Particle-Based Imaging Tools Revealing Water Flows in Maize Nodal Vascular Plexus
Abstract
1. Introduction
2. Results
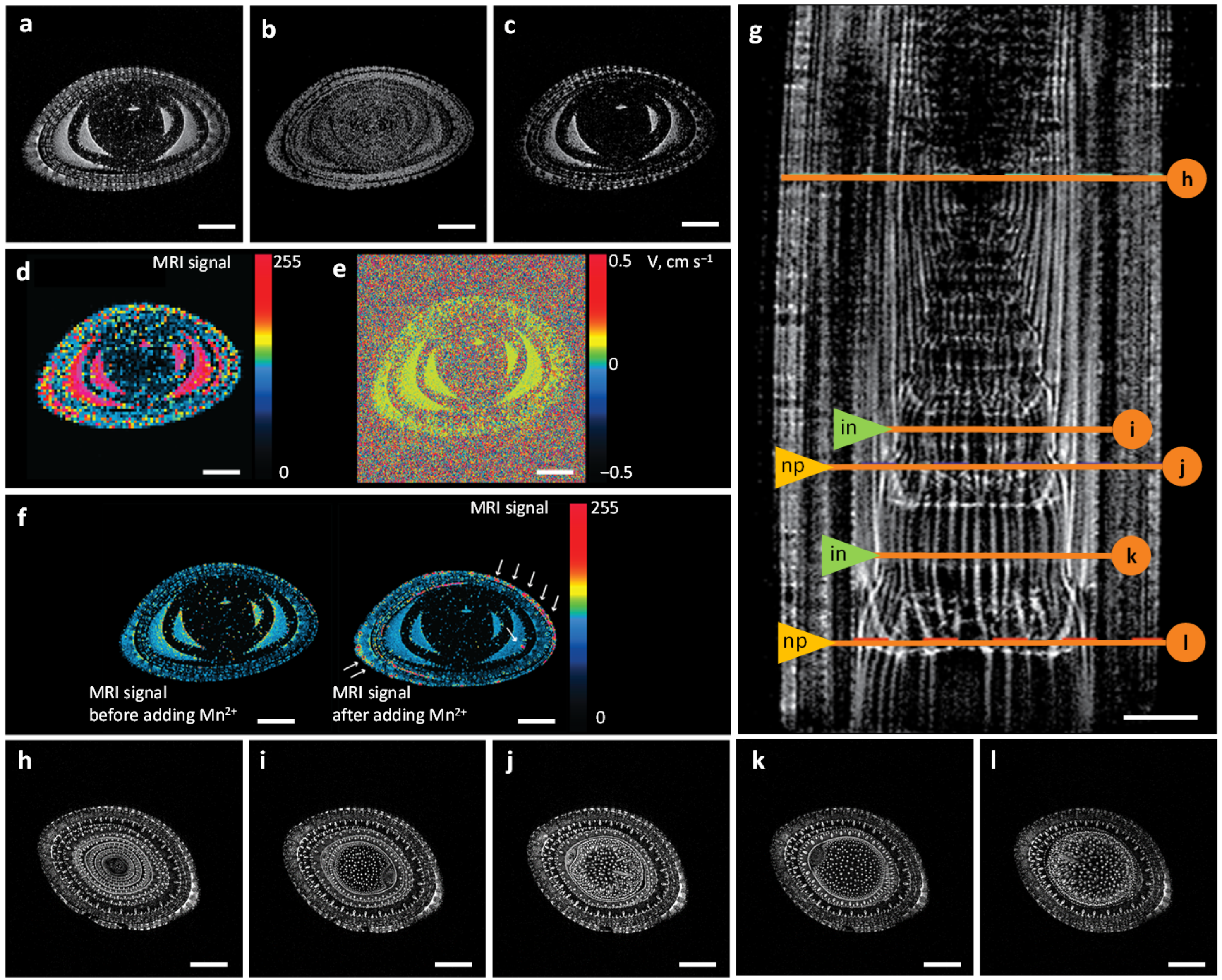
2.1. MRI Approaches for Water Visualization inside the Xylem Vascular Bundle System
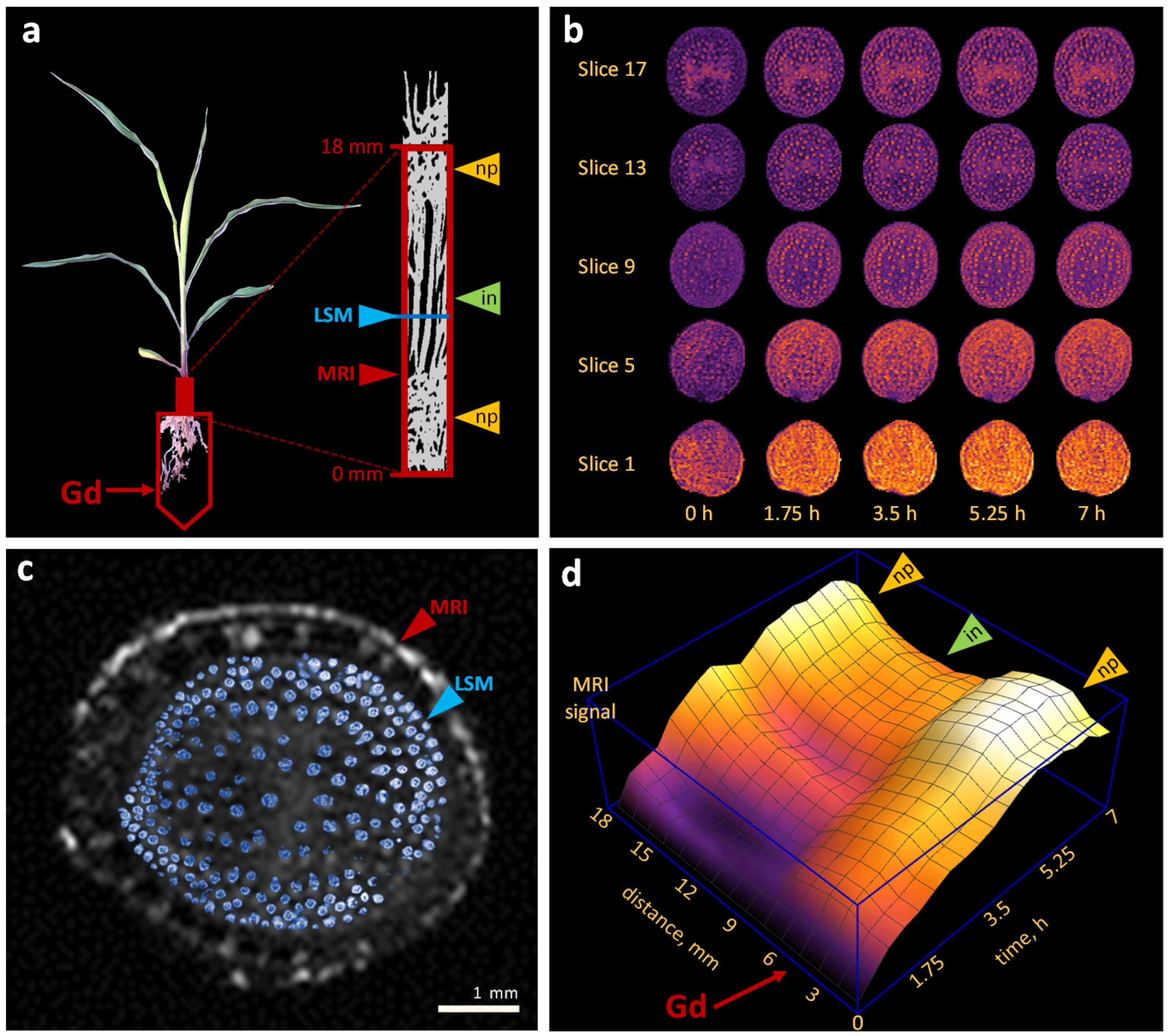
2.2. Water Flows in Internodes and Nodal Plexuses Derived from Contrast-Enhanced MRI
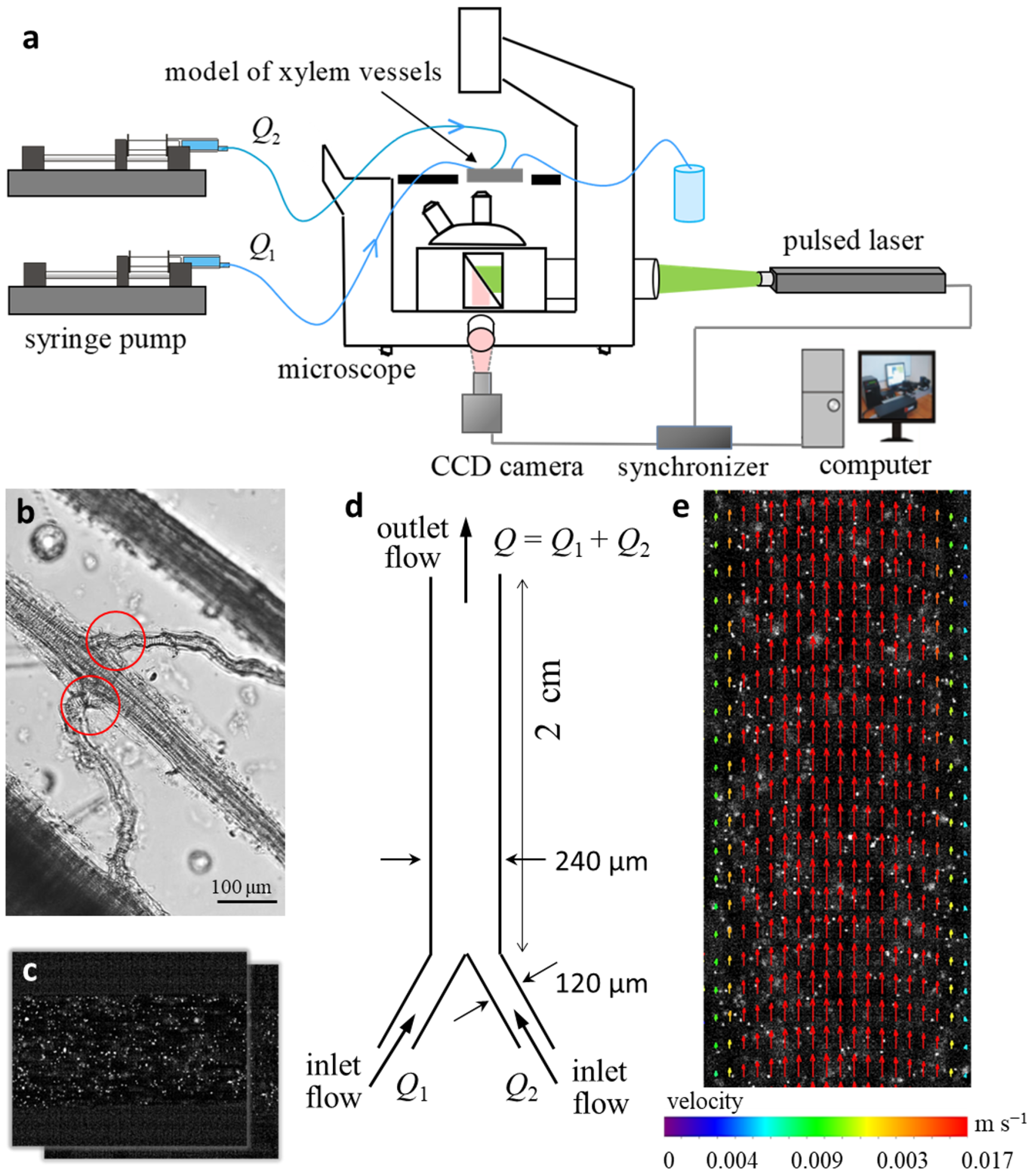
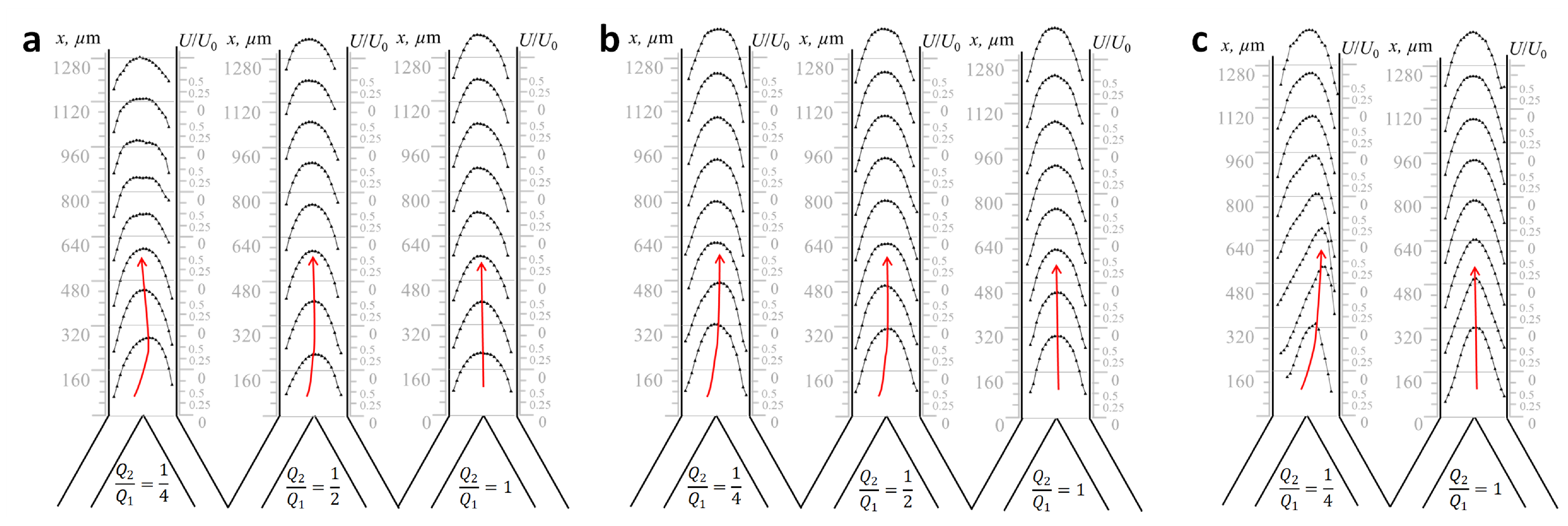
2.3. Physical Modeling of Flows in Microchannel Prototype “Y-Type Xylem Vascular Connection”
2.4. Reynolds-Number-Based Matching between Xylem Vessels in Maize Stem and Microchannels in Lab-on-a-Chip
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Laser Scanning Microscopy (LSM)
4.3. Magnetic Resonance Imaging
4.4. Particle Image Velocimetry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| micro-CT | X-ray micro computed tomography |
| PET | Positron emission tomography |
| LSM | Laser scanning microscopy |
| PI | Propidium iodide |
| DAPI | 4′,6-diamidino-2-phenylindole |
| PIV | Particle Image Velocimetry |
| micro-PIV | micro Particle Image Velocimetry |
| Reynolds number |
References
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.R.; Helariutta, Y.; He, X.Q.; Fukuda, H.; Kang, J.; Brady, S.M.; et al. The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef] [PubMed]
- Lobet, G.; Couvreur, V.; Meunier, F.; Javaux, M.; Draye, X. Plant water uptake in drying soils. Plant Physiol. 2014, 164, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W. Xylem: Differentiation, Water Transport and Ecology. eLS 2017, 1–7. [Google Scholar] [CrossRef]
- Holbrook, N.M.; Zwieniecki, M.A.; Melcher, P.J. The dynamics of “dead wood”: Maintenance of water transport through plant stems. Integr. Comp. Biol. 2002, 42, 492–496. [Google Scholar] [CrossRef]
