Enhanced Cd-Accumulation in Typha latifolia by Interaction with Pseudomonas rhodesiae GRC140 under Axenic Hydroponic Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plant Authentication
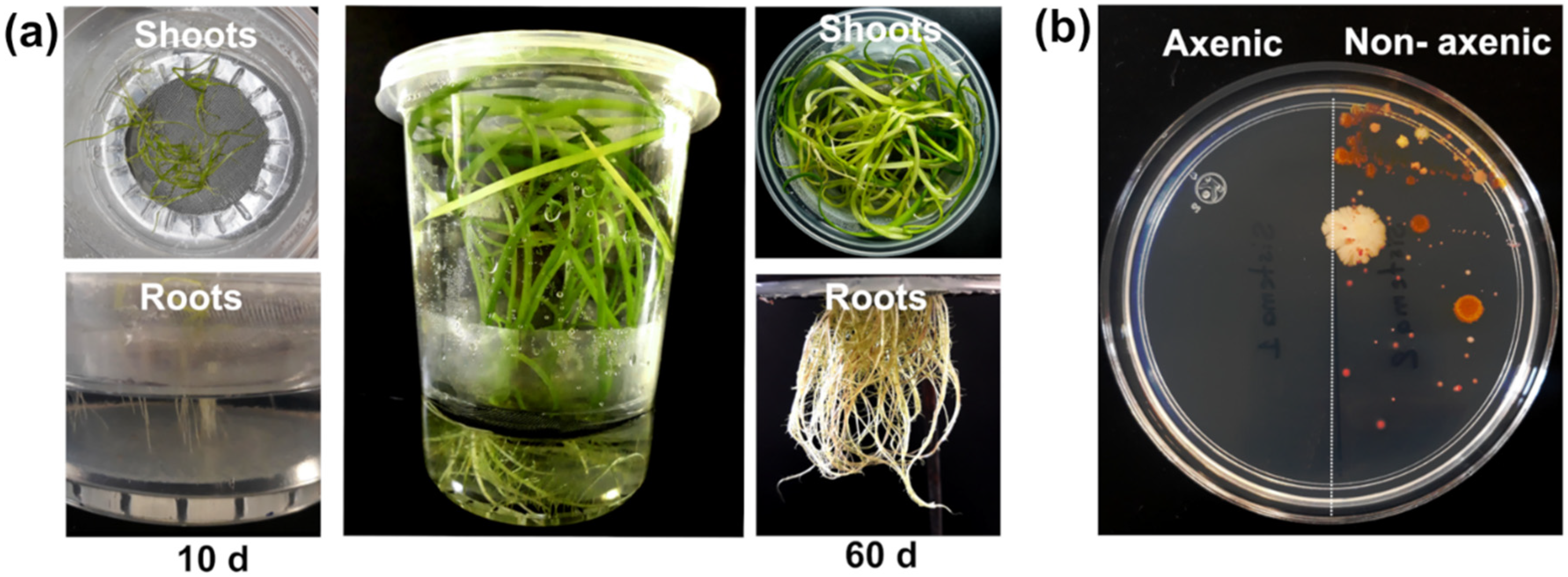
2.2. T. latifolia Culture under Axenic Hydroponic Conditions
2.3. Chemical Modeling of Cd Bioavailability in Hydroponics without Glucose
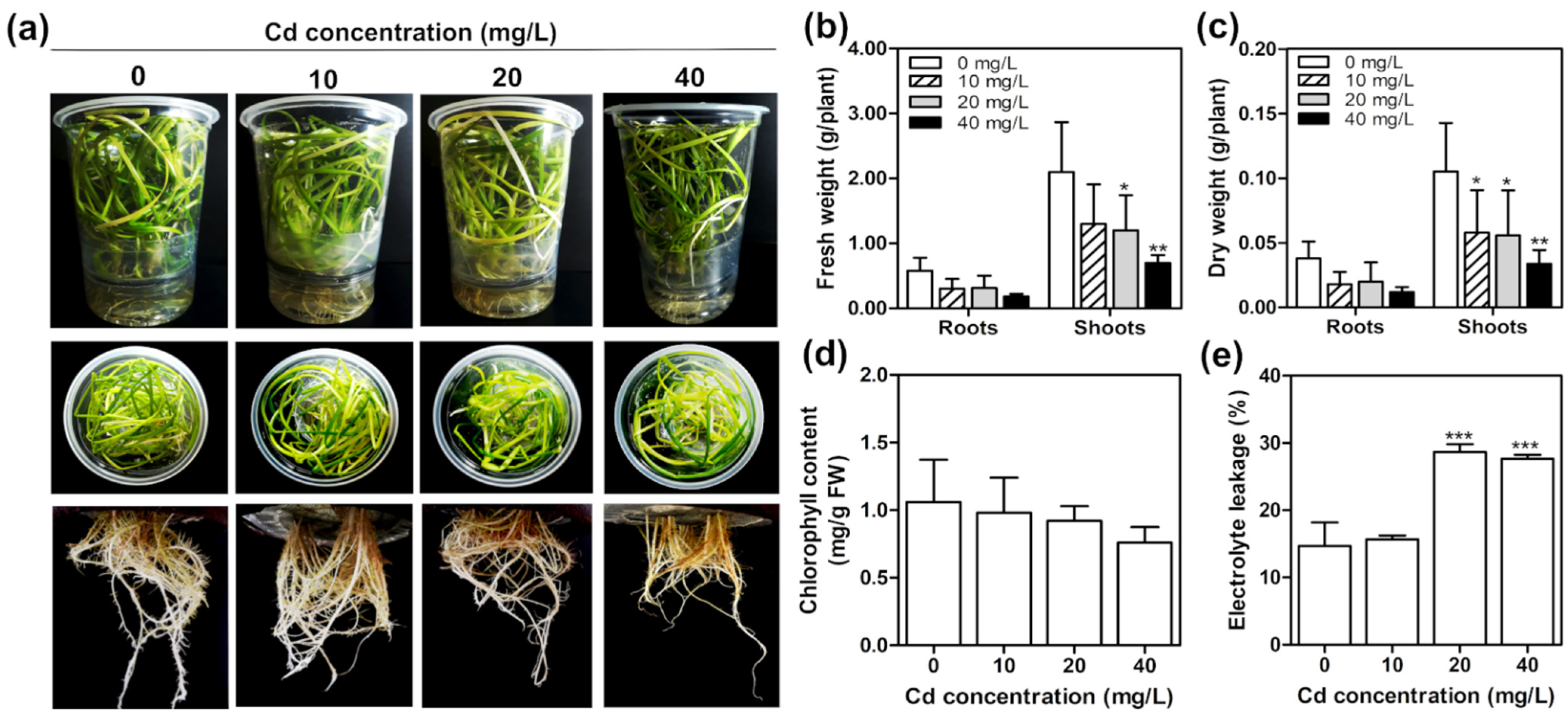
2.4. Cadmium Toxicity Effects on T. latifolia in Hydroponic Culture
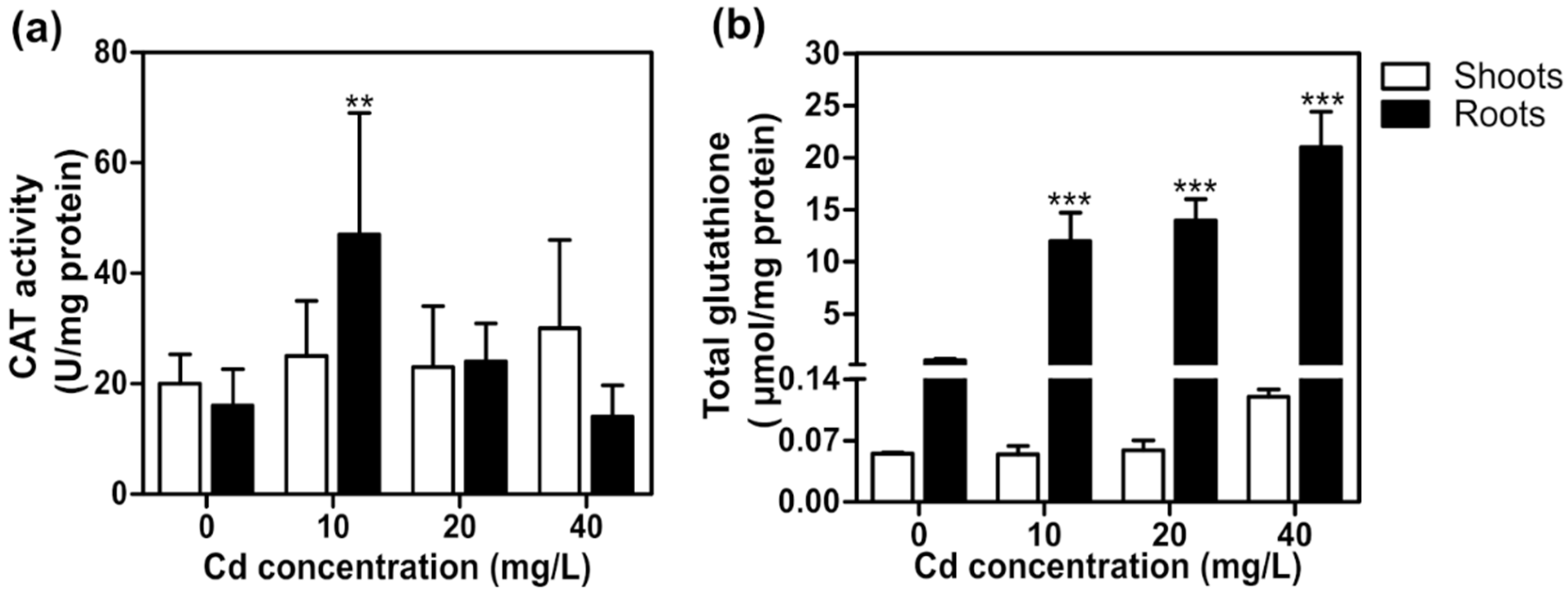
2.5. Cd Accumulation and Removal by T. latifolia Plants in the Hydroponic System
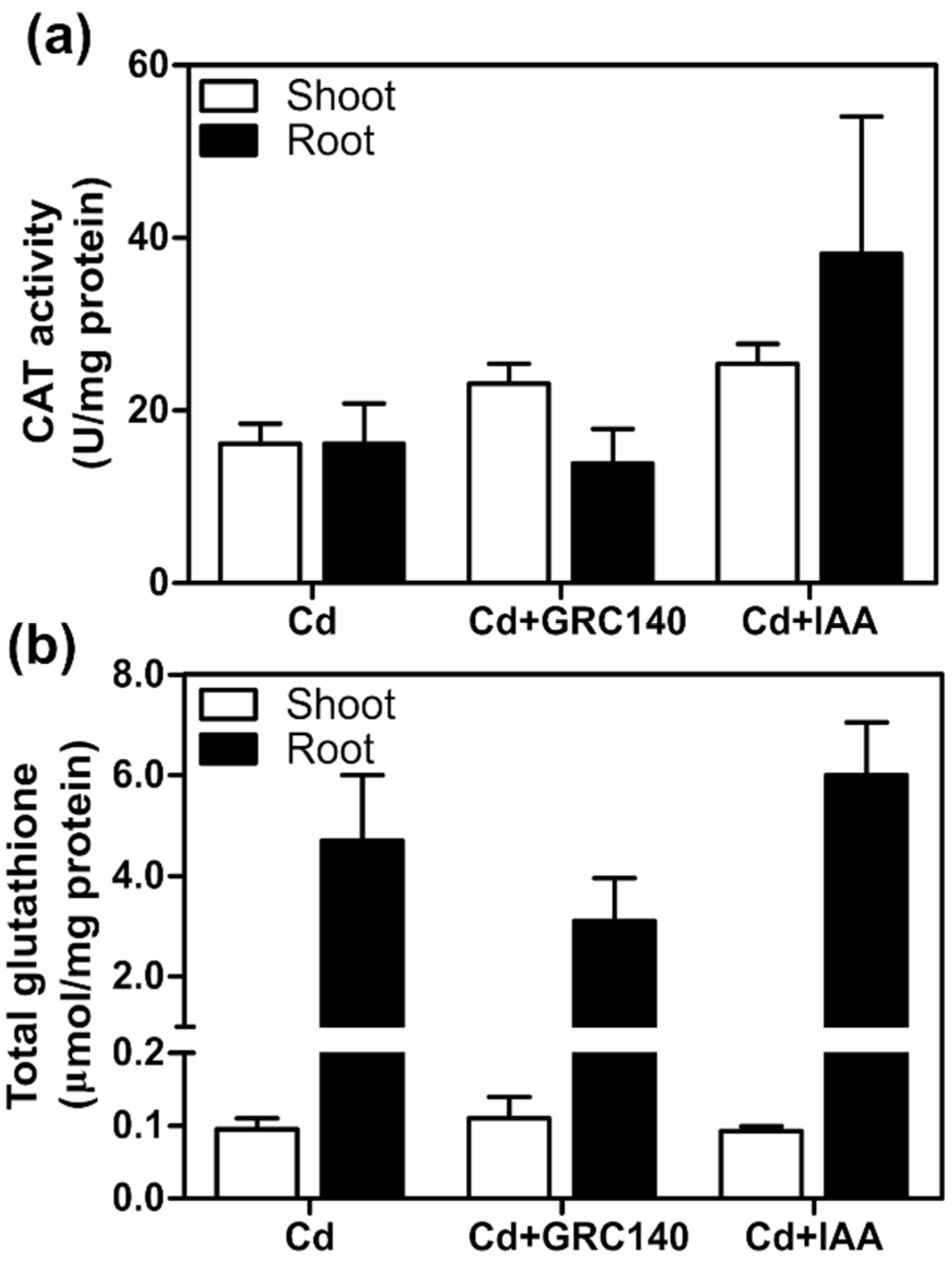
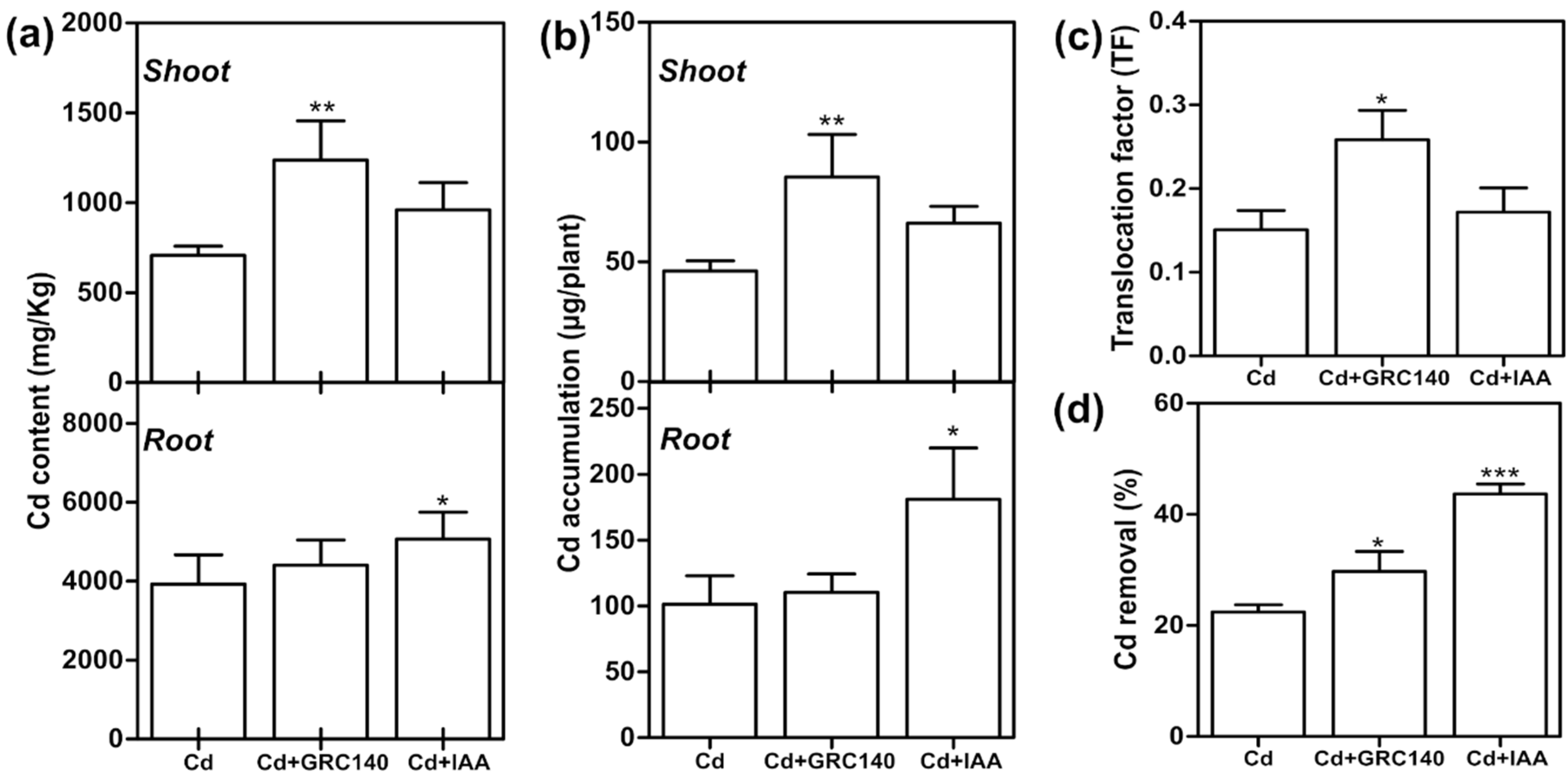
2.6. Effect of P. rhodesiae GRC140 and IAA on T. latifolia Exposed to Cd
2.7. Effect of P. rhodesiae GRC140 and IAA on Cd Uptake
3. Materials and Methods
3.1. Plant Material Sampling and Authentication
3.2. Establishment and Cultivation of Axenic T. latifolia Hydroponic Culture
3.3. Cadmium Plant Treatments and Chemical Modeling of Cd Bioavailability
3.4. P. rhodesiae GRC140 and IAA Treatments in Plants Exposed to Cd
3.5. Chlorophyll Quantification
3.6. Electrolyte Leakage
3.7. Catalase Activity and Total Glutathione Content
3.8. Cadmium Analysis
3.9. Bacteria Growth in the Hydroponic Systems and Root Colonization
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Chen, J.; Lu, S.; Yang, L.; Qian, J.; Cao, S. Increased lead and cadmium tolerance of Typha angustifolia from Huaihe River is associated with enhanced phytochelatin synthesis and improved antioxidative capacity. Environ. Technol. 2016, 37, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Rehman, M.Z.u.; Maqbool, A. A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Zhang, X.H.; Tapia, M.; Webb, J.B.; Huang, Y.H.; Miao, S. Molecular signatures of two cattail species, Typha domingensis and Typha latifolia (Typhaceae), in South Florida. Mol. Phylogenetics Evol. 2008, 49, 368–376. [Google Scholar] [CrossRef]
