Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities
Abstract
1. Introduction
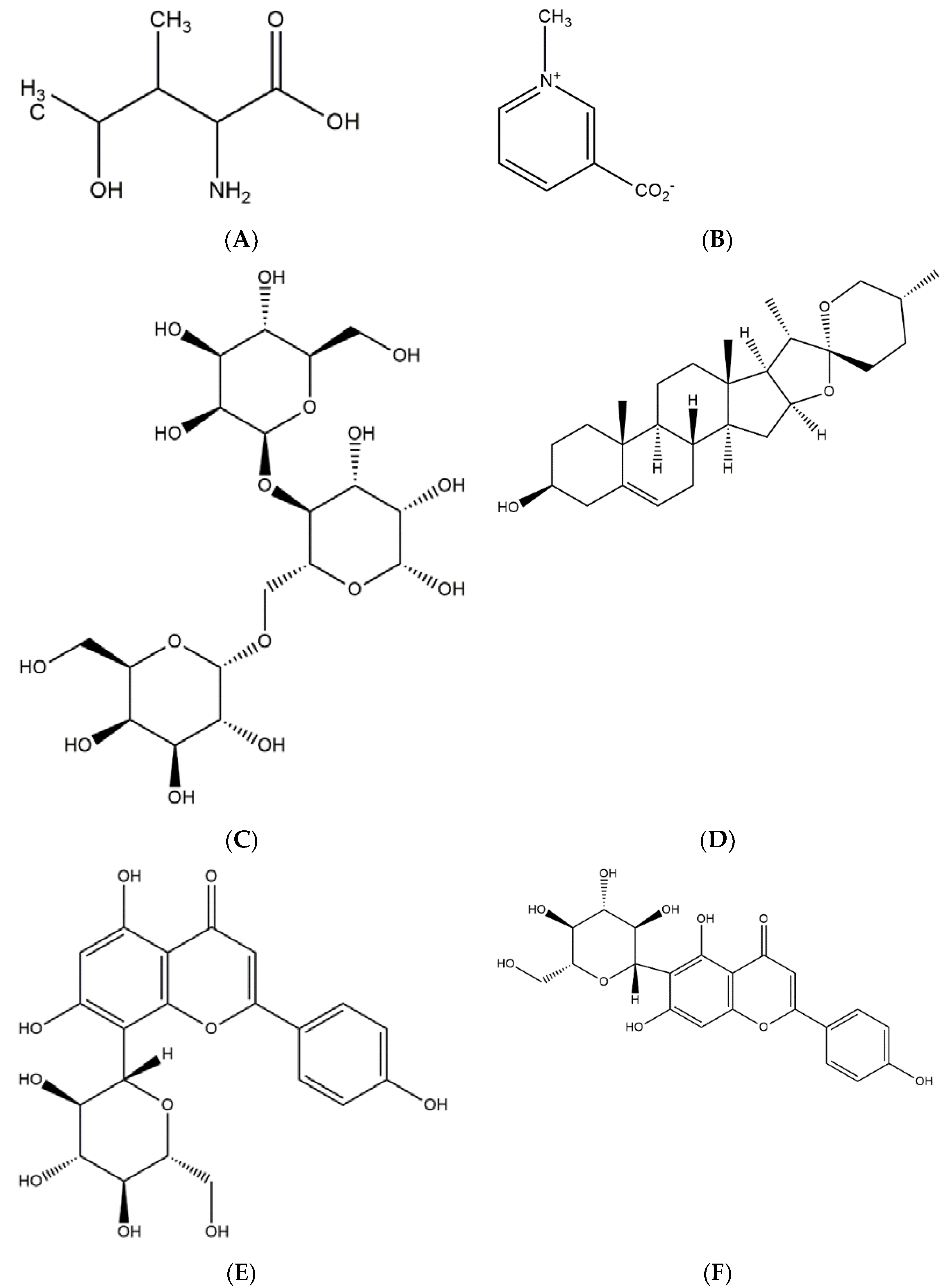
2. Physical and Chemical Properties of T. foenum-graecum
3. Traditional Uses of T. foenum-graecum
4. Pharmacological Uses of T. foenum-graecum
4.1. Hypoglycaemic Effects
4.2. Hypocholesterolemic Effects
4.3. Immunomodulatory
4.4. Antimicrobial Activity
4.5. Anticancer Activity
4.6. Antioxidant Property
4.7. Hormonal Effects
4.8. Regulation of Fat Metabolism
4.9. Neuroprotective Effect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, L.-B.; Liu, X.-N.; Liu, H.-M.; Pang, H.-L.; Qin, G.-Y. Extraction of Fenugreek (Trigonella foenum-graceum L.) Seed Oil Using Subcritical Butane: Characterization and Process Optimization. Molecules 2017, 22, 228. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.S.A.A.; Wankhade, M.V.; Saifuddin, M.S.S.K.; Sudarshan, M.A.R. Physico-Chemical Properties of Fenugreek (Trigonella foenum-graceum L.) Seeds. Int. J. Eng. Res. 2015, V4, 68–70. [Google Scholar] [CrossRef]
- Meghwal, M.; Goswami, T.K. A Review on the Functional Properties, Nutritional Content, Medicinal Utilization and Potential Application of Fenugreek. J. Food Process. Technol. 2012, 3, 1–10. [Google Scholar] [CrossRef]
- Mawahib, E.; Ammar, M.; Badr Eldin, A. Antimicrobial Activities of Phytochemical Screening of Callus and Seeds Extracts of Fenugreek (Trigonella foenum-graceum). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 147–157. [Google Scholar]
- Srinivasan, K. Fenugreek (Trigonella foenum-graceum): A Review of Health Beneficial Physiological Effects. Food Rev. Int. 2006, 22, 203–224. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Singh, V.; Garg, A. Availability of essential trace elements in Indian cereals, vegetables and spices using INAA and the contribution of spices to daily dietary intake. Food Chem. 2006, 94, 81–89. [Google Scholar] [CrossRef]
- Sowmya, P.; Rajyalakshmi, P. Hypocholesterolemic effect of germinated fenugreek seeds in human subjects. Mater. Veg. 1999, 53, 359–365. [Google Scholar] [CrossRef]
- Singh, P.; Bajpai, V.; Gond, V.; Kumar, A.; Tadigoppula, N.; Kumar, B. Determination of Bioactive Compounds of Fenugreek (Trigonella foenum-graceum) Seeds Using LC-MS Techniques. In Legume Genomics; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2107, pp. 377–393. [Google Scholar]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graceum) Crude Seeds by HPLC–DAD–ESI/MS Analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. Extraction, characterization and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed oil. Mater. Sci. Energy Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Bano, D.; Tabassum, H.; Ahmad, A.; Mabood, A.; Ahmad, I.Z. The medicinal significance of the bioactive compounds of Trigonella foenum-graceum: A review. Inter. J. Res. Ayurveda Pharma. 2016, 7, 84–91. [Google Scholar] [CrossRef]