- André, J.P. Vascular Organization of Angiosperms: A New Vision; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Kraehmer, H. On vascular bundle modifications in nodes and internodes of selected grass species. Sci. Agric. Bohem. 2017, 48, 112–121. [Google Scholar] [CrossRef][Green Version]
- Shane, M.; McCully, M.E.; Canny, M. The vascular system of maize stems revisited: Implications for water transport and xylem safety. Ann. Bot. 2000, 86, 245–258. [Google Scholar] [CrossRef]
- Shane, M.; Cully, M.M.; Canny, M. Architecture of branch-root junctions in maize: Structure of the connecting xylem and the porosity of pit membranes. Ann. Bot. 2000, 85, 613–624. [Google Scholar] [CrossRef][Green Version]
- Pascut, F.C.; Couvreur, V.; Dietrich, D.; Leftley, N.; Reyt, G.; Boursiac, Y.; Calvo-Polanco, M.; Casimiro, I.; Maurel, C.; Salt, D.E.; et al. Non-invasive hydrodynamic imaging in plant roots at cellular resolution. Nat. Commun. 2021, 12, 4682. [Google Scholar] [CrossRef]
- Tötzke, C.; Kardjilov, N.; Hilger, A.; Rudolph-Mohr, N.; Manke, I.; Oswald, S.E. Three-dimensional in vivo analysis of water uptake and translocation in maize roots by fast neutron tomography. Sci. Rep. 2021, 11, 10578. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Guo, X.; Ma, L.; Shao, M.; Pan, X.; Zhao, C. Micron-scale phenotyping quantification and three-dimensional microstructure reconstruction of vascular bundles within maize stalks based on micro-CT scanning. Funct. Plant Biol. 2016, 44, 10–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Pan, X.; Wang, J.; Guo, X.; Du, J. Micron-scale phenotyping techniques of maize vascular bundles based on X-ray microcomputed tomography. JoVE (J. Vis. Exp.) 2018, 9, e58501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, J.; Wang, J.; Ma, L.; Lu, X.; Pan, X.; Guo, X.; Zhao, C. High-throughput micro-phenotyping measurements applied to assess stalk lodging in maize (Zea mays L.). Biol. Res. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, L.; Wang, J.; Wang, X.; Guo, X.; Du, J. Phenotyping analysis of maize stem using micro-computed tomography at the elongation and tasseling stages. Plant Methods 2020, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.G.; Ryu, J.; Lee, S.J. Vulnerability of protoxylem and metaxylem vessels to embolisms and radial refilling in a vascular bundle of maize leaves. Front. Plant Sci. 2016, 7, 941. [Google Scholar] [CrossRef]
- Anselmucci, F.; Andò, E.; Viggiani, G.; Lenoir, N.; Arson, C.; Sibille, L. Imaging local soil kinematics during the first days of maize root growth in sand. Sci. Rep. 2021, 11, 22262. [Google Scholar] [CrossRef]
- Vita, R.S.; Menezes, N.L.; Pellegrini, M.O.; Melo-de Pinna, G.F. A new interpretation on vascular architecture of the cauline system in Commelinaceae (Commelinales). PLoS ONE 2019, 14, e0218383. [Google Scholar] [CrossRef]
- Borisjuk, L.; Rolletschek, H.; Neuberger, T. Surveying the plant’s world by magnetic resonance imaging. Plant J. 2012, 70, 129–146. [Google Scholar] [CrossRef]
- Windt, C.W.; Vergeldt, F.J.; De Jager, P.A.; Van As, H. MRI of long-distance water transport: A comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant Cell Environ. 2006, 29, 1715–1729. [Google Scholar] [CrossRef]
- Clearwater, M.; Clark, C. In vivo magnetic resonance imaging of xylem vessel contents in woody lianas. Plant Cell Environ. 2003, 26, 1205–1214. [Google Scholar] [CrossRef]
- Van de Wal, B.A.; Windt, C.W.; Leroux, O.; Steppe, K. Heat girdling does not affect xylem integrity: An in vivo magnetic resonance imaging study in the tomato peduncle. New Phytol. 2017, 215, 558–568. [Google Scholar] [CrossRef]
