Reallocation of Soluble Sugars and IAA Regulation in Association with Enhanced Stolon Growth by Elevated CO2 in Creeping Bentgrass
Abstract
:1. Introduction
2. Results
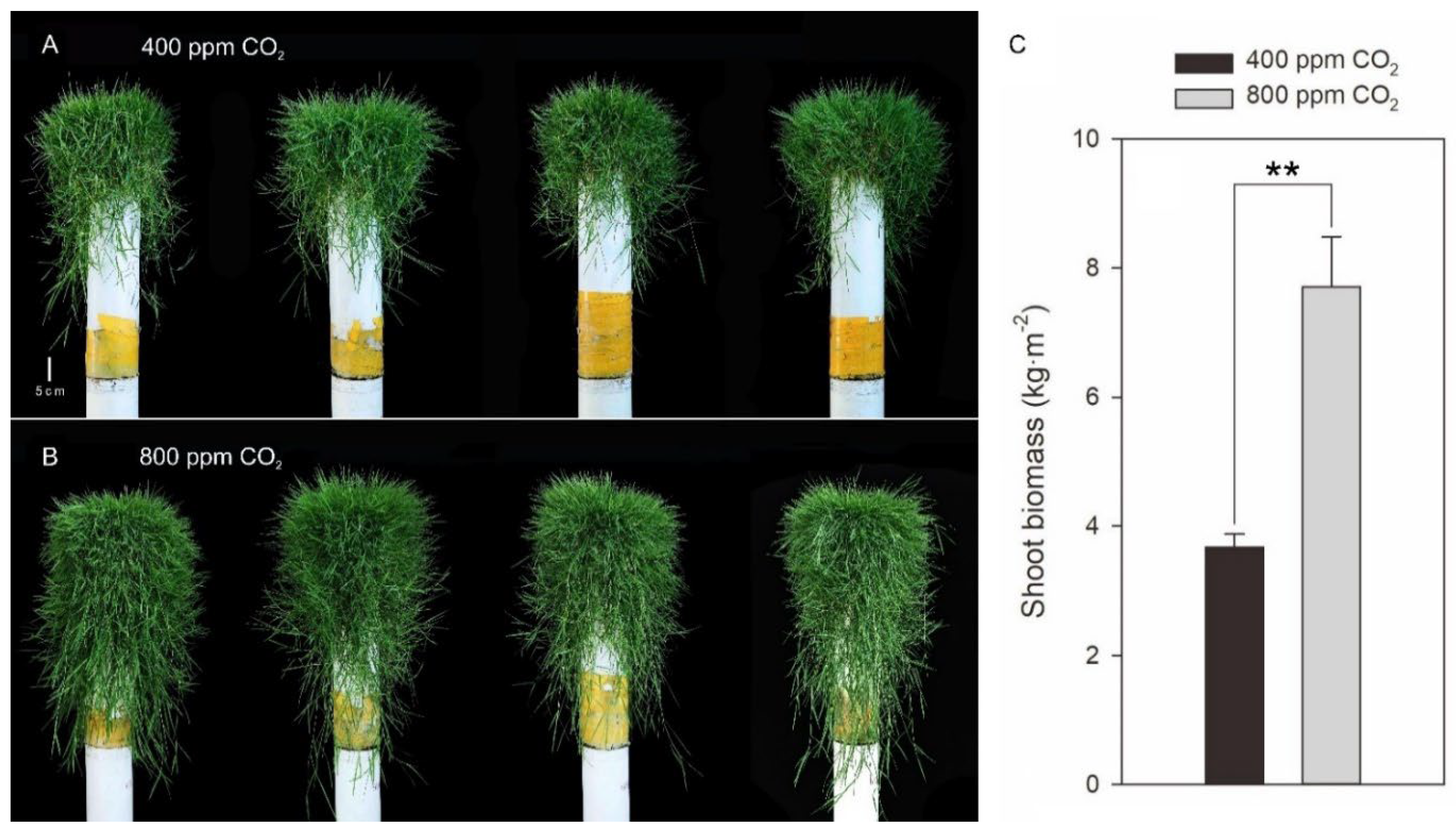
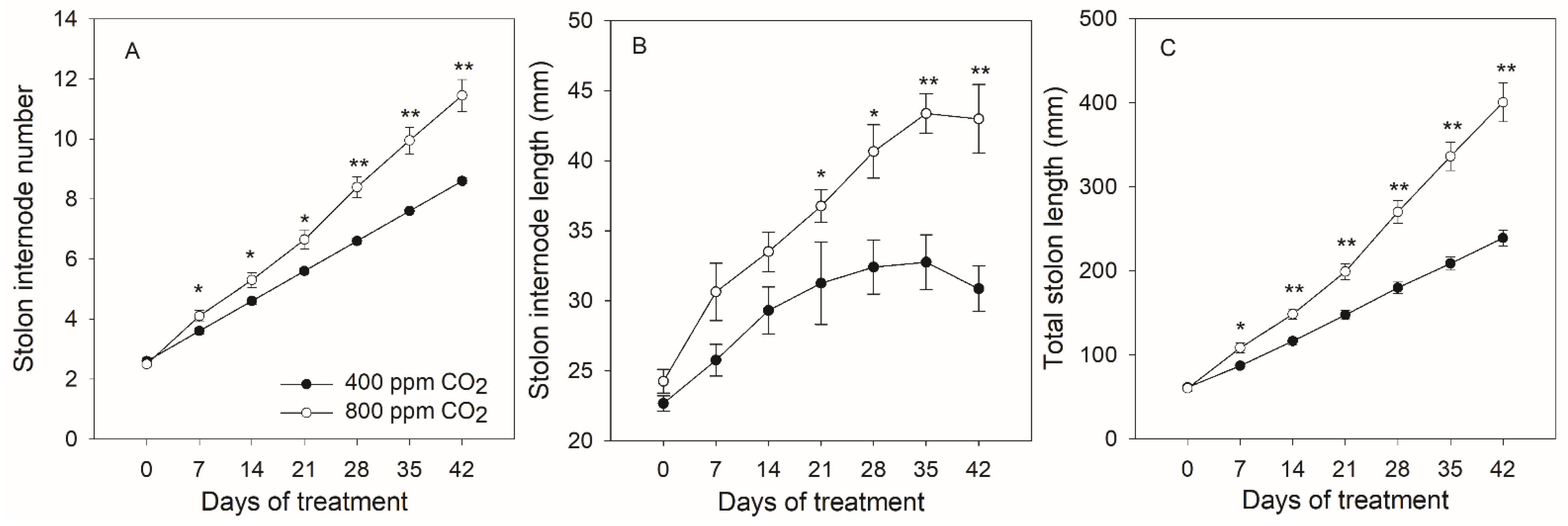
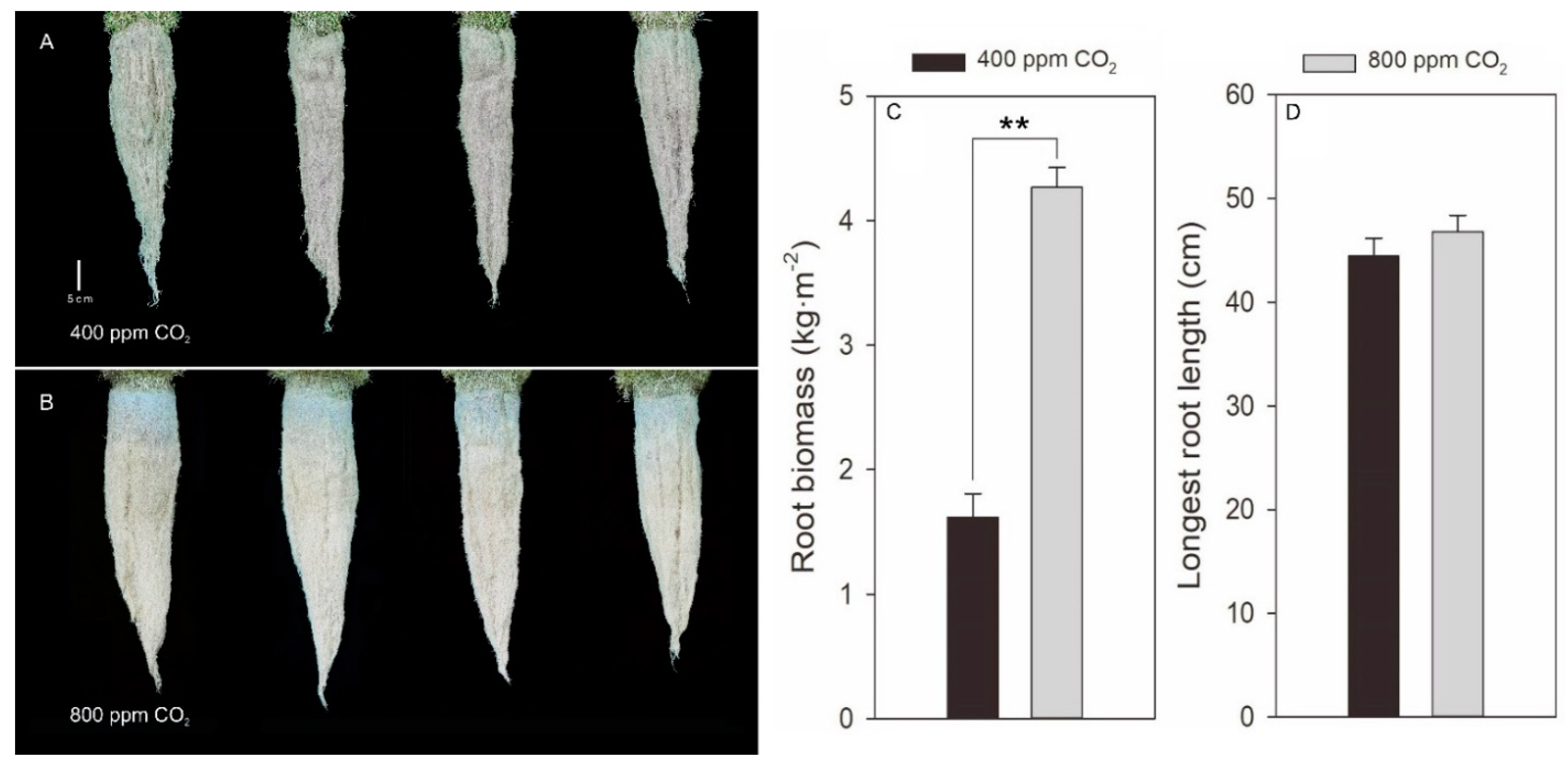
2.1. Effects of Elevated CO2 on Morphological Parameters in Creeping Bentgrass
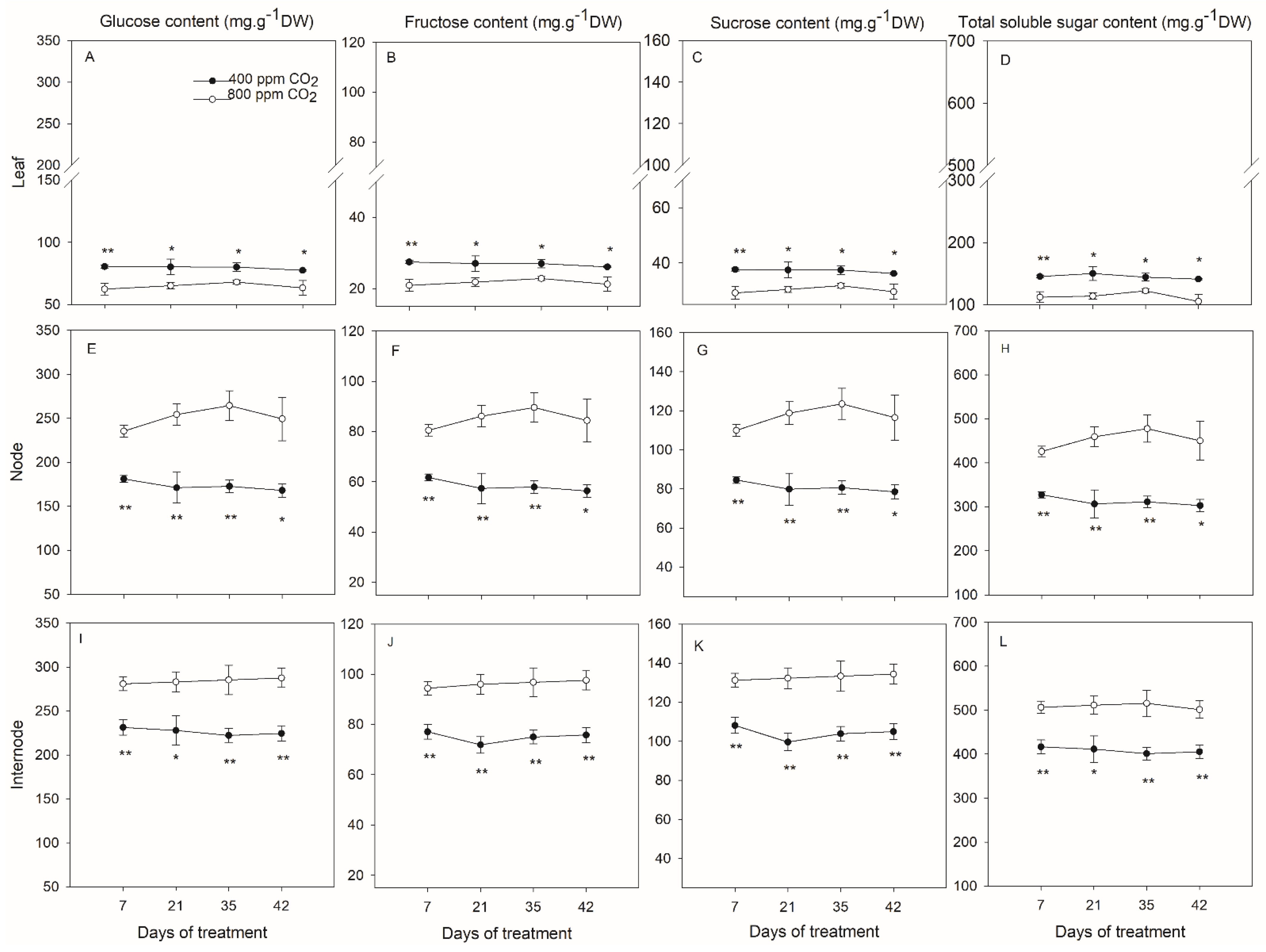
2.2. Effects of Elevated CO2 on Shoot Soluble Sugars
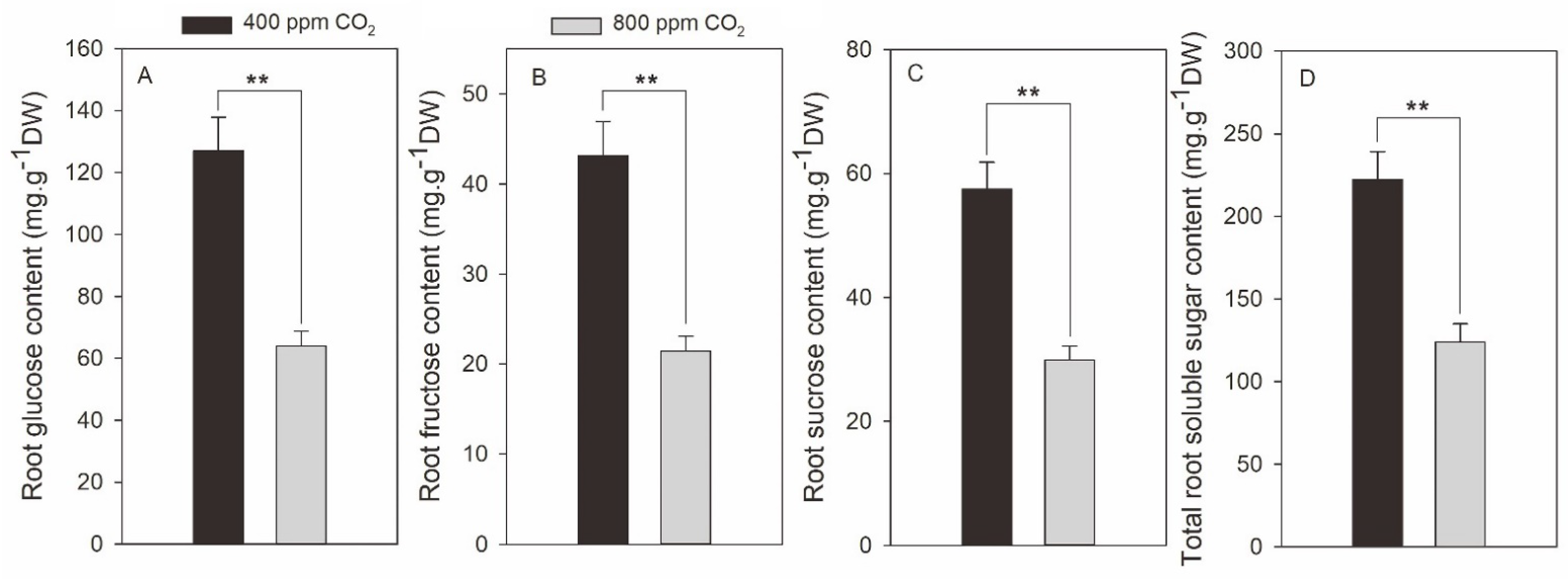
2.3. Effects of Elevated CO2 on Root Soluble Sugars
2.4. Effects of Elevated CO2 on Endogenous Hormone Content
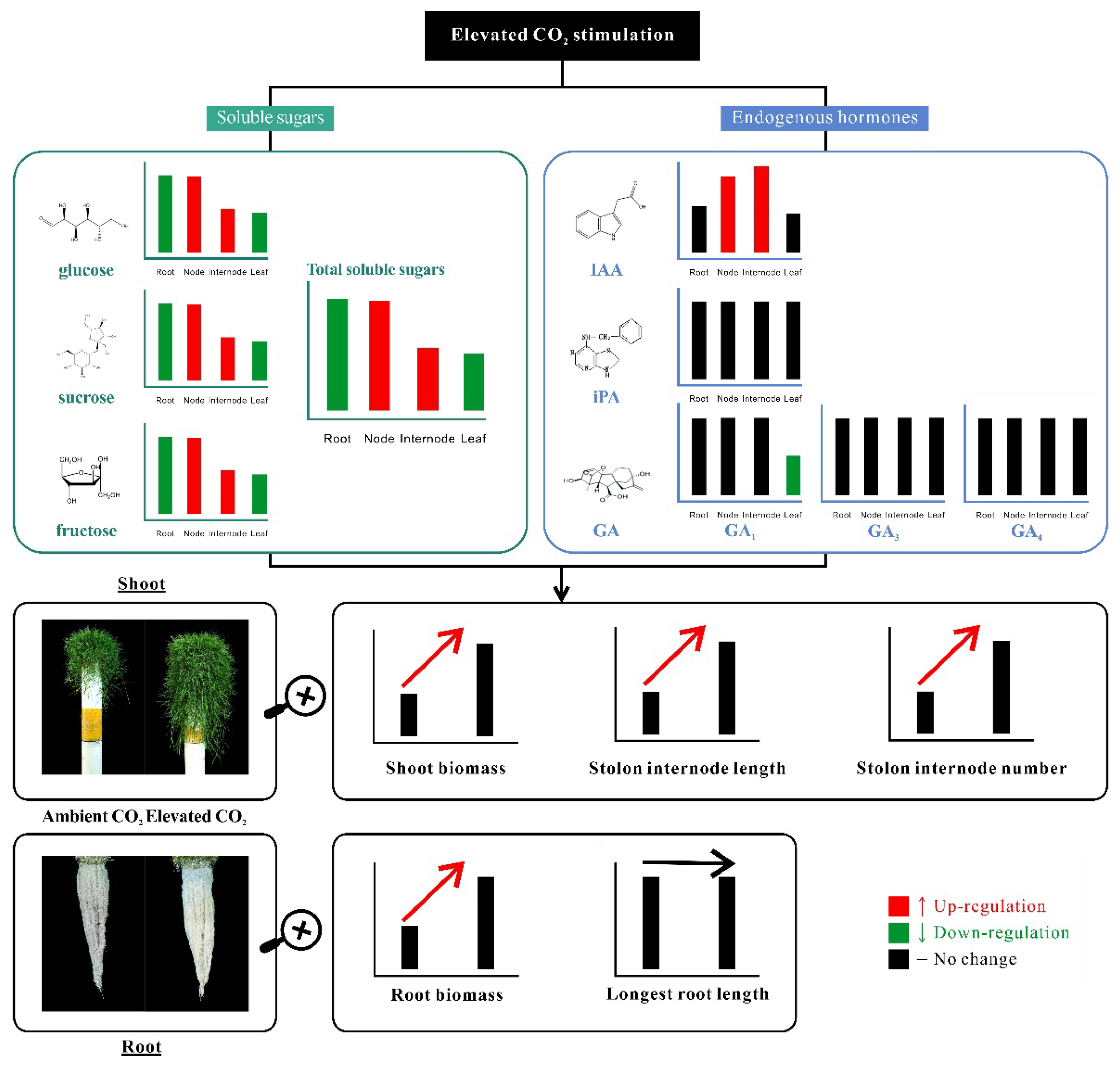
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Experimental Design and Treatments
4.3. Growth and Physiological Measurements
4.4. Sugar Extraction and Quantification
4.5. Hormone Measurement
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savini, G.; Giorgi, V.; Scarano, E.; Neri, D. Strawberry plant relationship through the stolon. Physiol. Plant. 2008, 134, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fan, N.; Zhuang, L.; Yu, J.; Huang, B. Enhanced stolon growth and metabolic adjustment in creeping bentgrass with elevated CO2 concentration. Environ. Exp. Bot. 2018, 155, 87–97. [Google Scholar] [CrossRef]
- Pitelka, L.E.; Ashmun, J.W. Physiology and integration of ramets in clonal plants. In Population Biology and Evolution of Clonal Organisms; Jackson, J.B.C., Ed.; Yale University Press: New Haven, CT, USA, 1985. [Google Scholar]
- Stuefer, J.F.; Huber, H. The role of stolon internodes for ramet survival after clone fragmentation in Potentilla anserina. Ecol. Lett. 1999, 2, 135–139. [Google Scholar] [CrossRef]
- Fahrig, L.; Coffin, D.P.; Lauenroth, W.K.; Shugart, H.H. The advantage of long-distance clonal spreading in highly disturbed habitats. Evol. Ecol. 1994, 8, 172–187. [Google Scholar] [CrossRef]
