Exogenous Paclobutrazol Reinforces the Antioxidant and Antimicrobial Properties of Lavender (Lavandula officinalis L.) Oil through Modulating Its Composition of Oxygenated Terpenes
Abstract
:1. Introduction
2. Results
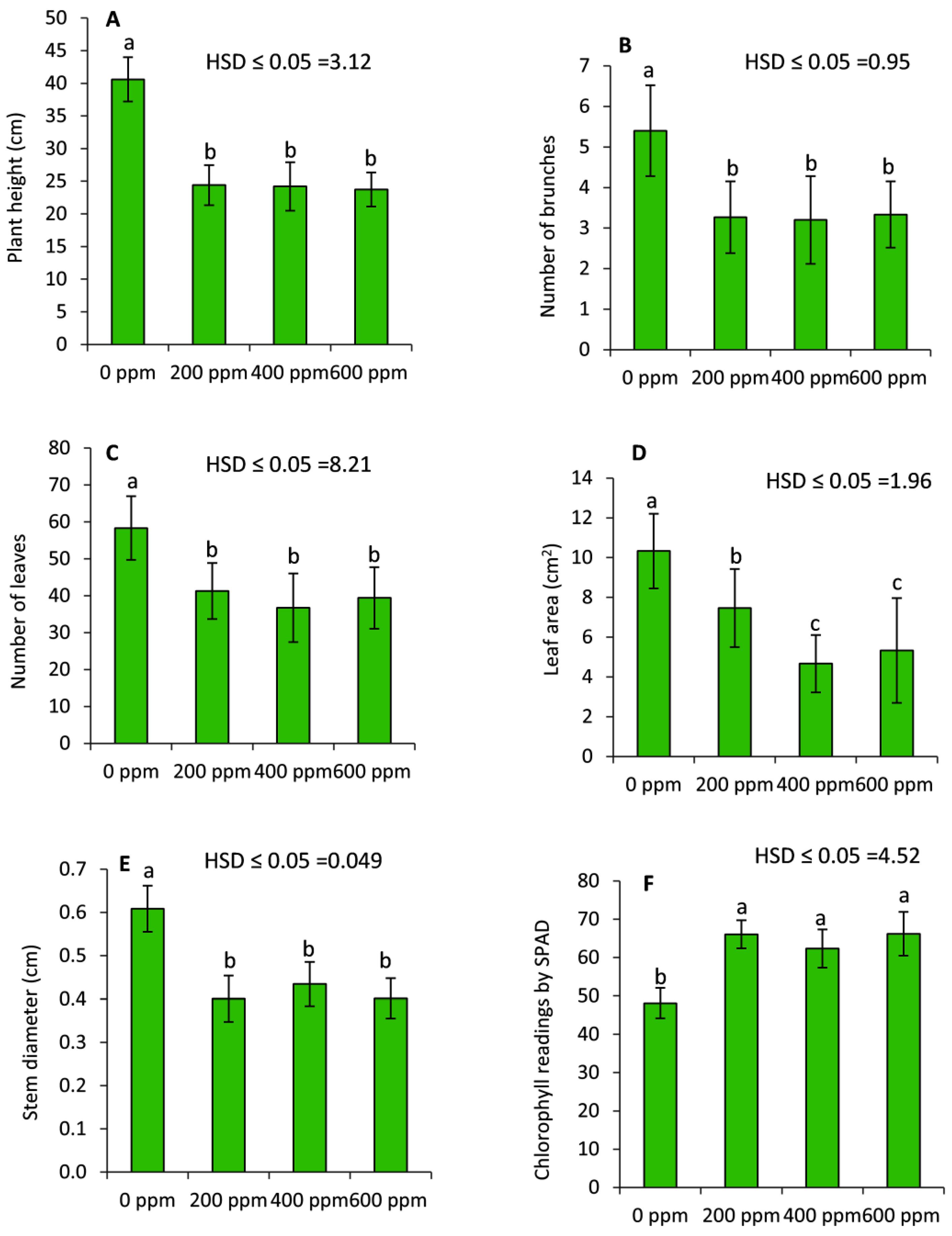
2.1. Effect of PBZ on the Vegetative Growth of Lavender Plants
2.2. Effect of PBZ on the Yield of Lavender Essential Oil
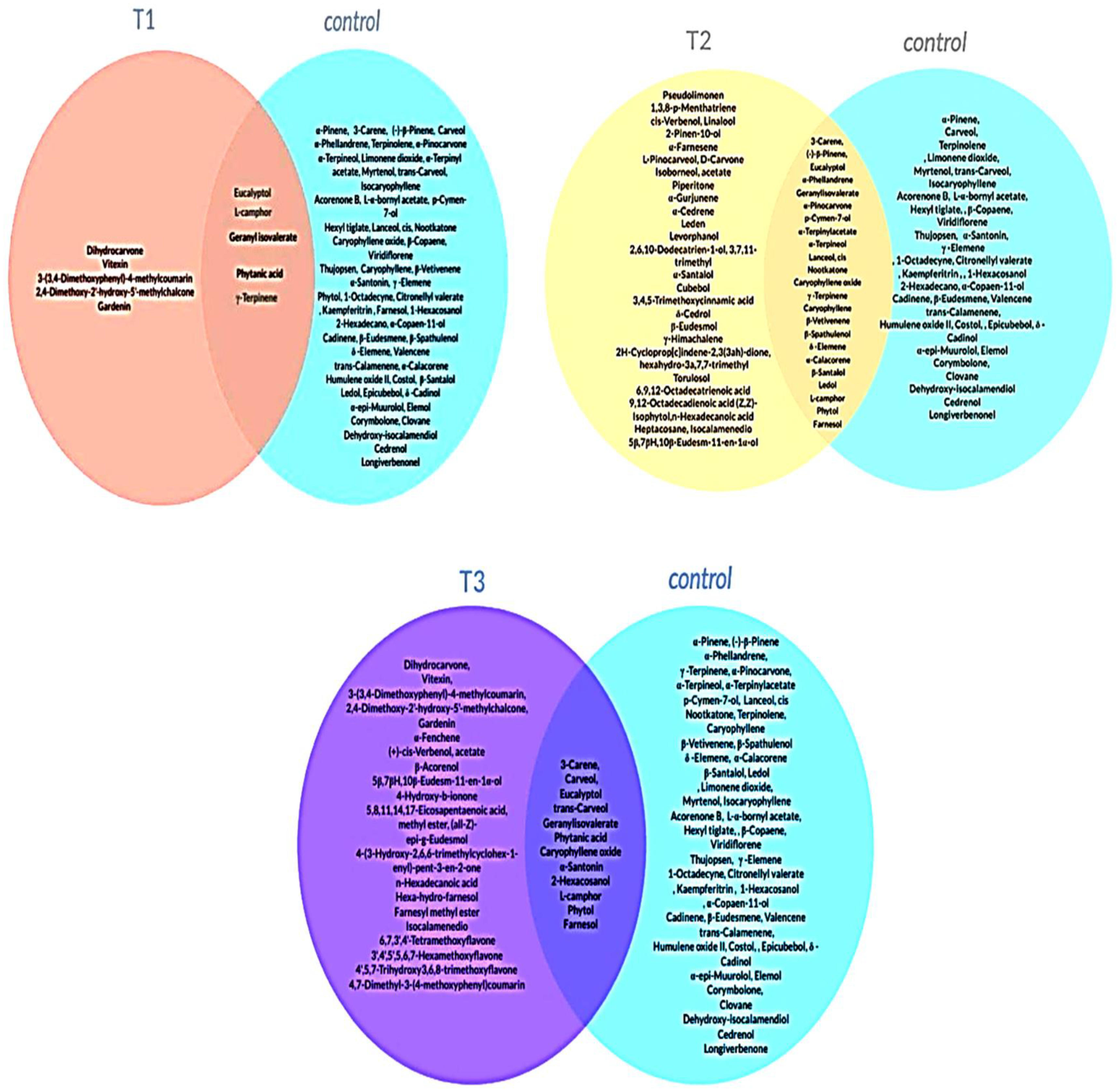
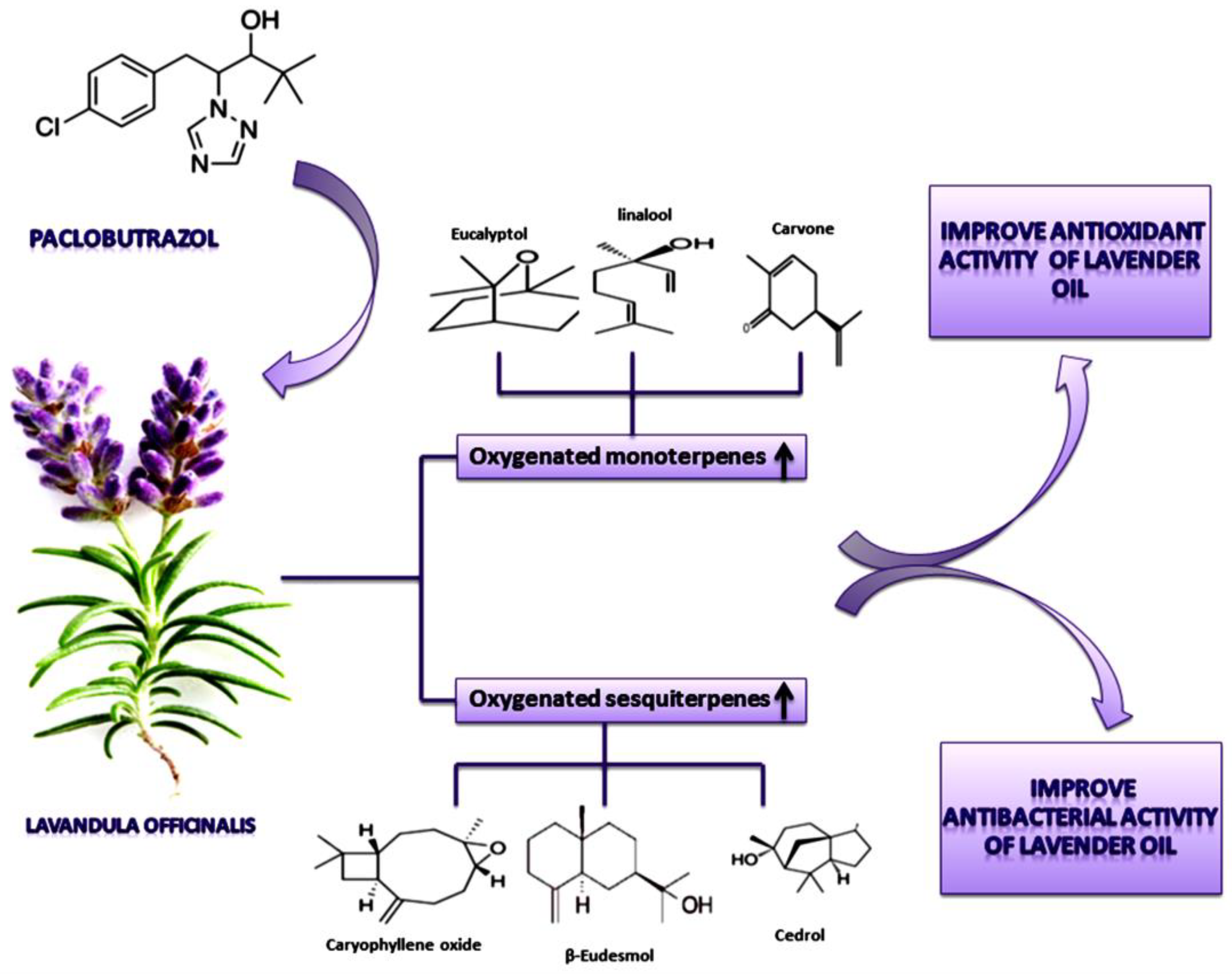
2.3. Effect of PBZ on Lavender Essential Oil Composition
2.4. Effect of PBZ on the Principal Component Analysis (PCA) of Lavender Essential Oil
2.5. Effect of PBZ on the Quantity of Monoterpene and Sesquiterpene Constituents
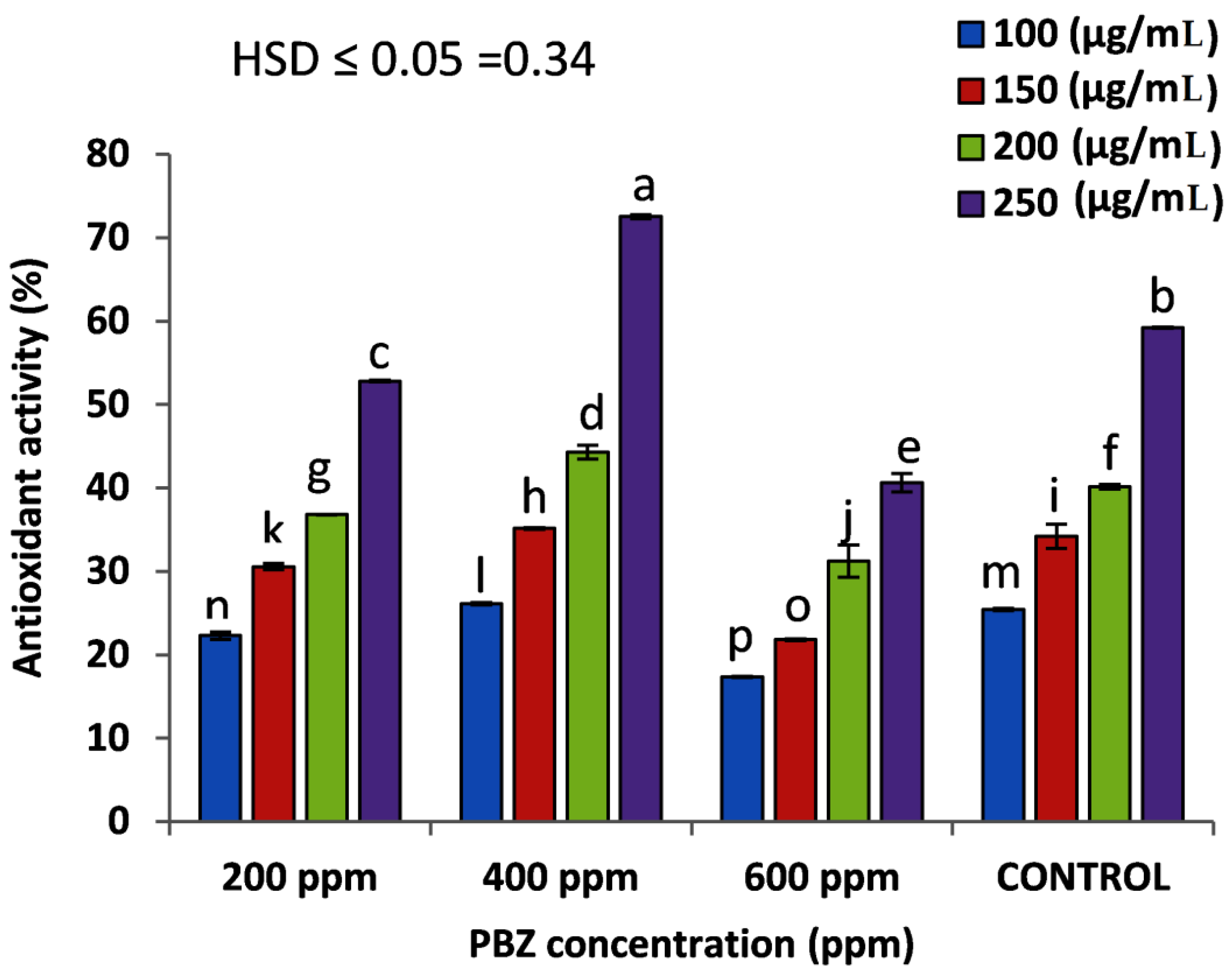
2.6. Effect of PBZ on the Antioxidant Activity
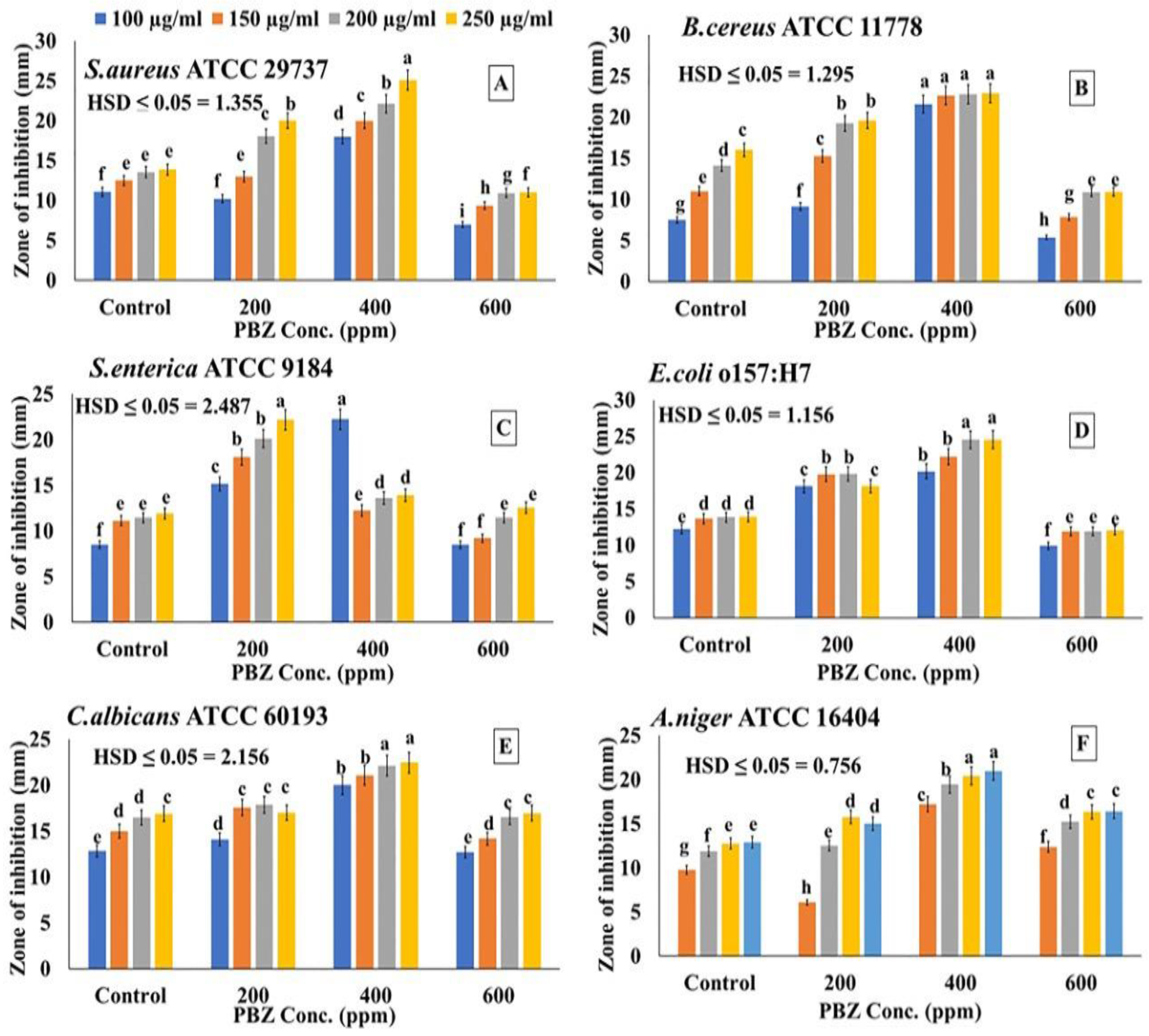
2.7. Effect of PBZ on the Antimicrobial Activity
3. Discussion
4. Material and Methods
4.1. Plant Material, Treatments and Growth Parameters
4.2. Extraction and Determination of Lavender Essential Oil Content
4.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4.4. DPPH Free Radical Scavenging Activity
4.5. Pathogenic Microbial Strains
4.6. Antimicrobial Activity of Lavendar Essential Oil Using Well Diffusion Method
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Mnayer, D.; Özçelik, B.; Altin, G.; Kasapoğlu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M.; Selamoglu, Z.; Acharya, K.; Sen, S. Plants of the genus Lavandula: From farm to pharmacy. Nat. Prod. Commun. 2018, 13, 1934578X1801301037. [Google Scholar] [CrossRef] [Green Version]
- Śmigielski, K.; Sikora, M.; Majewska, M.; Raj, A. The application of essentials oils to natural and organic cosmetics. Pol. J. Cosmetol. 2008, 11, 89–107. [Google Scholar]