- Scheenen, T.; Heemskerk, A.; De Jager, A.; Vergeldt, F.; Van As, H. Functional imaging of plants: A nuclear magnetic resonance study of a cucumber plant. Biophys. J. 2002, 82, 481–492. [Google Scholar] [CrossRef]
- Jahnke, S.; Menzel, M.I.; Van Dusschoten, D.; Roeb, G.W.; Bühler, J.; Minwuyelet, S.; Blümler, P.; Temperton, V.M.; Hombach, T.; Streun, M.; et al. Combined MRI–PET dissects dynamic changes in plant structures and functions. Plant J. 2009, 59, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Padmanabhan, P.; Gulyás, B.Z. Gadolinium (iii) based nanoparticles for T1-weighted magnetic resonance imaging probes. RSC Adv. 2016, 6, 60945–60966. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, L.; Kuang, Y.; Xiong, D.; Pei, R. Gadolinium-based nanoscale MRI contrast agents for tumor imaging. J. Mater. Chem. B 2017, 5, 3431–3461. [Google Scholar] [CrossRef] [PubMed]
- Gussoni, M.; Greco, F.; Vezzoli, A.; Paleari, M.A.; Moretti, V.M.; Lanza, B.; Zetta, L. Osmotic and aging effects in caviar oocytes throughout water and lipid changes assessed by 1H NMR T1 and T2 relaxation and MRI. Magn. Reson. Imaging 2007, 25, 117–128. [Google Scholar] [CrossRef]
- Snaar, J.; Van As, H. Probing water compartments and membrane permeability in plant cells by 1H NMR relaxation measurements. Biophys. J. 1992, 63, 1654–1658. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. Effect of trehalose and ultrasound-assisted osmotic dehydration on the state of water and glass transition temperature of broccoli (Brassica oleracea L. var. botrytis L.). J. Food Eng. 2013, 119, 640–647. [Google Scholar] [CrossRef]
- Comtet, J.; Jensen, K.H.; Turgeon, R.; Stroock, A.D.; Hosoi, A. Passive phloem loading and long-distance transport in a synthetic tree-on-a-chip. Nat. Plants 2017, 3, 17032. [Google Scholar] [CrossRef]