- Song, Y.B.; Yu, F.H.; Li, J.M.; Keser, L.H.; Fischer, M.; Dong, M.; Kleunen, M.V. Plant invasiveness is not linked to the capacity of regeneration from small fragments: An experimental test with 39 stoloniferous species. Biol. Invasions 2013, 15, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Hanna, W.W. Centipedegrass—Diversity and vulnerability. Crop Sci. 1995, 35, 332–334. [Google Scholar] [CrossRef]
- Pessarakli, M. Handbook of Turfgrass Management and Physiology; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Turgeon, A. Turfgrass Management, 8th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Zheng, Y.; Li, F.; Hao, L.; Yu, J.; Guo, L.; Zhou, H.; Ma, C.; Zhang, X.; Xu, M. Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrated and nitrogen content of soybean. BMC Plant Biol. 2019, 19, 255. [Google Scholar] [CrossRef]
- Pedersen, J.; Santos, F.D.; Vuuren, D.V.; Gupta, J.; Swart, R. An assessment of the performance of scenarios against historical global emissions for IPCC reports. Global Environ. Chang. 2021, 66, 102199. [Google Scholar] [CrossRef]
- Huang, B.; Xu, Y. Cellular and molecular mechanisms for elevated CO2-regulation of plant growth and stress adaptation. Crop Sci. 2015, 55, 1405–1424. [Google Scholar] [CrossRef]
- Yu, J.J.; Chen, L.H.; Xu, M.; Huang, B.R. Effects of elevated CO2 on physiological responses of tall fescue to elevated temperature, drought stress, and the combined stresses. Crop Sci. 2012, 52, 1848–1858. [Google Scholar] [CrossRef]
- Yu, J.J.; Yang, Z.M.; Jespersen, D.; Huang, B.R. Photosynthesis and protein metabolism associated with elevated CO2-mitigation of heat stress damages in tall fescue. Environ. Exp. Bot. 2014, 99, 75–85. [Google Scholar] [CrossRef]
- Yu, J.; Fan, N.; Li, R.; Zhuang, L.; Xu, Q.; Huang, B. Proteomic profiling for metabolic pathways involved in interactive effects of elevated carbon dioxide and nitrogen on leaf growth in a perennial grass species. J. Proteome Res. 2019, 18, 2446–2457. [Google Scholar] [CrossRef]