- Khole, S.; Chatterjee, S.; Variyar, P.; Sharma, A.; Devasagayam, T.; Ghaskadbi, S. Bioactive constituents of germinated fenugreek seeds with strong antioxidant potential. J. Funct. Foods 2014, 6, 270–279. [Google Scholar] [CrossRef]
- Dharajiya, D.; Jasani, H.; Khatrani, T.; Kapuria, M.; Pachchigar, K.; Patel, P. Evaluation of Antibacteria and Antifungal Activity of Fenugreek (Trigonella foenum-graceum) Extracts. Int. J. Pharm. Pharm. Sci. 2016, 8, 212–217. [Google Scholar]
- Premanath, R.; Sudisha, J.; Devi, N.L.; Aradhya, S. Antibacterial and Anti-oxidant Activities of Fenugreek (Trigonella foenum graecum L.) Leaves. Res. J. Med. Plant 2011, 5, 695–705. [Google Scholar] [CrossRef][Green Version]
- Yadav, R.; Kaushik, R. A Study of Phytochemical Constituents and Pharmacological Actions of T. foenum-graecum: A Review. Int. J. Pharm. Technol. 2011, 3, 1022–1028. [Google Scholar]
- Raghuram, T.C.; Sharma, R.D.; Sivakumar, B.; Sahay, B.K. Effect of fenugreek seeds on intravenous glucose disposition in non-insulin dependent diabetic patients. Phytotherapy Res. 1994, 8, 83–86. [Google Scholar] [CrossRef]
- Snehlata, H.; Payal, D. Fenugreek (Trigonella foenum-graceum L): An overview. Int. J. Curr. Pharm. 2012, 2, 169–187. [Google Scholar]
- Abdel-Barry, J.A.; Abdel-Hassan, I.A.; Al-Hakiem, M.H. Hypoglycaemic and antihyperglycaemic effects of Trigonella foenum-graceum leaf in normal and alloxan induced diabetic rats. J. Ethnopharmacol. 1997, 58, 149–155. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R.; Lal, B. Effect of Trigonella foenum-graceum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: A double blind placebo controlled study. J. Assoc. Physicians India 2001, 49, 1057–1061. [Google Scholar]
- Kannappan, S.; Anuradha, C.V. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin metformin in a rat model. Indian J. Med. Res. 2009, 129, 401–408. [Google Scholar]
- Hannan, J.M.A.; Ali, L.; Rokeya, B.; Khaleque, J.; Akhter, M.; Flatt, P.; Abdel-Wahab, Y.H.A. Soluble dietary fibre fraction of Trigonella foenum-graceum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 2007, 97, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, N.S.; Morsi, A.; Ahmed, Y.M.; Hassan, H.M.; AboulMagd, A.M. Ecological HPLC method for analyzing an antidiabetic drug in real rat plasma samples and studying the effects of concurrently administered fenugreek extract on its pharmacokinetics. RSC Adv. 2021, 11, 4740–4750. [Google Scholar] [CrossRef]
- Muraki, E.; Hayashi, Y.; Chiba, H.; Tsunoda, N.; Kasono, K. Dose-dependent effects, safety and tolerability of fenugreek in diet-induced metabolic disorders in rats. Lipids Health Dis. 2011, 10, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Madar, Z. The effect of an ethanol extract derived from fenugreek (Trigonella foenum-graceum) on bile acid absorption and cholesterol levels in rats. Br. J. Nutr. 1993, 69, 277–287. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, P.; Rani, A. Antimicrobial Activity of Trigonella foenum-graceum L. (Fenugreek). Eur. J. Exp. Biol. 2017, 7. [Google Scholar] [CrossRef]
- Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella foenum-graceum L.) on Hyperlipidemia in Diabetic Patients. J. Diabetes Res. 2019, 2019, 8507453. [Google Scholar] [CrossRef] [PubMed]
- Heshmat-Ghahdarijani, K.; Mashayekhiasl, N.; Amerizadeh, A.; Jervekani, Z.T.; Sadeghi, M. Effect of fenugreek consumption on serum lipid profile: A systematic review and meta-analysis. Phytotherapy Res. 2020, 34, 2230–2245. [Google Scholar] [CrossRef]
- Bin-Hafeez, B.; Haque, R.; Parvez, S.; Pandey, S.; Sayeed, I.; Raisuddin, S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int. Immunopharmacol. 2003, 3, 257–265. [Google Scholar] [CrossRef]
- Tripathi, S.; Maurya, A.; Kaul, A.; Kahrana, M.; Sahu, R. Immunomodulatory property of ethanolic extract of Trigonella foenum-graeceum leaves on mice. Der Pharmacia Lettre 2012, 4, 708–713. [Google Scholar]
- Hassan, N.; Withycombe, C.; Ahluwalia, M.; Thomas, A.; Morris, K. A methanolic extract of Trigonella foenum-graceum (fenugreek) seeds regulates markers of macrophage polarization. Funct. Foods Health Dis. 2015, 5, 417–426. [Google Scholar] [CrossRef][Green Version]
- Shesharao, M.K.P.; Rao, M.L.S.; Sathyanarayana, N.; Shridhar, S.; Byregowda, S.; Ramachandra, G. Evaluation of immunomodulatory cells CD4+and CD8+and their ratio using alcoholic seed extract of Trigonella foenum graecum and alcoholic leaves extract of Coccinia indica by flow cytometry in streptozotocin-induced diabetic rats. J. Pharmacogn. Phytochem. 2020, 9, 2943–2947. [Google Scholar]
- Haouala, R.; Hawala, S.; El-Ayeb, A.; Khanfir, R.; Boughanmi, N. Aqueous and organic extracts of Trigonella foenum-graceum L. inhibit the mycelia growth of fungi. J. Environ. Sci. 2008, 20, 1453–1457. [Google Scholar] [CrossRef]
- Chalghoumi, R.; Mabrouki, S.; Abdouli, H.; Line, J. Antibacterial Activity of Fenugreek Seeds (Trigonella foenum-graceum) Crude Extracts Against a Rabbit Escherichia coli Isolate. Acad. J. Microbiol. 2016, 3, 139–144. [Google Scholar]
- Shabbeer, S.; Sobolewski, M.; Anchoori, R.K.; Kachhap, S.; Hidalgo, M.; Jimeno, A.; Davidson, N.E.; Carducci, M.; Khan, S.R. Fenugreek: A naturally occurring edible spice as an anticancer agent. Cancer Biol. Ther. 2009, 8, 272–278. [Google Scholar] [CrossRef]
- AlSemari, A.; Alkhodairy, F.; Aldakan, A.; Al-Mohanna, M.; Bahoush, E.; Shinwari, Z.; Alaiya, A. The selective cytotoxic anti-cancer properties and proteomic analysis of Trigonella foenum-graceum. BMC Complement. Altern. Med. 2014, 14, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Singh, S.K.; Mathur, A. In vitro cytotoxicity of Calotropis procera and Trigonella foenum-graceum against human cancer cell lines. J. Chem. Pharm. Res. 2010, 2, 861–865. [Google Scholar]
- Sebastian, K.; Thampan, R.V. Differential effects of soybean and fenugreek extracts on the growth of MCF-7 cells. Chem. Interact. 2007, 170, 135–143. [Google Scholar] [CrossRef]