- Erland, L.A.; Mahmoud, S.S. Lavender (Lavandula angustifolia) oils. In Essential Oils in Food Preservation, Flavor And Safety; Academic Press: Amsterdam, The Netherlands, 2016; pp. 501–508. [Google Scholar]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Héral, B.; Stierlin, É.; Fernandez, X.; Michel, T. Phytochemicals from the genus Lavandula: A review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Denner, S.S. Lavandula angustifolia miller: English lavender. Holist. Nurs. Pract. 2009, 23, 57–64. [Google Scholar] [CrossRef]
- Tarek, N.; Hassan, H.M.; Abdel Ghani, S.M.; Radwan, I.; Hammouda, O.; El-Gendy, A.O. Comparative chemical and antimicrobial study of nine essential oils obtained from medicinal plants growing in Egypt. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Djenane, D.; Aïder, M.; Yangüela, J.; Idir, L.; Gómez, D.; Roncalés, P. Antioxidant and antibacterial effects of Lavandula and Mentha essential oils in minced beef inoculated with E. coli O157: H7 and S. aureus during storage at abuse refrigeration temperature. Meat Sci. 2012, 92, 667–674. [Google Scholar] [CrossRef]
- Romeo, F.V.; De Luca, S.; Piscopo, A.; Poiana, M. Antimicrobial effect of some essential oils. J. Essent. Oil Res. 2008, 20, 373–379. [Google Scholar] [CrossRef]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula angustifolia and Lavandula latifolia essential oils from Spain: Aromatic profile and bioactivities. Planta Med. 2016, 82, 163–170. [Google Scholar]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia Mill. Essential oil exerts antibacterial and anti-inflammatory effect in macrophage mediated immune response to Staphylococcus aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Insawang, S.; Pripdeevech, P.; Tanapichatsakul, C.; Khruengsai, S.; Monggoot, S.; Nakham, T.; Artrod, A.; D’Souza, P.E.; Panuwet, P. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem. Biodivers. 2019, 16, e1900371. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Al-Shaheen, S.; Kaskoos, R.; Ahamad, J.; Jameel, M.; Mir, S. Essential oil composition and antioxidant activity of Lavandula angustifolia from Iraq. Int. Res. J. Pharm. 2013, 4, 117–120. [Google Scholar]
