Respiratory and Photosynthetic Responses of Antarctic Vascular Plants Are Differentially Affected by CO2 Enrichment and Nocturnal Warming
Abstract
1. Introduction
2. Results
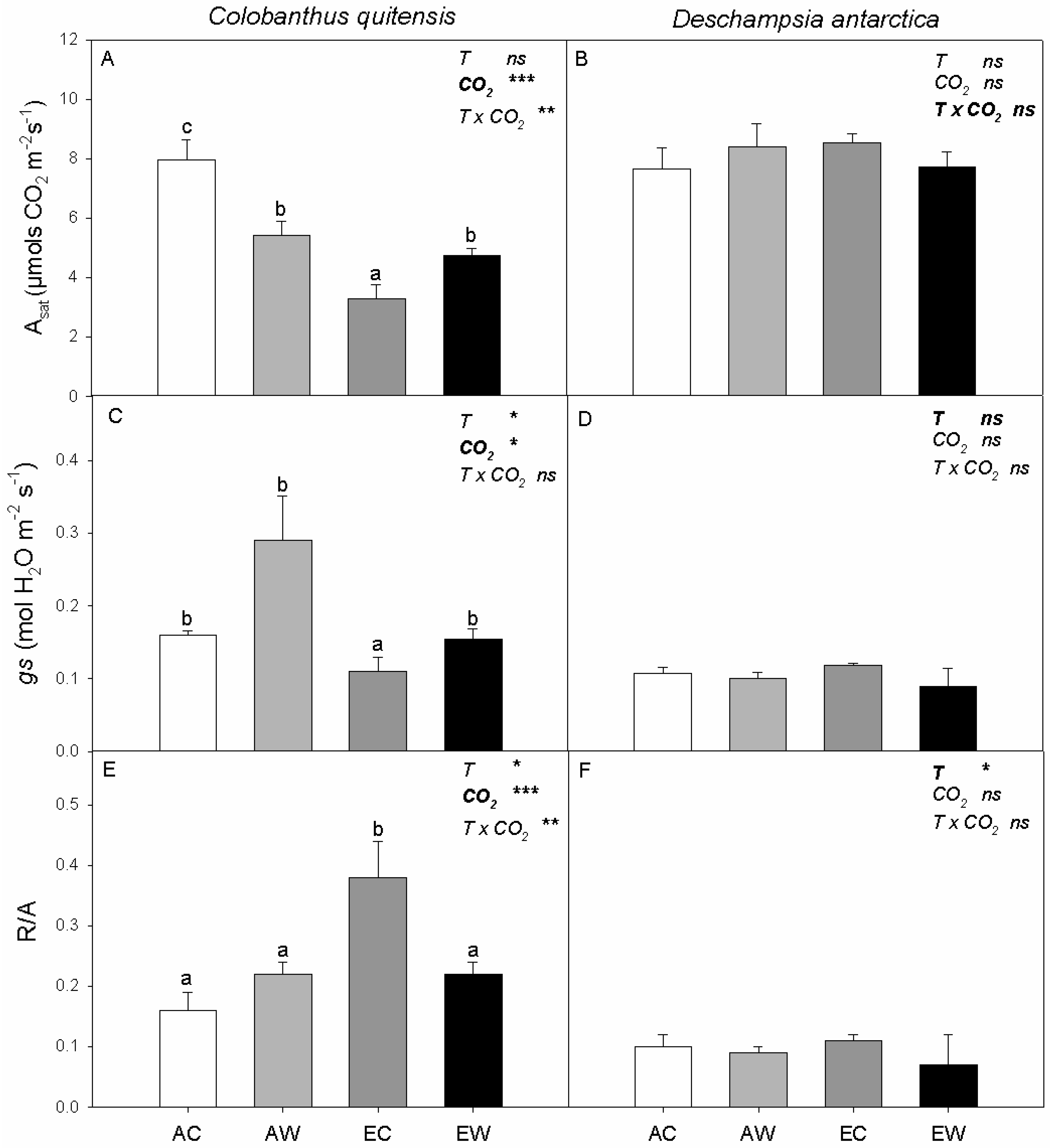
2.1. Gas Exchange and Carbon Balance
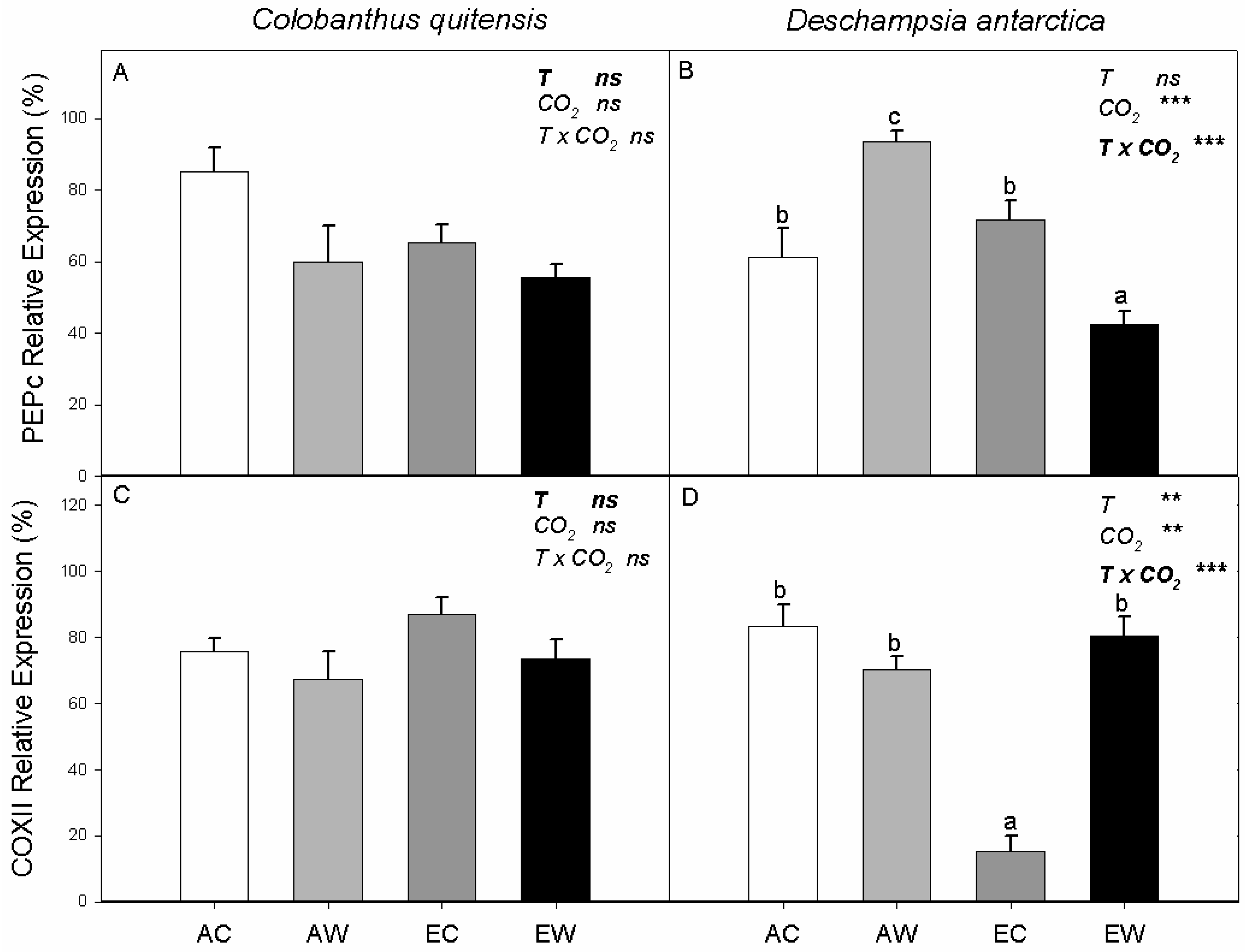
2.2. Non-Structural Carbohydrates, Relative Abundances of PEPc and COX-II
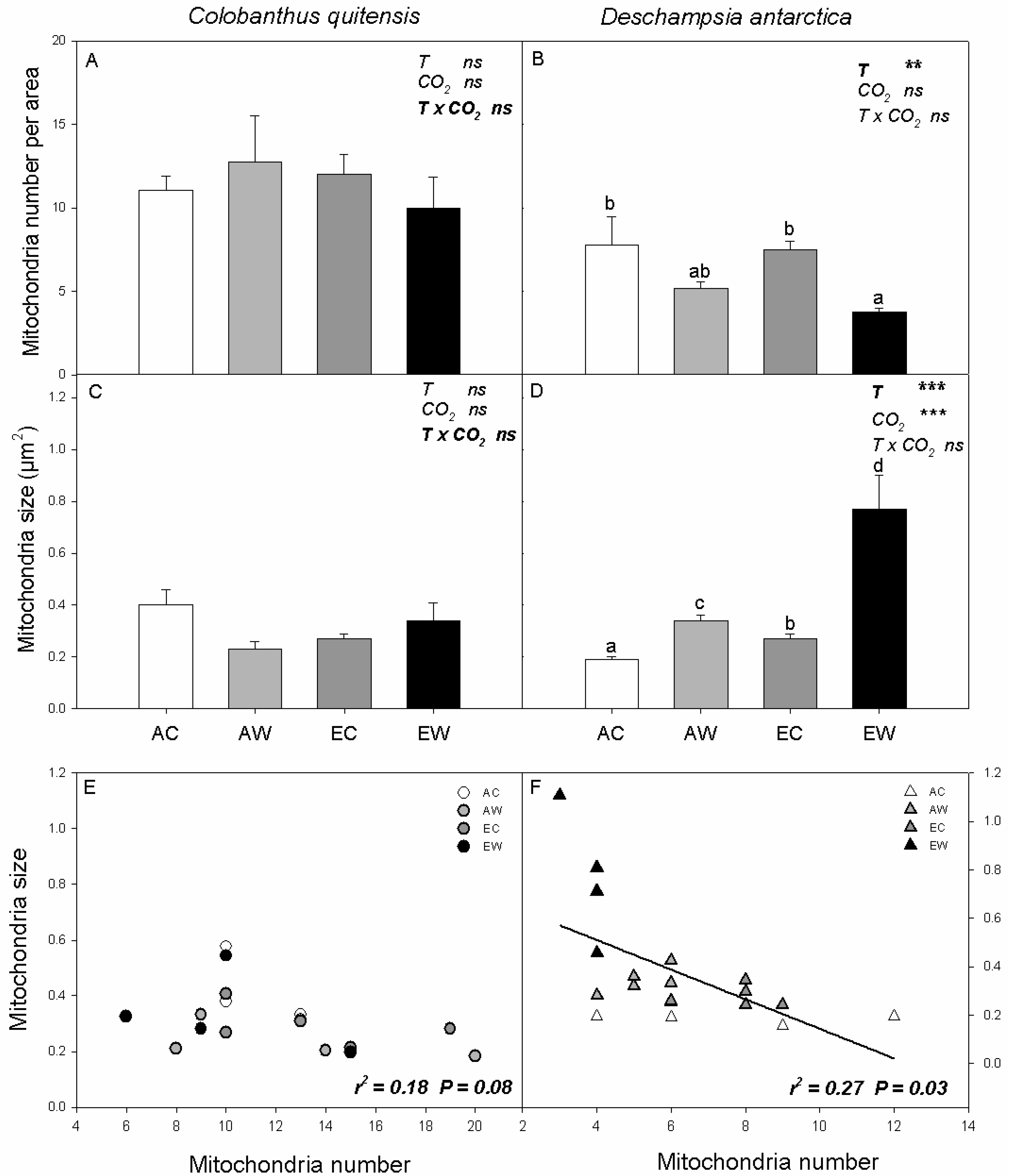
2.3. Mitochondrial Traits
3. Discussion
3.1. Elevated CO2 and Nocturnal Warming Differentially Affected the Photosynthetic Performance of the Two Antarctic Species
