Enhanced Synthesis of Foreign Nuclear Protein Stimulates Viral Reproduction via the Induction of γ-Thionin Expression
Abstract
1. Introduction
2. Results
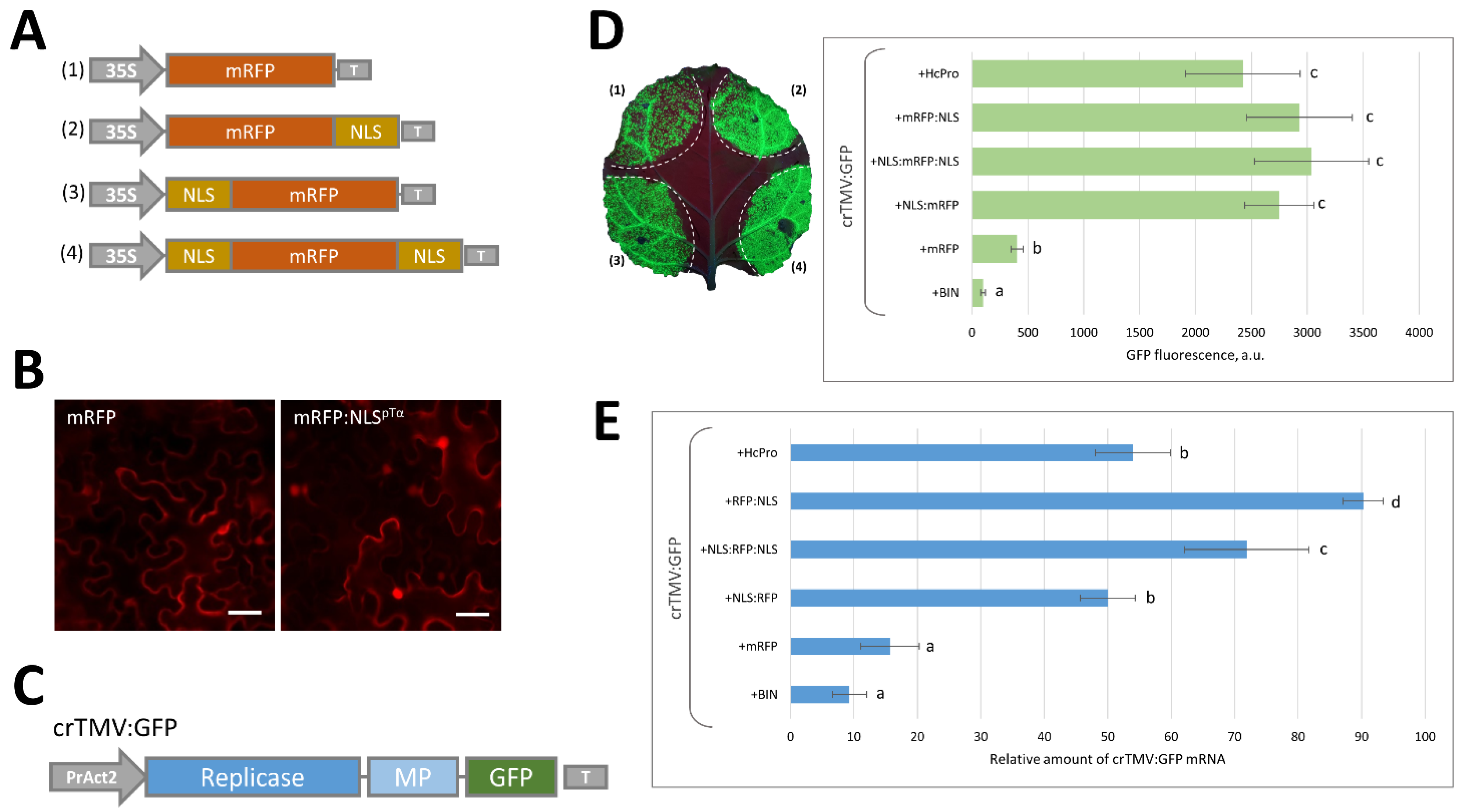
2.1. Massive Synthesis of a Model NLS-Containing Protein Stimulates Reproduction of a crTMV-Based Viral Vector
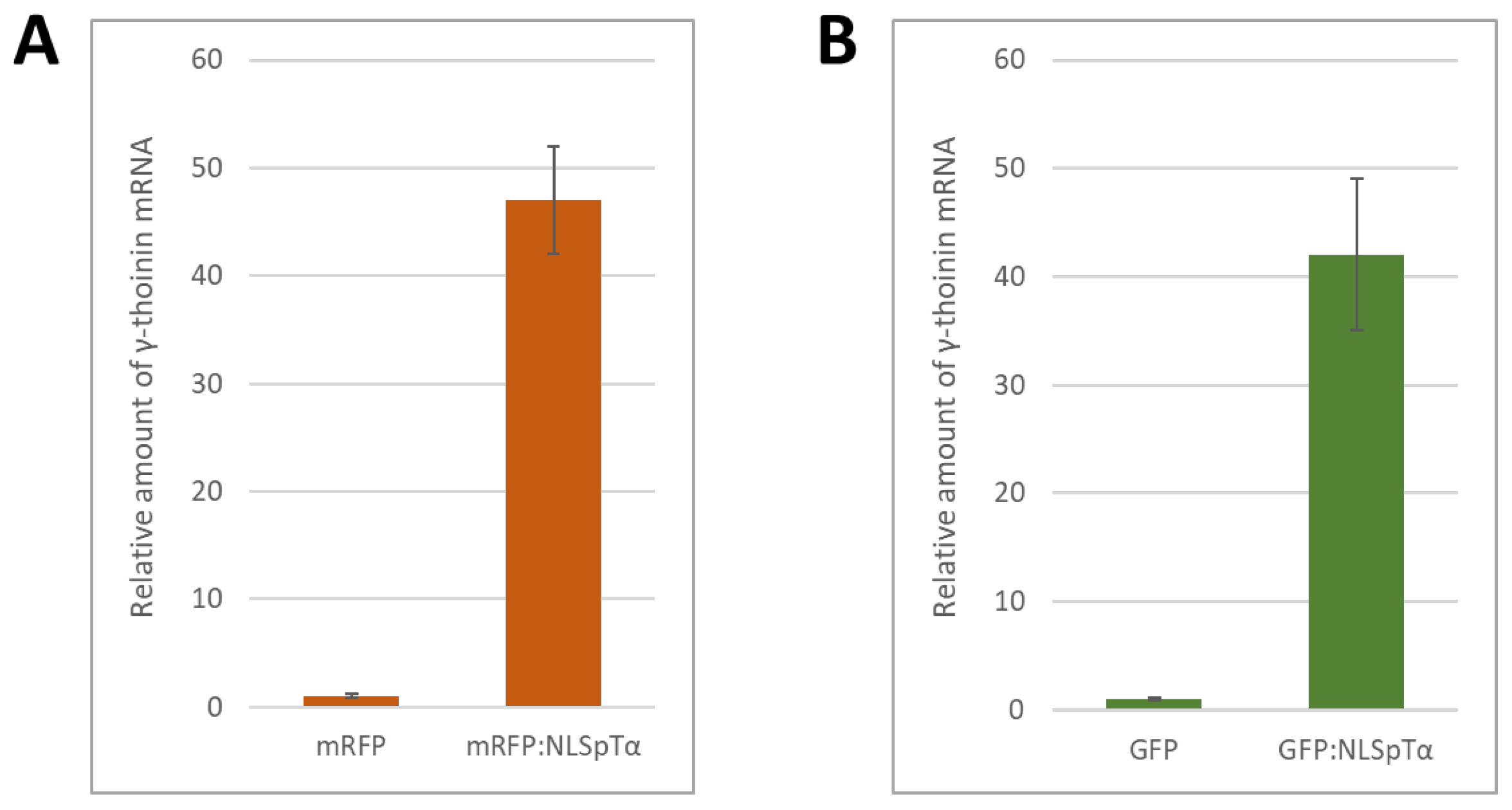
2.2. NbγThio Expression Is Activated in Response to a Model NLS-Containing Protein
2.3. The NbγThio Promoter (PrγThio) Is Sensitive to the Intensive Accumulation of the Model Nuclear Protein
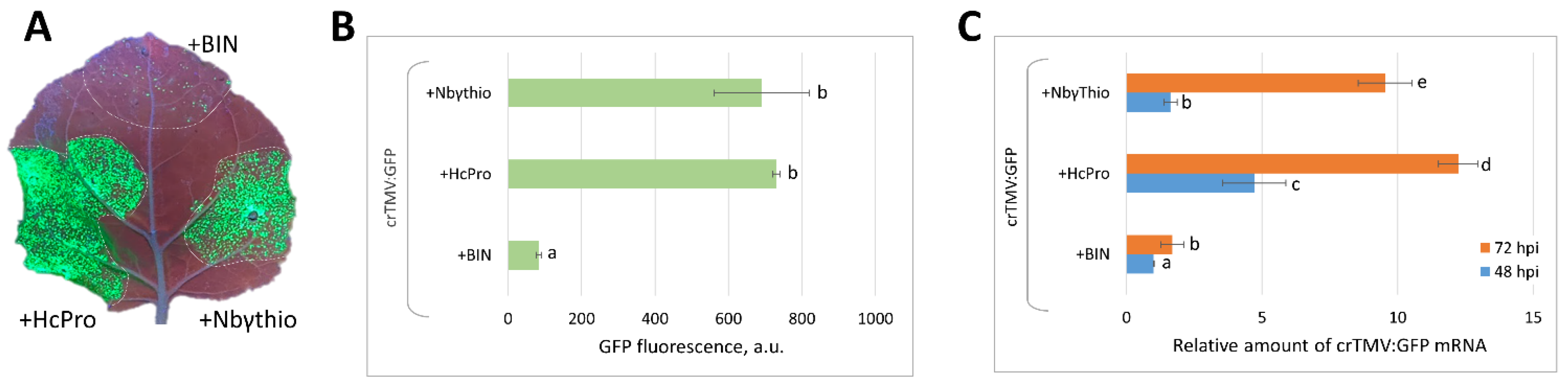
2.4. Co-Expression of crTMV:GFP Vector with 35S-NbγThio Resulted in the Increased Accumulation of Viral RNA and Enhanced GFP Production
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Plasmid Constructs
4.3. Isolation of the γ-Thionin Promoter Region (PrγThio)
4.4. Construction of SSH cDNA Libraries
4.5. Agroinfiltration Experiments
4.6. GFP and mRFP Imaging
4.7. GFP Fluorescence Measurement
4.8. GUS Activity Measurement
4.9. Quantitative Real-Time PCR (qRT-PCR) Analysis of Transcript Concentrations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopertekh, L.; Schiemann, J. Transient Production of Recombinant Pharmaceutical Proteins in Plants: Evolution and Perspectives. Curr. Med. Chem. 2019, 26, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Fu, J.; Ma, J.; Wang, X.; Gao, C.; Zhuang, C.; Wan, J.; Jiang, L. Isolation, Culture, and Transient Transformation of Plant Protoplasts. Curr. Protoc. Cell Biol. 2014, 63, 2.8.1–2.8.17. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, J.; Vidal, J.; Reisch, B. Stable Transformation of Plant Cells by Particle Bombardment/Biolistics. In Transgenic Plants: Methods and Protocols. Methods in Molecular BiologyTM; Peña, L., Ed.; Humana Press: Totowa, NJ, USA, 2005; Volume 286, pp. 61–78. ISBN 978-1-59259-827-4. [Google Scholar]