- Srivastava, S.; Lal, R.; Yadav, K.; Pant, Y.; Bawitlung, L.; Kumar, P.; Mishra, A.; Gupta, P.; Pal, A.; Rout, P. Chemical composition of phenylpropanoid rich chemotypes of Ocimum basilicum L. and their antimicrobial activities. Ind. Crops Prod. 2022, 183, 114978. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, C.C.; Da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Moro, I.J.; Gondo, G.D.G.A.; Pierri, E.G.; Pietro, R.C.L.R.; Soares, C.P.; Sousa, D.P.d.; Santos, A.G.d. Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz. J. Pharm. Sci. 2018, 53, e00076. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, M.; Mehdizadeh, L. Chemistry of essential oils and factors influencing their constituents. In Soft Chemistry and Food Fermentation; Ferdowsi University of Mashhad: Mashhad, Iran, 2017; pp. 379–419. [Google Scholar]
- De Azeredo, G.A.; Stamford, T.L.M.; Nunes, P.C.; Neto, N.J.G.; De Oliveira, M.E.G.; De Souza, E.L. Combined application of essential oils from Origanum vulgare L. and Rosmarinus officinalis L. to inhibit bacteria and autochthonous microflora associated with minimally processed vegetables. Food Res. Int. 2011, 44, 1541–1548. [Google Scholar] [CrossRef] [Green Version]
- Salem, N.; Bachrouch, O.; Sriti, J.; Msaada, K.; Khammassi, S.; Hammami, M.; Selmi, S.; Boushih, E.; Koorani, S.; Abderraba, M. Fumigant and repellent potentials of Ricinus communis and Mentha pulegium essential oils against Tribolium castaneum and Lasioderma serricorne. Int. J. Food Prop. 2017, 20, S2899–S2913. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.-Y.; Li, R.; Jiang, Z.-T.; Wang, Y.; Tan, J.; Tang, S.-H.; Zhang, Y. Screening and evaluation of radical scavenging active compounds in the essential oil from Magnolia biondii Pamp by electronic nose coupled with chemical methodology. Ind. Crops Prod. 2020, 144, 112060. [Google Scholar] [CrossRef]