3.2. Dark Respiration Showed Differential Sensitivity and Thermal Acclimation to Elevated CO2 and Nocturnal Warming
3.3. The Determining Factor to Maintain the Carbon Balance in Antarctic Species Appears to Be the Maintenance of the Photosynthetic Rate
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Gas Exchange
4.4. Quantification of Thermal Acclimation of Respiration
4.5. Biochemical Analyses
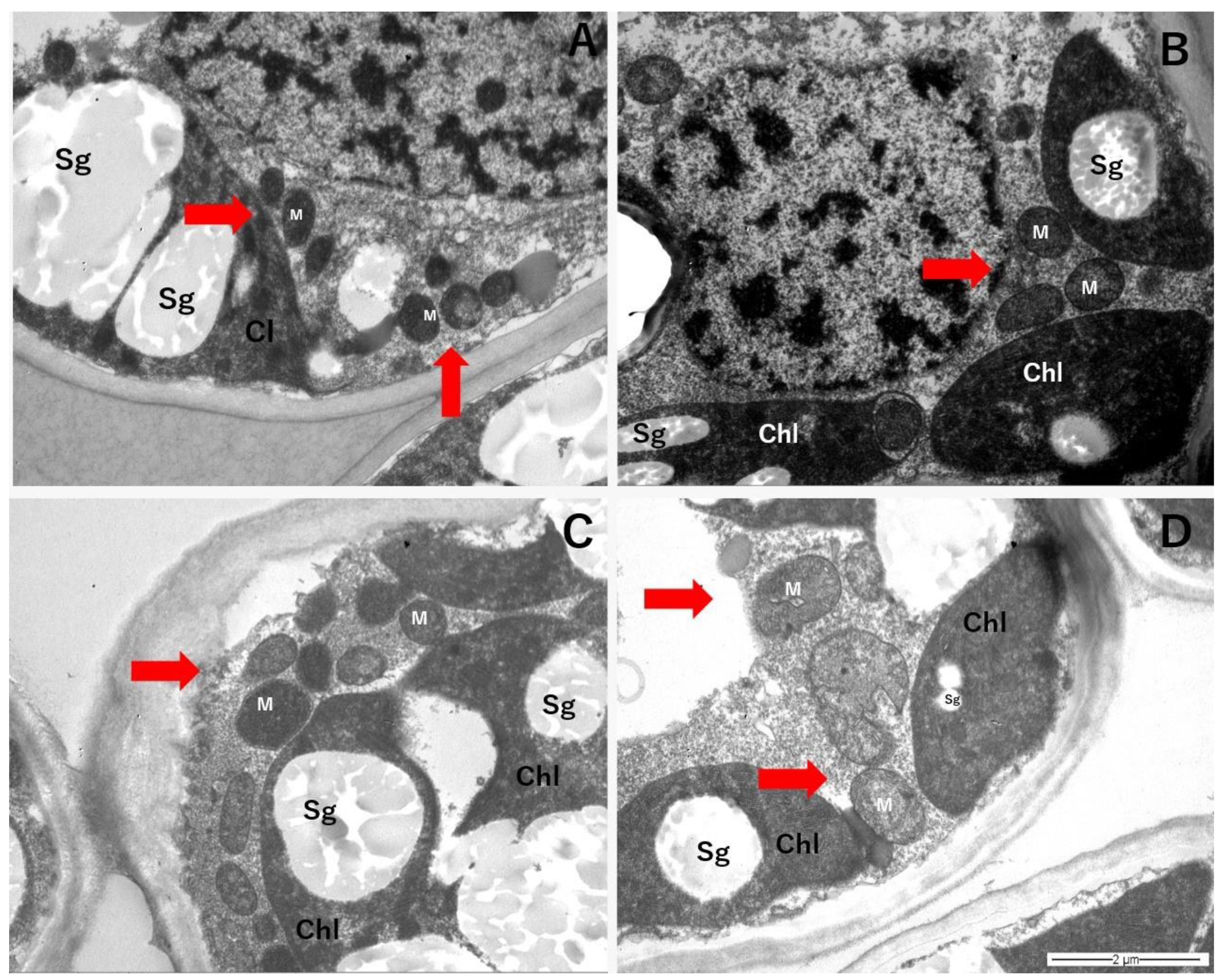
4.6. Transmission Electron Microscopy of Leaf Mesophyll
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloaway, J.; Heimann, M.; et al. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernamental Panel on Climate Change; Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 465–570. [Google Scholar]
- Keeling, R.F.; Powell, F.L.; Shaffer, G.; Robbins, P.A.; Simonson, T.S. Impacts of Changes in Atmospheric O2 on Human Physiology. Is There a Basis for Concern? Front. Plant Physiol. 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R.S., et al., Eds.; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Bracegirdle, T.J.; Barrand, N.E.; Kusahara, K.; Wainer, I. Predicting Antarctic climate using climate models. Antarct. Environ. Portal 2016. Available online: http://nora.nerc.ac.uk/id/eprint/513739 (accessed on 6 September 2020).