- Ueki, S.; Magori, S.; Lacroix, B.; Citovsky, V. Transient Gene Expression in Epidermal Cells of Plant Leaves by Biolistic DNA Delivery. Methods Mol. Biol. Clifton NJ 2013, 940, 17–26. [Google Scholar] [CrossRef]
- Sanagala, R.; Moola, A.K.; Bollipo Diana, R.K. A Review on Advanced Methods in Plant Gene Targeting. J. Genet. Eng. Biotechnol. 2017, 15, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient Expression Vectors for Functional Genomics, Quantification of Promoter Activity and RNA Silencing in Plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef]
- Tyurin, A.A.; Suhorukova, A.V.; Kabardaeva, K.V.; Goldenkova-Pavlova, I.V. Transient Gene Expression Is an Effective Experimental Tool for the Research into the Fine Mechanisms of Plant Gene Function: Advantages, Limitations, and Solutions. Plants 2020, 9, 1187. [Google Scholar] [CrossRef]
- Wroblewski, T.; Tomczak, A.; Michelmore, R. Optimization of Agrobacterium-Mediated Transient Assays of Gene Expression in Lettuce, Tomato and Arabidopsis. Plant Biotechnol. J. 2005, 3, 259–273. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Siemiatkowska, B.; Toleco, M.R.; Jing, Y.; Strotmann, V.; Zhang, J.; Stahl, Y.; Fernie, A.R. A Highly Efficient Agrobacterium-Mediated Method for Transient Gene Expression and Functional Studies in Multiple Plant Species. Plant Commun. 2020, 1, 100028. [Google Scholar] [CrossRef]
- Gleba, Y.; Klimyuk, V.; Marillonnet, S. Magnifection—A New Platform for Expressing Recombinant Vaccines in Plants. Vaccine 2005, 23, 2042–2048. [Google Scholar] [CrossRef]
- Gleba, Y.Y.; Tusé, D.; Giritch, A. Plant Viral Vectors for Delivery by Agrobacterium. Curr. Top. Microbiol. Immunol. 2014, 375, 155–192. [Google Scholar] [CrossRef]
- Marillonnet, S.; Thoeringer, C.; Kandzia, R.; Klimyuk, V.; Gleba, Y. Systemic Agrobacterium Tumefaciens-Mediated Transfection of Viral Replicons for Efficient Transient Expression in Plants. Nat. Biotechnol. 2005, 23, 718–723. [Google Scholar] [CrossRef]
