Foliar Spraying of Solanum tuberosum L. with CaCl2 and Ca(NO3)2: Interactions with Nutrients Accumulation in Tubers
Abstract
:1. Introduction
2. Results
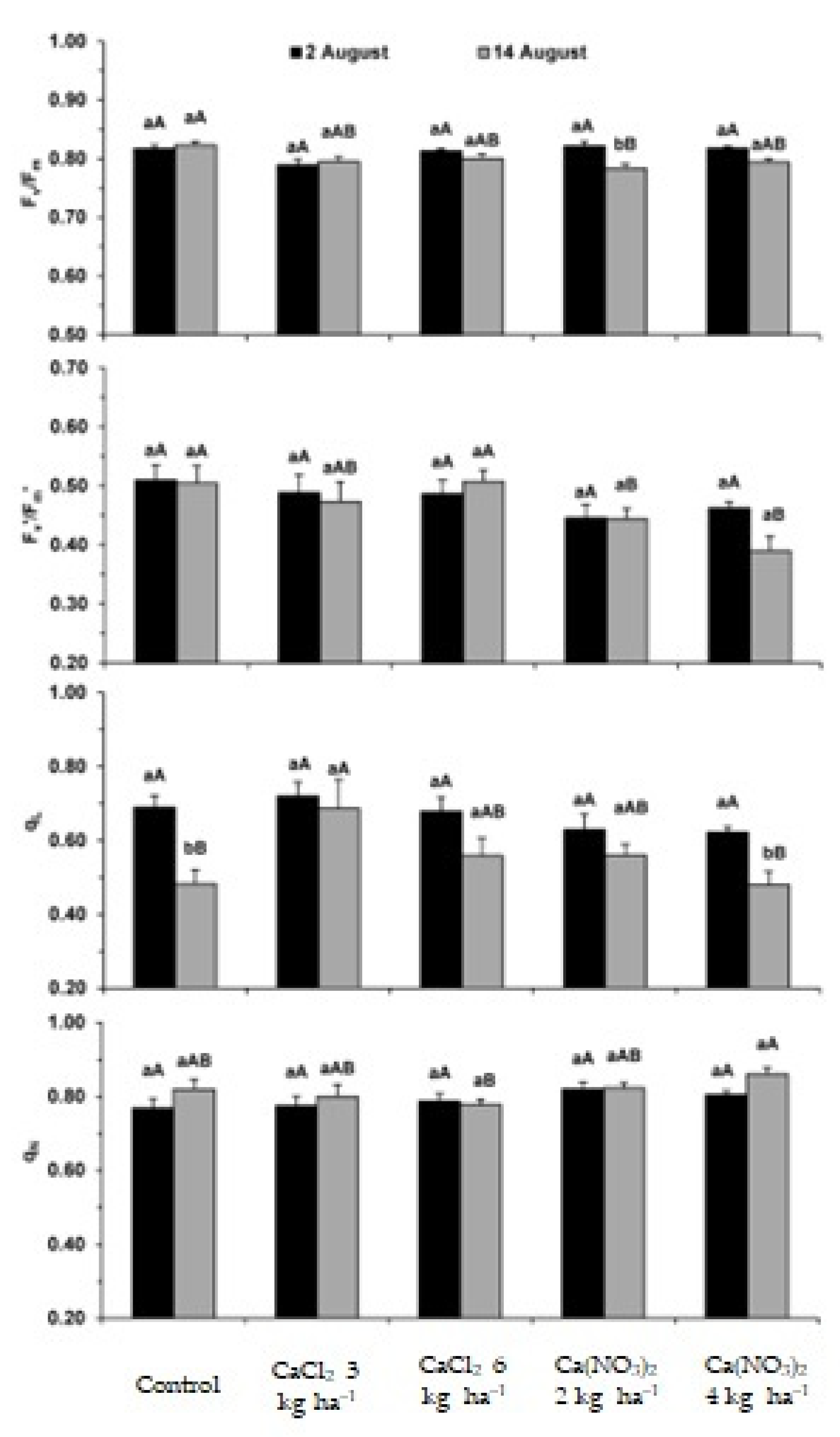
2.1. Photosynthetic Apparatus Performance
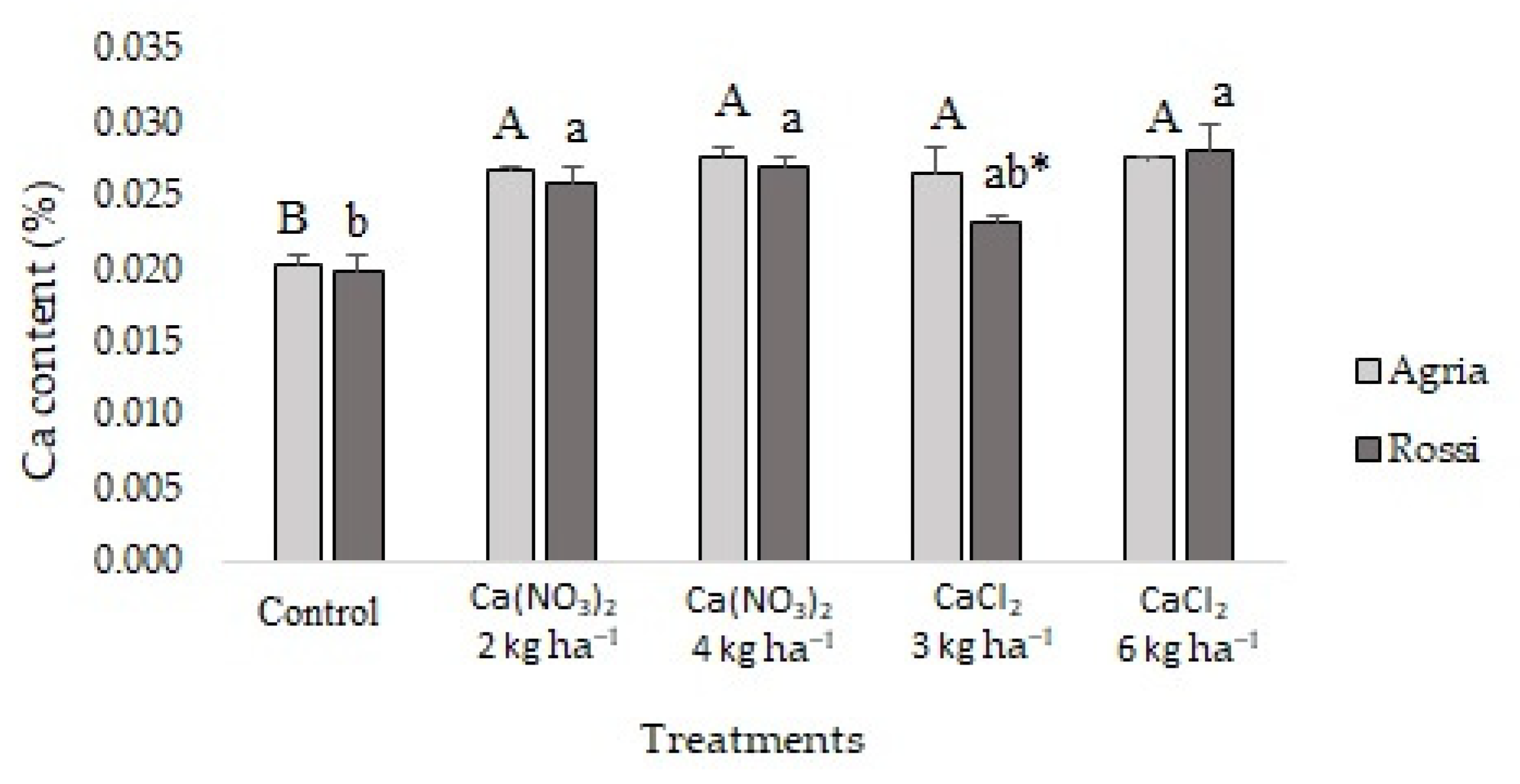
2.2. Mineral Content in Tubers
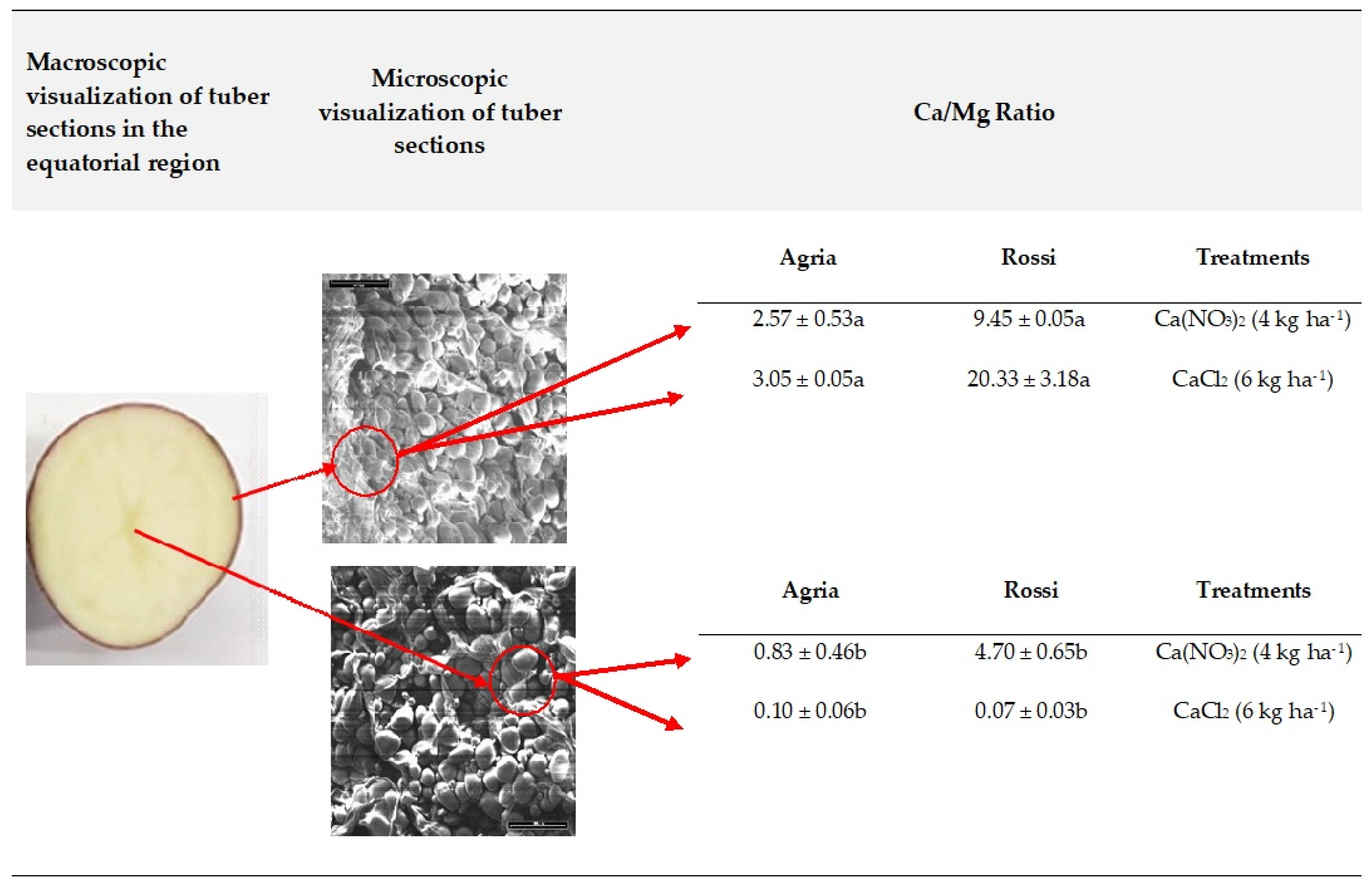
2.3. Tissue Localization of Mineral Elements in Tubers
2.4. Size and Colorimetric Characteristics
3. Discussion
3.1. Impact on the Performance of the Photosynthetic Apparatus
3.2. Calcium Accumulation and Interaction with Other Nutrients
3.3. Accumulation in Tuber Tissue
4. Materials and Methods
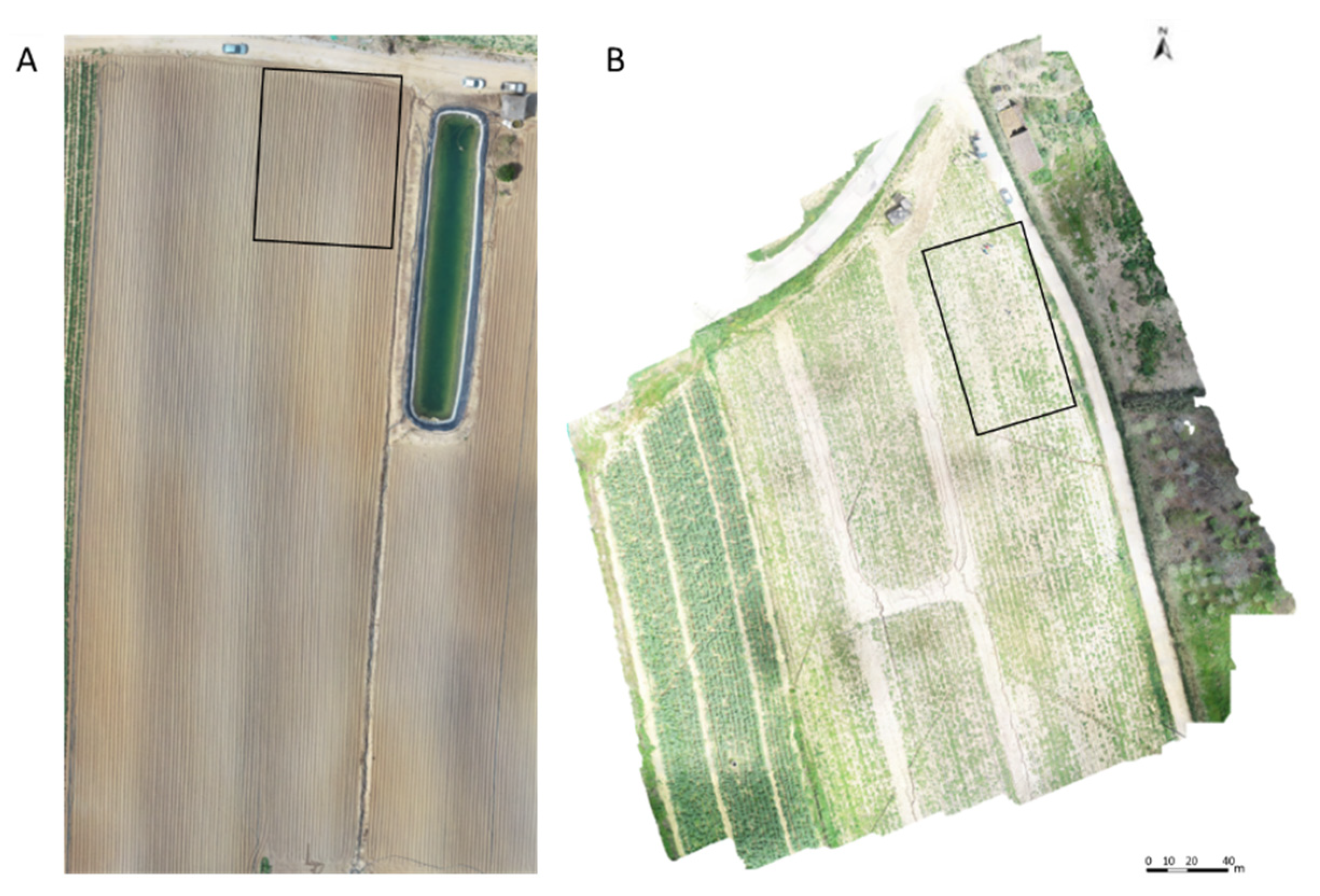
4.1. Experimental Fields
4.2. Chlorophyll a Fluorescence Parameters
4.3. Analysis of Total Nutrients by Atomic Absorption Spectrophotometry
4.4. Analysis of Ca/Mg by Scanning Electron Microscope Coupled to X-ray Dispersive Energy Spectroscopy
4.5. Analysis of Nutrients in Tissues through Fluorescence Detection
4.6. Height, Diameter, and Colorimetric Parameters
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, S.; Qin, G.; Zhang, H.; Tao, S.; Wu, J.; Wang, S.; Zhang, S. Calcium treatments promote the aroma volatiles emission of pear (Pyrus ussuriensis “Nanguoli”) fruit during post-harvest ripening process. Sci. Hortic. 2017, 215, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates, Inc.: Sunderland, UK, 2002; p. 665. [Google Scholar]
- White, P.J. The pathways of calcium movement to the xylem. J. Exp. Bot. 2001, 52, 891–899. [Google Scholar] [CrossRef]
- Moore, C.A.; Bowen, H.C.; Scrase-Field, S.; Knight, M.R.; White, P.J. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevation in root endodermal cells subjected to cooling. Plant J. 2002, 30, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.M.; Shuman, L. Micronutrient nutrition of plants. CRC Crit. Rev. Plant Sci. 1995, 14, 49–82. [Google Scholar] [CrossRef]
- Barthakur, N.N.; Donnelly, D.J.; Habib, A. Transfer of strontium-90 and Ca-45 from medium to plant and their translocation in micropropagated potato. In Proceedings of the International Congress on the Radioecology and Ecotoxicology of Continental and Estuarine Environments, Aix en Provence, France, 1 September 2001–31 January 2003; pp. 3–7. [Google Scholar]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar] [CrossRef]
- Ziegler, H. Nature of transported substances. In Transport in Plants I: Phloem Transport. Encyclopedia of Plant Physiology; Zimmermann, M.H., Milburn, J.A., Eds.; Springer: New York, NY, USA, 1975; Volume 1, pp. 59–100. [Google Scholar] [CrossRef]
- Welch, R.M. Importance of seed mineral nutrition reserves in crop growth and development. In Mineral Nutrition of Crops: Fundamental Mechanisms and Implications; Rengel, Z., Ed.; Food Products Press: New York, NY, USA, 1999; pp. 2005–2226. [Google Scholar]
- Ho, L.; White, P.J. A cellular hypothesis for the induction of blossom and rot in tomato fruit. Ann. Bot. 2005, 95, 571–581. [Google Scholar] [CrossRef] [Green Version]