- IPCC. Technical Summary. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2017. [Google Scholar]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Yang, B.; Peng, C.; Harrison, S.P.; Wei, H.; Wang, H.; Zhu, Q.; Wang, M. Allocation mechanisms of non-structural carbohydrates of Robinia pseudoacacia L. seedlings in response to drought and waterlogging. Forests 2018, 9, 754. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Tjoelker, M.G.; Vanderklein, D.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct. Ecol. 1998, 12, 395–405. [Google Scholar] [CrossRef]
- Whitehead, D.; Griffin, K.L.; Turnbull, M.H.; Tissue, D.T.; Engel, V.C.; Brown, K.J.; Schuster WS, F.; Walkroft, A.S. Response of total night-time respiration to differences in total daily photosynthesis for leaves in a Quercus rubra L. canopy: Implications for modelling canopy CO2 exchange. Glob. Change Biol. 2004, 10, 925–938. [Google Scholar] [CrossRef]
- Atkin, O.K.; Scheurwater, I.; Pons, T.L. Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol. 2007, 174, 367–380. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Björkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Way, D.A.; Yamori, W. Thermal acclimation of photosynthesis: On the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth. Res. 2014, 119, 89–100. [Google Scholar] [CrossRef]
- Wang, J.; Cheung, M.; Rasooli, L.; Amirsadeghi, S.; Vanlerberghe, G.C. Plant respiration in a high CO2 world: How will alternative oxidase respond to future atmospheric and climatic conditions? Can. J. Plant Sci. 2014, 94, 1091–1101. [Google Scholar] [CrossRef]
- Arp, W.J. Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 1991, 14, 869–875. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Oleksyn, J.; Reich, P.B. Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Glob. Change Biol. 1999, 5, 679–691. [Google Scholar] [CrossRef]
- Crous, K.Y.; Zaragoza-Castells, J.; Ellsworth, D.S.; Duursma, R.A.; Löw, M.; Tissue, D.T.; Atkin, O.K. Light inhibition of leaf respiration in field-grown Eucalyptus saligna in whole-tree chambers under elevated atmospheric CO2 and summer drought. Plant Cell Environ. 2012, 35, 966–981. [Google Scholar] [CrossRef]
- Tan, K.; Zhou, G.; Ren, S. Response of leaf dark respiration of winter wheat to changes in CO2 concentration and temperature. Chin. Sci. Bull. 2013, 58, 1795–1800. [Google Scholar] [CrossRef][Green Version]
- Kroner, Y.; Way, D.A. Carbon fluxes acclimate more strongly to elevated growth temperatures than to elevated CO2 concentrations in a northern conifer. Glob. Change Biol. 2016, 22, 2913–2928. [Google Scholar] [CrossRef]
- Slot, M.; Kitajima, K. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 2015, 177, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.G.; Dukes, J.S. Short-term acclimation to warmer temperatures accelerates leaf carbon exchange processes across plant types. Glob. Change Biol. 2017, 23, 4840–4853. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Atkin, O.K.; Bruhn, D.; Hurry, V.M.; Tjoelker, M.G. The hot and the cold: Unravelling the variable response of plant respiration to temperature. Funct. Plant Biol. 2005, 32, 87–105. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, M.; Shen, R.; Wan, S. Acclimation of leaf dark respiration to nocturnal and diurnal warming in a semiarid temperate steppe. Funct. Plant Biol. 2013, 40, 1159–1167. [Google Scholar] [CrossRef]
- Miroslavov, E.A.; Kravkina, I.M. Comparative analysis of chloroplasts and mitochondria in leaf chlorenchyma from mountain plants grown at different altitudes. Ann. Bot. 1991, 68, 195–200. [Google Scholar] [CrossRef]
- Amthor, J.S.; Koch, G.W.; Bloom, A.J. CO2 inhibits respiration in leaves of Rumex crispus L. Plant Physiol. 1992, 98, 757–760. [Google Scholar] [CrossRef]
- Lambers, H.; Ribas-Carbó, M. Plant Respiration: From Cell to Ecosystem; Springer Science & Business Media: Dordrecht, The Netherlands, 2005; Volume 18. [Google Scholar]
