Diaporthe citri: A Fungal Pathogen Causing Melanose Disease
Abstract
:1. Introduction
1.1. Major Fungal Diseases on Citrus
1.2. Diaporthe Species Associated with Citrus
1.3. Identification and Molecular Diagnostics
1.3.1. Morphological Characteristics
1.3.2. Molecular Identification
1.3.3. Molecular Diagnosis
1.3.4. Genetic Populations
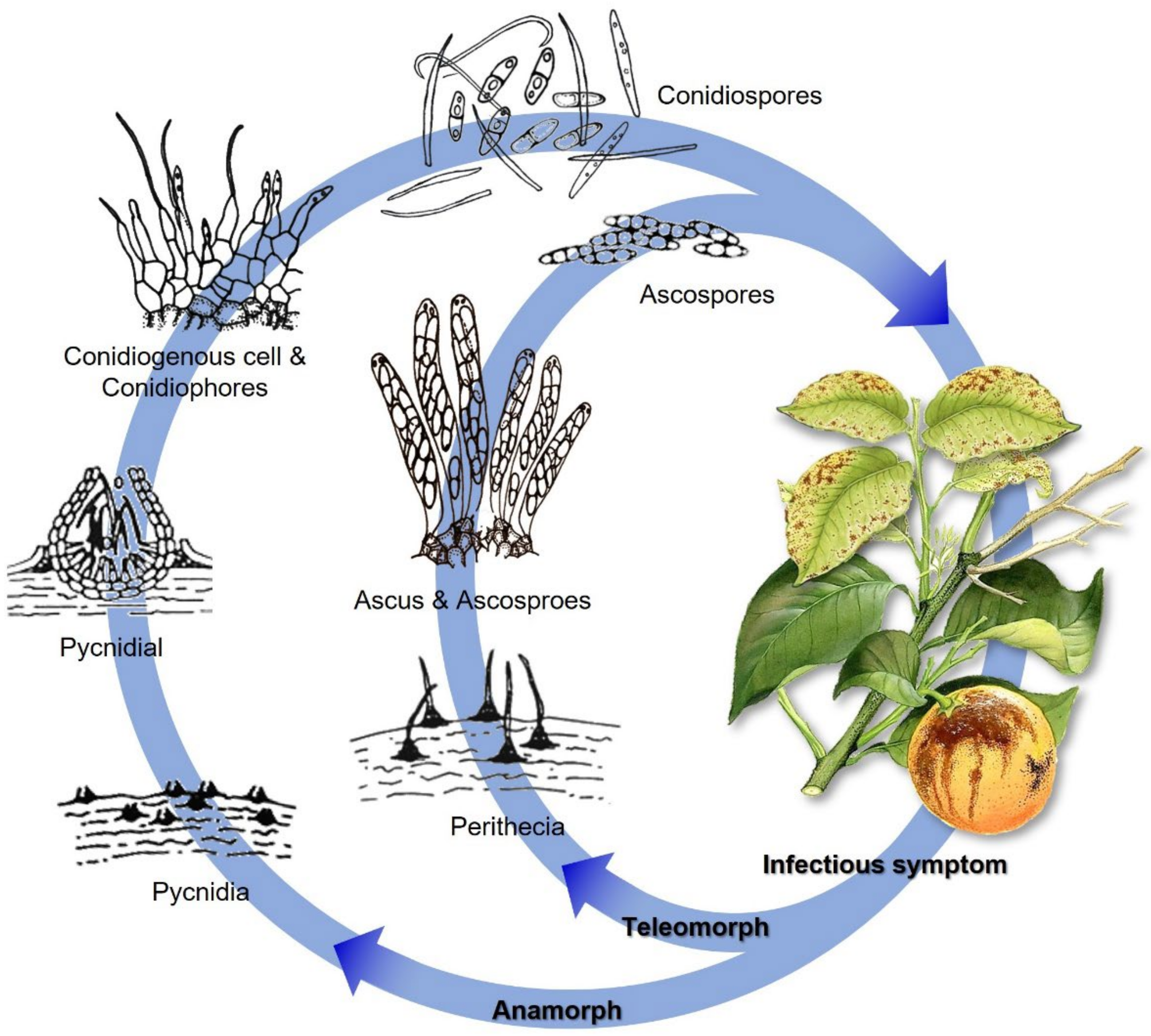
2. Epidemiology, Life Cycle, and Symptomatology
3. Geographic Distribution and Host Associations
4. Main Management Approaches of Melanose Disease
4.1. Chemical Control
4.2. Biological Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BeZerra, J.D.P.; Crous, P.W.; Aiello, D.; Gullino, M.L.; Polizzi, G.; Guarnaccia, V. Genetic diversity and pathogenicity of Botryosphaeriaceae species associated with symptomatic citrus plants in Europe. Plants 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.E.; Wang, W.; Crous, P.W.; Wang, H.K.; Jiao, C.; Huang, F.; Pu, Z.X.; Zhu, Z.R.; Li, H.Y. Species of Botryosphaeriaceae associated with citrus branch diseases in China. Persoonia 2021, 47, 106–135. [Google Scholar] [CrossRef]
- Chung, K.R. Elsinoë fawcettii and Elsinoë australis: The fungal pathogens causing citrus scab. Mol. Plant Pathol. 2011, 12, 123–135. [Google Scholar] [CrossRef]
- Fan, X.L.; Barreto, R.W.; Groenewald, J.Z.; Bezerra, J.D.; Pereira, O.L.; Cheewangkoon, R.; Mostert, L.; Tian, C.M.; Crous, P.W. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Stud. Mycol. 2017, 87, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Govindarajulu, B.; Ramana, K.T.V.; Kumar, C.S.K.; Gopi, V.; Sankar, T.G.; Lakshmi, L.M.; Lakshmi, T.N.; Sarada, G. Citrus scab (Elsinoë fawcettii): A review. J. Agric. Allied Sci. 2014, 3, 49–58. [Google Scholar]
- Hyun, J.W.; Yi, S.H.; MacKenzie, S.J.; Timmer, L.W.; Kim, K.S.; Kang, S.K.; Kwon, H.M.; Lim, H.C. Pathotypes and genetic relationship of worldwide collections of Elsinoë spp. causing scab disease of citrus. Phytopathology 2009, 99, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Miles, A.K.; Tan, Y.P.; Shivas, R.G.; Drenth, A. Novel pathotypes of Elsinoë australis associated with Citrus australasica and Simmondsia chinensis in Australia. Trop. Plant Pathol. 2015, 40, 26–34. [Google Scholar] [CrossRef]
- Timmer, L.W.; Priest, M.; Broadbent, P.; Tan, M.K. Morphological and pathological characterization of species of Elsinoë causing scab diseases of citrus. Phytopathology 1996, 86, 1032–1038. [Google Scholar] [CrossRef]
- Whiteside, J.O. Biological characteristics of Elsinoë fawcetti pertaining to the epidemiology of sour orange scab. Phytopathology 1975, 65, 1170–1177. [Google Scholar] [CrossRef]
- Peever, T.L.; Carpenter-Boggs, L.; Timmer, L.W.; Carris, L.M.; Bhatia, A. Citrus black rot is caused by phylogenetically distinct lineages of Alternaria alternata. Phytopathology 2005, 95, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Timmer, L.W.; Peever, T.L.; Solel, Z.; Akimitsu, K. Alternaria diseases of citrus—Novel pathosystems. Phytopathol. Mediterr. 2003, 42, 99–112. [Google Scholar]
- Timmer, L.W.; Solel, Z.; Orozco-Santos, M. Alternaria Brown Spot of Mandarins. In Compendium of Citrus Diseases; Timmer, L.W., Garnsey, S.M., Graham, J.H., Eds.; The American Phytopathological Society Press: Saint Paul, MI, USA, 2000. [Google Scholar]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretorius, M.C.; Crous, P.W.; Groenewald, J.Z.; Braun, U. Phylogeny of some cercosporoid fungi from Citrus. Sydowia 2003, 55, 286–305. [Google Scholar]
- Huang, F.; Groenewald, J.Z.; Zhu, L.; Crous, P.W.; Li, H.Y. Cercosporoid diseases of Citrus. Mycologia 2015, 107, 1151–1171. [Google Scholar] [CrossRef] [Green Version]
- Bragança, C.A.D.; Damm, U.; Baroncelli, R.; Júnior, N.S.M.; Crous, P.W. Species of the Colletotrichum acutatum complex associated with anthracnose diseases of fruit in Brazil. Fungal Biol. 2016, 120, 547–561. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Johnston, P.R.; Weir, B.S.; Tan, Y.P.; Shivas, R.G.; Crous, P.W. The Colletotrichum boninense species complex. Stud. Mycol. 2012, 73, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 2009, 39, 45–87. [Google Scholar]
- Guarnaccia, V.; Groenewald, J.Z.; Polizzi, G.; Crous, P.W. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 2017, 39, 32–50. [Google Scholar] [CrossRef]
- Honger, J.O.; Offei, S.K.; Oduro, K.A.; Odamtten, G.T.; Nyaku, S.T. Identification and molecular characterisation of Colletotrichum species from avocado, citrus and pawpaw in Ghana. S. Afr. J. Plant Soil 2016, 33, 177–185. [Google Scholar] [CrossRef]
- Huang, F.; Chen, G.Q.; Hou, X.; Fu, Y.S.; Cai, L.; Hyde, K.D.; Li, H.Y. Colletotrichum species associated with cultivated citrus in China. Fungal Divers. 2013, 61, 61–74. [Google Scholar] [CrossRef]
- Peng, L.J.; Yang, Y.L.; Hyde, K.D.; Bahkali, A.H.; Liu, Z.Y. Colletotrichum species on citrus leaves in Guizhou and Yunnan provinces, China. Cryptogam. Mycol. 2012, 33, 267–283. [Google Scholar]
- Wang, W.; de Silva, D.D.; Moslemi, A.; Edwards, J.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Colletotrichum species causing anthracnose of citrus in Australia. J. Fungi 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, G.J.; Eckert, J.W.; Pitt, J.I. Revised description of Penicillium ulaiense and its role as a pathogen of citrus fruits. Phytopathology 1994, 84, 719–727. [Google Scholar] [CrossRef]
- Tashiro, N.; Manabe, K.; Ide, Y. First report of whisker mold, a postharvest disease on citrus caused by Penicillium ulaiense (in Japan). J. Gen. Plant Pathol. 2012, 78, 140–144. [Google Scholar] [CrossRef]