- Chen, Y.J.; Yu, J.J.; Huang, B.R. Effects of elevated CO2 concentration on water relations and photosynthetic responses to drought stress and recovery during rewatering in tall fescue. J. Am. Soc. Hortic. Sci. 2015, 140, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.L.; Yu, J.J.; Huang, B. Elevated CO2-mitigation of high temperature stress associated with maintenance of positive carbon balance and carbohydrate accumulation in kentucky bluegrass. PLoS ONE 2014, 9, e89725. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.; Yang, Z.; Fan, N.; Yu, J.; Huang, B. Metabolomic changes associated with elevated CO2-regulation of salt tolerance in Kentucky bluegrass. Environ. Exp. Bot. 2019, 165, 129–138. [Google Scholar] [CrossRef]
- Yu, J.; Li, R.; Fan, N.; Yang, Z.; Huang, B. Metabolic pathways involved in carbon dioxide enhanced heat tolerance in bermudagrass. Front. Plant Sci. 2017, 8, 1506. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Sun, L.; Fan, N.; Yang, Z.; Huang, B. Physiological factors involved in positive effects of elevated carbon dioxide concentration on bermudagrass tolerance to salinity stress. Environ. Exp. Bot. 2015, 115, 20–27. [Google Scholar] [CrossRef]
- Burgess, P.; Huang, B. Growth and physiological responses of creeping bentgrass (Agrostis stolonifera) to elevated carbon dioxide concentrations. Hortic. Res. 2014, 1, 14021. [Google Scholar] [CrossRef] [Green Version]
- Kinmonth-Schultz, H.; Kim, S.H. Carbon gain, allocation, and storage in rhizomes in response to elevated CO2 and fertilization in an invasive perennial C3 grass, Phalaris arundinacea. Func. Plant Biol. 2011, 38, 797–807. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An emerging regulator of tuber and storage root development. Plant Sci. 2021, 306, 110854. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Wu, X.; Chen, F.; Yuan, L.; Mi, G. Gibberellins synthesis is involved in the reduction of cell flux and elemental growth rate in maize leaf under low nitrogen supply. Environ. Exp. Bot. 2018, 150, 198–208. [Google Scholar] [CrossRef]