- Klimyuk, V.; Pogue, G.; Herz, S.; Butler, J.; Haydon, H. Production of Recombinant Antigens and Antibodies in Nicotiana Benthamiana Using “magnifection” Technology: GMP-Compliant Facilities for Small- and Large-Scale Manufacturing. Curr. Top. Microbiol. Immunol. 2014, 375, 127–154. [Google Scholar] [CrossRef]
- Holtz, B.R.; Berquist, B.R.; Bennett, L.D.; Kommineni, V.J.M.; Munigunti, R.K.; White, E.L.; Wilkerson, D.C.; Wong, K.-Y.I.; Ly, L.H.; Marcel, S. Commercial-Scale Biotherapeutics Manufacturing Facility for Plant-Made Pharmaceuticals. Plant Biotechnol. J. 2015, 13, 1180–1190. [Google Scholar] [CrossRef]
- Komarova, T.V.; Baschieri, S.; Donini, M.; Marusic, C.; Benvenuto, E.; Dorokhov, Y.L. Transient Expression Systems for Plant-Derived Biopharmaceuticals. Expert Rev. Vaccines 2010, 9, 859–876. [Google Scholar] [CrossRef]
- Peyret, H.; Lomonossoff, G.P. The PEAQ Vector Series: The Easy and Quick Way to Produce Recombinant Proteins in Plants. Plant Mol. Biol. 2013, 83, 51–58. [Google Scholar] [CrossRef]
- Giritch, A.; Klimyuk, V.; Gleba, Y. 125 Years of Virology and Ascentof Biotechnologies Based on Viral Expression. Tsitol. Genet. 2017, 51, 19–39. [Google Scholar]
- Abrahamian, P.; Hammond, R.W.; Hammond, J. Plant Virus-Derived Vectors: Applications in Agricultural and Medical Biotechnology. Annu. Rev. Virol. 2020, 7, 513–535. [Google Scholar] [CrossRef]
- Ding, S.-W.; Voinnet, O. Antiviral Immunity Directed by Small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef]
- Csorba, T.; Pantaleo, V.; Burgyán, J. RNA Silencing: An Antiviral Mechanism. Adv. Virus Res. 2009, 75, 35–71. [Google Scholar] [CrossRef]
- Scholthof, H.B. Heterologous Expression of Viral RNA Interference Suppressors: RISC Management. Plant Physiol. 2007, 145, 1110–1117. [Google Scholar] [CrossRef][Green Version]
- Qu, F.; Morris, T.J. Suppressors of RNA Silencing Encoded by Plant Viruses and Their Role in Viral Infections. FEBS Lett. 2005, 579, 5958–5964. [Google Scholar] [CrossRef]