- Louw, J.P.; Korsten, L. Pathogenicity and host susceptibility of Penicillium spp. on citrus. Plant Dis. 2015, 99, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by species of Diaporthe. IMA Fungus 2017, 8, 317–334. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Hou, X.; Dewdney, M.M.; Fu, Y.S.; Chen, G.Q.; Hyde, K.D.; Li, H.Y. Diaporthe species occurring on citrus in China. Fungal Divers. 2013, 61, 237–250. [Google Scholar] [CrossRef]
- Huang, F.; Udayanga, D.; Wang, X.H.; Hou, X.; Mei, X.F.; Fu, Y.S.; Hyde, K.D.; Li, H.Y. Endophytic Diaporthe associated with citrus: A phylogenetic reassessment with seven new species from China. Fungal Biol. 2015, 119, 331–347. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.Y.; Lin, Y.; Li, J.B.; Xiong, B.; Luo, C.X. Phylogenetic analysis and development of molecular tool for detection of Diaporthe citri causing melanose disease of citrus. Plants 2020, 9, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danggomen, A.; Visarathanonth, N.; Manoch, L.; Piasai, O. Morphological studies of endophytic and plant pathogenic Phomopsis liquidambaris and Diaporthe phaseolorum (P. phaseoli anamorph) from healthy plants and diseased fruits. Thai J. Agric. Sci. 2013, 46, 157–164. [Google Scholar]
- Dong, Z.Y.; Manawasinghe, I.S.; Huang, Y.H.; Shu, Y.X.; Phillips, A.J.; Dissanayake, A.J.; Hyde, K.D.; Xiang, M.M.; Luo, M. Endophytic Diaporthe associated with Citrus grandis cv. Tomentosa in China. Front. Microbiol. 2021, 11, 609387. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 2013, 31, 1–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.P.; Edwards, J.; Grice, K.R.E.; Shivas, R.G. Molecular phylogenetic analysis reveals six new species of Diaporthe from Australia. Fungal Divers. 2013, 61, 251–260. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Hyde, K.D. Species limits in Diaporthe: Molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 2014, 32, 83–101. [Google Scholar] [CrossRef] [Green Version]
- Fawcett, H.S. The cause of stem-end rot of citrus fruits (Phomopsis citri n. sp.). Phytopathology 1912, 2, 109–113. [Google Scholar]
- Wolf, F.A. The perfect stage of the fungus which causes melanose of citrus. J. Agric. Res. 1926, 33, 621–625. [Google Scholar]
- Chen, G.Q.; Jiang, L.Y.; Xu, F.S.; Li, H.Y. In vitro and in vivo screening of fungicides for controlling citrus melanose caused by Diaporthe citri. J. Zhejiang Univ. (Agric. Life Sci.) 2010, 36, 440–444. (In Chinese) [Google Scholar]
- Douanla-Meli, C.; Langer, E.; Mouafo, F.T. Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon. Fungal Ecol. 2013, 6, 212–222. [Google Scholar] [CrossRef]
- Habib, W.; Gerdes, E.; Antelmi, I.; Baroudy, F.; Choueiri, E.; Nigro, F. Diaporthe foeniculina associated with severe shoot blight of lemon in Lebanon. Phytopathol. Mediterr. 2015, 54, 149–150. [Google Scholar]
- Jiang, L.Y.; Xu, F.S.; Huang, Z.D.; Huang, F.; Chen, G.Q.; Li, H.Y. Occurrence and control of citrus melanose caused by Diaporthe citri. Acta Agric. Zhejiangensis 2012, 24, 647–653. (In Chinese) [Google Scholar]
- Mondal, S.N.; Vicent, A.; Reis, R.F.; Timmer, L.W. Saprophytic colonization of citrus twigs by Diaporthe citri and factors affecting pycnidial production and conidial survival. Plant Dis. 2007, 91, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, T.S.; Suryanarayanan, T.S.; Geeta, R. Endophytic Phomopsis species: Host range and implications for diversity estimates. Can. J. Microbiol. 2006, 52, 673–680. [Google Scholar] [CrossRef]
- Xiong, T.; Zeng, Y.T.; Wang, W.; Li, P.D.; Gai, Y.P.; Jiao, C.; Zhu, Z.R.; Xu, J.P.; Li, H.Y. Abundant genetic diversity and extensive differentiation among geographic populations of the citrus pathogen Diaporthe citri in Southern China. J. Fungi 2021, 7, 749. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Crous, P.W. Species of Diaporthe on Camellia and Citrus in the Azores Islands. Phytopathol. Mediterr. 2018, 57, 307–319. [Google Scholar]
- Yang, Q.; Jiang, N.; Tian, C.M. New species and records of Diaporthe from Jiangxi province, China. MycoKeys 2021, 77, 41–64. [Google Scholar] [CrossRef]
- Vakalounakis, D.J.; Ntougias, S.; Kavroulakis, N.; Protopapadakis, E. Neofusicoccum parvum and Diaporthe foeniculina associated with twig and shoot blight and branch canker of citrus in Greece. J. Phytopathol. 2019, 167, 527–537. [Google Scholar] [CrossRef]
- Tekiner, N.; Tozlu, E.; Guarnaccia, V. First report of Diaporthe foeniculina causing fruit rot of lemon in Turkey. J. Gen. Plant Pathol. 2020, 102, 277. [Google Scholar] [CrossRef] [Green Version]
- Juybari, H.Z.; Tajick Ghanbary, M.A.; Karimi, H.; Arzanlou, K.; Seasonal, M. Seasonal, tissue and age influences on frequency and biodiversity of endophytic fungi of Citrus sinensis in Iran. For. Pathol. 2019, 6, e12559. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.Y.; Yin, W.X.; Luo, C.X.; Lin, Y. Morphology characterization, molecular phylogeny, and pathogenicity of Diaporthe passifloricola on Citrus reticulata cv. Nanfengmiju in Jiangxi province, China. Plants 2021, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.J.; Wei, X.; Xia, P.L.; Yi, J.P.; Yu, Z.H.; Deng, J.X.; Li, Q.L. Diaporthe taoicola and D. siamensis, two new records on Citrus sinensis in China. Mycobiology 2021, 49, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Chung, W.C.; Huang, H.C.; Chung, W.H.; Chung, W.H. Identification of endophytic fungi of medicinal herbs of Lauraceae and Rutaceae with antimicrobial property. Taiwania 2012, 57, 229–241. [Google Scholar]
