UVB Irradiation-Induced Transcriptional Changes in Lignin- and Flavonoid Biosynthesis and Indole/Tryptophan-Auxin-Responsive Genes in Rice Seedlings
Abstract
:1. Introduction
2. Results
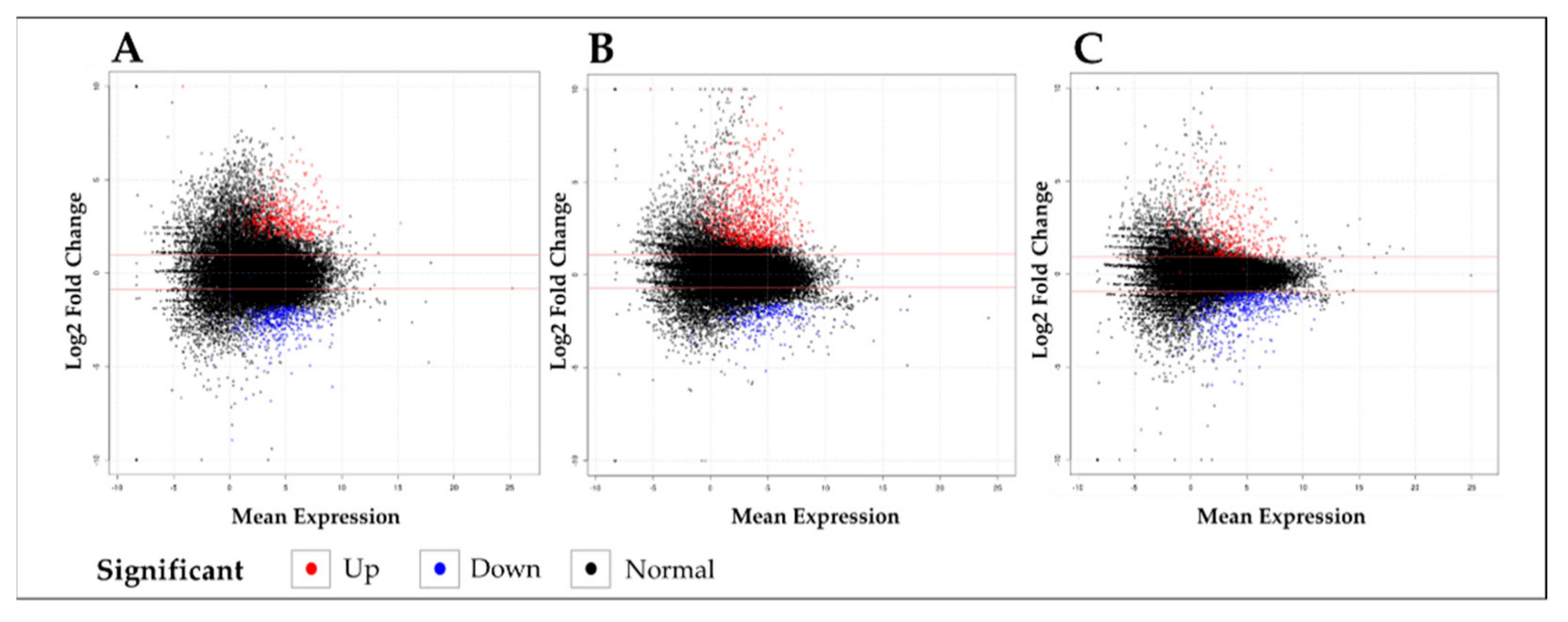
2.1. RNA-Seq and De Novo Assembly of the O. sativa Transcriptome
2.2. Gene Ontology (GO) and KEGG Analysis
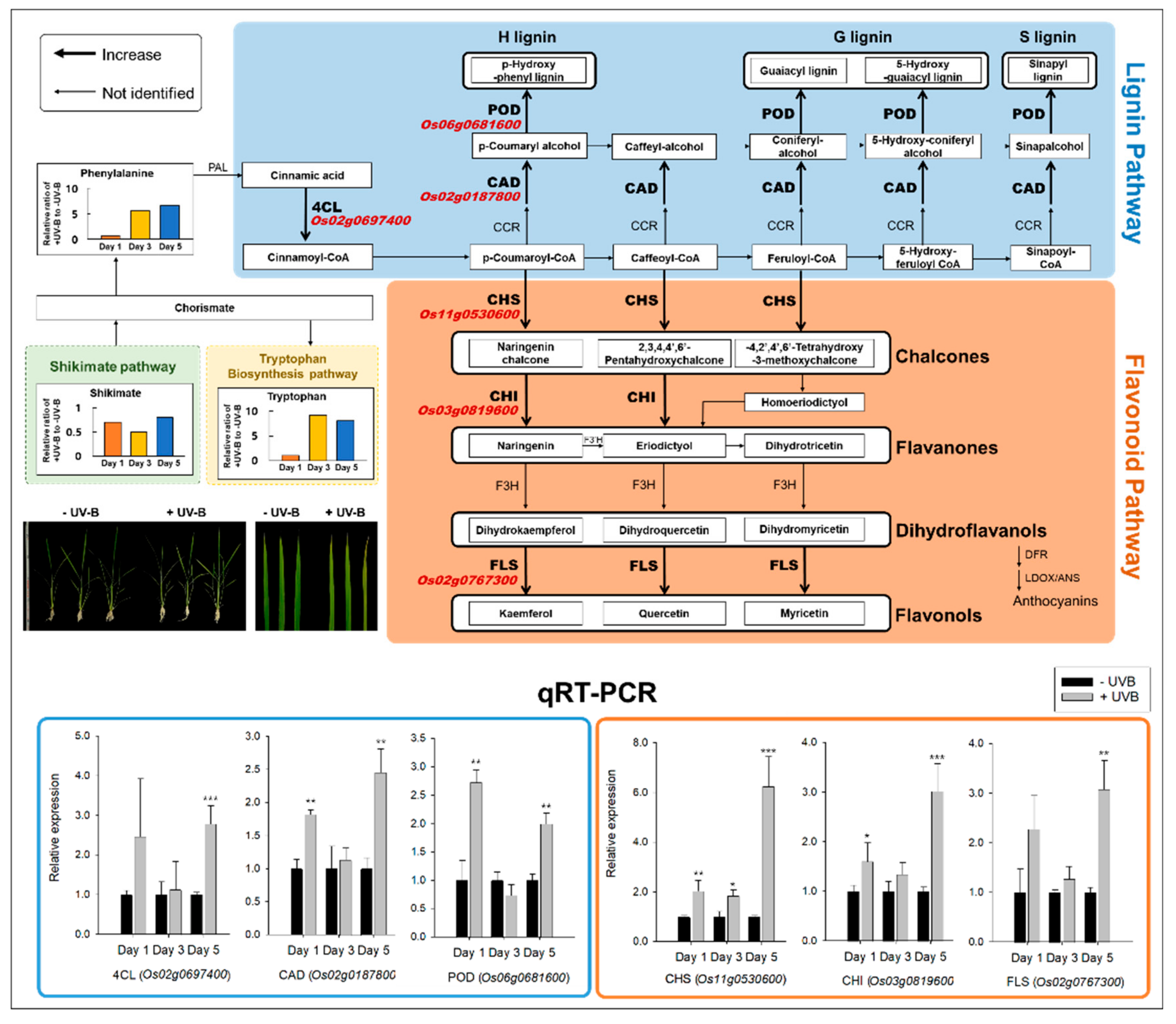
2.3. Lignin and Flavonoid Biosynthesis Responses by RNA-Seq and qRT-PCR
2.4. Responses of Indole/Tryptophan Biosynthesis- and Auxin-Related Genes
3. Discussion
3.1. Lignin and Flavonoid Pathways against UVB Irradiation
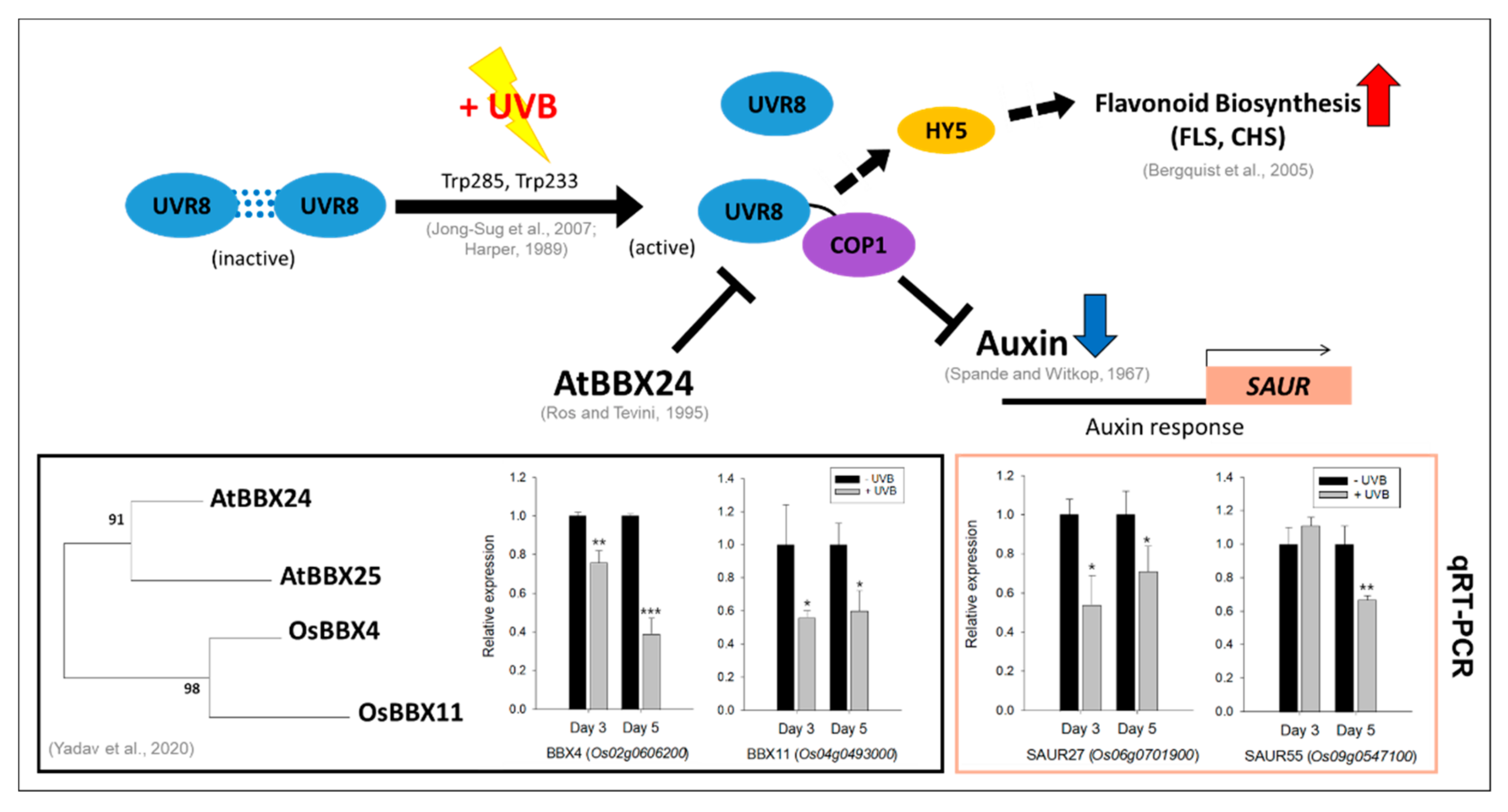
3.2. Responses of Indole/Tryptophan Biosynthesis and Auxin-Related Genes
4. Materials and Methods
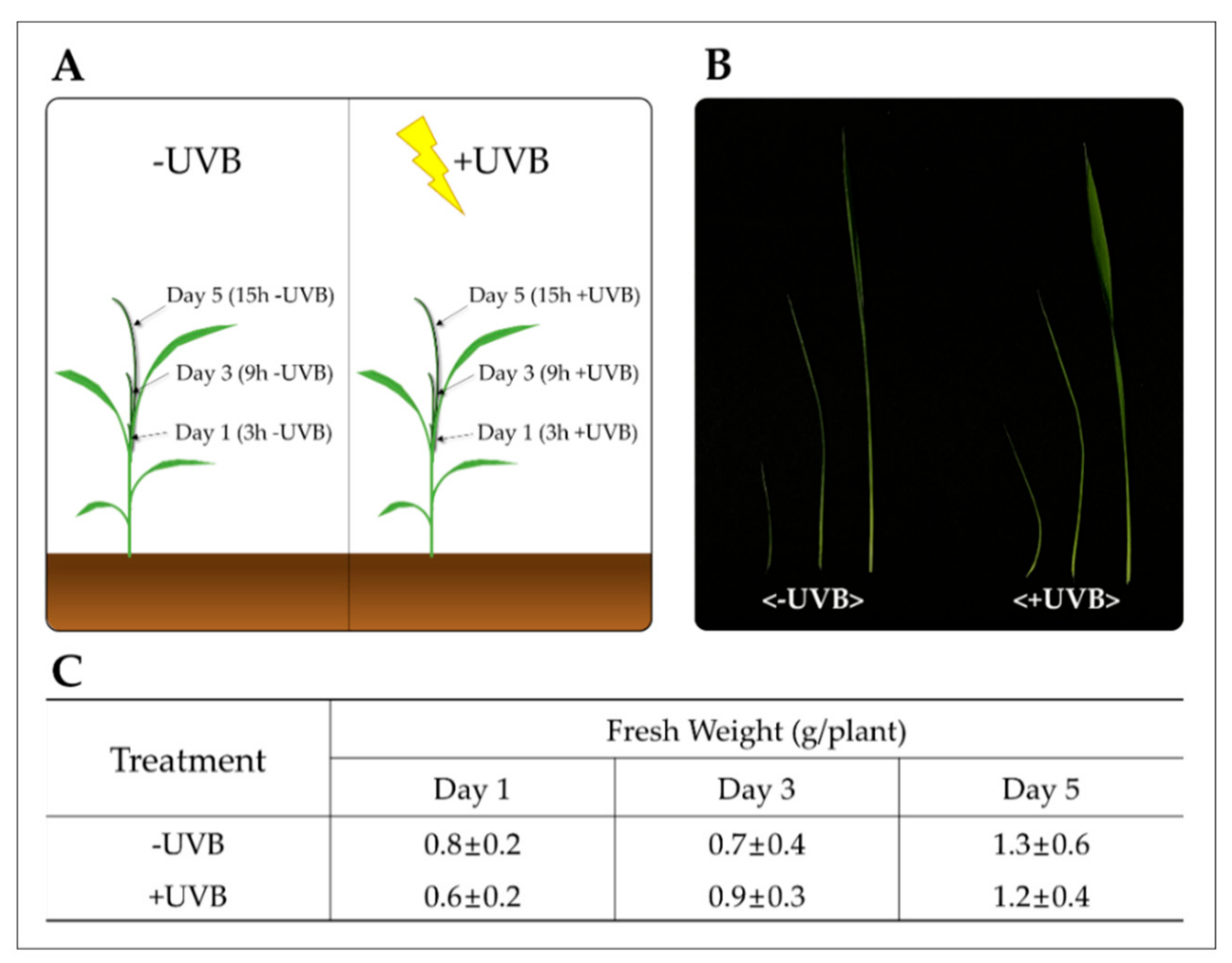
4.1. Plant Material and UVB Irradiation
4.2. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
4.3. RNA-Seq Library Construction, Sequencing, and DEGs Analysis
4.4. Metabolite Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blumthaler, M.; Ambach, W. Indication of increasing solar ultraviolet-B radiation flux in alpine regions. Science 1990, 248, 206–208. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Wise, R.R.; Naylor, A.W. Chilling-enhanced photooxidation: Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987, 83, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asada, K. Production and action of active oxygen species in photosynthetic tissues. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; CRC Press: Boca Raton, FL, USA, 2019; pp. 77–104. [Google Scholar]
- Foyer, C.H.; Harbinson, J. Oxygen metabolism and the regulation of photosynthetic electron transport. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–42. [Google Scholar]
- Pandey, N.; Pandey-Rai, S. Modulations of physiological responses and possible involvement of defense-related secondary metabolites in acclimation of Artemisia annua L. against short-term UV-B radiation. Planta 2014, 240, 611–627. [Google Scholar] [CrossRef]