- Komarova, T.V.; Schwartz, A.M.; Frolova, O.Y.; Zvereva, A.S.; Gleba, Y.Y.; Citovsky, V.; Dorokhov, Y.L. Pol II-Directed Short RNAs Suppress the Nuclear Export of MRNA. Plant Mol. Biol. 2010, 74, 591–603. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Komarova, T.V.; Petrunia, I.V.; Frolova, O.Y.; Pozdyshev, D.V.; Gleba, Y.Y. Airborne Signals from a Wounded Leaf Facilitate Viral Spreading and Induce Antibacterial Resistance in Neighboring Plants. PLoS Pathog. 2012, 8, e1002640. [Google Scholar] [CrossRef] [PubMed]
- Hanford, H.E.; Von Dwingelo, J.; Abu Kwaik, Y. Bacterial Nucleomodulins: A Coevolutionary Adaptation to the Eukaryotic Command Center. PLoS Pathog. 2021, 17, e1009184. [Google Scholar] [CrossRef] [PubMed]
- Le, L.H.M.; Ying, L.; Ferrero, R.L. Nuclear Trafficking of Bacterial Effector Proteins. Cell. Microbiol. 2021, 23, e13320. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, Y.P.; Zolotukhin, A.S.; Vorobjev, I.A.; Chichkova, N.V.; Pavlov, N.A.; Karger, E.M.; Evstafieva, A.G.; Felber, B.K.; Vartapetian, A.B. Mutational Analysis of Human Prothymosin α Reveals a Bipartite Nuclear Localization Signal. FEBS Lett. 1997, 413, 135–141. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Ivanov, P.A.; Komarova, T.V.; Skulachev, M.V.; Atabekov, J.G. An Internal Ribosome Entry Site Located Upstream of the Crucifer-Infecting Tobamovirus Coat Protein (CP) Gene Can Be Used for CP Synthesis In Vivo. J. Gen. Virol. 2006, 87, 2693–2697. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.K.; Carrington, J.C. Silencing on the Spot. Induction and Suppression of RNA Silencing in the Agrobacterium-Mediated Transient Expression System. Plant Physiol. 2001, 126, 930–938. [Google Scholar] [CrossRef]
- Bierne, H.; Cossart, P. When Bacteria Target the Nucleus: The Emerging Family of Nucleomodulins. Cell. Microbiol. 2012, 14, 622–633. [Google Scholar] [CrossRef]