- Liu, D.; Xu, M.; Hu, Y.; Wang, R.; Tong, J.; Xiao, L. Dynamic changes of key plant hormones during potato tuber development. Mol. Plant Breed. 2019, 6, 1998–2003. [Google Scholar]
- Wu, W.; Kang, D.; Kang, X.; Wei, H. The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef]
- Burgess, P.; Chapman, C.; Zhang, X.; Huang, B. Stimulation of growth and alteration of hormones by elevated carbon dioxide for creeping bentgrass exposed to drought. Crop Sci. 2019, 59, 1672–1680. [Google Scholar] [CrossRef]
- Elgersma, A.; Li, F. Effects of cultivar and cutting frequency on dynamics of stolon growth and leaf appearance in white clover in mixed swards. Grass Forage Sci. 1997, 52, 370–380. [Google Scholar] [CrossRef]
- Tworkoski, T.J.; Benassi, T.E.; Takeda, F. The effect of nitrogen on stolon and ramet growth in four genotypes of Fragaria chiloensis L. Sci. Hortic. 2001, 88, 106. [Google Scholar] [CrossRef]
- O’Neal, S.W.; Prince, J.S. Seasonal effects of light, temperature, nutrient concentration and salinity on the physiology and growth of Caulerpa paspaloides (Chlorophyceae). Marine Biol. 1988, 97, 17–24. [Google Scholar] [CrossRef]
- Dong, B.C.; Yu, G.L.; Wei, G.; Zhang, M.X.; Dong, M.; Yu, F.H. How internode length, position and presence of leaves affect survival and growth of Alternanthera philoxeroides after fragmentation? Ecol. Evol. 2010, 24, 1447–1461. [Google Scholar] [CrossRef]
- Dong, B.; Liu, R.; Zhang, Q.; LI, H.; Zhang, M.; Lei, G.; Yu, F. Burial depth and stolon internode length independently affect survival of small clonal fragments. PLoS ONE 2011, 6, e23942. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.; Burgess, P.; Huang, B. Effects of elevated carbon dioxide on drought tolerance and post-drought recovery involving rhizome growth in kentucky bluegrass (Poa pratensis L.). Crop Sci. 2021, 61, 3219–3231. [Google Scholar] [CrossRef]