- Gould, K.S.; Markham, K.R.; Smith, R.H.; Goris, J.J. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J. Exp. Bot. 2000, 51, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Close, D.C.; McArthur, C. Rethinking the role of many plant phenolics–protection from photodamage not herbivores? Oikos 2002, 99, 166–172. [Google Scholar] [CrossRef]
- Thomas, T.; Puthur, J.T. UV-B priming enhances specific secondary metabolites in Oryza sativa (L.) empowering to encounter diverse abiotic stresses. Plant Growth Regul. 2020, 92, 169–180. [Google Scholar] [CrossRef]
- Tao, Y.; Ferrer, J.-L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 133, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, S.; Velanis, C.N.; Jenkins, G.I.; Franklin, K.A. UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc. Natl. Acad. Sci. USA 2014, 111, 11894–11899. [Google Scholar] [CrossRef] [Green Version]
- Mazza, C.A.; Ballaré, C.L. Photoreceptors UVR 8 and phytochrome B cooperate to optimize plant growth and defense in patchy canopies. N. Phytol. 2015, 207, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Queval, G.; Noctor, G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal. Biochem. 2007, 363, 58–69. [Google Scholar] [CrossRef]
- Csepregi, K.; Coffey, A.; Cunningham, N.; Prinsen, E.; Hideg, É.; Jansen, M.A. Developmental age and UV-B exposure co-determine antioxidant capacity and flavonol accumulation in Arabidopsis leaves. Environ. Exp. Bot. 2017, 140, 19–25. [Google Scholar] [CrossRef]
