Exploring the Relationship between Trichome and Terpene Chemistry in Chrysanthemum
Abstract
:1. Introduction
2. Results
2.1. Morphological Observation of Trichomes in C. morifilium cultivars and C. indicum accessions
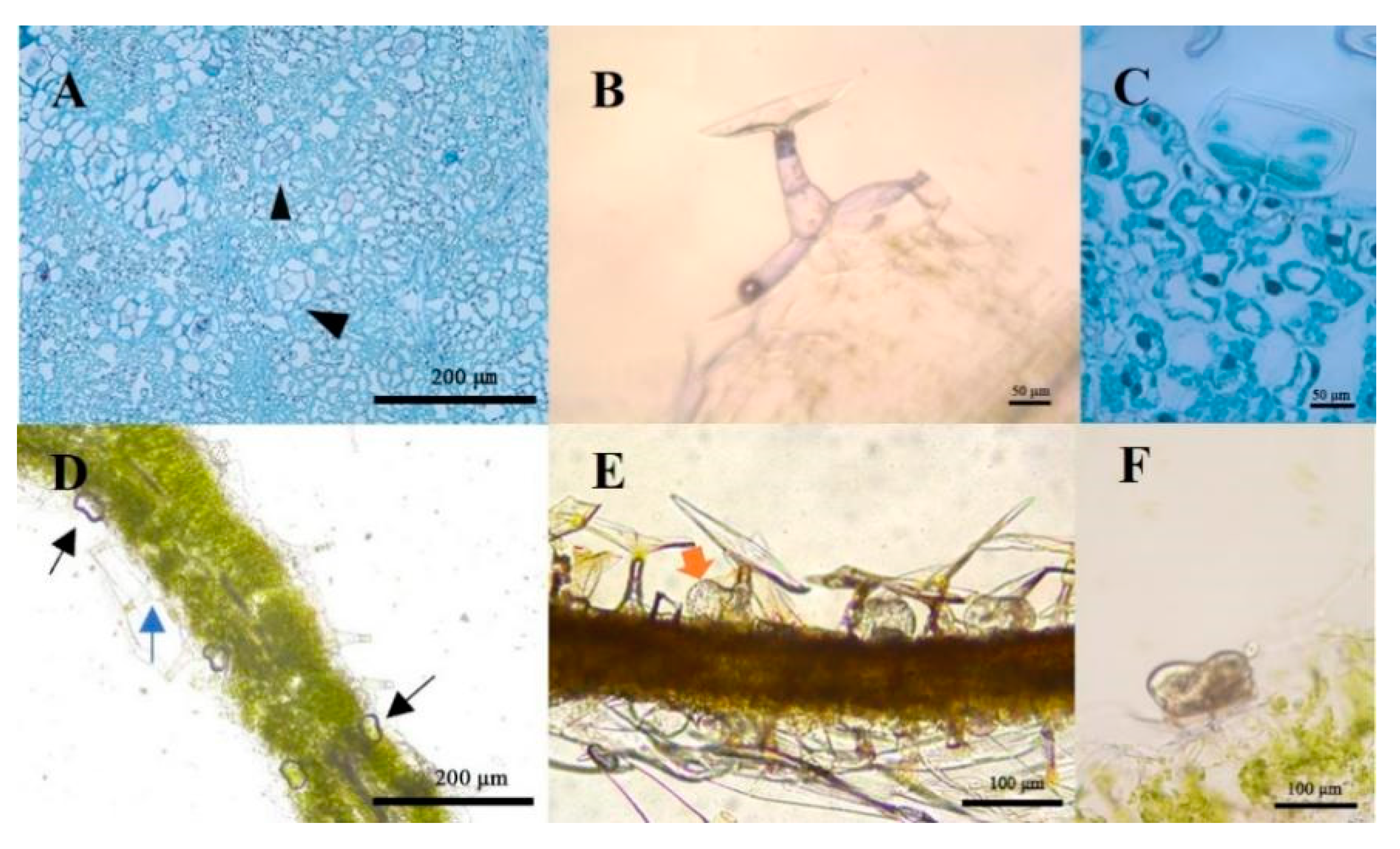
2.2. Structure Observation and Histochemistry of Glandular Trichomes and T-Shaped Trichomes
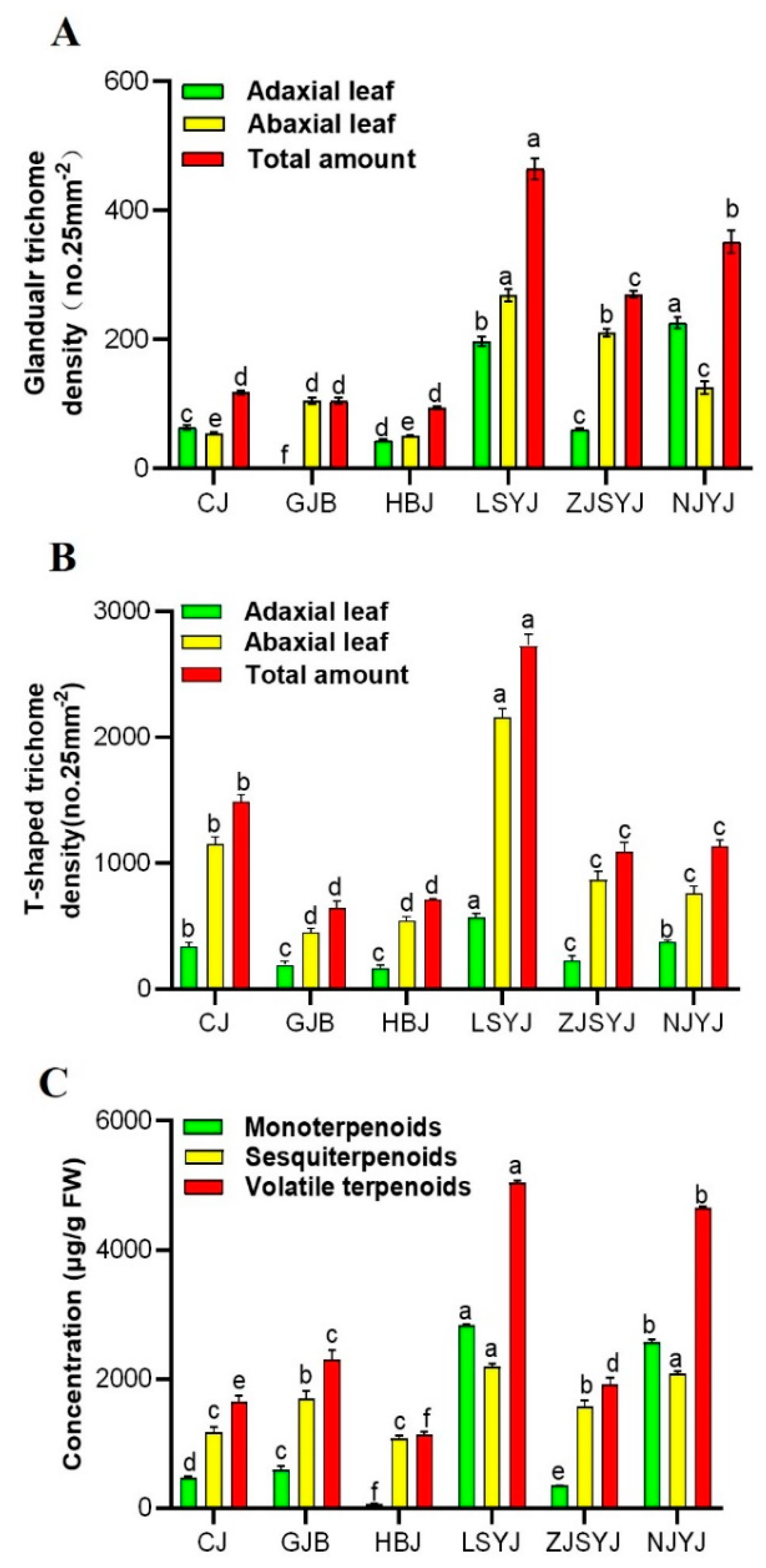
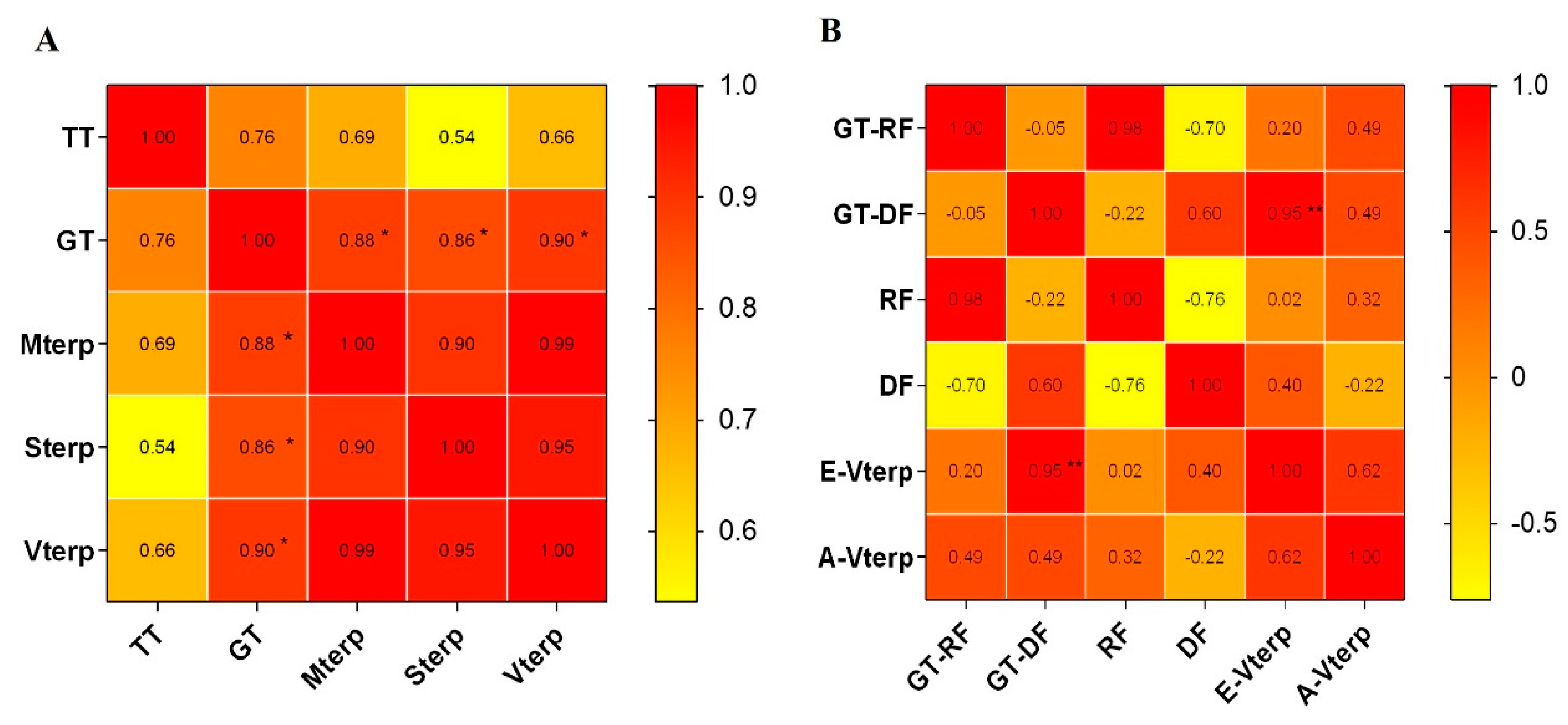
2.3. Correlation of the Trichome Density and Volatile Terpenoid Content from Leaves of C. morifilium cultivars and C. indicum accessions
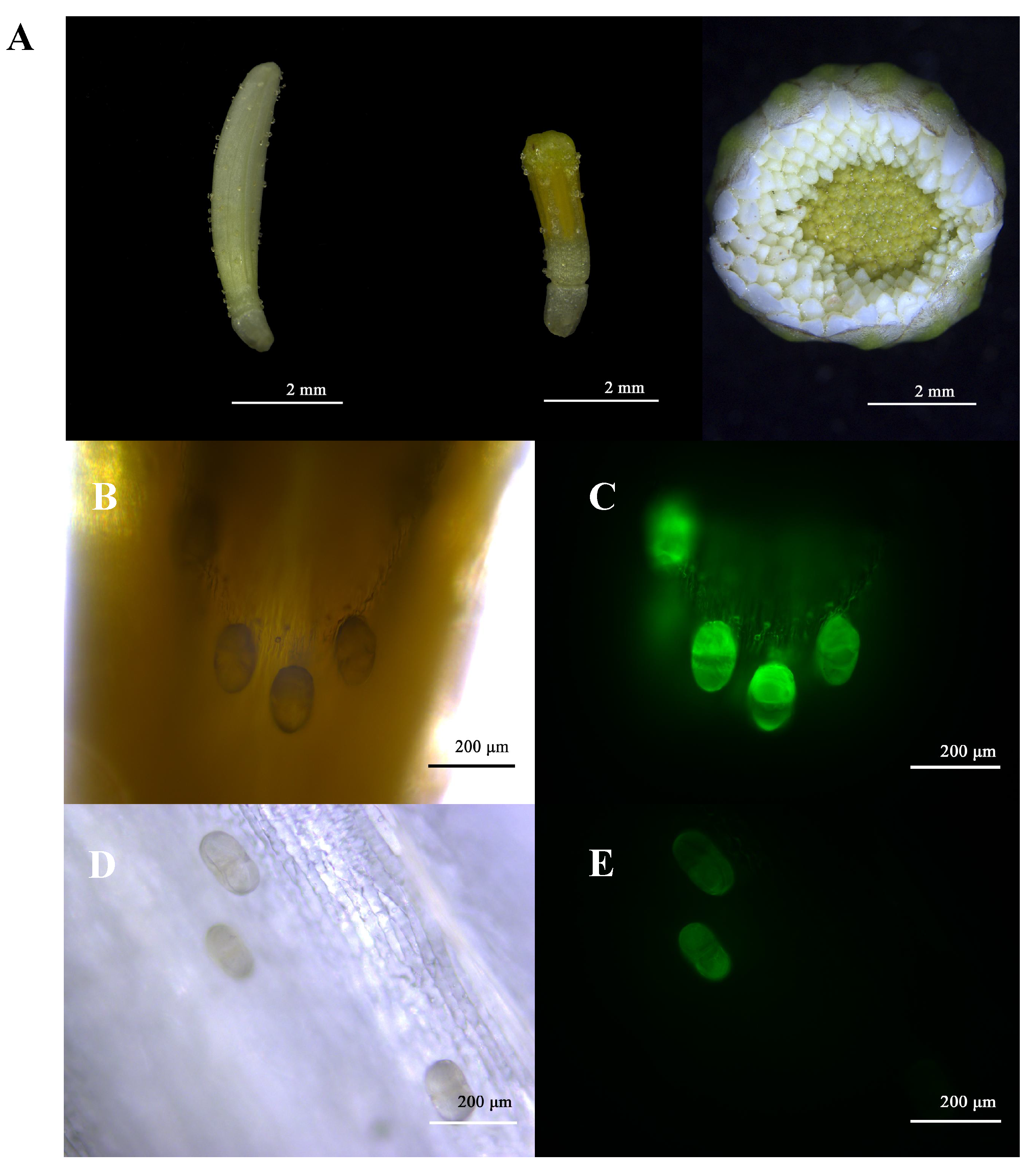
2.4. Flowerhead Morphology and Glandular Trichome Types of the Six Chrysanthemum cultivars\accessions at the Break Bud Stage
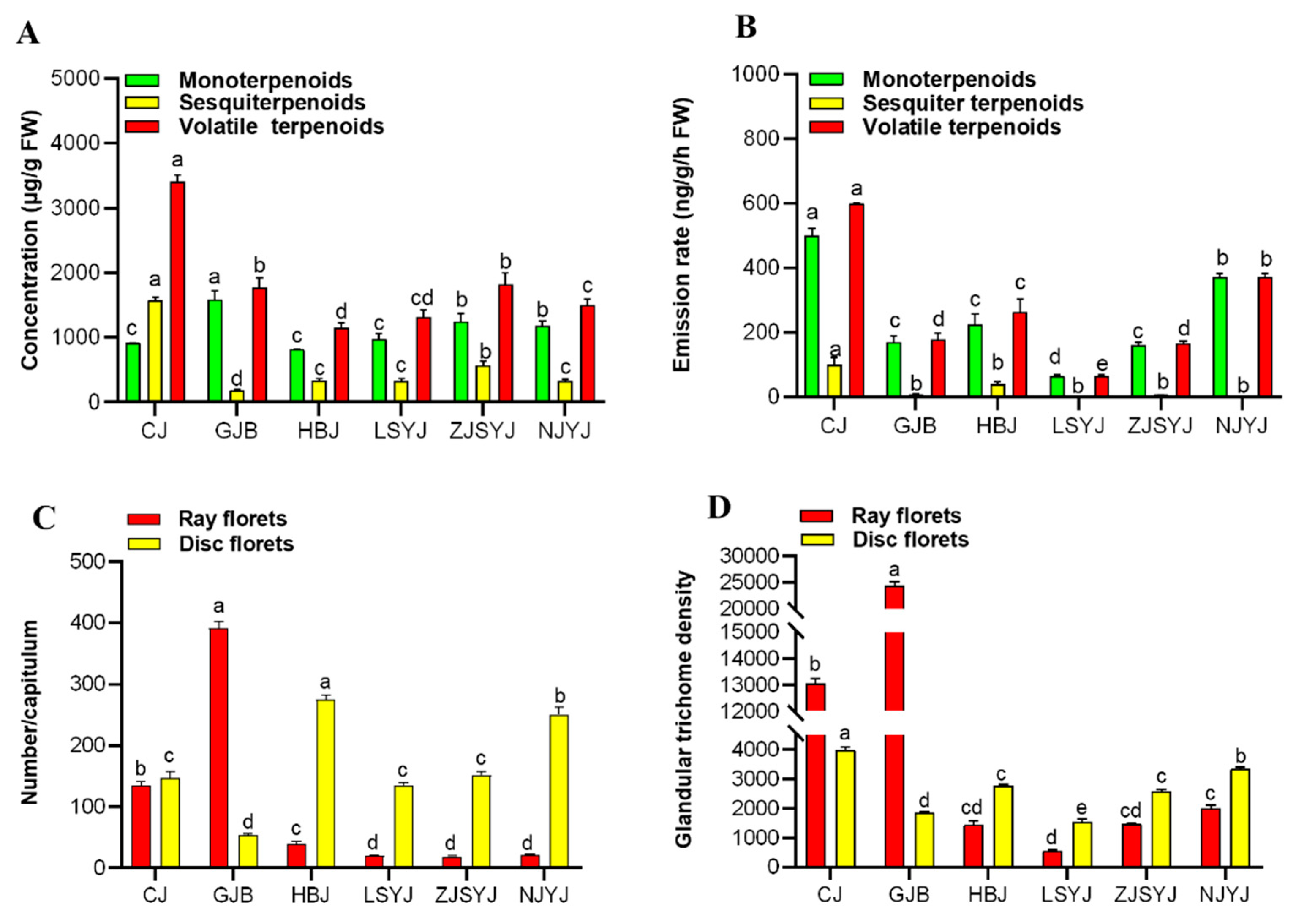
2.5. The Correlation of Terpenoid Production and Emission and Trichome Density in the Flowerheads of Six Chrysanthemum cultivars\accessions
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Organic Extraction of the VOCs
4.3. Headspace Collection of Volatile Terpenoids from Flowerheads
4.4. Identification of Volatiles by GC-MS
4.5. Scanning Electron Microscopy (SEM) Observation
4.6. Glandular Trichome Density Measurement
4.7. Histochemical Characterization
4.8. Paraffin Section
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, A.; Zhu, X.; Chen, F.; Gao, H.; Jiang, J.; Chen, S. A Chrysanthemum Heat Shock Protein Confers Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2014, 15, 5063–5078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Harima, S.; Yoshikawa, M. Absolute Stereostructures of Two New Flavanone Glycosides and a Phenylbutanoid Glycoside from the Flowers of Chrysanthemum indicum L. Their Inhibitory Activities for Rat Lens Aldose Reductase. Chem. Pharm. Bull. 2002, 50, 972–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Sun, Y.; Li, D.; Chen, Y. Chrysanthemum indicum L.: A