Different Concentrations of Potassium Silicate in Nutrient Solution Affects Selected Growth Characteristics and Mineral Composition of Barley (Hordeum vulgare L.)
Abstract
:1. Introduction
2. Results
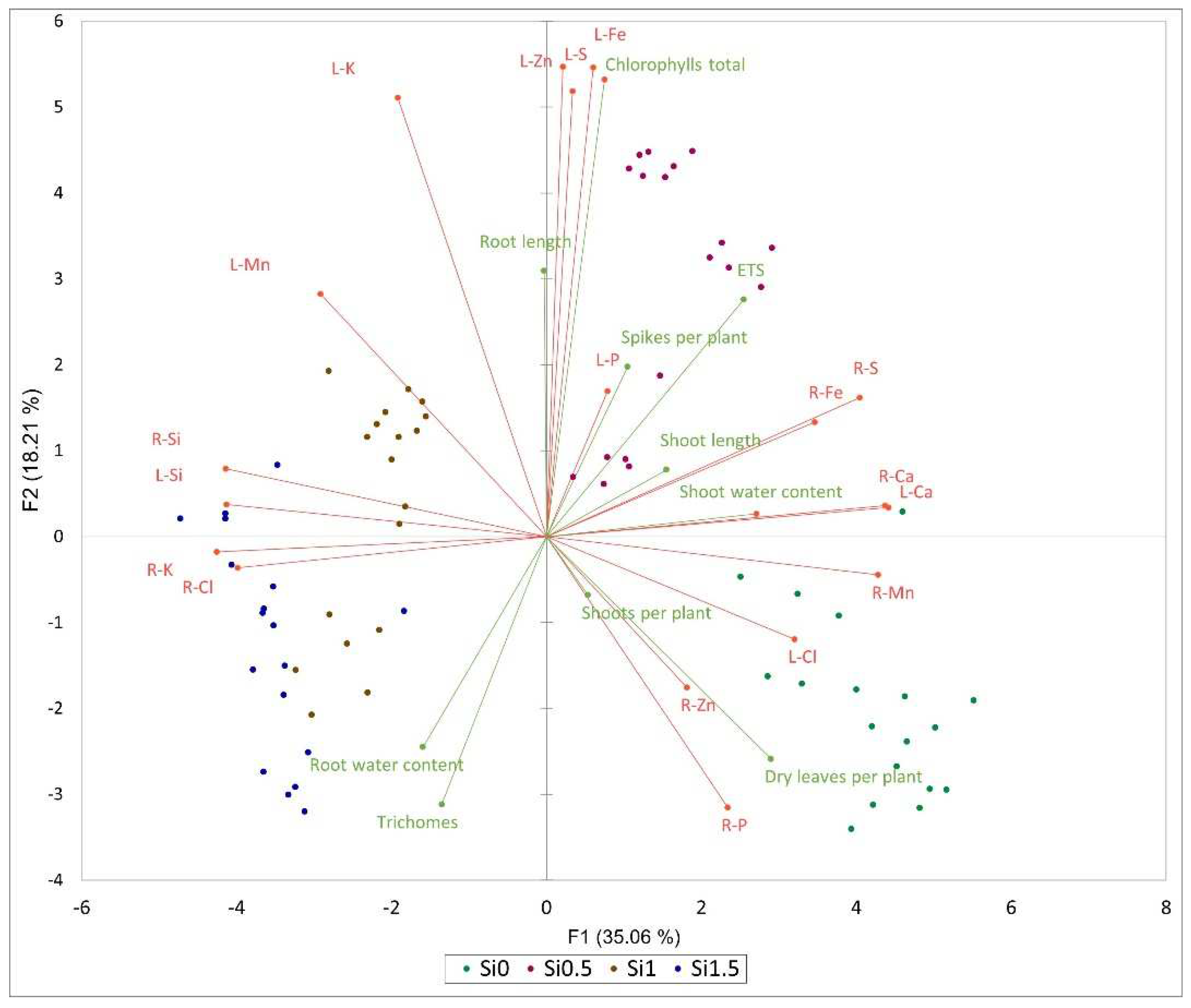
2.1. Morphological, Biochemical and Physiological Characteristics of Barley Plants
2.2. Elemental Composition
3. Discussion
3.1. Morphological, Biochemical and Physiological Characteristics of Barley Plants
3.2. Elemental Composition
4. Materials and Methods
4.1. Morphological and Anatomical Properties
4.2. Physiological Analysis
4.3. Biochemical Analysis
4.4. Elemental Composition
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hans Wedepohl, K. The Composition of the Continental Crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Matichenkov, V.V.; Bocharnikova, E.A. The Relationship between Silicon and Soil Physical and Chemical Properties. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 209–219. [Google Scholar]
- Exley, C. Silicon in Life: A Bioinorganic Solution to Bioorganic Essentiality. J. Inorg. Biochem. 1998, 69, 139–144. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic Variation in the Silicon Composition of Plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F.; Higashitani, A.; Sato, K.; Takeda, K. Genotypic Variation in Silicon Concentration of Barley Grain. Plant Soil 2003, 249, 383–387. [Google Scholar]
- Mitani, N.; Chiba, Y.; Yamaji, N.; Ma, J.F. Identification and Characterization of Maize and Barley Lsi2-like Silicon Efflux Transporters Reveals a Distinct Silicon Uptake System from That in Rice. Plant Cell 2009, 21, 2133–2142. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Takahashi, E. Silicon-Accumulating Plants in the Plant Kingdom. In Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002; pp. 63–71. ISBN 9780444511669. [Google Scholar]
- Liang, Y.; Shen, Q.; Shen, Z.; Ma, T. Effects of Silicon on Salinity Tolerance of Two Barley Cultivars. J. Plant Nutr. 1996, 19, 173–183. [Google Scholar] [CrossRef]
- Wu, J.W.; Shi, Y.; Zhu, Y.X.; Wang, Y.C.; Gong, H.J. Mechanisms of Enhanced Heavy Metal Tolerance in Plants by Silicon: A Review. Pedosphere 2013, 23, 815–825. [Google Scholar] [CrossRef]
- Zellner, W.; Tubaña, B.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Plant Stress Reduction and Why This Element Is Not Used Routinely for Managing Plant Health. Plant Dis. 2021, 105, 2033–2049. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef] [Green Version]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felisberto, G.; de Mello Prado, R.; de Oliveira, R.L.L.; de Carvalho Felisberto, P.A. Are Nanosilica, Potassium Silicate and New Soluble Sources of Silicon Effective for Silicon Foliar Application to Soybean and Rice Plants? Silicon 2021, 13, 3217–3228. [Google Scholar] [CrossRef]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of Silicon Supplementation on Growth and Metabolism of Strawberry Plants at Three Developmental Stages. N. Z. J. Crop Hortic. Sci. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon With Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon Availability Modifies Nutrient Use Efficiency and Content, C:N:P Stoichiometry, and Productivity of Winter Wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Brackhage, C.; Schaller, J.; Bäucker, E.; Dudel, E.G. Silicon Availability Affects the Stoichiometry and Content of Calcium and Micro Nutrients in the Leaves of Common Reed. Silicon 2013, 5, 199–204. [Google Scholar] [CrossRef]