- Bonanno, G.; Cirelli, G.L. Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicol. Environ. Saf. 2017, 143, 92–101. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-DAIM, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Ma, L.; Liu, Q.; Vestergård, M.; Topalovic, O.; Wang, Q.; Zhou, Q.; Huang, L.; Yang, X.; Feng, Y. The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J. Hazard. Mater. 2020, 395, 122661. [Google Scholar] [CrossRef]
- Rolón-Cárdenas, G.A.; Arvizu-Gómez, J.L.; Soria-Guerra, R.E.; Pacheco-Aguilar, J.R.; Alatorre-Cobos, F.; Hernández-Morales, A. The role of auxins and auxin-producing bacteria in the tolerance and accumulation of cadmium by plants. Environ. Geochem. Health 2022. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, J.; Wang, Y.; Lin, L.; Liao, M.a.; Wang, J.; Deng, Q.; Tang, Y.; Wang, X.; Wang, J. Effects of exogenous indole acetic acid on growth and cadmium accumulation of Cyphomandra betacea seedlings. Int. J. Environ. Anal. Chem. 2020, 102, 771–779. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Rolón-Cárdenas, G.A.; Arvizu-Gómez, J.L.; Pacheco-Aguilar, J.R.; Vázquez-Martínez, J.; Hernández-Morales, A. Cadmium-tolerant endophytic Pseudomonas rhodesiae strains isolated from Typha latifolia modify the root architecture of Arabidopsis thaliana Col-0 in presence and absence of Cd. Braz. J. Microbiol. 2020, 52, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Alatorre-Cobos, F.; Calderón-Vázquez, C.; Ibarra-Laclette, E.; Yong-Villalobos, L.; Pérez-Torres, C.-A.; Oropeza-Aburto, A.; Méndez-Bravo, A.; González-Morales, S.-I.; Gutiérrez-Alanís, D.; Chacón-López, A.; et al. An improved, low-cost, hydroponic system for growing Arabidopsis and other plant species under aseptic conditions. BMC Plant Biol. 2014, 14, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, A.; Okada, S.; Zhang, C.; Delhaize, E.; Mathesius, U.; Richardson, A.E.; Watt, M.; Gilliham, M.; Ryan, P.R. A sterile hydroponic system for characterising root exudates from specific root types and whole-root systems of large crop plants. Plant Methods 2018, 14, 114. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.; Verheust, Y.; Bonarrigo, G.; Van Hulle, S. Closed hydroponic systems: Operational parameters, root exudates occurrence and related water treatment. Rev. Environ. Sci. Biotechnol. 2017, 16, 59–79. [Google Scholar] [CrossRef]
- Golan-Goldhirsh, A.; Barazani, O.; Nepovim, A.; Soudek, P.; Smrcek, S.; Dufkova, L.; Krenkova, S.; Yrjala, K.; Schröder, P.; Vanek, T. Plant response to heavy metals and organic pollutants in cell culture and at whole plant level. J. Soils Sediments 2004, 4, 133–140. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, M.C.; Bonifas, I.; Alfaro-De la Torre, M.C.; Flores-Flores, J.L.; Bañuelos-Hernández, B.; Patiño-Rodríguez, O. Increased accumulation of cadmium and lead under Ca and Fe deficiency in Typha latifolia: A study of two pore channel (TPC1) gene responses. Environ. Exp. Bot. 2015, 115, 38–48. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Y.; Xie, L.; Zeng, G.; Ma, K. Beilschmiedia turbinata: A newly recognized but dying species of lauraceae from tropical asia based on morphological and molecular data. PLoS ONE 2013, 8, e67636. [Google Scholar] [CrossRef]
- Czakó, M.; Feng, X.; He, Y.; Liang, D.; Márton, L. Genetic modification of wetland grasses for phytoremediation. Z. Für Nat. C 2005, 60, 285–291. [Google Scholar] [CrossRef]
- Estime, L.; O’Shea, M.; Borst, M. Suspension culture and plant regeneration of Typha latifolia. Hort. Sci. 2002, 37, 406–408. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.D.; Beech, J.; Sarma, K.S. Shoot regeneration and plant acclimatization of the wetland monocot Cattail (Typha latifolia). Plant Cell Rep. 1998, 18, 71–75. [Google Scholar] [CrossRef]
- Rogers, S.M.D. Tissue culture and wetland establishment of the freshwater monocots Carex, Juncus, Scirpus, and Typha. In Vitro Cell Dev. Biol. Plant 2003, 39, 1–5. [Google Scholar] [CrossRef]