Comprehensive Review of Its Botany, Phytochemistry and Pharmacology. Am. J. Chin. Med. 2020, 48, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, W.; Chen, X.; Wang, W.; Köllner, T.G.; Chen, S.; Chen, F.; Chen, F. Diversity and Biosynthesis of Volatile Terpenoid Secondary Metabolites in the Chrysanthemum Genus. Crit. Rev. Plant Sci. 2021, 40, 422–445. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Y.; Chen, S.; Chen, F.; Chen, F. Concentration-Dependent Emission of Floral Scent Terpenoids from Diverse Cultivars of Chrysanthemum Morifolium and Their Wild Relatives. Plant Sci 2021, 309, 110959. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.Y.; Zheng, C.S.; Wang, C.; Yoo, Y.K. Studies of Aroma Compounds in Chrysanthemum in Different Florescence and Inflorescence Parts and Aroma Releasing. ActaBot. Boreali-Occident. Sin. 2012, 32, 722–730. [Google Scholar]
- Chang, K.-M.; Kim, G.-H. Volatile Aroma Constituents of Gukhwa (Chrysanthemum morifolium R.). Food. Sci. Biotechnol. 2013, 22, 659–663. [Google Scholar] [CrossRef]
- Jian, L.; Sun, M.; Zhang, Q.X. Analysis on Aroma Compositions in Flowers, Stems and Leaves of Chrysanthemum indicum Var. aromaticum. J. Northwest. A F Univ. 2014, 42, 87–92. [Google Scholar]
- Guo, Y.; Zhang, T.; Zhong, J.; Ba, T.; Xu, T.; Zhang, Q.; Sun, M. Identification of the Volatile Compounds and Observation of the Glandular Trichomes in Opisthopappus taihangensis and Four Species of Chrysanthemum. Plants 2020, 9, 855. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.P.; Bhakuni, R.S. Secondary Metabolites of Chrysanthemum Genus and Their Biological Activities. Curr. Sci. 2005, 20, 1489–1501. [Google Scholar]