- Alrumaihi, F.A.; Khan, M.A.; Allemailem, K.S.; Alsahli, M.A.; Almatroudi, A.; Younus, H.; Alsuhaibani, S.A.; Algahtani, M.; Khan, A. Methanolic Fenugreek Seed Extract Induces p53-Dependent Mitotic Catastrophe in Breast Cancer Cells, Leading to Apoptosis. J. Inflamm. Res. 2021, 14, 1511–1535. [Google Scholar] [CrossRef]
- Almalki, D.A.; Naguib, D.M. Anticancer Activity of Aqueous Fenugreek Seed Extract Against Pancreatic Cancer, Histological Evidence. J. Gastrointest. Cancer 2021, 1–4. [Google Scholar] [CrossRef]
- Bukhari, S.; Bhanger, M.; Memon, S. Antioxidant activity from the extract of fenugreek seeds. Pak. J. Anal. Environ. Chem. 2008, 9, 78–83. [Google Scholar]
- Bhatia, K.; Kaur, M.; Atif, F.; Ali, M.; Rehman, H.; Rahman, S.; Raisuddin, S. Aqueous extract of Trigonella foenum-graceum L. ameliorates additive urotoxicity of buthionine sulfoximine and cyclophosphamide in mice. Food Chem. Toxicol. 2006, 44, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, M.; Mandal, M.; Murthy, S. Comparative analysis of antioxidant and antibacterial properties of Aegle marmelos, Coriandrum sativum and Trigonella foenum graecum. Acta Biol. Indica 2012, 1, 105–108. [Google Scholar]
- Tewari, D.; Jóźwik, A.; Łysek-Gładysińska, M.; Grzybek, W.; Adamus-Białek, W.; Bicki, J.; Strzałkowska, N.; Kamińska, A.; Horbańczuk, O.; Atanasov, A. Fenugreek (Trigonella foenum-graceum L.) Seeds Dietary Supplementation Regulates Liver Antioxidant Defense Systems in Aging Mice. Nutrition 2020, 12, 2552. [Google Scholar] [CrossRef]
- Proctor, M.; Farquhar, C. Diagnosis and management of dysmenorrhoea. Br. Med. J. 2006, 332, 1134–1138. [Google Scholar] [CrossRef]
- Younesy, S.; Amiraliakbari, S.; Esmaeili, S.; Alavimajd, H.; Nouraei, S. Effects of Fenugreek Seed on the Severity and Systemic Symptoms of Dysmenorrhea. J. Reprod. Infertil. 2014, 15, 41–48. [Google Scholar]
- Inanmdar, W.; Sultana, A.; Mubeen, U.; Rahman, K. Clinical efficacy of Trigonella foenum graecum (Fenugreek) and dry cupping therapy on intensity of pain in patients with primary dysmenorrhea. Chin. J. Integr. Med. 2016, 1–8. [Google Scholar] [CrossRef]
- Anjaneyulu, K.; Bhat, K.M.; Srinivasa, S.R.; Devkar, R.A.; Henry, T. Beneficial Role of Hydro-alcoholic Seed Extract of Trigonella foenum graecum on Bone Structure and Strength in Menopause Induced Osteopenia. Ethiop. J. Health Sci. 2018, 28, 787–794. [Google Scholar] [CrossRef]
- Sevrin, T.; Alexandre-Gouabau, M.-C.; Castellano, B.; Aguesse, A.; Ouguerram, K.; Ngyuen, P.; Darmaun, D.; Boquien, C.-Y.; Gouabau, A. Impact of Fenugreek on Milk Production in Rodent Models of Lactation Challenge. Nutrition 2019, 11, 2571. [Google Scholar] [CrossRef]
- Sevrin, T.; Boquien, C.-Y.; Gandon, A.; Grit, I.; De Coppet, P.; Darmaun, D.; Alexandre-Gouabau, M.-C. Fenugreek Stimulates the Expression of Genes Involved in Milk Synthesis and Milk Flow through Modulation of Insulin/GH/IGF-1 Axis and Oxytocin Secretion. Genes 2020, 11, 1208. [Google Scholar] [CrossRef]
- McCarthy, M. Genomics, Type 2 Diabetes, and Obesity. N. Engl. J. Med. 2010, 363, 2339–2350. [Google Scholar] [CrossRef]