- Min, X.; Kim, W.S. Artificial Xylem Chip: A Three-Dimensionally Printed Vertical Digital Microfluidic Platform. Langmuir 2020, 36, 14841–14848. [Google Scholar] [CrossRef]
- Shi, W.; Dalrymple, R.M.; McKenny, C.J.; Morrow, D.S.; Rashed, Z.T.; Surinach, D.A.; Boreyko, J.B. Passive water ascent in a tall, scalable synthetic tree. Sci. Rep. 2020, 10, 230. [Google Scholar] [CrossRef]
- Lee, M.; Lim, H.; Lee, J. Fabrication of artificial leaf to develop fluid pump driven by surface tension and evaporation. Sci. Rep. 2017, 7, 14735. [Google Scholar] [CrossRef]
- de Araujo, D.S.; de Moraes, D.H.M.; Mesquita, M.; Flores, R.A.; Battisti, R.; Santos, G.G.; de Deus, F.P.; Ferrarezi, R.S. Numerical Modeling of Microfluid Dynamics in Xylem Vessels of Khaya grandifoliola. Water 2021, 13, 2723. [Google Scholar] [CrossRef]
- Tan, J.N.; Neild, A. Microfluidic mixing in a Y-junction open channel. AIP Adv. 2012, 2, 032160. [Google Scholar] [CrossRef]
- Kravtsova, A.; Ianko, P.; Meshalkin, Y.; Bilsky, A. Influence of external periodic perturbation on the flow in T-microchannel. AIP Conf. Proc. 2018, 2027, 040084. [Google Scholar] [CrossRef]
- Callaghan, P.T. Principles of Nuclear Magnetic Resonance Microscopy; Oxford University Press on Demand: Oxford, UK, 1993. [Google Scholar]
- Edzes, H.T.; Van Dusschoten, D.; Van As, H. Quantitative T2 imaging of plant tissues by means of multi-echo MRI microscopy. Magn. Reson. Imaging 1998, 16, 185–196. [Google Scholar] [CrossRef]
- Köckenberger, W.; Pope, J.; Xia, Y.; Jeffrey, K.; Komor, E.; Callaghan, P. A non-invasive measurement of phloem and xylem water flow in castor bean seedlings by nuclear magnetic resonance microimaging. Planta 1997, 201, 53–63. [Google Scholar] [CrossRef]