- Mahadevakumar, S.; Yadav, V.; Tejaswini, G.S.; Sandeep, S.N.; Janardhana, G.R. First report of Phomopsis citri associated with dieback of Citrus lemon in India. Plant Dis. 2014, 98, 1281. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Samsampour, D.; Seyahooei, M.A.; Bagheri, A.; Soltani, J. Diversity and spatiotemporal distribution of fungal endophytes associated with Citrus reticulata cv. Siyahoo. Curr. Microbiol. 2019, 76, 279–289. [Google Scholar] [CrossRef]
- Hongsanan, S.; Maharachchikumbura, S.S.N.; Hyde, K.D.; Samarakoon, M.C.; Jeewon, R.; Zhao, Q.; Al-Sadi, A.M.; Bahkali, A.H. An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers. 2017, 84, 25–41. [Google Scholar] [CrossRef]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; de Cock, A.W.A.M.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important phytopathogenic genera: I (2014). Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef] [Green Version]
- Jayasiri, S.C.; Hyde, K.D.; Ariyawansa, H.A.; Bhat, J.; Buyck, B.; Cai, L.; Dai, Y.C.; Abd-Elsalam, K.A.; Ertz, D.; Hidayat, I.; et al. The faces of fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 2015, 74, 3–18. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Bhat, J.D.; Dayarathne, M.C.; Huang, S.K.; Norphanphoun, C.; Senanayake, I.C.; Perera, R.H.; et al. Families of Sordariomycetes. Fungal Divers. 2016, 79, 1–317. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Huang, S.K.; Abdel-Wahab, M.A.; Daranagama, D.A.; Dayarathne, M.; D’souza, M.J.; Goonasekara, I.D.; et al. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 2015, 72, 199–301. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Crous, P.W.; Groenewald, J.Z.; Maharachchikumbura, S.S.N.; Jeewon, R.; Phillips, A.J.L.; Bhat, J.D.; Perera, R.H.; Li, Q.R.; Li, W.J.; et al. Families of Diaporthales based on morphological and phylogenetic evidence. Stud. Mycol. 2017, 86, 217–296. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, D.; Liu, X.Z.; McKenzie, E.H.C.; Chukeatirote, E.; Bahkali, A.H.A.; Hyde, K.D. The genus Phomopsis: Biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011, 50, 189–225. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Rajeshkumar, K.C.; Hawksworth, D.L.; Madrid, H.; Kirk, P.M.; Braun, U.; Singh, R.V.; Crous, P.W.; Kukwa, M.; et al. Notes for genera: Ascomycota. Fungal Divers. 2017, 86, 1–594. [Google Scholar]
- Nitschke, T.R.J. Pyrenomycetes germanici. In Die Kernpilze Deutschlands Bearbeitet von dr. Th. Nitschke; Eduard Trewendt: Breslau, Germany, 1870; Volume 2, pp. 161–320. [Google Scholar]
- Diogo, E.L.F.; Santos, J.M.; Phillips, A.J.L. Phylogeny, morphology and pathogenicity of Diaporthe and Phomopsis species on almond in Portugal. Fungal Divers. 2010, 44, 107–115. [Google Scholar] [CrossRef]
- Farr, D.F.; Castlebury, L.A.; Rossman, A.Y.; Putnam, M.L. A new species of Phomopsis causing twig dieback of Vaccinium vitis-idaea (lingonberry). Mycol. Res. 2002, 106, 745–752. [Google Scholar] [CrossRef]
- Gao, Y.H.; Liu, F.; Duan, W.J.; Crous, P.W.; Cai, L. Diaporthe is paraphyletic. IMA Fungus 2017, 8, 153–187. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Sun, S.K. Phomopsis fruit rot of subtropical peach in Taiwan. Plant Pathol. Bull. 2003, 12, 212–214. [Google Scholar]
- Santos, J.M.; Phillips, A.J.L. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Divers. 2009, 34, 111–125. [Google Scholar]
- Brayford, D. Variation in Phomopsis isolates from Ulmus species in the British Isles and Italy. Mycol. Res. 1990, 94, 691–697. [Google Scholar] [CrossRef]
- Chi, P.K.; Jiang, Z.D.; Xiang, M.M. Flora Fungorum Sinicorum; Science Press: Beijing, China, 2007; Volume 34. (In Chinese) [Google Scholar]
- Mostert, L.; Crous, P.W.; Kang, J.C.; Phillips, A.J.L. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: Morphological, cultural, molecular and pathological characterization. Mycologia 2001, 93, 146–167. [Google Scholar] [CrossRef]
- Uecker, F.A. A world list of Phomopsis names with notes on nomenclature, morphology and biology. Mycol. Mem. 1988, 13, 1–231. [Google Scholar]
- Timmer, L.W.; Mondal, S.N.; Peres, N.A.R.; Bhatia, A. Fungal Diseases of Fruit and Foliage of Citrus Trees. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Springer: Dordrecht, The Netherlands, 2004; Volume 1, pp. 247–290. [Google Scholar]
- Wehmeyer, L.E. The Genus Diaporthe Nitschke and Its Segregates; Science Series; University of Michigan Studies: Ann Arbor, MI, USA, 1933; Volume 9, pp. 1–349. [Google Scholar]
- Whiteside, J.O.; Garnsey, S.M.; Timmer, L.W. Melanose. In Compendium of Citrus Diseases; Whiteside, J.O., Ed.; APS Press: St. Paul, MN, USA, 1988; pp. 20–21. [Google Scholar]
- Muntanola-Cvetković, M.; Mihaljčević, M.; Petrov, M. On the identity of the causative agent of a serious Phomopsis-Diaporthe disease in sunflower plants. Nova Hedwig. 1981, 34, 417–435. [Google Scholar]
- Punithalingam, E. Phomopsis anacardii. CMI Descr. Pathog. Fungi Bact. 1985, 826, 1–2. [Google Scholar]
- Cristescu, C. A new species of Phomopsis Sacc. (Mitosporic fungi) in Romania. Rom. J. Biol.-Plant Biol. 2003, 48, 45–49. [Google Scholar]
- Rehner, S.A.; Uecker, F.A. Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycete phomopsis. Can. J. Bot. 1994, 72, 1666–1674. [Google Scholar] [CrossRef]
- Cristescu, C. The morphology and anatomy of structure somatic and reproductive of species of Phomopsis (Sacc.) Bubák. Bul. Grădinii Bot. Iaşi Tomul 2007, 14, 19–27. [Google Scholar]