- Davies, H.V.; Millard, P. Fractionation and distribution of calcium in sprouting and non-sprouting potato tubers. Ann. Bot. 1985, 56, 745–754. [Google Scholar] [CrossRef]
- Oparka, K.J.; Davies, H.V. Subcellular localization of calcium in potato tubers. Potato Res. 1988, 31, 297–304. [Google Scholar] [CrossRef]
- Nelson, D.P.; Pan, W.L.; Franceschi, V.R. Xylem and phloem transport of mineral nutrients from Solanum tuberosum roots. J. Exp. Bot. 1990, 41, 1143–1148. [Google Scholar] [CrossRef]
- Bonomelli, C.; Gil, P.M.; Schaffer, B. Effect of soil type on calcium absorption and partitioning in young avocado (Persea americana Mill.) trees. Agronomy 2019, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- Coelho, A.R.F.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Luís, I.C.; Caleiro, J.C.; Simões, M.; Kullberg, J.; Legoinha, P.; Brito, G.; et al. Can foliar pulverization with CaCl2 and Ca(NO3)2 trigger Ca enrichment in Solanum Tuberosum L. tubers? Plants 2021, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Lundergardh, H. Mineral Nutrition of Plants. Annu. Rev. Biochem. 1934, 3, 485–498. [Google Scholar] [CrossRef]
- Wall, M.E. The role of potassium in plants: III. Nitrogen and carbohydrate metabolism in potassium-deficient plants supplied with either nitrate or ammonium nitrogen. Soil Sci. 1940, 49, 393–408. [Google Scholar] [CrossRef]
- Ishizuka, Y.; Tanaka, A. Studies on the metabolism of nutritional elements in rice plants. J. Sci. Soil Manure 1960, 31, 491–494. [Google Scholar]
- Soliman, M.F.; Kostandi, S.F.; van Beusichem, M.L. Influence of sulfur and nitrogen fertilizer on the uptake of iron, manganese, and zinc by corn plants grown in calcareous soil. Commn. Soil Sci. Plant Anal. 1992, 23, 1289–1300. [Google Scholar] [CrossRef]
- Kawasaki, T. Metabolism and physiology of calcium and magnesium. Sci. Rice Plant. Tokyo Food Agric. Policy Res. Cent. 1995, 2, 412–419. [Google Scholar]
- Fageria, N.K. Ionic interactions in rice plants from dilute solutions. Plant Soil 1983, 70, 309–316. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Growth and nutrient concentrations of common bean, lowland rice, corn soybean, and wheat at different soil pH on an Inceptisol. J. Plant Nutr. 1999, 22, 1495–1507. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wang, Y.; Yang, S.; Qu, H. The changes of intracellular calcium concentration and distribution in the hard end pear (Pyrus pyrifolia cv. “Whangkeumbae”) fruit. Cell Calcium 2018, 71, 15–23. [Google Scholar] [CrossRef] [PubMed]
- de Valença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Sec. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Ozgen, S.; Palta, J.P. Supplemental calcium application influences potato tuber number and size. HortScience 2004, 40, 102–105. [Google Scholar] [CrossRef] [Green Version]
- PEI-Nutrient Management, Prince Edward Island, Canada. 2014. Available online: www.gov.pe.ca/agriculture/labservices (accessed on 1 June 2021).
- El-Hadidi, E.M.; El-Dissoky, R.A.; Abdelhafez, A.A.H. Foliar calcium and magnesium application effect on potato crop grown in clay loam soils. JSSAE 2017, 8, 1–8. [Google Scholar] [CrossRef]