- Lawson, A.R.; Kelly, K.B.; Sale, P. Defoliation frequency and cultivar effects on the storage and utilisation of stolon and root reserves in white clover. Aust. J. Agr. Res. 2000, 51, 1039–1046. [Google Scholar] [CrossRef]
- Goulas, E.; Le Dily, F.; Teissedre, L.; Corbel, G.; Robin, C.; Christophe, R.; Ourry, A. Vegetative storage proteins in white clover (Trifolium repens L.): Quantitative and qualitative features. Ann. Bot. 2001, 88, 789–795. [Google Scholar]
- Huang, Q.; Shen, Y.; Li, X.; Zhang, G.; Huang, D.; Fan, Z. Regeneration capacity of the small clonal fragments of the invasive Mikania micrantha H.B.K.: Effects of the stolon thickness, internode length and presence of leaves. Weed Biol. Manag. 2014, 15, 70–77. [Google Scholar] [CrossRef]
- Urbonaviciute, A.; Samuoliene, G.; Sakalauskaite, J.; Duchovskis, P.; Brazaityte, A.; Siksnianiene, J.B.; Ulinskaite, R.; Sabajeviene, G.; Baranauskis, K. The effect of elevated CO2 concentrations on leaf carbohydrate, chlorophyll contents and photosynthesis in radish. Pol. J. Environ. Stud. 2006, 15, 921–925. [Google Scholar]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in Hordeum vulgare. Photosynth. Res. 2012, 111, 269–283. [Google Scholar] [CrossRef]
- Faria, T.; Wilkins, D.; Besford, R.T.; Vaz, M.; Pereira, J.S.; Chaves, M.M. Growth at elevated CO2 leads to down-regulation of photosynthesis and altered response to high temperature in Quercus suber L. seedlings. J. Exp. Bot. 1996, 47, 1755–1761. [Google Scholar] [CrossRef] [Green Version]
- Jach, M.E.; Ceulemans, R. Effects of elevated atmospheric CO2 on phenology, growth and crown structure of Scots pine (Pinus sylvestris) seedlings after two years of exposure in the field. Tree Physiol. 1999, 19, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shen, Y.; Huang, Q.; Fan, Z.; Huang, D. Regeneration capacity of small clonal fragments of the invasive Mikania micrantha H.B.K.: Effects of burial depth and stolon internode length. PLoS ONE 2013, 8, e84657. [Google Scholar] [CrossRef]