- Vaishnavi, B.C.; Ananya, S.J.; Jitendra, N.Y.; Rajendra, B.S. Strategies to Improve Stability of Essential Oils. J. Pharm. Sci. Res. 2021, 13, 416–425. [Google Scholar]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Cavalcante, Í.H.L.; Nogueira e Silva, G.J.; Cavacini, J.A.; Araújo e Amariz, R.; Tonetto de Freitas, S.; Oliveira de Sousa, K.Â.; Almeida da Silva, M.; Gomes da Cunha, J. Metconazole on inhibition of gibberellin biosynthesis and flowering management in mango. Erwerbs-Obstbau 2020, 62, 89–95. [Google Scholar] [CrossRef]
- Zhu, L.-H.; van de Peppel, A.; Li, X.-Y.; Welander, M. Changes of leaf water potential and endogenous cytokinins in young apple trees treated with or without paclobutrazol under drought conditions. Sci. Hortic. 2004, 99, 133–141. [Google Scholar] [CrossRef]
- Opio, P.; Tomiyama, H.; Saito, T.; Ohkawa, K.; Ohara, H.; Kondo, S. Paclobutrazol elevates auxin and abscisic acid, reduces gibberellins and zeatin and modulates their transporter genes in Marubakaido apple (Malus prunifolia Borkh. var. ringo Asami) rootstocks. Plant Physiol. Biochem. 2020, 155, 502–511. [Google Scholar] [CrossRef]
- Davis, T.D.; Curry, E.A.; Steffens, G.L. Chemical regulation of vegetative growth. Crit. Rev. Plant Sci. 1991, 10, 151–188. [Google Scholar] [CrossRef]
- Ghasemi Soluklui, A.A.; Ershadi, A.; Tabatabaee, Z.E.; Fallahi, E. Paclobutrazol-induced biochemical changes in pomegranate (Punica granatum L.) cv. ‘Malas Saveh’ under freezing stress. Int. J. Hortic. Sci. Technol. 2014, 1, 181–190. [Google Scholar]
- Mehmood, M.Z.; Qadir, G.; Afzal, O.; Din, A.M.U.; Raza, M.A.; Khan, I.; Hassan, M.J.; Awan, S.A.; Ahmad, S.; Ansar, M. Paclobutrazol improves sesame yield by increasing dry matter accumulation and reducing seed shattering under rainfed conditions. Int. J. Plant Prod. 2021, 15, 337–349. [Google Scholar] [CrossRef]
- El-Hai, K. Controlling of Alternaria leaf spot disease on faba bean using some growth substances. Asian J. Plant Pathol. 2015, 9, 124–134. [Google Scholar]