- Liu, H.; Hao, N.; Jia, Y.; Liu, X.; Ni, X.; Wang, M.; Liu, W. The ethylene receptor regulates Typha angustifolia leaf aerenchyma morphogenesis and cell fate. Planta 2019, 250, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Application of plant tissue cultures in phytoremediation research: Incentives and limitations. Biotechnol. Bioeng. 2009, 103, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. In Reviews of Environmental Contamination and Toxicology Volume 241; de Voogt, P., Gunther, F.A., Eds.; Springer International Publishing: Cham, Swizerland, 2017; pp. 73–137. [Google Scholar]
- Santos-Díaz, M.d.S.; Barrón-Cruz, M.d.C. Lead, chromium and manganese removal by in vitro root cultures of two aquatic macrophytes species: Typha Latifolia L. and Scirpus Americanus Pers. Int. J. Phytoremediation 2011, 13, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Piotto, F.A.; Carvalho, M.E.A.; Souza, L.A.; Rabêlo, F.H.S.; Franco, M.R.; Batagin-Piotto, K.D.; Azevedo, R.A. Estimating tomato tolerance to heavy metal toxicity: Cadmium as study case. Environ. Sci. Pollut. Res. 2018, 25, 27535–27544. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Takashima, K.; Ahmad Nor, A.S.b.; Ando, S.; Takahashi, H.; Kaneko, T. Evaluation of plant stress due to plasma-generated reactive oxygen and nitrogen species using electrolyte leakage. Jpn. J. Appl. Phys. 2020, 60, 010504. [Google Scholar] [CrossRef]
- Demidchik, V. ROS-Activated ion channels in plants: Biophysical characteristics, physiological functions and molecular nature. Int. J. Mol. Sci. 2018, 19, 1263. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Qin, Z.; Li, X.; Wang, L.; Wang, Y.; Zhang, J.; Huang, Y.; Yang, S. Selenite antagonizes the phytotoxicity of Cd in the cattail Typha angustifolia. Ecotoxicol. Environ. Saf. 2020, 189, 109959. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, R. Regulations of reactive oxygen species in plants abiotic stress: An integrated overview. In Plant Life under Changing Environment; Tripathi, D.K., Pratap Singh, V., Chauhan, D.K., Sharma, S., Prasad, S.M., Dubey, N.K., Ramawat, N., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 323–353. [Google Scholar]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xiao, J.; Zhao, Y.; Zhang, Y.; Jie, Y.; Shen, D.; Yue, C.; Huang, J.; Hua, Y.; Zhou, T. Comparative physiological and transcriptomic analyses reveal ascorbate and glutathione coregulation of cadmium toxicity resistance in wheat genotypes. BMC Plant Biol. 2021, 21, 459. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Zhang, T.; Zhang, R.; Liu, R.; Chen, Y. Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int. J. Biol. Macromol. 2015, 77, 59–67. [Google Scholar] [CrossRef]
- Li, G.-Z.; Chen, S.-J.; Li, N.-Y.; Wang, Y.-Y.; Kang, G.-Z. Exogenous glutathione alleviates cadmium toxicity in wheat by influencing the absorption and translocation of cadmium. Bull. Environ. Contam. Toxicol. 2021, 107, 320–326. [Google Scholar] [CrossRef]
- Xu, W.; Shi, W.; Yan, F.; Zhang, B.; Liang, J. Mechanisms of cadmium detoxification in cattail (Typha angustifolia L.). Aquat. Bot. 2011, 94, 37–43. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Carranza-Alvarez, C.; Alfaro-De la Torre, M.C.; Chavez-Guerrero, L.; Garcia-De la Cruz, R.F. Removal and accumulation of cadmium and lead by Typha latifolia exposed to single and mixed metal solutions. Arch. Environ. Contam. Toxicol. 2009, 57, 688–696. [Google Scholar] [CrossRef]
- Woraharn, S.; Meeinkuirt, W.; Phusantisampan, T.; Chayapan, P. Rhizofiltration of cadmium and zinc in hydroponic systems. Water Air Soil Pollut. 2021, 232, 204. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, N.; Mao, X.; Yan, Z.; Wang, J.; Tan, W.; Li, X.; Liu, H.; Wang, L.; Xi, B. Cadmium tolerance and accumulation characteristics of wetland emergent plants under hydroponic conditions. RSC Adv. 2018, 8, 33383–33390. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, G.P.S.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytoremediation of lead by a wild, non-edible Pb accumulator Coronopus didymus (L.) Brassicaceae. Int. J. Phytoremediation 2018, 20, 483–489. [Google Scholar] [CrossRef]