- Rosskopf, E.N.; Charudattan, R.; Shabana, Y.M.; Benny, G.L. Phomopsis amaranthicola, a new species from Amaranthus sp. Mycologia 2000, 92, 114–122. [Google Scholar] [CrossRef]
- Rosskopf, E.N.; Charudattan, R.; Valero, J.T.; Stall, W.M. Field evaluation of Phomopsis amaranthicola, a biological control agent of Amaranthus spp. Plant Dis. 2000, 84, 1225–1230. [Google Scholar] [CrossRef] [Green Version]
- Sutton, B.C. The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Kew, Surrey, UK, 1980. [Google Scholar]
- Punithalingam, E. Studies on Sphaeropsidales in culture, II. Mycol. Pap. 1974, 136, 1–63. [Google Scholar]
- Rodeva, R.; Stoyanova, Z.; Pandeva, R. A new fruit disease of pepper in Bulgaria caused by Phomopsis capsici. Acta Hortic. 2009, 830, 551–556. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chaisiri, C.; Lin, Y.; Yin, W.X.; Luo, C.X. Whole-genome sequence of Diaporthe citri isolate NFHF-8-4 the causal agent of citrus melanose. Mol. Plant Microbe. Interact. 2021, 34, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.P.; Xiong, T.; Xiao, X.; Li, P.D.; Zeng, Y.T.; Li, L.; Riely, B.K.; Li, H.Y. The genome sequence of the citrus melanose pathogen Diaporthe citri and two citrus-related Diaporthe species. Phytopathology 2021, 111, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic species recognition and species concepts in fungi. Fungal Ecol. 2000, 31, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wang, M.; Damm, U.; Crous, P.W.; Cai, L. Species boundaries in plant pathogenic fungi: A Colletotrichum case study. BMC Evol. Biol. 2016, 16, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achari, S.R.; Kaur, J.; Dinh, Q.; Mann, R.; Sawbridge, T.; Summerell, B.A.; Edwards, J. Phylogenetic relationship between australian Fusarium oxysporum isolates and resolving the species complex using the multispecies coalescent model. BMC Genom. 2020, 21, 248. [Google Scholar] [CrossRef] [Green Version]
- Hilário, S.; Gonçalves, M.F.M.; Alves, A. Using genealogical concordance and coalescent-based species delimitation to assess species boundaries in the Diaporthe eres complex. J. Fungi 2021, 7, 507. [Google Scholar] [CrossRef]
- Hilário, S.; Santos, L.; Alves, A. Diaporthe amygdali, a species complex or a complex species? Fungal Biol. 2021, 125, 505–518. [Google Scholar] [CrossRef]
- Kubatko, L.S.; Degnan, J.H. Inconsistency of phylogenetic estimates from concatenated data under coalescence. Syst. Biol. 2007, 56, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.E.; Timmer, L.W.; Lawrence, C.B.; Pryor, B.M.; Peever, T.L. Discord between morphological and phylogenetic species boundaries: Incomplete lineage sorting and recombination results in fuzzy species boundaries in an asexual fungal pathogen. BMC Evol. Biol. 2014, 14, 38. [Google Scholar] [CrossRef]
- Castlebury, L. The Diaporthe vaccinii complex of fruit pathogens. Inoculum 2005, 56, 12. [Google Scholar]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Wingfield, M.J.; Akulov, A.; Carnegie, A.J.; Cheewangkoon, R.; Gramaje, D.; Groenewald, J.Z.; Guarnaccia, V.; Halleen, F.; et al. Genera of phytopathogenic fungi: GOPHY 2. Stud. Mycol. 2019, 92, 47–133. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Correia, V.G.; Phillips, A.J.L.; Spatafora, J.W. Primers for mating-type diagnosis in Diaporthe and Phomopsis: Their use in teleomorph induction in vitro and biological species definition. Fungal Biol. 2010, 114, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Farr, D.F.; Castlebury, L.A.; Rossman, A.Y. Morphological and molecular characterization of Phomopsis vaccinii and additional isolates of phomopsis from blueberry and cranberry in the Eastern United States. Mycologia 2002, 94, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. Insights into the genus Diaporthe: Phylogenetic species delimitation in the D. eres species complex. Fungal Divers. 2014, 67, 203–229. [Google Scholar] [CrossRef] [Green Version]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. The Diaporthe sojae species complex: Phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biol. 2015, 119, 383–407. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Liu, M.; Zhang, W.; Chen, Z.; Udayanga, D.; Chukeatirote, E.; Li, X.H.; Yan, J.Y.; Hyde, K.D. Morphological and molecular characterisation of Diaporthe species associated with grapevine trunk disease in China. Fungal Biol. 2015, 119, 283–294. [Google Scholar] [CrossRef]
- Santos, L.; Alves, A.; Alves, R. Evaluating multi-locus phylogenies for species boundaries determination in the genus Diaporthe. PeerJ 2017, 5, e3120. [Google Scholar] [CrossRef] [Green Version]
- Van Niekerk, J.M.; Groenewald, J.Z.; Farr, D.F.; Fourie, P.H.; Halleen, F.; Crous, P.W. Reassessment of Phomopsis species on grapevine. Australas. Plant Pathol. 2005, 34, 27–39. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.Y.; Lin, Y.; Fu, Y.P.; Zhu, F.X.; Luo, C.X. Phylogenetic and haplotype network analyses of Diaporthe eres species in China based on sequences of multiple loci. Biology 2021, 10, 179. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Farr, D.F.; Rossman, A.Y. Phylogenetic distinction of Phomopsis isolates from cucurbits. Inoculum 2001, 52, 25. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for desianing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Tacke, B.K.; Casper, H.C. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl. Acad. Sci. USA 2000, 97, 7905–7910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.M.; Castlebury, L.A.; Rossman, A.Y.; White, J.F. New molecular markers for fungal phylogenetics: Two genes for species-level systematic in the Sordariomycetes (Ascomycota). Mol. Phylogenet. Evol. 2012, 64, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Myllys, L.; Stenroos, S.; Thell, A. New genes for phylogenetic studies of lichenized fungi: Glyceraldehyde-3-phosphate dehydrogenase and betatubulin genes. Lichenologist 2002, 34, 237–246. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Risède, J.M.; Simoneau, P.; Hywel-Jones, N.L. Calonectria species and their Cylindrocladium anamorphs: Species with clavate vesicles. Stud. Mycol. 2004, 50, 415–430. [Google Scholar] [CrossRef] [Green Version]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microb. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Pecchia, S.; Mercatelli, E.; Vannacci, G. Intraspecific diversity within Diaporthe helianthi: Evidence from rDNA intergenic spacer (IGS) sequence analysis. Mycopathologia 2004, 157, 317–326. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Choi, C.W.; Jung, K.E.; Kim, M.J.; Yoon, S.H.; Park, S.M.; Jin, S.B.; Hyun, J.W. Development of molecular marker to detect citrus melanose caused by Diaporthe citri from citrus melanose-like symptoms. Plant Pathol. J. 2021, 37, 681–686. [Google Scholar] [CrossRef]
- Crous, P.W.; Schoch, C.L.; Hyde, K.D.; Wood, A.R.; Gueidan, C.; de Hoog, G.S.; Groenewald, J.Z. Phylogenetic lineages in the Capnodiales. Stud. Mycol. 2009, 64, 17–47. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Crytococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilário, S.; Amaral, I.A.; Gonçalves, M.F.M.; Lopes, A.; Santos, L.; Alves, A. Diaporthe species associated with twig blight and dieback of Vaccinium corymbosum in Portugal, with description of four new species. Mycologia 2020, 112, 293–308. [Google Scholar] [CrossRef]
- Li, K.N.; Rouse, D.I.; German, T.L. PCR primers that allow intergenetic differentiation of ascomycetes and their application to Verticillium spp. Appl. Environ. Microb. 1994, 60, 4324–4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; Verkley, G.J.M.; Gruyter, J.; Perelló, A.; Woudenberg, J.H.C.; Groenewald, J.Z.; Crous, P.W. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 2009, 101, 363–382. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Baroncelli, R.; Cacciola, S.O.; Pane, C.; Monti, M.M.; Firrao, G.; Vergara, M.; Lio, G.M.S.; Vannacci, G.; Scala, F. Polyketide synthases of Diaporthe helianthi and involvement of DHPKS1 in virulence on sunflower. BMC Genom. 2018, 19, 27. [Google Scholar] [CrossRef]
- Says-Lesage, V.; Roeckel-Drevet, P.; Viguié, A.; Tourvieille, J.; Nicolas, P.; Labrouhe, D.T. Molecular variability within Diaporthe/Phomopsis helianthi from France. Phytopathology 2002, 92, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Croll, D.; McDonald, B.A. The genetic basis of local adaptation for pathogenic fungi in agricultural ecosystems. Mol. Ecol. 2017, 26, 2027–2040. [Google Scholar] [CrossRef]
- Stukenbrock, E.H.; McDonald, B.A. The origins of plant pathogens in agro-ecosystems. Annu. Rev. Phytopathol. 2008, 46, 75–100. [Google Scholar] [CrossRef] [Green Version]
- Xu, J. Evolutionary Genetics of Fungi; Horizon Scientific Press: Norfolk, UK, 2005. [Google Scholar]
- Timmer, L.W.; Garnsey, S.M.; Graham, J.H. Scab Diseases, revised edition: 31–32 ed.; American Phytopathological Society Press: St. Paul, MN, USA, 2000; p. 92. [Google Scholar]
- Kuhara, S. The Application of the Epidemiologic Simulation Model “Melan” to Control Citrus Melanose Caused by Diaporthe Citri (Faw.) Wolf; ASPAC Food and Fertilizer Technology Center: Taipei, China, 1999; p. 481. [Google Scholar]
- Kucharek, T.; Whiteside, J.; Brown, E. Melanose and Phomopsis Stem-End Rot of Citrus. In Plant Pathology Fact Sheet, Florida Cooperative Extension Service; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2000; pp. 26–30. [Google Scholar]
- Bach, W.J.; Wolf, F.A. The isolation of the fungus that causes citrus melanose and the pathological anatomy of the host. J. Agric. Res. 1928, 37, 243–252. [Google Scholar]
- Agostini, J.P.; Bushong, P.M.; Bhatia, A.; Timmer, L.W. Influence of environmental factors on severity of citrus scab and melanose. Plant Dis. 2003, 87, 1102–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnett, H.C. Melanose Diaporthe Citri (Faw.) Wolf; Florida Department of Agriculture and Consumer Services, Divition of Plant Industry: Gainesville, FL, USA, 1962; p. 1. [Google Scholar]
- Hardy, S.; Donovan, N. Managing Melanose in Citrus; Profitable & sustainable primary industries, New South Wales Department of Primary Industries: Orange, NSW, Australia, 2007; Volume 751, pp. 1–3. [Google Scholar]
- Nelson, S. Citrus Melanose. In Plant Disease; Cooperative Extension Service, College of tropical Agriculture and Human Resources, University of Hawaii: Honolulu, HI, USA, 2008; pp. 1–5. [Google Scholar]
- Barkley, P.B.; Schubert, T.; Schutte, G.C.; Godfrey, K.; Hattingh, V.; Telford, G.; Beattie, G.A.C.; Hoffman, K.M. Invasive Pathogens in Plant Biosecurity. Case Study: Citrus Biosecurity; Springer: Dordrecht, The Netherlands, 2014; pp. 547–592. [Google Scholar]
- Rehman, F.U.; Kalsoom, M.; Sultan, A.; Adnan, M.; Junaid, S.; Akram, H.; Tariq, M.A.; Shafique, T.; Zafar, M.I. Citrus melanose and quality degradation of fruit by this disease: A review. J. Biogeneric Sci. Res. 2020, 3, 1–4. [Google Scholar] [CrossRef]
- Dewdney, M.M.; Timmer, L.W. Florida Citrus Pest Management Guide. Available online: http://edis.ifas.ufl.edu (accessed on 19 August 2021).