- El-Zohiri, S.S.M.; Asfour, H.E. Effects of foliar sprays of potassium, magnesium and calcium on yield, quality and storageability of potato. In Proceedings of the Fifth International Congress of Suastain Agriculture Development 2009, Fayoum, Egypt, 21–23 December 2009; Faculty of Agriculture, Fayoum University: Faiyum, Egypt, 2009; pp. 57–71. [Google Scholar]
- Seifu, Y.W.; Deneke, S. Effect of calcium chloride and calcium nitrate on potato (Solanum tuberosum L.) growth and yield. J. Hortic 2017, 4, 207. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef] [Green Version]
- Urban, O.; Hlaváčová, M.; Klem, K.; Novotná, K.; Rapantová, B.; Smutná, P.; Horáková, V.; Hlavinka, P.; Trnka, M. Combined effects of drought and high temperature on photosynthetic characteristics in four winter wheat genotypes. Field Crop. Res. 2018, 223, 137–149. [Google Scholar] [CrossRef]
- Dubberstein, D.; Lidon, F.C.; Rodrigues, A.P.; Semedo, J.N.; Marques, I.; Rodrigues, W.P.; Gouveia, D.; Armengaud, J.; Semedo, M.C.; Martins, S.; et al. Resilient and sensitive key points of the photosynthetic machinery of Coffea spp. to the single and superimposed exposure to severe drought and heat stresses. Front. Plant Sci. 2020, 11, 1049. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rebelo, M.C.; Santos, M.E.; Antunes, M.L.; Nunes, M.A. Effects of calcium deficiency on Coffea arabica. Nutrient changes and correlation of calcium levels with some photosynthetic parameters. Plant Soil 1995, 172, 87–96. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trimpelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta 2015, 1847, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hao, L.; Zhu, B.; Jiang, Z. Plant Calcium signaling in response to potassium deficiency. Int. J. Mol. Sci. 2019, 19, 3456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) fluorometry and saturation pulse method: An overview. Chlorophyll Fluoresc. 2004, 19, 279–319. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Zlatev, Z.S.; Leitão, A.E.; Pais, I.P.; Fortunato, A.; Lidon, F.C. Moderate water stress causes different stomatal and non-stomatal changes in the photosynthetic functioning of Phaseolus vulgaris L. genotypes. Plant Biol. 2014, 16, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwell, F.; Goulao, L.; et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Glob. Chang. Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef]
- Coelho, A.R.F.; Pessoa, C.C.; Marques, A.C.; Luís, I.C.; Daccak, D.; Silva, M.M.; Simões, M.; Reboredo, F.H.; Pessoa, M.F.; Legoinha, P.; et al. Natural mineral enrichment in Solanum tuberosum L. cv. Agria: Accumulation of Ca and interaction with other nutrients by XRF analysis. Biol. Life Sci. Forum 2021, 4, 77. [Google Scholar] [CrossRef]
- Coelho, A.; Marques, A.; Campos Pessoa, C.; Daccak, D.; Carmo Luís, I.; Silva, M.; Simões, M.; Reboredo, F.; Pessoa, M.; Legoinha, P.; et al. Natural enrichment of Solanum tuberosum L. with calcium—Monitorization of mineral interactions in Plant Tissues. Biol. Life Sci. Forum 2022, 11, 28. [Google Scholar] [CrossRef]