- Xue, H.H.; Jiang, Y.F.; Zhao, H.W.; Kollner, T.G.; Chen, S.M.; Chen, F.D.; Chen, F. Characterization of Composition and Antifungal Properties of Leaf Secondary Metabolites from Thirteen Cultivars of Chrysanthemum Morifolium Ramat. Molecules 2019, 24, 4202. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Nam, B.; Kim, B.R.; Kim, S.H.; Jo, Y.D.; Ahn, J.W.; Kim, J.B.; Jin, C.H.; Han, A.R. Comparative Analysis of Phytochemical Composition of Gamma-Irradiated Mutant Cultivars of Chrysanthemum morifolium. Molecules 2019, 24, 3003. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Nuerbiye, A.; Cheng, P.; Wang, J.H.; Li, H. Analysis of Floral Volatile Components and Antioxidant Activity of Different Varieties of Chrysanthemum Morifolium. Molecules 2017, 22, 1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, X.; Jiang, Q.; Sun, H.; Jiang, J.; Chen, S.; Guan, Z.; Fang, W.; Chen, F. Gc-Ms Analysis of the Volatile Constituents in the Leaves of 14 Compositae Plants. Molecules 2018, 23, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichersky, E.; Raguso, R.A. Why Do Plants Produce So Many Terpenoid Compounds? New Phytol 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Mao, J.; Yu, L.; Stoopen, G.; Wang, M.; Mumm, R.; de Ruijter, N.C.A.; Dicke, M.; Jongsma, M.A.; et al. Defense of Pyrethrum Flowers: Repelling Herbivores and Recruiting Carnivores by Producing Aphid Alarm Pheromone. New Phytol 2019, 223, 1607–1620. [Google Scholar] [CrossRef]
- Piesik, D.; Miler, N.; Lemańczyk, G.; Bocianowski, J.; Buszewski, B. Botrytis Cinerea Infection in Three Cultivars of Chrysanthemum in ‘Alchimist’ and Its Mutants: Volatile Induction of Pathogen-Infected Plants. Sci. Hortic. 2015, 193, 127–135. [Google Scholar] [CrossRef]
- Kuang, C.L.; Lv, D.; Shen, G.H.; Li, S.S.; Luo, Q.Y.; Zhang, Z.Q. Chemical Composition and Antimicrobial Activities of Volatile Oil Extracted from Chrysanthemum morifolium Ramat. J. Food Sci. Technol. 2018, 55, 2786–2794. [Google Scholar] [CrossRef]