- Thomas, R.B.; Griffin, K.L. Direct and indirect effects of atmospheric carbon dioxide enrichment on leaf respiration of Glycine max (L.). Merr. Plant Physiol. 1994, 104, 355–361. [Google Scholar] [CrossRef]
- Amthor, J.S. Direct effect of elevated CO2 on nocturnal in situ leaf respiration in nine temperate deciduous tree species is small. Tree Physiol. 2000, 20, 139–144. [Google Scholar] [CrossRef]
- Baker, J.T.; Allen, L.H.; Boote, K.J.; Pickering, N.B. Direct effects of atmospheric carbon dioxide concentration on whole canopy dark respiration of rice. Glob. Change Biol. 2000, 6, 275–286. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Thomas, R.B.; De Lucia, E.H. Direct and indirect effects of elevated CO2 on leaf respiration in a forest ecosystem. Plant Cell Environ. 2001, 2, 975–982. [Google Scholar] [CrossRef]
- Gonzàlez-Meler, M.A.; Ribas-Carbó, M.; Siedow, J.N.; Drake, B.G. Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol. 1996, 112, 1349–1355. [Google Scholar] [CrossRef]
- Gonzàlez-Meler, M.A.; Blanc-Betes, E.; Flower, C.E.; Ward, J.K.; Gómez-Casanovas, N. Plastic and adaptive responses of plant respiration to changes in atmospheric CO2 concentration. Physiol. Plant. 2009, 137, 473–484. [Google Scholar] [CrossRef]
- Ayub, G.; Zaragoza-Castells, J.; Griffin, K.L.; Atkin, O.K. Leaf respiration in darkness and in the light under pre-industrial, current and elevated atmospheric CO2 concentrations. Plant Sci. 2014, 226, 120–130. [Google Scholar] [CrossRef]
- Griffin, K.L.; Anderson, O.R.; Gastrich, M.D.; Lewis, J.D.; Lin, G.; Schusteri, W.; Seemann, J.R.; Tissue, D.T.; Turnbull, M.H.; Whitehead, D. Plant growth in elevated CO2 alters mitochondrial number and chloroplast fine structure. Proc. Natl. Acad. Sci. USA 2001, 98, 347–353. [Google Scholar] [CrossRef]
- Wang, X.; Lewis, J.D.; Tissue, D.T.; Seemann, J.R.; Griffin, K.L. Effects of elevated atmospheric CO2 concentration on leaf dark respiration of Xanthium strumarium in light and in darkness. Proc. Natl. Acad. Sci. USA 2001, 98, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Lamba, S.; Hall, M.; Räntfors, M.; Chaudhary, N.; Linder, S.; Way, D.; Uddling, J.; Wallin, J. Physiological acclimation dampens initial effects of elevated temperature and atmospheric CO2 concentration in mature boreal Norway spruce. Plant Cell Environ. 2018, 41, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Norby, R.J.; Luo, Y.Q. Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multifactor world. New Phytol. 2004, 162, 281–293. [Google Scholar] [CrossRef]
- Lee, J.R.; Raymond, B.; Braceggirdle, T.J.; Chadés, I.; Fuller, R.A.; Shaw, J.D.; Terauds, A. Climate change drives expansion of Antarctic ice-free habitat. Nature 2017, 547, 49–54. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.L.; Engelbrecht, F.; et al. Impacts of 1.5 °C global warming on natural and human systems in Global Warming of 1.5 °C. In An IPCC Special Report on the Impacts of Global Warming of 1.5 °C; Masson-Delmotte, P., Zhai, H.O., Pörtner, D., Roberts, J., Skea, P.R., Shukla, A., Pirani, W., Moufouma-Okia, C., Péan, R., Pidcock, S., et al., Eds.; UWA School of Agriculture and Environment Department of Geography: Perth, Australia, 2018. [Google Scholar]
- Li, C.; Michel, C.; Seland Graff, L.; Bethke, I.; Zappa, G.; Bracegirdle, T.J.; Fischer, E.; Harvey, B.J.; Iversen, T.; King, M.P.; et al. Mid latitude atmospheric circulation responses under 1.5 and 2.0 °C warming and implications for regional impacts. Earth Syst. Dyn. 2018, 9, 359–382. [Google Scholar] [CrossRef]
- NOAA National Center for Environmental Information. State of the Climate: Global Climate Report for Annual. 2015. Available online: https://www.ncdc.noaa.gov/sotc/global/201513 (accessed on 30 June 2020).
- Berwyn, B. Antarctica’s CO2 Level Tops 400 PPM for First Time in Perhaps 4 Million Years. Inside Climate News. 2016. Available online: https://insideclimatenews.org/news/16062016/antarctica-co2-level-tops-400-ppm-first-time-perhaps-4-million-years-british-antarctic-survey-global-warming (accessed on 10 August 2020).