- Bierne, H.; Pourpre, R. Bacterial Factors Targeting the Nucleus: The Growing Family of Nucleomodulins. Toxins 2020, 12, 220. [Google Scholar] [CrossRef]
- Pelegrini, P.B.; Franco, O.L. Plant γ-Thionins: Novel Insights on the Mechanism of Action of a Multi-Functional Class of Defense Proteins. Int. J. Biochem. Cell Biol. 2005, 37, 2239–2253. [Google Scholar] [CrossRef]
- Shafee, T.M.A.; Lay, F.T.; Hulett, M.D.; Anderson, M.A. The Defensins Consist of Two Independent, Convergent Protein Superfamilies. Mol. Biol. Evol. 2016, 33, 2345–2356. [Google Scholar] [CrossRef]
- Sathoff, A.E.; Samac, D.A. Antibacterial Activity of Plant Defensins. Mol. Plant-Microbe Interact. 2019, 32, 507–514. [Google Scholar] [CrossRef]
- Siebert, P.D.; Chenchik, A.; Kellogg, D.E.; Lukyanov, K.A.; Lukyanov, S.A. An Improved PCR Method for Walking in Uncloned Genomic DNA. Nucleic Acids Res. 1995, 23, 1087–1088. [Google Scholar] [CrossRef]
- Lacroix, B.; Vaidya, M.; Tzfira, T.; Citovsky, V. The VirE3 Protein of Agrobacterium Mimics a Host Cell Function Required for Plant Genetic Transformation. EMBO J. 2005, 24, 428–437. [Google Scholar] [CrossRef]
- Fischer, R.; Buyel, J.F. Molecular Farming—The Slope of Enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef]
- Schillberg, S.; Finnern, R. Plant Molecular Farming for the Production of Valuable Proteins—Critical Evaluation of Achievements and Future Challenges. J. Plant Physiol. 2021, 258–259, 153359. [Google Scholar] [CrossRef]
- Spiegel, H.; Stöger, E.; Twyman, R.M.; Buyel, J.F. Current Status and Perspectives of the Molecular Farming Landscape. In Molecular Pharming; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–23. ISBN 978-1-118-80151-2. [Google Scholar]
- Höng, K.; Austerlitz, T.; Bohlmann, T.; Bohlmann, H. The Thionin Family of Antimicrobial Peptides. PLoS ONE 2021, 16, e0254549. [Google Scholar] [CrossRef]