- Driscoll, P.J. Copper toxicity on florida citrus—Why did it happen? Proc. Fla. State Hort. Soc. 2004, 117, 124–127. [Google Scholar]
- Narciso, J.; Widmer, W.; Ference, C.; Ritenour, M.; Diaz, R. Use of carnauba based carrier for copper sprays reduces infection by Xanthomonas citri subsp. citri and Diaporthe citri in Florida commercial grapefruit groves. Agric. Sci. 2012, 3, 962–970. [Google Scholar]
- Stover, E.D.; Ciliento, S.; Albrigo, G. Copper fungicide spray timings for melanose control in grapefruit: Comparison of computer modelling of copper residues vs. calendar sprays. Hortscience 2004, 39, 886A-886. [Google Scholar] [CrossRef]
- Timmer, L.W.; Zitko, S.E. Evaluation of copper fungicides and rates of metallic copper for control of melanose on grapefruit in florida. Plant Dis. 1996, 80, 166–169. [Google Scholar] [CrossRef]
- Bushong, P.M.; Timmer, L.W. Evaluation of postinfection control of citrus scab and melanose with benomyl, fenbuconazole, and azoxystrobin. Plant Dis. 2007, 84, 1246–1249. [Google Scholar] [CrossRef] [Green Version]
- Inuma, T. Decreasing the frequency of control of citrus melanose by using the fungicides dithianon for satsuma mandarin cultivation (in Japanese). Ann. Rept. Kansai Pl. Prot. 2014, 56, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Idrees, M.; Naz, S.; Ehetisham-Ul-Haq, M.; Mehboob, S.; Kamran, M.; Ali, S.; Iqbal, M. Protectant and curative efficacy of different fungicides against citrus melanose caused by Phomopsis citri under in vivo conditions. Int. J. Biosci. 2019, 15, 194–199. [Google Scholar]
- Anwar, U.; Mubeen, M.; Iftikhar, Y.; Zeshan, M.A.; Shakeel, Q.; Sajid, A.; Umer, M.; Abbas, A. Efficacy of different fungicides against citrus melanose disease in Sargodha, Pakistan. Pak. L. Phytopathol. 2021, 33, 67–74. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.S.; Mei, X.F.; Jiang, L.Y.; Han, G.X.; Li, H.Y. Sensitivity evaluation of Diaporthe citri populations to mancozeb and screening of alternative fungicides for citrus melanose control. J. Plant Protec. 2018, 45, 373–381. (In Chinese) [Google Scholar]
- Qin, S.Y.; Zhou, X.Y.; Jiang, Y.L. Screening of fungicides for controlling citrus melanose in lab. Guangdong Agric. Sci. 2012, 39, 77–79. (In Chinese) [Google Scholar]
- Ko, Y.J.; Kang, S.Y.; Jeun, Y.C. Suppression of citrus melanose on the leaves treated with rhizobacterial strains after inoculation with Diaporthe citri. Res. Plant Dis. 2012, 18, 331–337. (In Korean) [Google Scholar] [CrossRef]
- Ko, Y.J.; Kim, J.S.; Kim, K.D.; Jeun, Y.C. Microscopical observation of inhibition-behaviors against Diaporthe citri by pre-treated with Pseudomonas putida strain THJ609-3 on the leaves of citrus plants. J. Microbiol. 2014, 52, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Ko, E.J.; Kim, S.J.; Hyun, H.N.; Jeun, Y.C. Suppression of melanose caused by Diaporthe citri on citrus leaves pretreated with bio-sulfur. Plant Pathol. J. 2019, 35, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Maung, C.E.H.; Choi, T.G.; Kim, K.Y. Large scale cultivation of Bacillus velezensis CE 100 and effect of its culture on control of citrus melanose caused by Diaporthe citri. Korean J. Soil Sci. Fert. 2021, 54, 297–310. [Google Scholar] [CrossRef]

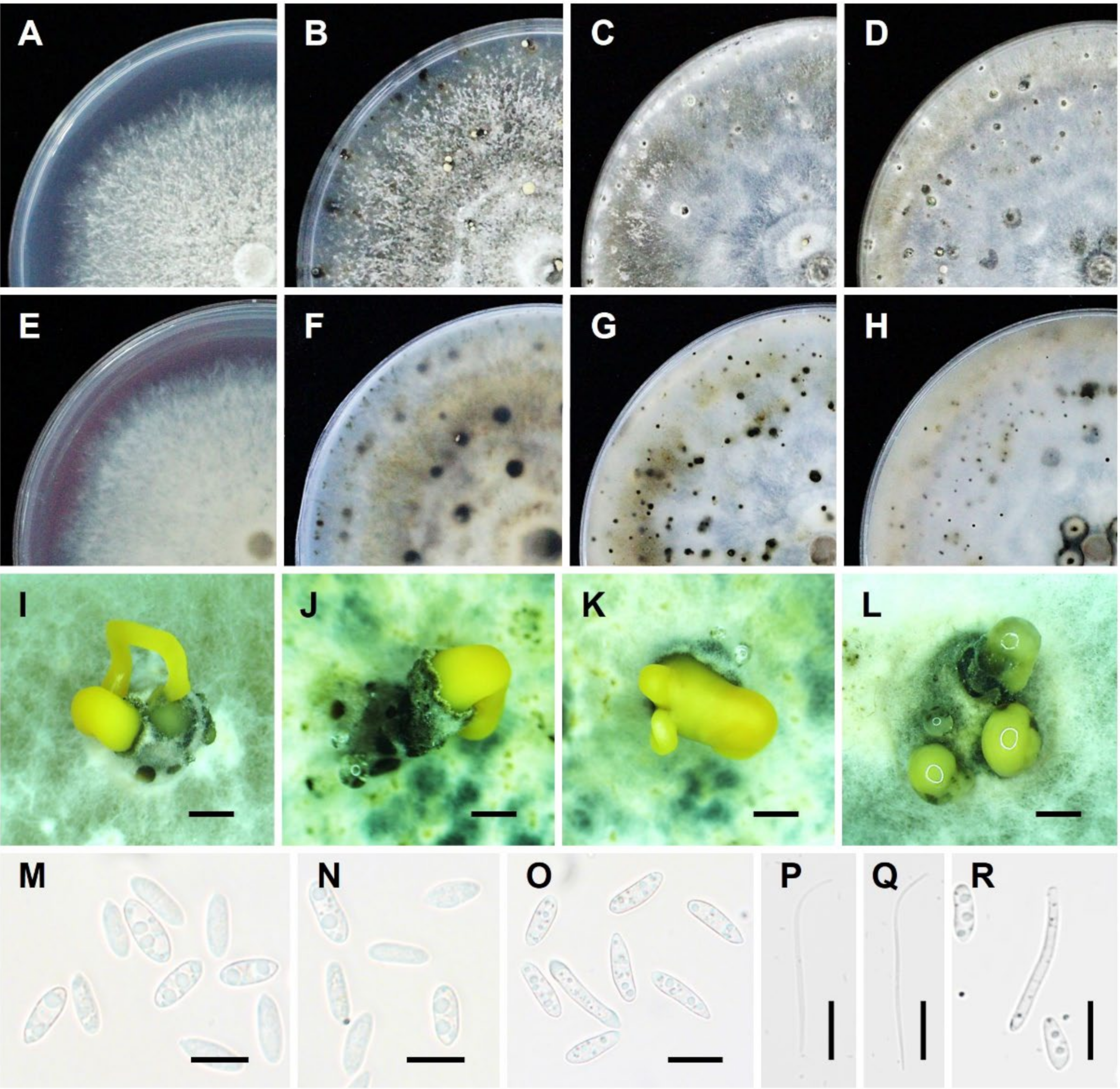

| Diaporthe Species | Citrus Host and Allied Genera | Locality Distribution | Symptom/Tissue | Reference(s) |
|---|---|---|---|---|
| D. apiculatum | Citrus grandis cv. Tomentosa | China | non-symptom/twig | [34] |
| D. aquatica | C. grandis cv. Tomentosa | China | non-symptom/fruit | [34] |
| D. arecae | C. grandis | China | non-symptom/twig, leaf | [31] |
| C. limon | China | non-symptom/branch | [31] | |
| C. reticulata | China | non-symptom/branch, twig | [31] | |
| C. sinensis | China | non-symptom/branch, twig | [31] | |
| C. sinensis | Suriname | Decaying/fruit | [35] | |
| C. unshiu | China | non-symptom/twig | [31] | |
| D. biconispora | C. grandis | China | non-symptom/branch | [31] |
| C. sinensis | China | non-symptom/branch | [31] | |
| Fortunella margarita | China | non-symptom/branch | [31] | |
| D. biguttulata | C. limon | China | non-symptom/branch | [31] |
| D. citri | C. reticulata | China | Melanose, stem-end rot, dead wood/fruit, leaf | [30,31,37,46] |
| C. reticulata | New Zealand | N.A./stem | [37] | |
| C. reticulata | Portugal (Azores) | Blight/shoot | [47] | |
| C. reticulata cv. Nanfengmiju | China | Melanose/fruit, leaf, twig | [32] | |
| C. sinensis | Brazil | N.A./fruit | [37] | |