- Kumar, P.; Bhandari, U.; Jamadagni, S. Fenugreek Seed Extract Inhibit Fat Accumulation and Ameliorates Dyslipidemia in High Fat Diet-Induced Obese Rats. BioMed Res. Int. 2014, 2014, 606021. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Sheikholeslami, M.A.; Ghafghazi, S.; Pouriran, R.; Parvardeh, S. Analgesic effect of α-terpineol on neuropathic pain induced by chronic constriction injury in rat sciatic nerve: Involvement of spinal microglial cells and inflammatory cytokines. Iran. J. Basic Med. Sci. 2019, 22, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.; Roshdy, H.; Kassem, S. The potential therapeutic role of Fenugreek saponin against Alzheimers disease: Evaluation of apoptotic and acetylcholinesterase inhibitory activities. J. Appl. Pharm. Sci. 2016, 6, 166–173. [Google Scholar] [CrossRef][Green Version]
- Foltynie, T.; Kahan, J. Parkinson’s disease: An update on pathogenesis and treatment. J. Neurol. 2013, 260, 1433–1440. [Google Scholar] [CrossRef]
| Particular | Plant part | Units | Value/100 g |
|---|---|---|---|
| Ascorbic acid | Seed | mg | 12–23 |
| Ascorbic acid | Leaves | mg | 52.0 |
| Pyridoxine | Seed | mg | 0.60 |
| Retinol | Seed | IU | 60–100 |
| Niacin | Seed | mg | 6.0 |
| β-carotene | Seed | µg | 96 |
| β-carotene | Leaves | mg | 2.3 |
| Thiamine | Seed | µg | 340 |
| Thiamine | Leaves | µg | 40 |
| Riboflavin | Seed | µg | 290 |
| Riboflavin | Leaves | µg | 310 |
| Folic acid | Seed | µg | 84 |
| Chemical Constituents of Fenugreek Seed | |
|---|---|
| Alkaloids | trimethylamine, neurin, trigonelline, choline, gentianine, carpaine betain |
| Amino acids | isoleucine, 4-hydroxyisoleucine, histidine, leucine, lysine, L-tryptophan, argenine |
| Saponins | graecunins, fenugrin B, fenugreekine, trigofoenosides A-G |
| Steroidal sapinogens | yamogenin, diosgenin, smilagenin, sarsasapogenin, tigogenin, neotigogenin, gitogenin, yuccagenin, saponaretin |
| Flavonoids | quercetin, rutin, vitexin, isovitexin |
| Fibres | gum, neutral detergent fibre |
| Lipids | triacylglycerols, diacylglycerols, monoacylglycerols, phosphatidylcholine, phosphatidylethanoamine, free fatty acids |
| Others | coumarin, lipids, vitamins, minerals. 28% mucilage; 22% proteins; 5% of a stronger-swelling, bitter fixed oil |
| Traditional Uses | Reference |
|---|---|
| Demulcent, lactation stimulant, and laxatives | [14] |
| Aid labour, period cramps, and tonic for metabolism | [15] |
| Increase milk production in breastfeeding mothers and relieve menstrual cramps, treat cellulitis, boils, and tuberculosis | [15] |
| Dysmenorrhoeal and postmenopausal symptoms | [6] |
| Topical effect in soothing irritation caused by eczema | [16] |
| Lower the amount of calcium oxalate, which is a crystal that causes the formation of kidney stones | [16] |
| Detoxifying agent in removing toxic wastes, dead cells, and trapped protein through the lymphatic system | [6] |
| Pharmaceutical Properties | Plant Part | Effects | Model | Reference |