- Mishra, T.; Pandey, V.C. Phytoremediation of Red Mud Deposits Through Natural Succession. In Phytomanagement of Polluted Sites; Pandey, V.C., Bauddh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–424. [Google Scholar]
- Sharma, P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: An update. Bioresour. Technol. 2021, 328, 124835. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheng, K.; Huang, G.; Chen, G.; Zhou, S.; Huang, Y.; Zhang, J.; Duan, H.; Fan, H. Effects of exogenous 3-indoleacetic acid and cadmium stress on the physiological and biochemical characteristics of Cinnamomum camphora. Ecotoxicol. Environ. Saf. 2020, 191, 109998. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Chen, R.; Ma, Y.; Yang, B.; Chen, Z.; Liang, Y.; Fang, J.; Xiao, Y. Role of two plant growth-promoting bacteria in remediating cadmium-contaminated soil combined with Miscanthus floridulus (Lab.). Plants 2021, 10, 912. [Google Scholar] [CrossRef]
- Pan, F.; Luo, S.; Shen, J.; Wang, Q.; Ye, J.; Meng, Q.; Wu, Y.; Chen, B.; Cao, X.; Yang, X.; et al. The effects of endophytic bacterium SaMR12 on Sedum alfredii Hance metal ion uptake and the expression of three transporter family genes after cadmium exposure. Environ. Sci. Pollut. Res. 2017, 24, 9350–9360. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; An, G. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ. 2009, 32, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Yu, G.; Wang, G.; Li, J.; Chi, T.; Wang, S.; Peng, H.; Chen, H.; Du, C.; Jiang, C.; Liu, Y.; et al. Enhanced Cd2+ and Zn2+ removal from heavy metal wastewater in constructed wetlands with resistant microorganisms. Bioresour. Technol. 2020, 316, 123898. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Remy, E.; Duque, P. Assessing tolerance to heavy-metal stress in Arabidopsis thaliana seedlings. In Environmental Responses in Plants: Methods and Protocols; Duque, P., Ed.; Springer: New York, NY, USA, 2016; pp. 197–208. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of Phtosynthetic Tissues: Chlorophylls and Carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Umnajkitikorn, K.; Fukudome, M.; Uchiumi, T.; Teaumroong, N. Elevated nitrogen priming induced oxinitro-responses and water deficit tolerance in rice. Plants 2021, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.; Elhindi, K.; El-Hendawy, S.; Al-Rashed, S.; Abd-ElGawad, A. Antioxidant system and biomolecules alteration in Pisum sativum under heavy metal stress and possible alleviation by 5-aminolevulinic acid. Molecules 2019, 24, 4194. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Carranza-Álvarez, C.; Alonso-Castro, A.J.; Alfaro-De La Torre, M.C.; García-De La Cruz, R.F. Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosí, México. Water Air Soil Pollut. 2008, 188, 297–309. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [Green Version]
- Balsanelli, E.; de Oliveira Pedrosa, F.; de Souza, E.M. Quantification of grass colonization by associative bacteria. Curr. Protoc. Plant Biol. 2017, 2, 108–123. [Google Scholar] [CrossRef]

| Species (%) | Cd Concentration (mg/L) | ||
|---|---|---|---|
| 10 | 20 | 40 | |
| Cd2+ | 66.70 | 75.86 | 79.70 |
| CdCl+ | 5.60 | 7.11 | 9.03 |
| CdCl2 (aq) | 0.03 | 0.04 | 0.06 |
| CdSO4 (aq) | 1.90 | 2.17 | 2.21 |
| CdNH32+ | 0.03 | 0.03 | 0.03 |
| CdNO3+ | 1.06 | 1.20 | 1.25 |
| CdHPO4 (aq) | 1.42 | 1.60 | 1.64 |
| CdEDTA2− | 23.14 | 11.94 | 6.06 |
| CdHEDTA− | 0.07 | 0.04 | 0.02 |
| Dissolved (%) | 100 | 100 | 100 |
| Precipitated (%) | 0 | 0 | 0 |
| Cd Concentration (mg/L) | Cd Content (mg/Kg DW) | BCF | TF | Cd Removal (%) | |
|---|---|---|---|---|---|
| Shoots | Roots | ||||
| 0 | nd | nd | nd | nd | nd |
| 10 | 406 ± 59 b | 2970 ± 65 c | 509 ± 96 a | 0.18 ± 0.04 a | 37 ± 3.8 a |