- Rokitta, M.; Peuke, A.; Zimmermann, U.; Haase, A. Dynamic studies of phloem and xylein flow in fully differentiated plants by fast nuclear-magnetic-resonance microimaging. Protoplasma 1999, 209, 126–131. [Google Scholar] [CrossRef]
- Nikolaev, S.; Zubairova, U. A computational model of the cereal leaves hydraulics. J. Phys. Conf. Ser. 2021, 2099, 012039. [Google Scholar] [CrossRef]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef]
- Wheeler, T.D.; Stroock, A.D. The transpiration of water at negative pressures in a synthetic tree. Nature 2008, 455, 208–212. [Google Scholar] [CrossRef]
- Noblin, X.; Mahadevan, L.; Coomaraswamy, I.; Weitz, D.A.; Holbrook, N.M.; Zwieniecki, M.A. Optimal vein density in artificial and real leaves. Proc. Natl. Acad. Sci. USA 2008, 105, 9140–9144. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Schlüter, M.; Räbiger, N. Experimental investigation of liquid–liquid mixing in T-shaped micro-mixers using μ-LIF and μ-PIV. Chem. Eng. Sci. 2006, 61, 2968–2976. [Google Scholar] [CrossRef]
- Silva, G.; Leal, N.; Semiao, V. Micro-PIV and CFD characterization of flows in a microchannel: Velocity profiles, surface roughness and Poiseuille numbers. Int. J. Heat Fluid Flow 2008, 29, 1211–1220. [Google Scholar] [CrossRef]
- Thomas, S.; Ameel, T.A. An experimental investigation of moderate Reynolds number flow in a T-Channel. Exp. Fluids 2010, 49, 1231–1245. [Google Scholar] [CrossRef]
- Zubairova, U.S.; Verman, P.Y.; Oshchepkova, P.A.; Elsukova, A.S.; Doroshkov, A.V. LSM-W 2: Laser scanning microscopy worker for wheat leaf surface morphology. BMC Syst. Biol. 2019, 13, 33–44. [Google Scholar] [CrossRef]
- Legland, D.; Arganda-Carreras, I.; Andrey, P. MorphoLibJ: Integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 2016, 32, 3532–3534. [Google Scholar] [CrossRef]