|---|---|---|---|---|
| Hypoglycaemic | Seed | Fenugreek improves peripheral glucose utilization and tolerance | Non-insulin-dependent diabetic patients | [4] |
| Improvement in glycaemic control among patients with mild type 2 diabetes mellitus | Patients with type II diabetes | [18] | ||
| Dialyzed fenugreek seed extract was comparable to that of insulin | Alloxan-induced diabetic mice | [19] | ||
| Improves glucose control as well as decreasing insulin resistance | Double-blind placebo study | [20] | ||
| Fenugreek seed polyphenols improved insulin signalling and sensitivity compared to metformin-treated rats | Fructose-fed rats | [21] | ||
| Reduction in serum glucose and an increase in liver glycogen | Type 2 diabetic rat | [22] | ||
| Concurrent administration of fenugreek increased the bioavailability of metformin | Rat animal model | [23] | ||
| Hypocholesterolemic | Seed | Reduction in total cholesterol and low-density lipoprotein (LDL) | Hypercholesterolemia patients | [8] |
| Lower blood lipids, total cholesterol, and triglycerides without affecting the high-density lipoprotein | Patients with coronary heart disease | [8] | ||
| 18 to 20% reduction in plasma and liver cholesterol | Ethanolic fenugreek seed-extract-fed rats | [25] | ||
| Lower LDL, total cholesterol, and triglycerides | Fenugreek-seed-powder-treated newly diagnosed type II diabetes patients | [27] | ||
| Immunomodulatory | Seed | Stimulatory effect on the body and organ weight, haemagglutinin titre, quantitative haemolysis assay, late-type hypersensitivity response, plaque-forming assay, phagocytic activity, and capacity of macrophages | Swiss albino mice treated with aqueous fenugreek extract | [29] |
| Stimulation of the humoral immunity and has anti-inflammatory properties | Mice treated with ethanolic fenugreek extract | [30] | ||
| Regulates the expression of pro-inflammatory marker and immunoregulator marker M1 and M2, respectively | THP-1 macrophages | [31] | ||
| Elevation of the CD4+ and CD8+ values | Streptozotocin-induced diabetic rats | [32] | ||
| Antimicrobial | Seed, Leaves and Stem | Methanolic extract had antibacterial affect, but the aqueous extract did not show any activity. The magnitude of effects differs with the plant parts and species of microorganism, as well as the extraction solvent used | Well diffusion involving E. coli, P. aeruginosa, and B. cereus, and various fungal strains | [26,34] |
| Anticancer | Seed | Potent cytotoxic effect of whole extract compared to purified compound | Prostate cancer cell lines, breast cancer cell lines, and pancreatic cancer cell lines | [35] |
| Selective cytotoxicity effect of fenugreek extract | T cell lymphoma | [36] | ||
| Alcoholic fenugreek extract showed in vitro cytotoxicity | IMR-32, a neuroblastoma cell line, and HT29, a cancer cell line | [37] | ||
| Decrease in cell viability and early apoptotic changes | MCF-7 cells, a breast cancer cell line | [38] | ||