- Shafaghat, A.; Larijani, K.; Salimi, F. Composition and Antibacterial Activity of the Essential Oil Ofchrysanthemum Partheniumflower from Iran. J. Essent. Oil Bear. Plants 2009, 12, 708–713. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, Y.; Zhao, H.; Kollner, T.G.; Chen, S.; Chen, F.; Chen, F. Diverse Terpenoids and Their Associated Antifungal Properties from Roots of Different Cultivars of Chrysanthemum morifolium Ramat. Molecules 2020, 25, 2083. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jiang, S.; Liu, Y.; Daniyal, M.; Jian, Y.; Peng, C.; Shen, J.; Liu, S.; Wang, W. The Flower Head of Chrysanthemum morifolium Ramat. (Juhua): A Paradigm of Flowers Serving as Chinese Dietary Herbal Medicine. J. Ethnopharmacol. 2020, 261, 113043. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Yang, M.S.; Supanjani, N.; Smith, D.L. Fertilizer Effect on the Yield and Terpene Components from the Flowerheads of Chrysanthemum boreale M. (Compositae). Agron. Sustain. Dev. 2005, 25, 205–211. [Google Scholar] [CrossRef]
- Werker, E. Trichome Diversity and Development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Schilmiller, A.; Schauvinhold, I.; Larson, M.; Xu, R.; Charbonneau, A.; Schmidt, A.; Wilkerson, C.; Last, R.; Pichersky, E. Monoterpenes in the Glandular Trichomes of Tomato Are Synthesized from a Neryl Diphosphate Precursor Rather Than Geranyl Diphosphate. Proc. Natl. Acad. Sci. USA 2009, 106, 10865–10870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulskamp, M. Plant Trichomes: A Model for Cell Differentiation. Nat. Rev. Mol. Cell Biol. 2004, 5, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Mahmoud, S.S.; Wildung, M.R.; Turner, G.W.; Davis, E.M.; Lange, I.; Baker, R.C.; Boydston, R.A.; Croteau, R.B. Improving Peppermint Essential Oil Yield and Composition by Metabolic Engineering. Proc. Natl. Acad. Sci. USA 2011, 108, 16944–16949. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Chen, M.; Shen, Q.; Li, L.; Fu, X.; Pan, Q.; Tang, Y.; Shi, P.; Lv, Z.; Jiang, W.; et al. Homeodomain Protein 1 Is Required for Jasmonate-Mediated Glandular Trichome Initiation in Artemisia Annua. New Phytol 2017, 213, 1145–1155. [Google Scholar] [CrossRef]