- Cavieres, L.A.; Sáez, P.; Sanhueza, C.; Sierra-Almeida, A.; Rabert, C.; Corcuera, L.J.; Bravo, L.A. Ecophysiological traits of Antarctic vascular plants: Their importance in the responses to climate change. Plant Ecol. 2016, 217, 343–358. [Google Scholar] [CrossRef]
- Fowbert, J.A.; Smith, R.I.L. Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct. Alp. Res. 1994, 26, 290–296. [Google Scholar] [CrossRef]
- Cannone, N.; Guglielmin, M.; Convey, P.; Worland, M.R.; Favero Longo, S.E. Vascular plant changes in extreme environments: Effects of multiple drivers. Clim. Change 2015, 134, 651–665. [Google Scholar] [CrossRef]
- Sáez, P.; Cavieres, L.A.; Galmés, J.; Peguero-Pina, J.J.; Sancho-Knapik, D.; Vivas, M.; Sanhueza, C.; Ramírez, C.F.; Rivera, B.K.; Corcuera, L.J.; et al. In situ warming in the Antarctica: Effects on growth and photosynthesis in the Antarctic vascular plants. New Phytol. 2018, 218, 1406–1418. [Google Scholar] [CrossRef]
- Sanhueza, C.; Fuentes, F.; Cortés, D.; Bascunan-Godoy, L.; Sáez, P.; Bravo, L.; Cavieres, L. Contrasting thermal acclimation of leaf dark respiration and photosynthesis of Antarctic vascular plant species exposed to nocturnal warming. Physiol. Plant. 2019, 167, 205–216. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress implants. Front. Plant Sci. 2015, 6, 701. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising (CO2): Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Makino, A.; Nakano, H.; Mae, T.; Shimada, T.; Yamamoto, N. Photosynthesis, plant growth and N allocation in transgenic rice plants with decreased Rubisco under CO2 enrichment. J. Exp. Bot. 2000, 51, 383–389. [Google Scholar] [CrossRef]
- Wattal, R.K.; Siddiqui, Z.H. Effect of Elevated Levels of Carbon Dioxide on the Activity of RuBisCO and Crop Productivity. In Crop Production and Global Environmental Issues; Hakeem, K., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Turnbull, M.H.; Murthy, R.; Griffin, K.L. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant Cell Environ. 2002, 25, 1729–1737. [Google Scholar] [CrossRef]
- Sadok, W.; Jagadish, K.S.V. The Hidden Costs of Nighttime Warming on Yields. Trends Plant Sci. 2020, 25, 644–651. [Google Scholar] [CrossRef]
- Schoppach, R.; Sadok, W. Transpiration sensitivities to evaporative demand and leaf areas vary with night and daywarming regimes among wheat genotypes. Funct. Plant Biol. 2013, 40, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Wang, D.; Zhu, C.; Chen, J. Plant Physiological, Morphological and Yield-Related Responses to Night Temperature Changes across Different Species and Plant Functional Types. Front. Plant Sci. 2016, 7, 1774. [Google Scholar] [CrossRef] [PubMed]
- Tombesi, S.; Cinceral Frioni, T.; Ughini, V.; Gatti, M.; Palliotti, A.; Poni, S. Relationship among night temperature, carbohydrate translocation and inhibition of grapevine leaf photosynthesis. Environ. Exp. Bot. 2019, 157, 293–298. [Google Scholar] [CrossRef]
- Day, T.; Ruhland, C.; Grobe, C.; Xiong, F. Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia 1999, 119, 24–35. [Google Scholar] [CrossRef]
- Florez-Sarasa, I.; Fernie, A.R.; Gupta, K.J. Does the alternative respiratory pathway offer protection against the adverse effects resulting from climate change? J. Exp. Bot. 2020, 71, 465–469. [Google Scholar] [CrossRef]
- Bui, V. Photosynthetic Acclimation to Warming and Elevated CO2 in two Antarctic Vascular Plant Species. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2016. Available online: https://ir.lib.uwo.ca/etd/3654 (accessed on 3 March 2020).