- Weinl, S.; Held, K.; Schlücking, K.; Steinhorst, L.; Kuhlgert, S.; Hippler, M.; Kudla, J. A plastid protein crucial for Ca2+ regulated stomatal responses. New Phytol. 2008, 179, 675–686. [Google Scholar] [CrossRef]
- Subramanian, N.K.; White, P.J.; Broadley, M.R.; Ramsay, G. The three-dimensional distribution of minerals in potato tubers. Ann. Bot. 2011, 107, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Drazeta, L.; Lang, A.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Causes and effects of changes in xylem functionality in apple fruit. Ann. Bot. 2004, 93, 275–282.0. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K. Nutrients interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Jacobsen, S.T. Interaction between phosphate and calcium in nutrient uptake by plant roots. Comm. Soil Sci. Plant Anal. 1979, 10, 141–152. [Google Scholar] [CrossRef]
- Robson, A.D.; Pitman, J.B. Interactions between Nutrients in Higher Plants; Springer: Berlin/Heidelberg, Germany, 1983; pp. 147–180. [Google Scholar]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Läuchli, A.; Polito, V.S. Displacement of Ca2+ by Na+ from the plasmalemma of root cells: A primary response to salt stress? Plant Physiol. 1985, 79, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengel, Z. The role of calcium in salt toxicity. Plant Cell Environ. 1992, 15, 625–632. [Google Scholar] [CrossRef]
- LaHaye, P.A.; Epstein, E. Salt toleration by plants: Enhancement with calcium. Science 1969, 166, 395–396. [Google Scholar] [CrossRef]
- Cramer, G. Sodium-calcium interactions under salinity stress. In Salinity: Environment—Plants—Molecules; Springer: Dordrecht, The Netherlands, 2002; pp. 205–227. [Google Scholar] [CrossRef]
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef]
- Coleman, J.E. Zinc enzymes. Curr. Opin. Chem. Biol. 1998, 2, 222–234. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, Y. Effects of Zn deficiency and bicarbonate on the growth and photosynthetic characteristics of four plant species. PLoS ONE 2017, 12, e0169812. [Google Scholar] [CrossRef]
- Madero, P.; Pequerul, A.; Perez, C.; Val, J.; Monge, E. Specificity of iron in some aspects of soybean (Glicine max L.). In Optimization of Plant Nutrition; Springer: Dordrecht, The Netherlands, 1993; pp. 497–502. [Google Scholar]
- Lu, A.; Jing, G.; Wu, P.; Ni, J.; Jiang, S.; Zhang, Y. Rice genotype differences in nutrient status under excessive ferric iron conditions. J. Plant Nutr. 1997, 20, 1361–1373. [Google Scholar] [CrossRef]
- van de Mortel, J.E.; Almar, V.L.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; Themaat, E.; Koornneef, M.; Aarts, M. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef] [Green Version]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.I.; Rubio, V.; Pérez-Pérez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 2010, 6, e1001102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jifon, J.L.; Lester, G.E. Foliar potassium fertilization improves fruit quality of field grown muskmelon on calcareous soils in South Texas. J. Sci. Food Agric. 2009, 89, 2452–2460. [Google Scholar] [CrossRef]