- Zhou, Y.; Lambrides, C.; Fukai, S. Associations between drought resistance, regrowth and quality in a perennial C4 grass. Eur. J. Agron. 2015, 65, 1–9. [Google Scholar] [CrossRef]
- He, L.; Xiao, X.; Zhang, X.; Jin, Y.; Pu, Z.; Lei, N.; He, X.; Chen, J. Clonal fragments of stoloniferous invasive plants benefit more from stolon storage than their congeneric native species. Flora 2021, 281, 151877. [Google Scholar] [CrossRef]
- Fry, J.D.; Lang, N.S.; Clifton, R.; Maier, F.P. Freezing tolerance and carbohydrate content of low-temperature-acclimated and nonacclimated centipedegrass. Crop Sci. 1993, 33, 1051–1055. [Google Scholar] [CrossRef]
- Patton, A.J.; Volenec, J.J.; Reicher, Z.J. Stolon growth and dry matter partitioning explain differences in zoysiagrass establishment rates. Crop Sci. 2007, 47, 1237–1245. [Google Scholar] [CrossRef]
- Chapman, C.; Burgess, P.; Huang, B. Responses to elevated carbon dioxide for postdrought recovery of turfgrass species differing in growth characteristics. Crop Sci. 2021, 61, 4436–4446. [Google Scholar] [CrossRef]
- Burgess, P.; Huang, B. Root protein metabolism in association with improved root growth and drought tolerance by elevated carbon dioxide in creeping bentgrass. Field Crop Res. 2014, 165, 80–91. [Google Scholar] [CrossRef]
- Li, T.; Di, Z.; Han, X.; Yang, X. Elevated CO2 improves root growth and cadmium accumulation in the hyperaccumulator Sedum alfredii. Plant Soil 2012, 354, 325–334. [Google Scholar] [CrossRef]
- Hiltpold, I.; Moore, B.; Johnson, S. Elevated atmospheric carbon dioxide concentrations alter root morphology and reduce the effectiveness of entomopathogenic nematodes. Plant Soil 2020, 477, 29–38. [Google Scholar] [CrossRef]
- Da Cruz, G.S.; Audus, L.J. Studies of hormone-directed transport in decapitated stolons of Saxifraga sarmentosa. Ann. Bot. 1978, 42, 1009–1027. [Google Scholar] [CrossRef]