- Roy, K.S.; Bhattacharyya, P.; Neogi, S.; Rao, K.S.; Adhya, T.K. Combined effect of elevated CO2 and temperature on dry matter production, net assimilation rate, C and N allocations in tropical rice (Oryza sativa L.). Field Crops Res. 2012, 139, 71–79. [Google Scholar] [CrossRef]
- Zhang, S.; Fua, W.; Zhanga, Z.; Fana, Y.; Liua, T. Effects of elevated CO2 concentration and temperature on some physiological characteristics of cotton (Gossypium hirsutum L.) leaves. Environ. Exp. Bot. 2017, 133, 108–117. [Google Scholar] [CrossRef]
- Turnbull, M.H.; Tissue, D.T.; Murthy, R.; Wang, X.; Sparrow, A.D.; Griffin, K.L. Nocturnal warming increases photosynthesis at elevated CO2 partial pressure in Populus deltoides. New Phytol. 2004, 161, 819–826. [Google Scholar] [CrossRef]
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Interactions of elevated [CO2] and night temperature on rice growth and yield. Agric. For. Meteorol. 2009, 149, 51–58. [Google Scholar] [CrossRef]
- Campbell, C.; Atkinson, L.; Zaragoza-Castells, J.; Lundmark, M.; Atkin, O.; Hurry, V. Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. New Phytol. 2007, 176, 375–389. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Oleksyn, J.; Lorenc-Plucinska, G.; Reich, P.B. Acclimation of respiratory temperature responses in northern and southern populations of Pinus banksiana. New Phytol. 2009, 181, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Tsunoda, T.; Miyagi, A.; Kawai-Yamada, M.; Sugiura, D.; Miyazawa, S.; Tokida, T.; Usui, Y.; Nakamura, H.; Sakai, H.; et al. Effects of Elevated Atmospheric CO2 on Respiratory Rates in Mature Leaves of Two Rice Cultivars Grown at a Free-Air CO2 Enrichment Site and Analyses of the Underlying Mechanisms. Plant Cell Physiol. 2018, 59, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Stangl, Z.R.; Ivanov, A.G.; Bui, V.; Mema, M.; Hüner, N.P.; Öquist, G.; Way, D.; Hurry, V. Contrasting acclimation abilities of two dominant boreal conifers to elevated CO2 and temperature. Plant Cell Environ. 2018, 41, 1331–1345. [Google Scholar] [CrossRef]
- Bruhn, D.; Egerton, J.J.G.; Loveys, B.R.; Ball, M.C. Evergreen leaf respiration acclimates to long-term nocturnal warming under field conditions. Glob. Change Biol. 2007, 13, 1216–1223. [Google Scholar] [CrossRef]
- Zaragoza-Castells, J.; Sánchez-Gómez, D.; Hartley, I.P.; Matesanz, S.; Valladares, F.; Lloyd, J.; Atkin, O.K. Climate-dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: The importance of acclimation in both high-light and shaded habitats. Funct. Ecol. 2008, 22, 172–184. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Badger, M.R.; Day, D.A.; Barthet, M.M.; Smith PM, C.; Millar, A.H.; Whelan, J.; Atkin, O.K. Dynamic changes in the mitochondrial electron transport chain underpinning cold acclimation of leaf respiration. Plant Cell Environ. 2008, 31, 1156–1169. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Vanlerberghe, G.C. Growth at Elevated CO2 Requires Acclimation of the Respiratory Chain to Support Photosynthesis. Plant Physiol. 2018, 178, 82–100. [Google Scholar] [CrossRef]
- Clemente-Moreno, M.J.; Omranian, N.; Sáez, P.; Figueroa, C.M.; Del-Saz, N.; Elso, M.; Poblete, L.; Orf, I.; Cuadros-Inostroza, A.; Cavieres, L.; et al. Cytochrome respiration pathway and sulphur metabolism sustain stress tolerance to low temperature in the Antarctic species Colobanthus quitensis. New Phytol. 2020, 225, 754–768. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Gomez, F.; Gergoff, G.; Gulaét, J.J.; Puntarulo, S. Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. J. Exp. Bot. 2005, 56, 1269–1276. [Google Scholar] [CrossRef]
- Way, D.A.; Sage, R.F. Elevated growth temperatures reduce the carbon gain of black spruce (Picea mariana (Mill.) B.S.P.). Glob. Change Biol. 2008, 14, 624–636. [Google Scholar] [CrossRef]
- Gianoli, E.; Inostroza, P.; Zúñiga-Feest, A.; Reyes-Díaz, M.; Cavieres, L.; Bravo, L.; Corcuera, L. Ecotypic Differentiation in Morphology and Cold Resistance in Populations of Colobanthus quitensis (Caryophyllaceae) from the Andes of Central Chile and the Maritime Antarctic. Arct. Antarct. Alp. Res. 2004, 36, 484–489. [Google Scholar] [CrossRef]
- Sierra-Almeida, A.; Casanova-Katny, M.A.; Bravo, L.; Corcuera, L.; Cavieres, L. Photosynthetic responses to temperature and light of Antarctic and Andean populations of Colobanthus quitensis (Caryophyllaceae). Rev. Chil. Hist. Nat. 2007, 80, 335–343. [Google Scholar] [CrossRef]