| C. sinensis | China | Melanose/twig, leaf | [32,46,48] | |
| C. sinensis | USA, Florida | Stem-end rot/fruit | [30] | |
| C. unshiu | China | non-symptom/twig | [31] | |
| C. unshiu var. Juwadeun | Korea | N.A./fruit | [37] | |
| Citrus sp. | USA, Florida | N.A./leaf | [37] | |
| D. citriasiana | C. grandis cv. Shatianyou | China | Anonymous spot/leaf | [31] |
| C. reticulata cv. Nanfengmiju | China | Melanose-like/leaf | [32] | |
| C. sinensis | China | Melanose-like/leaf | [32] | |
| C. unshiu | China | Dead wood, non-symptom /branch, leaf | [30,31] | |
| D. citrichinensis | C. grandis | China | non-symptom/branch | [31] |
| C. unshiu | China | Dead wood, scab/branch, leaf | [30,31] | |
| Fortunella margarita | China | non-symptom/branch | [31] | |
| D. cytosporella | C. limon | Spain | N.A./fruit | [37] |
| C. limonia | Italy | N.A. | [37] | |
| C. sinensis | USA, California | N.A./twig | [37] | |
| D. discoidispora | C. reticulata cv. Nanfengmiju | China | Melanose-like/fruit, leaf | [32] |
| C. sinensis | China | non-symptom/twig | [30,31] | |
| C. unshiu | China | non-symptom/twig | [31] | |
| D. endocitricola | C. grandis cv. Tomentosa | China | non-symptom/fruit | [34] |
| D. endophytica | C. unshiu | China | Scab/leaf | [31] |
| D. eres | C. reticulata cv. Nanfengmiju | China | Melanose-like/twig, fruit, leaf | [32] |
| C. unshiu | China | Non-symptom/twig | [31] | |
| Fortunella margarita | China | Non-symptom/branch | [31] | |
| Citrus sp. | China | Non-symptom/branch, fruit | [31] | |
| D. foeniculina | C. aurantiifolia | Greece | Blight, canker/shoot, branch | [49] |
| C. aurantiifolia-limon | Greece | Blight, canker/shoot, branch | [49] | |
| C. bergamia | Greece | Canker/branch | [29] | |
| C. japonica | Malta | Dieback/twig | [29] | |
| C. latifolia | USA, California | N.A./truck | [37] | |
| C. limon | Greece | Blight, canker/shoot, branch | [29,49] | |
| C. limon | Italy | Canker/trunk | [29] | |
| C. limon | Malta | Canker/trunk | [29] | |
| C. limon | New Zealand | N.A. | [37] | |
| C. limon | Portugal | Dieback/twig | [29] | |
| C. limon | Spain | Dieback/twig | [29,37] | |
| C. limon | Turkey | Rot/fruit | [50] | |
| C. limon | USA, California | N.A./branch | [29,37] | |
| C. limon | Lebanon | Blight/shoot | [42] | |
| C. maxima | Greece | Canker/branch | [29] | |
| C. maxima | Italy | Canker/branch | [29] | |
| C. medica | Greece | Blight, canker/shoot, branch | [49] | |
| C. mitis | Italy | Canker, dieback/branch, twig | [29] | |
| C. paradisi | Italy | Canker/branch | [29] | |
| C. paradisi | Malta | Canker/trunk | [29] | |
| C. paradisi | Portugal | Canker/branch | [29] | |
| C. reticulata | Greece | Dieback/twig | [29] | |
| C. reticulata | Italy | Dieback/twig | [29] | |
| C. reticulata | Spain | Dieback/twig | [29] | |
| C. sinensis | Iran | Non-symptom/leaf | [51] | |
| C. sinensis | Italy | Canker/branch, trunk | [29] | |
| C. sinensis | Malta | Canker/branch | [29] | |
| C. sinensis | Portugal | Canker, dieback/branch, twig | [29] | |
| Microcitrus australasica | Italy | Dieback/twig | [29] | |
| Poncirus trifoliate × C. paradisi | Greece | Blight, canker/shoot, branch | [49] | |
| D. foeniculina (D. baccae) | C. limon | Italy | Blight, canker/shoot, branch | [29] |
| C. paradisi | Italy | Canker/branch | [29] | |
| C. reticulata | Italy | Canker/trunk | [29] | |
| C. sinensis | Italy | Canker, dieback/trunk, twig | [29] | |
| D. guangdongensis | C. grandis cv. Tomentosa | China | non-symptom/fruit | [34] |
| D. hongkongensis | C. grandis | China | Non-symptom/twig | [31] |
| C. reticulata | China | Scab/leaf | [31] | |
| C. reticulata cv. Nanfengmiju | China | Non-symptom/twig | [31] | |
| C. sinensis | China | Non-symptom/twig | [31] | |
| C. unshiu | China | Scab/leaf | [31] | |
| D. infertilis | C. sinensis | Suriname | Decaying/fruit | [29,35] |
| D. limonicola | C. grandis cv. Tomentosa | China | non-symptom/fruit | [34] |
| C. limon | Malta | Canker/branch, trunk | [29] | |
| D. masirevicii | C. grandis cv. Tomentosa | China | non-symptom/fruit, twig | [34] |
| D. melitensis | C. limon | Malta | Canker/branch | [29] |
| D. multigutullata | C. grandis | China | Non-symptom/branch | [31] |
| C. maxima | China | Symptomatic/branches | [48] | |
| D. novem | C. aurantiifolia | Italy | Dieback/twig | [29] |
| C. japonica | Italy | Dieback/twig | [29] | |
| D. ovalispora | C. limon | China | non-symptom/twig | [31] |
| D. passifloricola | C. grandis cv. Tomentosa | China | non-symptom/fruit, twig | [34] |