- Kang, J.H.; Liu, G.; Shi, F.; Jones, A.D.; Beaudry, R.M.; Howe, G.A. The Tomato Odorless-2 Mutant Is Defective in Trichome-Based Production of Diverse Specialized Metabolites and Broad-Spectrum Resistance to Insect Herbivores. Plant Physiol 2010, 154, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Shi, F.; Jones, A.D.; Marks, M.D.; Howe, G.A. Distortion of Trichome Morphology by the Hairless Mutation of Tomato Affects Leaf Surface Chemistry. J. Exp. Bot. 2010, 61, 1053–1064. [Google Scholar] [CrossRef]
- Wagner, G. Secreting Glandular Trichomes More Than Just Hairs. Plant Physiol 1991, 96, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Appezzato-da-Glória, B.; Da Costa, F.B.; da Silva, V.C.; Gobbo-Neto, L.; Rehder, V.L.G.; Hayashi, A.H. Glandular Trichomes on Aerial and Underground Organs in Chrysolaena Species (Vernonieae—Asteraceae): Structure, Ultrastructure and Chemical Composition. Flora 2012, 207, 878–887. [Google Scholar] [CrossRef]
- Szakiel, A.; Ruszkowski, D.; Janiszowska, W. Saponins in Calendula officinalis L.—Structure, Biosynthesis, Transport and Biological Activity. Phtyochem. Rev. 2005, 4, 151–158. [Google Scholar] [CrossRef]
- Shunying, Z.; Yang, Y.; Huaidong, Y.; Yue, Y.; Guolin, Z. Chemical Composition and Antimicrobial Activity of the Essential Oils of Chrysanthemum indicum. J. Ethnopharmacol. 2005, 96, 151–158. [Google Scholar] [CrossRef]
- Tissier, A. Glandular Trichomes: What Comes after Expressed Sequence Tags? Plant J. 2012, 70, 51–68. [Google Scholar] [CrossRef]
- Dmitruk, M.; Sulborska, A.; Żuraw, B.; Stawiarz, E.; Weryszko-Chmielewska, E. Sites of Secretion of Bioactive Compounds in Leaves of Dracocephalum moldavica L.: Anatomical, Histochemical, and Essential Oil Study. Braz. J. Bot. 2019, 42, 701–715. [Google Scholar] [CrossRef] [Green Version]