- Searle, S.Y.; Turnbull, M.H. Seasonal variation of leaf respiration and the alternative pathway in field-grown Populus× canadensis. Physiol. Plant. 2011, 141, 332–342. [Google Scholar] [CrossRef]
- Kruse, J.; Rennenberg, H.; Adams, M.A. Steps towards a mechanistic understanding of respiratory temperature responses. New Phytol. 2011, 189, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Loveys, B.R.; Atkinson, L.J.; Sherlock, D.J.; Roberts, R.L.; Fitter, A.H.; Atkin, O.K. Thermal acclimation of leaf and root respiration, an investigation comparing inherently fast- and slow-growing plant species. Glob. Change Biol. 2003, 9, 895–910. [Google Scholar] [CrossRef]
- Marquis, R.J.; Newell, E.A.; Villegas, A.C. Non-structural carbohydrate accumulation and use in an understorey rain-forest shrub and relevance for the impact of leaf herbivory. Funct. Ecol. 1997, 11, 636–643. [Google Scholar] [CrossRef]
- Dickinson, R.E. Analytical procedures for the sequential extraction of 14 Clabeled constituents from leaves, bark and Wood of Cottonwood plants. Physiol. Plant. 1979, 45, 480–488. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Von Caemmerer, S. Effect of Rubisco activase deficiency on the temperature response of CO2 assimilation rate and Rubisco activation state: Insights from transgenic tobacco with reduced amounts of Rubisco activase. Plant Physiol. 2009, 151, 2073–2082. [Google Scholar] [CrossRef] [PubMed]



| C. quitensis | p Value | η2 | ||||
| Response Variable | T | CO2 | T x CO2 | T | CO2 | T x CO2 |
| Asat | 0.30 | <0.001 | 0.00 | 0.02 | 0.32 | 0.21 |
| gs | 0.02 | 0.01 | 0.59 | 0.20 | 0.24 | 0.01 |
| R10 | 0.60 | 0.22 | 0.06 | 0.01 | 0.07 | 0.16 |
| E0 | 0.49 | 0.90 | 0.09 | 0.03 | 0.00 | 0.14 |
| Q10 | 0.51 | 0.82 | 0.09 | 0.02 | 0.00 | 0.15 |
| Acclimset-temp | - | 0.14 | - | - | 0.20 | - |
| R/A | 0.02 | <0.001 | <0.001 | 0.12 | 0.29 | 0.27 |
| TSS | 0.79 | 0.96 | 0.41 | 0.00 | 0.00 | 0.04 |
| Starch | 0.13 | 0.10 | 0.22 | 0.10 | 0.12 | 0.07 |
| PEPc | 0.06 | 0.49 | 0.39 | 0.27 | 0.04 | 0.06 |
| COXII | 0.09 | 0.16 | 0.69 | 0.16 | 0.11 | 0.01 |
| Mit. Number | 0.58 | 0.82 | 0.24 | 0.02 | 0.00 | 0.09 |
| Mit.Size | 0.09 | 0.78 | 0.05 | 0.14 | 0.00 | 0.18 |
| D. antarctica | p Value | η2 | ||||
| Response Variable | T | CO2 | T x CO2 | T | CO2 | T x CO2 |
| Asat | 0.96 | 0.86 | 0.22 | 0.00 | 0.00 | 0.11 |
| gs | 0.23 | 0.99 | 0.48 | 0.08 | 0.00 | 0.03 |
| R10 | 0.26 | 0.76 | 0.09 | 0.06 | 0.00 | 0.14 |
| E0 | 0.17 | 0.02 | 0.08 | 0.07 | 0.19 | 0.11 |
| Q10 | 0.21 | 0.04 | 0.10 | 0.06 | 0.16 | 0.11 |
| Acclimset-temp | 0.06 | - | 0.27 | - | ||
| R/A | 0.04 | 0.75 | 0.98 | 0.24 | 0.01 | 0.00 |
| TSS | 0.11 | 0.05 | 0.32 | 0.12 | 0.16 | 0.05 |
| Starch | 0.01 | 0.67 | 0.62 | 0.26 | 0.01 | 0.01 |
| PEPc | 0.79 | <0.001 | <0.001 | 0.00 | 0.19 | 0.35 |
| COXII | <0.001 | <0.001 | <0.001 | 0.17 | 0.20 | 0.32 |
| Mit. Number | 0.00 | 0.32 | 0.48 | 0.32 | 0.03 | 0.02 |
| Mit.Size | <0.001 | <0.001 | 0.06 | 0.41 | 0.24 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhueza, C.; Cortes, D.; Way, D.A.; Fuentes, F.; Bascunan-Godoy, L.; Fernandez Del-Saz, N.; Sáez, P.L.; Bravo, L.A.; Cavieres, L.A. Respiratory and Photosynthetic Responses of Antarctic Vascular Plants Are Differentially Affected by CO2 Enrichment and Nocturnal Warming. Plants 2022, 11, 1520. https://doi.org/10.3390/plants11111520
Sanhueza C, Cortes D, Way DA, Fuentes F, Bascunan-Godoy L, Fernandez Del-Saz N, Sáez PL, Bravo LA, Cavieres LA. Respiratory and Photosynthetic Responses of Antarctic Vascular Plants Are Differentially Affected by CO2 Enrichment and Nocturnal Warming. Plants. 2022; 11(11):1520. https://doi.org/10.3390/plants11111520
Chicago/Turabian StyleSanhueza, Carolina, Daniela Cortes, Danielle A. Way, Francisca Fuentes, Luisa Bascunan-Godoy, Nestor Fernandez Del-Saz, Patricia L. Sáez, León A. Bravo, and Lohengrin A. Cavieres. 2022. "Respiratory and Photosynthetic Responses of Antarctic Vascular Plants Are Differentially Affected by CO2 Enrichment and Nocturnal Warming" Plants 11, no. 11: 1520. https://doi.org/10.3390/plants11111520
APA StyleSanhueza, C., Cortes, D., Way, D. A., Fuentes, F., Bascunan-Godoy, L., Fernandez Del-Saz, N., Sáez, P. L., Bravo, L. A., & Cavieres, L. A. (2022). Respiratory and Photosynthetic Responses of Antarctic Vascular Plants Are Differentially Affected by CO2 Enrichment and Nocturnal Warming. Plants, 11(11), 1520. https://doi.org/10.3390/plants11111520