- Lu, Q.; Weng, Y.; You, Y.; Xu, Q.; Li, H.; Li, Y.; Liu, H.; Du, S. Inoculation with abscisic acid (ABA)-catabolizing bacteria can improve phytoextraction of heavy metal in contaminated soil. Environ. Pollut. 2020, 257, 113497. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription Factors Interact with ABA through Gene Expression and Signaling Pathways to Mitigate Drought and Salinity Stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- El-Yazied, A.A.; Ibrahim, M.F.; Ibrahim, M.A.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M. Melatonin Mitigates Drought Induced Oxidative Stress in Potato Plants through Modulation of Osmolytes, Sugar Metabolism, ABA Homeostasis and Antioxidant Enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef]
- Lv, C.; Li, F.; Ai, X.; Bi, H. H2O2 participates in ABA regulation of grafting-induced chilling tolerance in cucumber. Plant Cell Rep. 2022, 41, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Syahputra, B.S.; Sinniah, U.R.; Omar SR, S.; Ismail, M.R. Changes in gibberellic acid (GA3) content in Oryza sativa due to paclobutrazol treatment. J. Food Pharm. Sci. 2013, 1, 1. [Google Scholar]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif. 2004, 37, 221–229. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Soumya, P.; Kumar, P.; Pal, M. Paclobutrazol: A novel plant growth regulator and multi-stress ameliorant. Indian J. Plant Physiol. 2017, 22, 267–278. [Google Scholar] [CrossRef]
- Abdelfattah, E.M.; Aimad, A.; Bourhia, M.; Chebbac, K.; Salamatullah, A.M.; Soufan, W.; Nafidi, H.-A.; Aboul-Soud, M.A.; Ouahmane, L.; Bari, A. Insecticidal and Antifungal Activities of Chemically-Characterized Essential Oils from the Leaves of Withania frutescens L. Life 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Adaszyńska-Skwirzyńska, M.; Swarcewicz, M.; Dobrowolska, A. The potential of use lavender from vegetable waste as effective antibacterial and sedative agents. Med. Chem. 2014, 4, 734–737. [Google Scholar]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Graßmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I. Antioxidant activity of Linalool. Eng. Technol. J. 2018, 36, 1. [Google Scholar]