- Lester, G.E.; Jifon, J.L.; Makus, D.J. Impact of potassium nutrition on postharvest fruit quality: Melon (Cucumis melo L.) case study. Plant Soil 2010, 335, 117–131. [Google Scholar] [CrossRef]
- Prajapati, J. The essential nutrient element for the crop. IJARW 2019, 1, 17–22. [Google Scholar]
- Pendias, A.K.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press LLC: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2010; ISBN 0-8493-1575-1. [Google Scholar]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Huang, W.; Zhang, S.; Cao, K. Cyclic electron flow plays an important role in photoprotection of tropical trees illuminated at temporal chilling temperature. Plant Cell Physiol. 2011, 52, 297–305. [Google Scholar] [CrossRef]
- Lidon, F.C.; Teixeira, M.G. Rice tolerance to excess Mn: Implications in the chloroplast lamellae and synthesis of a novel Mn protein. PPB 2000, 38, 969–978. [Google Scholar] [CrossRef]
- Foy, C.D. Plant adaptation to mineral stress problem in soils. ISJR 1983, 57, 339–392. [Google Scholar]
- Nazrul-Islam, A.K.M. Effects of interaction of calcium and manganese on the growth and nutrition of Epilobium hirsutum. Soil Sci. Plant Nutr. 1986, 32, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Ram, H.; Cakmak, I. Micronutrients: Soil to SeedI. In Biofortification of Staple Crops; Kumar, S., Dikshit, H.K., Mishara, G.P., Singh, A., Eds.; Springer: Singapore, 2022; pp. 519–549. [Google Scholar] [CrossRef]
- Lidon, F.C.; Henriques, F.S. Effects of increasing concentrations of Cu on metal uptake kinetics and biomass yields. Soil Sci. 1992, 154, 44–49. [Google Scholar] [CrossRef]
- Lidon, F.C.; Henriques, F.S. Changes in the thylakoid membrane polypeptide patterns triggered by excess Cu in rice. Photosynthetica 1993, 28, 109–117. [Google Scholar]
- Morsch, L.; Somavilla, L.M.; Trentin, E.; Silva, K.R.; Oliveiraa, J.M.S.; Brunetto, G.; Simão, D.G. Root system structure as a criterion for the selection of grapevine genotypes that are tolerant to excess copper and the ability of phosphorus to mitigate toxicity. Plant Physiol. Biochem. 2022, 171, 147–156. [Google Scholar] [CrossRef]
- Lidon, F.C.; Henriques, F.S. Subcellular localisation of copper and partial isolation of copper proteins in roots from rice plants exposed to excess copper. Funct. Plant Biol. 1994, 21, 427–436. [Google Scholar] [CrossRef]
- Mulder, E.G. Mineral nutrition in relation to the biochemistry and physiology of potatoes: I. Effect of nitrogen, phosphate, potassium, magnesium, and copper nutrition on the tyrosine content and tyrosinase activity with particular reference to blackening of the tubers. Plant Soil 1949, 1, 59–121. [Google Scholar] [CrossRef]
- Kramer, D.; Johnson, G.; Kiirats, O.; Edwards, G. New flux parameters for the determination of QA redox state and excitation fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Krause, G.H.; Jahns, P. Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: Characterization and function. In Papageorgiou GC, Govindjee, Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; pp. 463–495. [Google Scholar]