- Coleman, J.S. Leaf development and leaf stress: Increased susceptibility associated with sink-source transition. Tree Physiol. 1986, 2, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.A.; Paul, M.J.; Foyer, C.H. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochim. Biophys. Acta BBA Bioenerg. 2016, 1857, 1715–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.-M.; Lo, S.-F.; Ho, T.-H.D. Source–sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Kusano, M.; Tohge, T.; Fukushima, A.; Kobayashi, M.; Hayashi, N.; Otsuki, H.; Kondou, Y.; Goto, H.; Kawashima, M.; Matsuda, F. Metabolomics reveals comprehensive reprogramming involving two independent metabolic responses of Arabidopsis to UV-B light. Plant J. 2011, 67, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ou-Lee, T.-M.; Raba, R.; Amundson, R.G.; Last, R.L. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 1993, 5, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Conklin, P.L.; Williams, E.H.; Last, R.L. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc. Natl. Acad. Sci. USA 1996, 93, 9970–9974. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.; Lim, S.; Kim, Y.X.; Lee, Y.; Lee, S.; Lee, D.; Park, K.; Sung, J. Diurnal changes in CN metabolism and response of rice seedlings to UV-B radiation. J. Plant Physiol. 2018, 228, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, R.; Campbell, B.; Fountain, D.; Jordan, B.; Greer, D.; Hunt, D.; Hunt, C. Multivariate analysis of intraspecific responses to UV-B radiation in white clover (Trifolium repens L.). Plant Cell Environ. 2001, 24, 917–927. [Google Scholar] [CrossRef]
- Kumagai, T.; Hidema, J.; Kang, H.-S.; Sato, T. Effects of supplemental UV-B radiation on the growth and yield of two cultivars of Japanese lowland rice (Oryza sativa L.) under the field in a cool rice-growing region of Japan. Agric. Ecosyst. Environ. 2001, 83, 201–208. [Google Scholar] [CrossRef]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.; Harter, K. The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007, 50, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.A.; Field, K.J.; Davey, M.P.; Beerling, D.J.; Lomax, B.H. Metabolomic and physiological responses reveal multi-phasic acclimation of Arabidopsis thaliana to chronic UV radiation. Plant Cell Environ. 