| 20 | 668 ± 73 a | 3275 ± 15 b | 340 ± 38 b | 0.24 ± 0.01 a | 43 ± 6.3 a |
| 40 | 739 ± 24 a | 3520 ± 105 a | 126 ± 16 c | 0.23 ± 0.03 a | 22 ± 5.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolón-Cárdenas, G.A.; Martínez-Martínez, J.G.; Arvizu-Gómez, J.L.; Soria-Guerra, R.E.; Alfaro-De la Torre, M.C.; Alatorre-Cobos, F.; Rubio-Santiago, J.; González-Balderas, R.d.M.; Carranza-Álvarez, C.; Macías-Pérez, J.R.; et al. Enhanced Cd-Accumulation in Typha latifolia by Interaction with Pseudomonas rhodesiae GRC140 under Axenic Hydroponic Conditions. Plants 2022, 11, 1447. https://doi.org/10.3390/plants11111447
Rolón-Cárdenas GA, Martínez-Martínez JG, Arvizu-Gómez JL, Soria-Guerra RE, Alfaro-De la Torre MC, Alatorre-Cobos F, Rubio-Santiago J, González-Balderas RdM, Carranza-Álvarez C, Macías-Pérez JR, et al. Enhanced Cd-Accumulation in Typha latifolia by Interaction with Pseudomonas rhodesiae GRC140 under Axenic Hydroponic Conditions. Plants. 2022; 11(11):1447. https://doi.org/10.3390/plants11111447
Chicago/Turabian StyleRolón-Cárdenas, Gisela Adelina, Joana Guadalupe Martínez-Martínez, Jackeline Lizzeta Arvizu-Gómez, Ruth Elena Soria-Guerra, Ma. Catalina Alfaro-De la Torre, Fulgencio Alatorre-Cobos, Jesús Rubio-Santiago, Regina de Montserrat González-Balderas, Candy Carranza-Álvarez, José Roberto Macías-Pérez, and et al. 2022. "Enhanced Cd-Accumulation in Typha latifolia by Interaction with Pseudomonas rhodesiae GRC140 under Axenic Hydroponic Conditions" Plants 11, no. 11: 1447. https://doi.org/10.3390/plants11111447
APA StyleRolón-Cárdenas, G. A., Martínez-Martínez, J. G., Arvizu-Gómez, J. L., Soria-Guerra, R. E., Alfaro-De la Torre, M. C., Alatorre-Cobos, F., Rubio-Santiago, J., González-Balderas, R. d. M., Carranza-Álvarez, C., Macías-Pérez, J. R., Aldaba-Muruato, L. R., & Hernández-Morales, A. (2022). Enhanced Cd-Accumulation in Typha latifolia by Interaction with Pseudomonas rhodesiae GRC140 under Axenic Hydroponic Conditions. Plants, 11(11), 1447. https://doi.org/10.3390/plants11111447