- Braun, J.; Kender, W. Correlative bud inhibition and growth habit of the strawberry as influenced by application of gibberellic acid, cytokinin, and chilling during short daylength. J. Am. Soc. Hortic. Sci. 1985, 110, 28–34. [Google Scholar]
- Tyagi, S.; Kumar, S. Exogenous supply of IAA, GA and cytokinin to salinity stressed seeds of chickpea improve the seed germination and seedling growth. Int. J. Plant Sci. 2016, 11, 88–92. [Google Scholar] [CrossRef]
- Kotov, A.A.; Kotova, L.M.; Romanov, G.A. Signaling network regulating plant branching: Recent advances and new challenges. Plant Sci. 2021, 307, 110880. [Google Scholar] [CrossRef]
- Adams, R.E.; Kerber, K.; Pfister, E.W.; Weiler, E.W. Studies on the action of the new growth retardant CGA 163′935 (Cimectacarb). In Progress in Plant Growth Regulation; Karssen, C.M., van Loon, L.C., Vreugdenhil, D., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; pp. 818–827. [Google Scholar]
- Reicher, Z.J.; Dernoeden, P.H.; Richmond, D.S. Insecticides, fungicides, herbicides, and growth regulators used in turfgrass systems. In Turfgrass: Biology, Use, and Management; Stier, J.C., Horgan, B.P., Bonos, S.A., Eds.; American Society of Agronomy Soil Science Society of America Crop Science Society of America, Inc.: Madison, WI, USA, 2013. [Google Scholar]
- McCann, S.E.; Huang, B. Effects of trinexapac-ethyl foliar application on creeping bentgrass responses to combined drought and heat stress. Crop Sci. 2007, 47, 2121–2128. [Google Scholar] [CrossRef]
- McCann, S.E.; Huang, B. Drought responses of Kentucky bluegrass and creeping bentgrass as affected by abscisic acid and trinexapac-ethyl. J. Am. Soc. Hortic. Sci. 2008, 133, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plans without Soil; California Agricultural Experiment Station; The College of Agriculture University of California Berkeley: Davis, CA, USA, 1950; Volume 347, pp. 1–32. [Google Scholar]