- Raju, C.S.K.; Cossmer, A.; Scharf, H.; Panne, U.; Lück, D. Speciation of gadolinium based MRI contrast agents in environmental water samples using hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2010, 25, 55–61. [Google Scholar] [CrossRef]
- Kravtsova, A.Y. Visualization of Flow Regimes in Symmetric Microchannel in Case of Different Inlet Flowrate Ratios for Fixed Outlet Reynolds Number. J. Eng. Thermophys. 2020, 29, 612–617. [Google Scholar] [CrossRef]
- Kravtsova, A.Y.; Markovich, D.; Pervunin, K.; Timoshevskiy, M.; Hanjalić, K. High-speed visualization and PIV measurements of cavitating flows around a semi-circular leading-edge flat plate and NACA0015 hydrofoil. Int. J. Multiph. Flow 2014, 60, 119–134. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubairova, U.S.; Kravtsova, A.Y.; Romashchenko, A.V.; Pushkareva, A.A.; Doroshkov, A.V. Particle-Based Imaging Tools Revealing Water Flows in Maize Nodal Vascular Plexus. Plants 2022, 11, 1533. https://doi.org/10.3390/plants11121533
Zubairova US, Kravtsova AY, Romashchenko AV, Pushkareva AA, Doroshkov AV. Particle-Based Imaging Tools Revealing Water Flows in Maize Nodal Vascular Plexus. Plants. 2022; 11(12):1533. https://doi.org/10.3390/plants11121533
Chicago/Turabian StyleZubairova, Ulyana S., Aleksandra Yu. Kravtsova, Alexander V. Romashchenko, Anastasiia A. Pushkareva, and Alexey V. Doroshkov. 2022. "Particle-Based Imaging Tools Revealing Water Flows in Maize Nodal Vascular Plexus" Plants 11, no. 12: 1533. https://doi.org/10.3390/plants11121533
APA StyleZubairova, U. S., Kravtsova, A. Y., Romashchenko, A. V., Pushkareva, A. A., & Doroshkov, A. V. (2022). Particle-Based Imaging Tools Revealing Water Flows in Maize Nodal Vascular Plexus. Plants, 11(12), 1533. https://doi.org/10.3390/plants11121533