2009, 32, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Strid, Å.; Chow, W.S.; Anderson, J.M. UV-B damage and protection at the molecular level in plants. Photosynth. Res. 1994, 39, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.; van de Staaij, J.; Björn, L.O.; Caldwell, M. UV-B as an environmental factor in plant life: Stress and regulation. Trends Ecol. Evol. 1997, 12, 22–28. [Google Scholar] [CrossRef]
- Ravindran, K.; Indrajith, A.; Balakrishnan, V.; Venkatesan, K.; Kulandaivelu, G. Determination of defense mechanism in, i> Phaseolus trilobus Ait. seedlings treated under UV-B radiation. Afr. Crop Sci. J. 2008, 16. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, J.; Abozeid, A.; Wu, K.-X.; Guo, X.-R.; Mu, L.-Q.; Tang, Z.-H. UV-B radiation largely promoted the transformation of primary metabolites to phenols in Astragalus mongholicus seedlings. Biomolecules 2020, 10, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Xue, S.; Chen, J.; Shang, S.; Xiao, H.; Zang, Y.; Tang, X. Effects of Different Short-Term UV-B Radiation Intensities on Metabolic Characteristics of Porphyra haitanensis. Int. J. Mol. Sci. 2021, 22, 2180. [Google Scholar] [CrossRef]
- Burchard, P.; Bilger, W.; Weissenböck, G. Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ. 2000, 23, 1373–1380. [Google Scholar] [CrossRef]
- Gil, M.; Pontin, M.; Berli, F.; Bottini, R.; Piccoli, P. Metabolism of terpenes in the response of grape (Vitis vinifera L.) leaf tissues to UV-B radiation. Phytochemistry 2012, 77, 89–98. [Google Scholar] [CrossRef]