- Lidon, F.C. Modulation of macronutrients uptake and translocation in Mn-treated rice in early stages of vegetative growth. J. Plant Nutr. 2001, 24, 61–74. [Google Scholar] [CrossRef]
- Lidon, F.C. Micronutrient uptake and translocation in Mn-treated rice. J. Plant Nutr. 2002, 25, 757–768. [Google Scholar] [CrossRef]
- Marques, A.C.; Lidon, F.C.; Coelho, A.R.F.; Pessoa, C.C.; Luís, I.C.; Campos, P.S.; Simões, M.; Almeida, A.S.; Pessoa, M.F.; Galhano, G.; et al. Effect of rice grain (Oryza sativa L.) enrichment with selenium on foliar leaf gas exchanges and accumulation of nutrients. Plants 2021, 10, 288. [Google Scholar] [CrossRef]
- Pessoa, C.C.; Lidon, F.C.; Coelho, A.R.F.; Caleiro, J.C.; Marques, A.C.; Luís, I.C.; Kullberg, J.C.; Legoinha, P.; Brito, M.G.; Ramalho, J.C.; et al. Calcium biofortification of Rocha pears, tissues accumulation and physicochemical implications in fresh and heat-treated fruits. Sci. Hortic. 2021, 277, 109834. [Google Scholar] [CrossRef]

| Treatments | P | K | Na | Fe | Zn | |
|---|---|---|---|---|---|---|
| % | % | ppm | ppm | ppm | ||
| Agria | ||||||
| Control | 0.588 ± 0.209a | 0.272 ± 0.006c | 87.5 ± 1.81a | 114 ± 2.97a | 53.6 ± 9.29a | |
| Ca(NO3)2 | 2 kg ha−1 | 0.845 ± 0.053a | 0.312 ± 0.003b | 76.6 ± 0.81b | 135 ± 3.22a | 31.8 ± 1.55b |
| 4 kg ha−1 | 0.448 ± 0.196a | 0.337 ± 0.010ab | 69.1 ± 2.79b | 134 ± 6.32a | 29.9 ± 1.83b | |
| CaCl2 | 3 kg ha−1 | 0.903 ± 0.114a | 0.370 ± 0.009a | 58.2 ± 1.38c | 123 ± 6.52a | 33.9 ± 0.81ab |
| 6 kg ha−1 | 1.08 ± 0.26a | 0.368 ± 0.004a | 63.9 ± 0.84b | 131 ± 5.35a | 31.4 ± 3.51b | |
| Rossi | ||||||
| Control | 0.433 ± 0.096a | 0.270 ± 0.006b | 86.0 ± 2.47a | 71 ± 15.8b | 26.5 ± 0.39a | |
| Ca(NO3)2 | 2 kg ha−1 | 0.820 ± 0.052a | 0.279 ± 0.006b | 80.9 ± 1.76ab | 106 ± 6.13b | 27.2 ± 1.39a |
| 4 kg ha−1 | 0.451 ± 0.064a | 0.286 ± 0.007b | 71.5 ± 1.57b | 107 ± 4.48b | 27.6 ± 0.43a | |
| CaCl2 | 3 kg ha−1 | 0.811 ± 0.140a | 0.340 ± 0.009a | 72.6 ± 1.58b | 108 ± 3.31b | 26.8 ± 0.72a |
| 6 kg ha−1 | 0.705 ± 0.284a | 0.362 ± 0.016a | 39.5 ± 3.25c | 183 ± 27.7a | 31.0 ± 1.59a | |
| Treatments | Regions | C | H | O | K | Fe | Mn | Zn | Cu | |
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | |||||||||
| Agria | ||||||||||
| Control | 1 | 419 ± 21Aa | 58.6 ± 2.9Aa | 465 ± 23Aa | 40.1 ± 2.0Bb | 1.12 ± 0.10Aa | 35.7 ± 1.8Ab | 17.5 ± 0.9Bc | 5.74 ± 0.29Cc | |
| 2 | 392 ± 20Aa | 54.8 ± 2.7Aa | 435 ± 22Aa | 74.2 ± 3.7Ab | 0.15 ± 0.00Ba | 6.10 ± 0.31Cb | 22.6 ± 1.1Bb | 7.03 ± 0.35Bbc | ||
| 3 | 386 ± 19Aa | 54.0 ± 2.7 Aa | 429 ± 21Aa | 72.4 ± 3.6Ab | 0.10 ± 0.00Ca | 8.43 ± 0.42Bc | 27.9 ± 1.4Ab | 9.90 ± 0.49Aab | ||
| Ca(NO3)2 | 2 kg ha−1 | 1 | 428 ± 21Aa | 59.9 ± 3.0Aa | 475 ± 24Aa | 27.9 ± 1.4Bc | 0.40 ± 0.00Ac | 37.23 ± 0.36Ab | 12.9 ± 0.7Bc | 4.17 ± 0.21Bc |
| 2 | 418 ± 21Aa | 58.4 ± 2.9Aa | 464 ± 23Aa | 50.2 ± 2.5Ac | 0.10 ± 0.00Bb | 3.27 ± 0.16Cc | 23.4 ± 1.2Ab | 8.01 ± 0.40Ab | ||
| 3 | 404 ± 20Aa | 56.4 ± 2.8 Aa | 448 ± 22Aa | 56.9 ± 2.9Ac | 0.10 ± 0.00Ba | 10.1 ± 0.5Bbc | 30.9 ± 1.5Ab | 10.1 ± 0.50Aab | ||