| Anti-metastatic effect, induced the inhibition of cell migration and increase in late apoptosis, upregulation of p53 | MCF-7 and SK-BR3 breast cancer cell lines | [39] | ||
| IC50 at 25 μg/mL, better pancreatic tissue, higher survival rate | BXPC-3 pancreatic cancer cell line and albino mice | [40] | ||
| Antioxidative | Seed | Radical scavenging activity | Biochemical assay | [41] |
| Protective effects on lipid peroxidation and enzymatic antioxidant | Cyclophosphamide-treated mice | [42] | ||
| Highest superoxide and free radical scavenging due to high phenolic compound | NBT assay and H2O2 scavenging | [43] | ||
| Positive effect in the regulation of hepatic enzymes | 12-month-old mice | [44] | ||
| Increase in antioxidant radical scavenging activity | DPPH and ABTS assays | [11] | ||
| Hormonal effects | Seed | Larger pain reduction and duration of pain decreased | Double-blind, randomized, placebo-controlled trial | [45] |
| Reduction in lower abdominal pain | Patients with primary dysmenorrhea | [47] | ||
| Improvement in bone structure and strength | Ovariectomised Wistar rats | [48] | ||
| Increase in milk production | Pregnant Sprague–Dawley rats | [49] | ||
| Modulation of the insulin/GH/IGF-1 axis, stimulation by insulin, and oxytocin secretion | Pregnant Sprague–Dawley rats | [50] | ||
| Fat metabolism | Seed | Helps to speed up weight reduction by improving digestion and metabolism | Fat-induced obese rat | [52] |
| Suppresses hunger by increasing the sense of fullness, which aids weight loss | Fat-induced obese rat | [52] | ||
| Accelerating cholesterol metabolism and reversing cholesterol transport, as well as blocking 3-hydroxy-3-methylglutaryl coenzyme A reductase in serum and liver | In vivo | [53] | ||
| Neuroprotective effects | Seed | Fenugreek-saponins-inhibited apoptosis and acetylcholinesterase (AChE) activity | Rats | [55] |
| Substantial neuroprotective impact | Aluminium-chloride-induced neurotoxicity mouse | [29] | ||
| Avoiding rotational behaviour and restoring SNC (substantia nigra compact) neuron and MDA (malondialdehyde) levels | Trigonella-fed mouse | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visuvanathan, T.; Than, L.T.L.; Stanslas, J.; Chew, S.Y.; Vellasamy, S. Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants 2022, 11, 1450. https://doi.org/10.3390/plants11111450
Visuvanathan T, Than LTL, Stanslas J, Chew SY, Vellasamy S. Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants. 2022; 11(11):1450. https://doi.org/10.3390/plants11111450
Chicago/Turabian StyleVisuvanathan, Theysshana, Leslie Thian Lung Than, Johnson Stanslas, Shu Yih Chew, and Shalini Vellasamy. 2022. "Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities" Plants 11, no. 11: 1450. https://doi.org/10.3390/plants11111450
APA StyleVisuvanathan, T., Than, L. T. L., Stanslas, J., Chew, S. Y., & Vellasamy, S. (2022). Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants, 11(11), 1450. https://doi.org/10.3390/plants11111450