- Valares Masa, C.; Sosa Díaz, T.; Alías Gallego, J.C.; Chaves Lobón, N. Quantitative variation of flavonoids and diterpenes in leaves and stems of Cistus ladanifer L. at different ages. Molecules 2016, 21, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baucher, M.; Bernard-vailhé, M.A.; Chabbert, B.; Besle, J.M.; Opsomer, C.; Montagu, M.V.; Botterman, J. Down-regulation of cinnamyl alcohol dehydrogenase in transgenic alfalfa (Medicago sativa L.) and the effect on lignin composition and digestibility. Plant Mol. Biol. 1999, 39, 437–447. [Google Scholar] [CrossRef]
- Provan, G.J.; Scobbie, L.; Chesson, A. Characterisation of lignin from CAD and OMT deficient bm mutants of maize. J. Sci. Food Agric. 1997, 73, 133–142. [Google Scholar] [CrossRef]
- Thorstensson, E.M.G. Apparent inhibition to digestion by lignin in normal and brown midrib stems. J. Sci. Food Agric. 1992, 59, 183–188. [Google Scholar] [CrossRef]
- Vailhe, M.A.B.; Provan, G.J.; Scobbie, L.; Chesson, A.; Maillot, M.P.; Cornu, A.; Besle, J.M. Effect of phenolic structures on the degradability of cell walls isolated from newly extended apical internode of tall fescue (Festuca arundinacea Schreb.). J. Agric. Food Chem. 2000, 48, 618–623. [Google Scholar] [CrossRef]
- Tevini, M. UV-B Effects on Terrestrial Plants and Aquatic Organisms. Prog. Bot. 1994, 55, 174–190. [Google Scholar]
- Drumm-Herrel, H. Blue/UV Light Effects on Anthocyanin Synthesis. In Blue Light Effects in Biological Systems; Senger, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 375–383. [Google Scholar]
- Teramura, A.H. Effects of ultraviolet-b radiation on the growth and yield of crop plants. Physiol. Plant. 1983, 58, 415–427. [Google Scholar] [CrossRef]
- Robinson, T. Organic Constituents of Higher Plants, 6th ed.; Cordus Press: North Amherst, MA, USA, 1991. [Google Scholar]
- Park, J.S.; Choung, M.G.; Kim, J.B.; Hahn, B.S.; Bae, S.C.; Roh, K.H.; Kim, Y.H.; Cheon, C.I.; Sung, M.K.; Cho, K.J. Genes up-regulated during red coloration in UV-B irradiated lecttuce leaves. Plant Cell Rep. 2007, 26, 507–516. [Google Scholar] [CrossRef]