- Komarova, T.V.; Sheshukova, E.V.; Dorokhov, Y.L. Cell Wall Methanol as a Signal in Plant Immunity. Front. Plant Sci. 2014, 5, 101. [Google Scholar] [CrossRef]
- Sheshukova, E.V.; Komarova, T.V.; Pozdyshev, D.V.; Ershova, N.M.; Shindyapina, A.V.; Tashlitsky, V.N.; Sheval, E.V.; Dorokhov, Y.L. The Intergenic Interplay between Aldose 1-Epimerase-like Protein and Pectin Methylesterase in Abiotic and Biotic Stress Control. Front. Plant Sci. 2017, 8, 1646. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Machleder, E.M.; Chenchik, A.; Li, R.; Siebert, P.D. Reverse Transcriptase Template Switching: A SMART Approach for Full-Length CDNA Library Construction. BioTechniques 2001, 30, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS Fusions: Beta-Glucuronidase as a Sensitive and Versatile Gene Fusion Marker in Higher Plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheshukova, E.V.; Ershova, N.M.; Lipskerov, F.A.; Komarova, T.V. Enhanced Synthesis of Foreign Nuclear Protein Stimulates Viral Reproduction via the Induction of γ-Thionin Expression. Plants 2022, 11, 1530. https://doi.org/10.3390/plants11121530
Sheshukova EV, Ershova NM, Lipskerov FA, Komarova TV. Enhanced Synthesis of Foreign Nuclear Protein Stimulates Viral Reproduction via the Induction of γ-Thionin Expression. Plants. 2022; 11(12):1530. https://doi.org/10.3390/plants11121530
Chicago/Turabian StyleSheshukova, Ekaterina V., Natalia M. Ershova, Fedor A. Lipskerov, and Tatiana V. Komarova. 2022. "Enhanced Synthesis of Foreign Nuclear Protein Stimulates Viral Reproduction via the Induction of γ-Thionin Expression" Plants 11, no. 12: 1530. https://doi.org/10.3390/plants11121530
APA StyleSheshukova, E. V., Ershova, N. M., Lipskerov, F. A., & Komarova, T. V. (2022). Enhanced Synthesis of Foreign Nuclear Protein Stimulates Viral Reproduction via the Induction of γ-Thionin Expression. Plants, 11(12), 1530. https://doi.org/10.3390/plants11121530