- Shaaban, H.A.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Juergens, L.J.; Tuleta, I.; Stoeber, M.; Racké, K.; Juergens, U.R. Regulation of monocyte redox balance by 1, 8-cineole (eucalyptol) controls oxidative stress and pro-inflammatory responses in vitro: A new option to increase the antioxidant effects of combined respiratory therapy with budesonide and formoterol? Synergy 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Ríos-Chávez, P.; Saavedra-Molina, A.; López-Meza, J.E.; Ochoa-Zarzosa, A.; Garciglia, R.S. Antioxidant activity of the essential oil and its major terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag. 2017, 13, S875. [Google Scholar]
- Mamadalieva, N.Z.; Sharopov, F.; Satyal, P.; Azimova, S.S.; Wink, M. Composition of the essential oils of three Uzbek Scutellaria species (Lamiaceae) and their antioxidant activities. Nat. Prod. Res. 2017, 31, 1172–1176. [Google Scholar] [CrossRef]
- Durazzo, A. Study approach of antioxidant properties in foods: Update and considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, M.E.; Marei, G.I.K.; Rabea, E.I.; Taktak, N.E. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Physiol. 2019, 158, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Matasyoh, L.G.; Matasyoh, J.C.; Wachira, F.N.; Kinyua, M.G.; Muigai, A.W.T.; Mukiama, T.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum gratissimum L. growing in Eastern Kenya. Afr. J. Biotechnol. 2007, 6, 760–765. [Google Scholar]
- Sourmaghi, M.H.S.; Kiaee, G.; Golfakhrabadi, F.; Jamalifar, H.; Khanavi, M. Comparison of essential oil composition and antimicrobial activity of Coriandrum sativum L. extracted by hydrodistillation and microwave-assisted hydrodistillation. J. Food Sci. Technol. 2015, 52, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonca, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar] [CrossRef]