| C. reticulata cv. Nanfengmiju | China | Stem-end rot/fruit | [52] | |
| D. perseae | C. grandis cv. Tomentosa | China | non-symptom/leaf | [34] |
| D. phaseolorum | C. limon | Cameroon | non-symptom/leaf | [41] |
| D. sennae | C. grandis cv. Tomentosa | China | non-symptom/fruit | [34] |
| D. siamensis | C. sinensis | China | Stem-end rot/fruit | [53] |
| D. sojae | C. limon | China | Non-symptom/twig | [31] |
| C. limon | Cameroon | non-symptom/leaf | [41] | |
| C. reticulata | China | Non-symptom/twig | [31] | |
| C. reticulata cv. Nanfengmiju | China | Melanose-like, scab/twig, fruit, leaf | [31,32] | |
| C. unshiu | China | Non-symptom/twig | [31] | |
| D. subclavata | C. grandis cv. Shatianyou | China | Unidentified symptom/fruit | [31] |
| C. unshiu | China | Scab/leaf | [31] | |
| D. taoicola | C. sinensis | China | Stem-end rot/fruit | [53] |
| D. unshiuensis | C. reticulata cv. Nanfengmiju | China | Melanose-like/fruit, twig | [32] |
| C. sinensis | China | Melanose-like/twig, leaf | [32] | |
| C. unshiu | China | Unidentified symptom/fruit | [31] | |
| Fortunella margarita | China | Non-symptom/branch | [31] | |
| Diaporthe sp. | C. aurantium | Taiwan | non-symptom/N.A. | [54] |
| C. limon | India | Dieback/shoot, branch | [55] | |
| C. limon | Cameroon | non-symptom/leaf | [41] | |
| C. reticulata | Iran | non-symptom/N.A. | [56] | |
| Fortunella margarita | China | Non-symptom/branch | [31] |
| Gene/Locus 1 | Primer Name | Primer Sequences (5′ to 3′) | Reference |
|---|---|---|---|
| ACT | ACT-512F | ATGTGCAAGGCCGGTTTCGC | [108] |
| ACT-783R | TACGAGTCCTTCTGGCCCAT | [108] | |
| ACT878R | ATCTTCTCC ATGTCGTCCCAG | [37] | |
| APN2 | apn2fw2 | GCMATGTTYGAMATYCTGGAG | [101] |
| apn2rw2 | CTTGGTCTCCCAGCAGGTGAAC | [101] | |
| CAL | CAL-228F | GAGTTCAAGGAGGCCTTCTCCC | [108] |
| CAL-737R | CATCTTCTGGCCATCATGG | [108] | |
| CL1 | GARTWCAAGGAGGCCTTCTC | [109] | |
| CL2A | TTTTTGCATCATGAGTTGGAC | [109] | |
| CAL563F | GACAAATCA CCACCAARGAGC | [37] | |
| FG1093 | FG1093 E1F1 | GCGCCACAMCAAGWCSCACRC | [110] |
| FG1093 E3R1 | TTCTBCGCTTGGCCTTCTCRS | [110] | |
| GAPDH | Gpd1-LM | ATTGGCCGCATCGTCTTCCGCAA | [111] |
| Gpd2-LM | CCCACTCGTTGTCGTACCA | [111] | |
| HIS3 | CYLH3F | AGGTCCACTGGTGGCAAG | [112] |
| H3-1b | GCGGGCGAGCTGGATGTCCTT | [113] | |
| IGS | IGS-12a | AGTCTGTGGATTAGTGGCCG | [114] |
| NS1R | GAGACAAGCATATGACTAC | [114] | |
| ITS | ITS1 | TCCGTAGGTGAACCTGCGG | [115] |
| ITS-1F | CTTGGTCATTTAGAGGAAGTAA | [116] | |
| ITS4 | TCCTCCGCTTATTGATATGC | [115] | |
| DcitriF | GTTTAACTACTGCGCTCGGGGTCCTG | [117] | |
| DcitriR | CTTACTGTTGCCTCGGCGCAGG | [117] | |
| LSU | LSU1Fd | GRATCAGGTAGGRATACCCG | [118] |
| LR5 | TCCTGAGGGAAACTTCG | [119] | |
| MAT1-1-1 | MAT1-1-1FW | GCAAMIGTKTIKACTCACA | [99] |
| MAT1-1-1RV | GTCTMTGACCARGACCATG | [99] | |
| MAT1 141F | GGTCAAGAAGAAGAAGTCC | [120] | |
| MAT1-2-1 | MAT1-2-1FW | GCCCKCCYAAYCCATTCATC | [99] |
| MAT1-2-1RV | TTGACYTCAGAAGACTTGCGTG | [99] | |
| MAT2 188F | CCAGCTCCATCACAAC | [120] | |
| MS204 | MS204 E1F1 | AAGGGCACCCTGGAGGGCCAC | [110] |
| MS204 E5R1 | GATGGTGACGGYGTTGATGTA | [110] | |
| SSU | NMS1 | CAGCAGTGAGGAATATTGGTCAATG | [121] |
| NMS2 | GCGGATCATCGAATTAAATAACAT | [121] | |
| TEF-α | EF1-728F | CATCGAGAAGTTCGAGAAGG | [108] |
| EF1-986R | TACTTGAAGGAACCCTTACC | [108] | |
| EF-2 | GGARGTACCAGTSATCATGTT | [122] | |
| TUB2 | Bt2a | GGTAACCAAATCGGTGCTGCTTTC | [113] |
| Bt2b | ACCCTCAGTGTAGTGACCCTTGGC | [113] | |
| TUBDcitri-F1 | CCATTTGACCATCTGCAACAT | [32] | |
| TUBD-R1 | CCTTGGCCCAGTTGTTTCC | [32] | |
| Dc-F | CCCTCGAGGCATCATTAC | [46] | |
| Dc-R | ATGTTGCAGATGGTCAAATGG | [46] | |
| Tub2FD | GTBCACCTYCARACCGGYCARTG | [123] | |
| T22 | TCTGGATGTTGTTGGGAATCC | [124] | |
| T1 | AACATGCGTGAGATTGTAAGT | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaisiri, C.; Liu, X.; Lin, Y.; Luo, C. Diaporthe citri: A Fungal Pathogen Causing Melanose Disease. Plants 2022, 11, 1600. https://doi.org/10.3390/plants11121600
Chaisiri C, Liu X, Lin Y, Luo C. Diaporthe citri: A Fungal Pathogen Causing Melanose Disease. Plants. 2022; 11(12):1600. https://doi.org/10.3390/plants11121600
Chicago/Turabian StyleChaisiri, Chingchai, Xiangyu Liu, Yang Lin, and Chaoxi Luo. 2022. "Diaporthe citri: A Fungal Pathogen Causing Melanose Disease" Plants 11, no. 12: 1600. https://doi.org/10.3390/plants11121600
APA StyleChaisiri, C., Liu, X., Lin, Y., & Luo, C. (2022). Diaporthe citri: A Fungal Pathogen Causing Melanose Disease. Plants, 11(12), 1600. https://doi.org/10.3390/plants11121600