| 4 kg ha−1 | 1 | 400 ± 20Aa | 56.0 ± 2.8Aa | 445 ± 22Aa | 76.2 ± 3.8Aa | 1.42 ± 0.10Aa | 52.1 ± 2.6Aa | 37.4 ± 1.9Aa | 9.67 ± 0.48Ab | |
| 2 | 328 ± 16Bb | 45.9 ± 2.3Aa | 365 ± 18Aa | 85.5 ± 4.3Ab | 0.10 ± 0.00Bb | 4.41 ± 0.22Cc | 25.1 ± 1.3Bb | 5.33 ± 0.27Bc | ||
| 3 | 369 ± 19Bab | 51.6 ± 2.6 Aa | 410 ± 21Aa | 72.1 ± 3.6Ab | 0.10 ± 0.00Ba | 6.71 ± 0.34Bc | 18.2 ± 0.9Cc | 7.83 ± 0.39Bc | ||
| CaCl2 | 3 kg ha−1 | 1 | 375 ± 19Aa | 52.5 ± 2.6Aa | 416 ± 21ABa | 50.0 ± 2.5Bb | 0.50 ± 0.00Ab | 22.1 ± 1.1Ac | 25.3 ± 1.3ABb | 7.45 ± 0.37Bb |
| 2 | 416 ± 21Aa | 58.3 ± 2.9Aa | 462 ± 23Aa | 55.1 ± 2.8Bc | 0.10 ± 0.00Bb | 10.7 ± 0.5Ba | 19.7 ± 1.0Bb | 8.67 ± 0.43Bb | ||
| 3 | 363 ± 18Aa | 50.7 ± 2.5 Aa | 402 ± 20Ba | 76.0 ± 3.8Ab | 0.10 ± 0.00Ba | 12.1 ± 0.6Bb | 29.8 ± 1.5Ab | 13.1 ± 0.70Aa | ||
| 6 kg ha−1 | 1 | 383 ± 19Aa | 53.6 ± 2.7Aa | 426 ± 21Aa | 78.2 ± 3.9Ba | 0.80 ± 0.00Ab | 38.0 ± 1.9Ab | 40.1 ± 2.0Aa | 25.0 ± 1.30Aa | |
| 2 | 363 ± 18Aab | 50.8 ± 2.5Aa | 403 ± 20Aa | 99.2 ± 5.0AABa | 0.12 ± 0.01Bb | 9.37 ± 0.5Ca | 37.6 ± 1.9Aa | 26.7 ± 1.30Aa | ||
| 3 | 344 ± 17Aa | 49.1 ± 2.4 Aa | 382 ± 19Aa | 110 ± 50BAa | 0.12 ± 0.01Ba | 18.3 ± 0.9Ba | 37.9 ± 1.9Aa | 10.7 ± 0.50Bab | ||
| Rossi | ||||||||||
| Control | 1 | 425 ± 21Aa | 59.5 ± 3.0Aa | 472 ± 24Aa | 30.4 ± 1.5Cc | 0.70 ± 0.03Ab | 13.8 ± 0.7Ad | 8.77 ± 0.4Cd | n.d. | |
| 2 | 394 ± 20Aa | 55.1 ± 2.8Aa | 438 ± 22Aa | 65.4 ± 3.3Bb | 0.07 ± 0.01Bb | 11.5 ± 0.6Ac | 23.7 ± 1.2Bb | 0.14 ± 0.01c | ||
| 3 | 379 ± 19Aa | 53.1 ± 2.7 Aa | 421 ± 21Aa | 103 ± 5.0Aa | 0.06 ± 0.01 Ba | 13.2 ± 0.7Ab | 35.5 ± 1.8Aa | n.d. | ||
| Ca(NO3)2 | 2 kg ha−1 | 1 | 426 ± 21Aa | 59.5 ± 3.0Aa | 472 ± 24Aa | 36.5 ± 1.8Bc | 0.63 ± 0.03Ab | 28.9 ± 1.4Ac | 18.3 ± 0.9ABc | 6.42 ± 0.32Bc |
| 2 | 420 ± 21Aa | 58.8 ± 3.0Aa | 467 ± 23Aa | 48.5 ± 2.4Bc | 0.04 ± 0.01Bb | 5.7 ± 0.28Cd | 15.6 ± 0.8Bc | 6.20 ± 0.31Bb | ||
| 3 | 371 ± 19a | 51.8 ± 2.6Aa | 411 ± 21Aa | 60.0 ± 3.0Ab | 0.08 ± 0.01Ba | 21.8 ± 1.1Ba | 25.6 ± 1.3Aa | 9.25 ± 0.46Aa | ||
| 4 kg ha−1 | 1 | 350 ± 18Aa | 49.0 ± 2.5Aa | 389 ± 19Aa | 88.0 ± 4.4Aa | 1.34 ± 0.07Ab | 71.1 ± 3.6Aa | 68.7 ± 3.4Aa | 23.00 ± 1.2Ab | |
| 2 | 370 ± 19Aa | 51.7 ± 2.6Aa | 410 ± 21Aa | 29.4 ± 1.5Bd | 0.08 ± 0.01Bb | 19.7 ± 1.0Bb | 16.3 ± 0.8Bc | 5.83 ± 0.29Bb | ||
| 3 | 371 ± 19Aa | 51.9 ± 2.6 Aa | 412 ± 21Aa | 28.1 ± 1.4Bd | 0.09 ± 0.01Ba | 25.9 ± 1.3Ba | 14.3 ± 0.7Bb | 7.67 ± 0.38Ba | ||
| CaCl2 | 3 kg ha−1 | 1 | 362 ± 18Aa | 50.6 ± 2.5Aa | 401 ± 20Aa | 80.1 ± 4.0Ba | 2.26 ± 0.11Aa | 53.2 ± 2.7Ab | 61.1 ± 3.1Aa | 32.60 ± 1.6Aa |
| 2 | 363 ± 18Aa | 50.7 ± 2.5Aa | 403 ± 20Aa | 117.0 ± 6.0Aa | 0.15 ± 0.01Ba | 57.0 ± 2.9Aa | 53.1 ± 2.7Aa | 21.10 ± 1.1Ba | ||
| 3 | 390 ± 20Aa | 54.6 ± 2.7 Aa | 433 ± 22Aa | 38.5 ± 1.9Ca | 0.06 ± 0.01Ca | 8.98 ± 0.45Bc | 18.5 ± 0.9Bb | 9.07 ± 0.50Ca | ||
| 6 kg ha−1 | 1 | 413 ± 21Aa | 57.8 ± 2.9Aa | 458 ± 23Aa | 51.3 ± 2.6Ab | 0.11 ± 0.01Ac | 13.0 ± 0.7Ad | 25.3 ± 0.0Ab | 9.83 ± 0.46Ac | |
| 2 | 363 ± 18Aa | 50.8 ± 2.5Aa | 403 ± 20Aa | 30.6 ± 1.5Bd | 0.09 ± 0.01Aab | 4.9 ± 0.25Cd | 13.3 ± 4.4Bc | 8.61 ± 0.43Ab | ||
| 3 | 382 ± 19Aa | 53.4 ± 2.7 Aa | 423 ± 21Aa | 36.1 ± 1.8Bc | 0.10 ± 0.01Aa | 8.9 ± 0.44Bc | 14.2 ± 0.7Bb | 9.22 ± 0.46Aa | ||