- Rahman, A.; Al-Reza, S.M.; Kang, S.C. Antifungal activity of essential oil and extracts of Piper chaba Hunter against phytopathogenic fungi. J. Am. Oil Chem. Soc. 2011, 88, 573–579. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Ciocarlan, A.; Lupascu, L.; Aricu, A.; Dragalin, I.; Popescu, V.; Geana, E.-I.; Ionete, R.E.; Vornicu, N.; Duliu, O.G.; Hristozova, G. Chemical composition and assessment of antimicrobial activity of lavender essential oil and some by-products. Plants 2021, 10, 1829. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control. 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Moussaoui, A.E.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Ouassou, H.; Elamine, Y.; Ullah, R.; Ezzeldin, E. Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: Correlation between total phenolic and flavonoid contents with antioxidant and antimicrobial activities of chemically characterized Extracts. Plants 2021, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Vidal, L.; Goldberg, E.; Leibovici, L.; Paul, M. Treatment of invasive candidal infections: Systematic review and meta-analysis. Mayo Clin. Proc. 2008, 83, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.; Travis, J. Local adaptation and the evolution of species’ ranges under climate change. J. Theor. Biol. 2010, 266, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, P.M.; Miranda, M.; Payrol, J.A.; Silva, M.; Hernández, V.; Peralta, E. Gas chromatography-mass spectrometry study from the leaves fractions obtained of Vernonanthura patens (Kunth) H. Rob. Int. J. Org. Chem. 2013, 3, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Gargouri, W.; Osés, S.M.; Fernández-Muiño, M.A.; Sancho, M.T.; Kechaou, N. Evaluation of bioactive compounds and biological activities of Tunisian propolis. LWT 2019, 111, 328–336. [Google Scholar] [CrossRef]
- Roberts, D.; Greenwood, M. Practical Food Microbiology; Public Health Laboratory Service: London, UK, 2003; Volume 2, pp. 55–68. [Google Scholar]
- M100-S27; Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Seventh Informational Supplement the Clinical Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2017.