- Bello, M.A.; Alvarez, I.; Torices, R.; Fuertes-Aguilar, J. Floral Development and Evolution of Capitulum Structure in Anacyclus (Anthemideae, Asteraceae). Ann. Bot. 2013, 112, 1597–1612. [Google Scholar] [CrossRef] [Green Version]
- Janet Vermeer, R.L.P. Glandular Trichomes on the Inflorescence of Chrysanthemum Morifolium Cvd. Ramatic. Can. J. Bot. 1979, 57, 8. [Google Scholar]
- Yan, A.; Pan, J.; An, L.; Gan, Y.; Feng, H. The Responses of Trichome Mutants to Enhanced Ultraviolet-B Radiation in Arabidopsis Thaliana. J. Photochem. Photobiol. B 2012, 113, 29–35. [Google Scholar] [CrossRef]
- Wang, G. Recent Progress in Secondary Metabolism of Plant Glandular Trichomes. Plant Biotechnol. J. 2014, 31, 353–361. [Google Scholar] [CrossRef]
- Ruan, C.-J.; da Silva, J.A.T. Adaptive Significance of Floral Movement. Crit. Rev. Plamt Sci. 2011, 30, 293–328. [Google Scholar] [CrossRef]
- Huss, E.; Bar Yosef, K.; Zaccai, M. Humans’ Relationship to Flowers as an Example of the Multiple Components of Embodied Aesthetics. Behav. Sci. 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Effmert, U.; Grosse, J.; Rose, U.S.; Ehrig, F.; Kagi, R.; Piechulla, B. Volatile Composition, Emission Pattern, and Localization of Floral Scent Emission in Mirabilis jalapa (Nyctaginaceae). Am. J. Bot. 2005, 92, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutaghane, N.; Kabouche, A.; El-Azzouny, A.M.; Kabouche, Z. Composition of the Essential Oil of Chrysanthemum Macrocarpum from Algeria. Chem. Nat. Compd. 2008, 44, 817–818. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant During the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Jiang, J.; Zhang, F.; Liu, Y.; Ding, L.; Chen, S.; Chen, F. Current Achievements and Future Prospects in the Genetic Breeding of Chrysanthemum: A Review. Hortic Res. 2019, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H.M. Therapeutic and Biomedical Potentialities of Terpenoids—A Review. J. Pure. Appl. Microbio. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Xu, J.; van Herwijnen, Z.O.; Drager, D.B.; Sui, C.; Haring, M.A.; Schuurink, R.C. Slmyc1 Regulates Type Vi Glandular Trichome Formation and Terpene Biosynthesis in Tomato Glandular Cells. Plant Cell 2018, 30, 2988–3005. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, D.; Hu, R.; Hua, C.; Ali, I.; Zhang, A.; Liu, B.; Wu, M.; Huang, L.; Gan, Y. Atgis, a C2h2 Zinc-Finger Transcription Factor from Arabidopsis Regulates Glandular Trichome Development through Ga Signaling in Tobacco. Biochem. Biophys. Res. Commun. 2017, 483, 209–215. [Google Scholar] [CrossRef]
- Xie, L.; Yan, T.; Li, L.; Chen, M.; Hassani, D.; Li, Y.; Qin, W.; Liu, H.; Chen, T.; Fu, X.; et al. An Hd-Zip-Myb Complex Regulates Glandular Secretory Trichome Initiation in Artemisia annua. New Phytol 2021, 231, 2050–2064. [Google Scholar] [CrossRef]
- Yan, T.; Li, L.; Xie, L.; Chen, M.; Shen, Q.; Pan, Q.; Fu, X.; Shi, P.; Tang, Y.; Huang, H.; et al. A Novel Hd-Zip Iv/Mixta Complex Promotes Glandular Trichome Initiation and Cuticle Development in Artemisia annua. New Phytol 2018, 218, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cao, C.; Zhang, C.; Zheng, S.; Wang, Z.; Wang, L.; Ren, Z. The Identification of Cucumis Sativus Glabrous 1 (Csgl1) Required for the Formation of Trichomes Uncovers a Novel Function for the Homeodomain-Leucine Zipper I Gene. J. Exp. Bot. 2015, 66, 2515–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, P.; Fu, X.; Shen, Q.; Liu, M.; Pan, Q.; Tang, Y.; Jiang, W.; Lv, Z.; Yan, T.; Ma, Y.; et al. The Roles of Aamixta1 in Regulating the Initiation of Glandular Trichomes and Cuticle Biosynthesis in Artemisia annua. New Phytol 2018, 217, 261–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.S.; Kollner, T.G.; Wiggins, G.; Grant, J.; Degenhardt, J.; Chen, F. Molecular and Genomic Basis of Volatile-Mediated Indirect Defense against Insects in Rice. Plant J. 2008, 55, 491–503. [Google Scholar] [CrossRef]