| Treatments | Height (cm) | Diameter (cm) | Color Parameters | |||
|---|---|---|---|---|---|---|
| L | a* | b* | ||||
| Agria | ||||||
| Control | 9.30 ± 0.19a | 5.60 ± 0.09a | 54.4 ± 1.31a | −2.89 ± 0.17a | 20.5 ± 0.22a | |
| Ca(NO3)2 | 2 kg ha−1 | 11.80 ± 0.31a | 5.47 ± 0.13a | 56.8 ± 0.86a | −2.90 ± 0.13a | 21.3 ± 0.49a |
| 4 kg ha−1 | 11.40 ± 0.91a | 4.77 ± 0.15a | 59.5 ± 0.36a | −3.31 ± 0.07a | 22.3 ± 0.33a | |
| CaCl2 | 3 kg ha−1 | 9.50 ± 0.19a | 5.70 ± 0.12a | 58.8 ± 0.50a | −2.95 ± 0.21a | 22.0 ± 0.29a |
| 6 kg ha−1 | 9.90 ± 0.68a | 4.73 ± 0.28a | 55.8 ± 1.29a | −2.85 ± 0.08a | 21.7 ± 1.39a | |
| Rossi | ||||||
| Control | 9.60 ± 0.32a | 6.24 ± 0.15a | 65.9 ± 1.19a | −2.52 ± 0.14c | 16.3 ± 0.30a | |
| Ca(NO3)2 | 2 kg ha−1 | 7.03 ± 0.53a | 4.50 ± 0.22a | 63.3 ± 0.73a | −2.64 ± 0.12c | 14.2 ± 0.27a |
| 4 kg ha−1 | 9.32 ± 1.58a | 5.50 ± 0.26a | 63.3 ± 1.12a | −2.75 ± 0.07c | 15.7 ± 0.24a | |
| CaCl2 | 3 kg ha−1 | 9.30 ± 0.32a | 5.65 ± 0.21a | 59.9 ± 2.55a | −2.15 ± 0.09a | 15.1 ± 0.77a |
| 6 kg ha−1 | 8.91 ± 1.17a | 5.61 ± 0.49a | 61.4 ± 1.88a | −2.34 ± 0.05b | 15.1 ± 0.65a | |
| Varieties | Planting | Foliar Applications | Harvest | Treatments | |||
|---|---|---|---|---|---|---|---|
| 1° | 2° | 3° | 4° | ||||
| Agria | 4 May 2018 | 6 July 2018 | 16 July 2018 | 26 July 2018 | 3 August 2018 | 4 September 2018 | CaCl2 (3 and 6 kg ha−1) or, Ca(NO3)2 (2 and 4 kg ha−1) |
| Rossi | 11 May 2018 | 25 July 2018 | 3 August 2018 | 14 August 2018 | 24 September 2018 | 24 September 2018 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, A.R.F.; Ramalho, J.C.; Lidon, F.C.; Marques, A.C.; Daccak, D.; Pessoa, C.C.; Luís, I.C.; Guerra, M.; Leitão, R.G.; Semedo, J.M.N.; et al. Foliar Spraying of Solanum tuberosum L. with CaCl2 and Ca(NO3)2: Interactions with Nutrients Accumulation in Tubers. Plants 2022, 11, 1725. https://doi.org/10.3390/plants11131725
Coelho ARF, Ramalho JC, Lidon FC, Marques AC, Daccak D, Pessoa CC, Luís IC, Guerra M, Leitão RG, Semedo JMN, et al. Foliar Spraying of Solanum tuberosum L. with CaCl2 and Ca(NO3)2: Interactions with Nutrients Accumulation in Tubers. Plants. 2022; 11(13):1725. https://doi.org/10.3390/plants11131725
Chicago/Turabian StyleCoelho, Ana Rita F., José Cochicho Ramalho, Fernando Cebola Lidon, Ana Coelho Marques, Diana Daccak, Cláudia Campos Pessoa, Inês Carmo Luís, Mauro Guerra, Roberta G. Leitão, José Manuel N. Semedo, and et al. 2022. "Foliar Spraying of Solanum tuberosum L. with CaCl2 and Ca(NO3)2: Interactions with Nutrients Accumulation in Tubers" Plants 11, no. 13: 1725. https://doi.org/10.3390/plants11131725
APA StyleCoelho, A. R. F., Ramalho, J. C., Lidon, F. C., Marques, A. C., Daccak, D., Pessoa, C. C., Luís, I. C., Guerra, M., Leitão, R. G., Semedo, J. M. N., Silva, M. M., Pais, I. P., Leal, N., Galhano, C., Rodrigues, A. P., Legoinha, P., Silva, M. J., Simões, M., Scotti Campos, P., ... Reboredo, F. H. (2022). Foliar Spraying of Solanum tuberosum L. with CaCl2 and Ca(NO3)2: Interactions with Nutrients Accumulation in Tubers. Plants, 11(13), 1725. https://doi.org/10.3390/plants11131725


















