; Audran, J.-C. Floral organ growth and carbohydrate content during pollen development in Lilium. Amer. J. Bot. 1996, 83, 459–469. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Jakhar, S.; Mukherjee, D. Chloroplast pigments, proteins, lipid peroxidation and activities of antioxidative enzymes during maturation and senescence of leaves and reproductive organs of Cajanus cajan L. Physiol. Mol. Biol. Plants. 2014, 20, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Lim, C.C.; Arora, R.; Townsend, E.C. Comparing Gompertz and Richards functions to estimate freezing injury in Rhododendron using electrolyte leakage. J. Am. Soc. Hortic. Sci. 1998, 123, 246–252. [Google Scholar] [CrossRef]






| Sample | Stage | Rutin | Narirutin | Naringin | Hesperidin | Neohesperidin | Nobiletin | Tangeretin | Total |
|---|---|---|---|---|---|---|---|---|---|
| Pulp | Control | 99.6 ± 6.4 d | 1034.6 ± 73.7 e | ND a | 1170.6 ± 48.3 d | ND | ND | ND | 2304.8 ± 122.5 d |
| I | 128.8 ± 11.8 c | 1319.2 ± 24.4 d | ND | 1215.0 ± 68.0 cd | ND | ND | ND | 2663.1 ± 92.0 c | |
| II | 163.7 ± 3.6 b | 1638.8 ± 33.1 b | ND | 1423.9 ± 20.9 bc | ND | ND | ND | 3226.4 ± 39.1 b | |
| III | 197.3 ± 1.3 a | 1850.4 ± 25.2 a | ND | 1584.1 ± 110.7 ab | ND | ND | ND | 3631.9 ± 114.4 a | |
| IV | 157.3 ± 2.4 b | 1501.7 ± 31.4 c | ND | 1683.3 ± 74.0 a | ND | ND | ND | 3342.3 ± 102.9 ab | |
| Peel | Control | 587.2 ± 51.9 e | 3134.4 ± 146.1 ab | 34.3 ± 2.0 a | 4615.1 ± 166.6 ab | Traces | 116.5 ± 2.6 a | 48.9 ± 0.3 a | 8536.3 ± 345.9 b |
| I | 721.4 ± 7.5 d | 2747.3 ± 64.0 b | 24.6 ± 3.4 ab | 3968.1 ± 288.0 b | Traces | 94.6 ± 4.1 b | 39.3 ± 1.2 b | 7595.3 ± 335.5 bc | |
| II | 1098.6 ± 26.2 b | 2830.3 ± 50.1 b | 23.2 ± 0.3 ab | 4386.2 ± 204.7 ab | Traces | 81.3 ± 1.7 c | 33.0 ± 0.6 c | 8452.6 ± 269.5 bc | |
| III | 1211.0 ± 39.3 a | 3469.0 ± 166.1 ab | 12.3 ± 6.2 bc | 4803.3 ± 214.8 a | Traces | 107.5 ± 2.3 a | 41.7 ± 1.0 b | 9644.9 ± 420.3 a | |
| IV | 980.4 ± 28.5 c | 2016.1 ± 175.7 c | 6.4 ± 6.4 c | 4297.0 ± 295.6 ab | Traces | 64.7 ± 4.6 d | 23.0 ± 1.6 d | 7387.6 ± 241.1 c |
| Treatment | TSS | Acidity (%) | Firmness | L* | a* | b* |
|---|---|---|---|---|---|---|
| Control | 9.0 ± 0.1 *** | 1.2 ± 0.2 *** | 8.0 ± 0.2 *** | 69.6 ± 0.2 *** | 18.4 ± 0.5 | 70.9 ± 0.3 *** |
| Sunburn | 8.6 ± 0.1 | 1.1 ± 0.2 | 12.5 ± 0.6 | 62.6 ± 0.8 | 16.8 ± 0.9 | 60.8 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Park, Y.; Yun, S.K.; Kim, S.S.; Joa, J.; Moon, Y.-E.; Do, G.-R. The Anatomical Differences and Physiological Responses of Sunburned Satsuma Mandarin (Citrus unshiu Marc.) Fruits. Plants 2022, 11, 1801. https://doi.org/10.3390/plants11141801
Kim M, Park Y, Yun SK, Kim SS, Joa J, Moon Y-E, Do G-R. The Anatomical Differences and Physiological Responses of Sunburned Satsuma Mandarin (Citrus unshiu Marc.) Fruits. Plants. 2022; 11(14):1801. https://doi.org/10.3390/plants11141801
Chicago/Turabian StyleKim, Misun, Yosup Park, Seok Kyu Yun, Sang Suk Kim, Jaeho Joa, Young-Eel Moon, and Gyung-Ran Do. 2022. "The Anatomical Differences and Physiological Responses of Sunburned Satsuma Mandarin (Citrus unshiu Marc.) Fruits" Plants 11, no. 14: 1801. https://doi.org/10.3390/plants11141801
APA StyleKim, M., Park, Y., Yun, S. K., Kim, S. S., Joa, J., Moon, Y.-E., & Do, G.-R. (2022). The Anatomical Differences and Physiological Responses of Sunburned Satsuma Mandarin (Citrus unshiu Marc.) Fruits. Plants, 11(14), 1801. https://doi.org/10.3390/plants11141801