- Singh, N.D.; Kumar, S.; Daniell, H. Expression of Beta-Glucosidase Increases Trichome Density and Artemisinin Content in Transgenic Artemisia annua Plants. Plant Biotechnol. J. 2016, 14, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Carde, R.D.P. Coloration Différentielle Des Inclusions Lipidiques Et Terpéniques Des Pseudophylles Du Pin Maritime Au Moyen Du Réactif Nadi. C. R. Acad. Sci. Paris Groupe 1964, 13, 1338–1340. [Google Scholar]
- Pearse, A.G. Histochemistry Theoretical and Applied; Churchill Livingston Press: London, UK, 1980. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw Hill Book Company Press: New York, NY, USA, 1940. [Google Scholar]
- Bukatsch, F. Bemerkungen Zur Doppelf~Irbung Astrablau—Safranin. Mikrokosmos 1972, 61, 255. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Chen, S.; Chen, F.; Chen, F.; Jiang, Y. Exploring the Relationship between Trichome and Terpene Chemistry in Chrysanthemum. Plants 2022, 11, 1410. https://doi.org/10.3390/plants11111410
Guan Y, Chen S, Chen F, Chen F, Jiang Y. Exploring the Relationship between Trichome and Terpene Chemistry in Chrysanthemum. Plants. 2022; 11(11):1410. https://doi.org/10.3390/plants11111410
Chicago/Turabian StyleGuan, Yaqin, Sumei Chen, Fadi Chen, Feng Chen, and Yifan Jiang. 2022. "Exploring the Relationship between Trichome and Terpene Chemistry in Chrysanthemum" Plants 11, no. 11: 1410. https://doi.org/10.3390/plants11111410
APA StyleGuan, Y., Chen, S., Chen, F., Chen, F., & Jiang, Y. (2022). Exploring the Relationship between Trichome and Terpene Chemistry in Chrysanthemum. Plants, 11(11), 1410. https://doi.org/10.3390/plants11111410