- SAS. SAS/STAT User’s Guide, Release 6.03 ed.; SAS Institute Inc.: Cary, NC, USA, 1988. [Google Scholar]



| Compound | Control | PBZ Treatments | ||||
|---|---|---|---|---|---|---|
| No. | 200 ppm | 400 ppm | 600 ppm | |||
| Monoterpene Hydrocarbons | ||||||
| 1 | α-Pinene | 1.07 | - | - | - | |
| 2 | β-Pinene | 0.95 | - | 0.69 | - | |
| 3 | 3-Carene | 0.38 | - | 0.84 | 0.22 | |
| 4 | γ -Terpinene | 0.89 | - | 0.92 | - | |
| 5 | Terpinolene | 0.91 | - | - | - | |
| Total (%) | 4.2 | - | 2.45 | 0.22 | ||
| Oxygenated monoterpenes | ||||||
| 1 | Carveol | 0.98 | - | - | 0.37 | |
| 2 | Eucalyptol | 21.55 | 29.89 | 22.95 | 0.64 | |
| 3 | α-Terpineol | 6.07 | - | 5.35 | - | |
| 4 | p-Cymen-7-ol | 0.79 | - | 0.71 | - | |
| 5 | Linalool | - | - | 2.25 | - | |
| 6 | L-Pinocarveol | - | - | 1 | - | |
| 7 | α-Pinocarvone | 1.52 | - | 1.65 | - | |
| 8 | cis-Verbenol | - | - | 0.24 | - | |
| 9 | 2-Pinen-10-ol | - | - | 1.25 | - | |
| 10 | trans-Carveol | 0.94 | - | - | 0.43 | |
| 11 | L-camphor | 17.56 | 13.76 | 16.67 | 9.98 | |
| 12 | D-Carvone | - | - | 1.93 | - | |
| Total (%) | 49.41 | 43.65 | 54 | 11.42 | ||
| Sesquiterpene Hydrocarbons | ||||||
| 1 | β-Copaene | 0.22 | - | - | - | |
| 2 | Caryophyllene | 0.47 | - | 0.61 | - | |
| 3 | α-Farnesene | - | - | 3.8 | - | |
| Total (%) | 0.69 | - | 4.41 | - | ||
| Oxygenated sesquiterpenes | ||||||
| 1 | β-Spathulenol | 0.69 | - | 0.43 | - | |
| 2 | Caryophyllene oxide | 0.23 | - | 0.25 | 9.94 | |
| 3 | β-Eudesmol | - | - | 1.28 | - | |
| 4 | Humulene oxide II | 0.64 | - | - | - | |
| 5 | δ-Cedrol | - | - | 3.2 | - | |
| 6 | Nootkatone | 0.37 | - | 0.52 | - | |
| Total (%) | 1.93 | - | 5.68 | 9.94 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, S.M.; Hassan, K.M.; Abdelhamid, A.N.; Yousef, E.E.; Abdellatif, Y.M.R.; Abu-Hussien, S.H.; Nasser, M.A.; Elshalakany, W.A.; Darwish, D.B.E.; Abdulmajeed, A.M.; et al. Exogenous Paclobutrazol Reinforces the Antioxidant and Antimicrobial Properties of Lavender (Lavandula officinalis L.) Oil through Modulating Its Composition of Oxygenated Terpenes. Plants 2022, 11, 1607. https://doi.org/10.3390/plants11121607
El-Sayed SM, Hassan KM, Abdelhamid AN, Yousef EE, Abdellatif YMR, Abu-Hussien SH, Nasser MA, Elshalakany WA, Darwish DBE, Abdulmajeed AM, et al. Exogenous Paclobutrazol Reinforces the Antioxidant and Antimicrobial Properties of Lavender (Lavandula officinalis L.) Oil through Modulating Its Composition of Oxygenated Terpenes. Plants. 2022; 11(12):1607. https://doi.org/10.3390/plants11121607
Chicago/Turabian StyleEl-Sayed, Salwa M., Karim. M. Hassan, Ahmed. N. Abdelhamid, Eman E. Yousef, Yasmin M. R. Abdellatif, Samah H. Abu-Hussien, Mohamed A. Nasser, Walaa. A. Elshalakany, Doaa Bahaa Eldin Darwish, Awatif M. Abdulmajeed, and et al. 2022. "Exogenous Paclobutrazol Reinforces the Antioxidant and Antimicrobial Properties of Lavender (Lavandula officinalis L.) Oil through Modulating Its Composition of Oxygenated Terpenes" Plants 11, no. 12: 1607. https://doi.org/10.3390/plants11121607
APA StyleEl-Sayed, S. M., Hassan, K. M., Abdelhamid, A. N., Yousef, E. E., Abdellatif, Y. M. R., Abu-Hussien, S. H., Nasser, M. A., Elshalakany, W. A., Darwish, D. B. E., Abdulmajeed, A. M., Alabdallah, N. M., Al-Qahtani, S. M., Al-Harbi, N. A., Dessoky, E. S., Ashour, H., & Ibrahim, M. F. M. (2022). Exogenous Paclobutrazol Reinforces the Antioxidant and Antimicrobial Properties of Lavender (Lavandula officinalis L.) Oil through Modulating Its Composition of Oxygenated Terpenes. Plants, 11(12), 1607. https://doi.org/10.3390/plants11121607