- Liu, N.; Shen, Y.; Huang, B. Osmoregulants involved in osmotic adjustment for differential drought tolerance in different bentgrass genotypes. J. Am. Soc. Hortic. Sci. 2015, 140, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]

| Total Stolon Length | Internode Length | Internode Number | Shoot Biomass | Root Biomass | Longest Root Length | |
|---|---|---|---|---|---|---|
| Total stolon length | 1 | 0.938 ** | 0.973 ** | 0.693 * | 0.939 ** | 0.121 |
| Internode length | 0.938 ** | 1 | 0.883 ** | 0.686 * | 0.831 ** | 0.121 |
| Internode number | 0.973 ** | 0.883 ** | 1 | 0.587 | 0.920 ** | 0.138 |
| Shoot biomass | 0.693 * | 0.686 * | 0.587 | 1 | 0.811 ** | 0.383 |
| Root biomass | 0.939 ** | 0.831 ** | 0.920 ** | 0.811 ** | 1 | 0.186 |
| Longest root | 0.121 | 0.121 | 0.138 | 0.383 | 0.186 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Li, M.; Li, Q.; Wang, R.; Li, R.; Yang, Z. Reallocation of Soluble Sugars and IAA Regulation in Association with Enhanced Stolon Growth by Elevated CO2 in Creeping Bentgrass. Plants 2022, 11, 1500. https://doi.org/10.3390/plants11111500
Yu J, Li M, Li Q, Wang R, Li R, Yang Z. Reallocation of Soluble Sugars and IAA Regulation in Association with Enhanced Stolon Growth by Elevated CO2 in Creeping Bentgrass. Plants. 2022; 11(11):1500. https://doi.org/10.3390/plants11111500
Chicago/Turabian StyleYu, Jingjin, Meng Li, Qiuguo Li, Ruying Wang, Ruonan Li, and Zhimin Yang. 2022. "Reallocation of Soluble Sugars and IAA Regulation in Association with Enhanced Stolon Growth by Elevated CO2 in Creeping Bentgrass" Plants 11, no. 11: 1500. https://doi.org/10.3390/plants11111500
APA StyleYu, J., Li, M., Li, Q., Wang, R., Li, R., & Yang, Z. (2022). Reallocation of Soluble Sugars and IAA Regulation in Association with Enhanced Stolon Growth by Elevated CO2 in Creeping Bentgrass. Plants, 11(11), 1500. https://doi.org/10.3390/plants11111500