- Harper, J.L. The value of a leaf. Oecologia 1989, 80, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Reifenrath, K.; Müller, C. Species-specific and leaf-age dependent effects of ultraviolet radiation on two Brassicaceae. Phytochemistry 2007, 68, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, S.Å.; Gertsson, U.E.; Knuthsen, P.; Olsson, M.E. Flavonoids in baby spinach (Spinacia oleracea L.): Changes during plant growth and storage. J. Agric. Food Chem. 2005, 53, 9459–9464. [Google Scholar] [CrossRef]
- Spande, T.T.; Witkop, B. [58] Determination of the tryptophan content of proteins with N-bromosuccinimide. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1967; Volume 11, pp. 498–506. [Google Scholar]
- Ros, J.; Tevini, M. Interaction of UV-radiation and IAA during growth of seedlings and hypocotyl segments of sunflower. J. Plant Physiol. 1995, 146, 295–302. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Day, T.A.; Neale, P.J. Effects of UV-B radiation on terrestrial and aquatic primary producers. Annu. Rev. Ecol. Syst. 2002, 33, 371–396. [Google Scholar] [CrossRef]
- Pego, J.V.; Kortstee, A.J.; Huijser, C.; Smeekens, S.C. Photosynthesis, sugars and the regulation of gene expression. J. Exp. Bot. 2000, 51 (Suppl. 1), 407–416. [Google Scholar] [CrossRef] [Green Version]
- Gill, R.T.; Katsoulakis, E.; Schmitt, W.; Taroncher-Oldenburg, G.; Misra, J.; Stephanopoulos, G. Genome-wide dynamic transcriptional profiling of the light-to-dark transition in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2002, 184, 3671–3681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Shao, X.; Li, J. Indole-3-glycerol phosphate, a branchpoint of indole-3-acetic acid biosynthesis from the tryptophan biosynthetic pathway in Arabidopsis thaliana. Plant J. 2000, 24, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Manmathan, H.; Sun, C.; Peebles, C.A. Examining the transcriptional response of overexpressing anthranilate synthase in the hairy roots of an important medicinal plant Catharanthus roseus by RNA-seq. BMC Plant Biol. 2016, 16, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Yang, B.; Zhang, D.; Chen, M.; Tian, J. Enhanced metabolic process to indole alkaloids in Clematis terniflora DC. after exposure to high level of UV-B irradiation followed by the dark. BMC Plant Biol. 2016, 16, 231. [Google Scholar] [CrossRef] [Green Version]
- Rizzini, L.; Favory, J.-J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Hu, Q.; Yan, Z.; Chen, W.; Yan, C.; Huang, X.; Zhang, J.; Yang, P.; Deng, H.; Wang, J. Structural basis of ultraviolet-B perception by UVR8. Nature 2012, 484, 214–219. [Google Scholar]
- Favory, J.J.; Stec, A.; Gruber, H.; Rizzini, L.; Oravecz, A.; Funk, M.; Albert, A.; Cloix, C.; Jenkins, G.I.; Oakeley, E.J. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 2009, 28, 591–601. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; McGhie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV light regulates flavonoid metabolism in apple (Malus x domestica). Plant Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef]
- Jenkins, G.I. Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ. 2017, 40, 2544–2557. [Google Scholar] [CrossRef] [Green Version]
- Podolec, R.; Wagnon, T.B.; Leonardelli, M.; Johansson, H.; Ulm, R. BBX proteins promote HY5-mediated UVR8 signaling in Arabidopsis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Li, Q.-F.; Björn, L.O.; He, J.-X.; Li, S.-S. Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 2012, 22, 1046–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbussche, F.; Tilbrook, K.; Fierro, A.C.; Marchal, K.; Poelman, D.; Van Der Straeten, D.; Ulm, R. Photoreceptor-mediated bending towards UV-B in Arabidopsis. Mol. Plant 2014, 7, 1041–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, D.H.; Park, S.G.; Kim, K.M.; Bae, W.S.; Lee, G.W.; Ha, B.S.; Ro, H.S.; Kim, M.K.; Ryoo, R.; Rhee, S.K.; et al. Whole genome de novo sequencing and genome annotation of the world popular cultivated edible mushroom, Lentinula edodes. J. Biotechnol. 2016, 223, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bibsonomy.org/bibtex/f230a919c34360709aa298734d63dca3 (accessed on 3 April 2022).
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.B.; Park, S.-Y.; Park, C.H.; Park, W.T.; Kim, S.-J.; Ha, S.-H.; Arasu, M.V.; Al-Dhabi, N.A.; Kim, J.K.; Park, S.U. Metabolomics of differently colored Gladiolus cultivars. Appl. Biol. Chem. 2016, 59, 597–607. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-E.; Kim, M.-S.; Sung, J. UVB Irradiation-Induced Transcriptional Changes in Lignin- and Flavonoid Biosynthesis and Indole/Tryptophan-Auxin-Responsive Genes in Rice Seedlings. Plants 2022, 11, 1618. https://doi.org/10.3390/plants11121618
Kim G-E, Kim M-S, Sung J. UVB Irradiation-Induced Transcriptional Changes in Lignin- and Flavonoid Biosynthesis and Indole/Tryptophan-Auxin-Responsive Genes in Rice Seedlings. Plants. 2022; 11(12):1618. https://doi.org/10.3390/plants11121618
Chicago/Turabian StyleKim, Ga-Eun, Me-Sun Kim, and Jwakyung Sung. 2022. "UVB Irradiation-Induced Transcriptional Changes in Lignin- and Flavonoid Biosynthesis and Indole/Tryptophan-Auxin-Responsive Genes in Rice Seedlings" Plants 11, no. 12: 1618. https://doi.org/10.3390/plants11121618
APA StyleKim, G.-E., Kim, M.-S., & Sung, J. (2022). UVB Irradiation-Induced Transcriptional Changes in Lignin- and Flavonoid Biosynthesis and Indole/Tryptophan-Auxin-Responsive Genes in Rice Seedlings. Plants, 11(12), 1618. https://doi.org/10.3390/plants11121618