- Bokor, B.; Bokorová, S.; Ondoš, S.; Švubová, R.; Lukačová, Z.; Hýblová, M.; Szemes, T.; Lux, A. Ionome and Expression Level of Si Transporter Genes (Lsi1, Lsi2, and Lsi6) Affected by Zn and Si Interaction in Maize. Environ. Sci. Pollut. Res. 2015, 22, 6800–6811. [Google Scholar] [CrossRef]
- Markovich, O.; Steiner, E.; Kouřil, Š.; Tarkowski, P.; Aharoni, A.; Elbaum, R. Silicon Promotes Cytokinin Biosynthesis and Delays Senescence in Arabidopsis and Sorghum. Plant Cell Environ. 2017, 40, 1189–1196. [Google Scholar] [CrossRef]
- Agarie, S. Involvement of Silicon in the Senescence of Rice Leaves. Plant Prod. Sci. 1998, 1, 104–105. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of Plant Silicon for Crops: A Review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A Review of Silicon in Soils and Plants and Its Role in Us Agriculture: History and Future Perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Čermelj Mavrič, A.; Golob, A.; Vogel-Mikuš, K.; Germ, M. Silicon Mitigates Negative Impacts of Drought and UV-B Radiation in Plants. Plants 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant Senescence and Crop Productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Grašič, M. Mnogotere Vloge Silicija Izboljšajo Uspevanje Rastlin. Acta Biol. Slov. 2019, 62, 3–56. [Google Scholar]
- Podar, D. Plant Growth and Cultivation. Methods Mol. Biol. 2013, 953, 23–45. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Wang, S.; Liu, P.; Deng, X.; Yin, L.; Zhang, S. Silicon Moderated the K Deficiency by Improving the Plant-Water Status in Sorghum. Sci. Rep. 2016, 6, 22882. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Chang, C.; Tucker, M.L. To Grow Old: Regulatory Role of Ethylene and Jasmonic Acid in Senescence. Front. Plant Sci. 2015, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Schaller, J.; Puppe, D.; Busse, J.; Paasch, S.; Katz, O.; Brunner, E.; Kaczoreck, D.; Sommer, M. Silicification Patterns in Wheat Leaves Related to Ontogeny and Soil Silicon Availability under Field Conditions. Plant Soil 2022, 1–15. [Google Scholar] [CrossRef]
- Golob, A.; Kavčič, J.; Stibilj, V.; Gaberščik, A.; Vogel-Mikuš, K.; Germ, M. The Effect of Selenium and UV Radiation on Leaf Traits and Biomass Production in Triticum Aestivum L. Ecotoxicol. Environ. Saf. 2017, 136, 142–149. [Google Scholar] [CrossRef]
- Klančnik, K.; Vogel-Mikuš, K.; Gaberščik, A. Silicified Structures Affect Leaf Optical Properties in Grasses and Sedge. J. Photochem. Photobiol. B Biol. 2014, 130, 1–10. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Gaberščik, A. UV-B Radiation and Selenium Affected Energy Availability in Green Alga Zygnema. Biologia 2009, 64, 676–679. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, C.G.; Gomez, F.; Gergoff, G.; Guiamét, J.J.; Puntarulo, S. Up-Regulation of the Mitochondrial Alternative Oxidase Pathway Enhances Photosynthetic Electron Transport under Drought Conditions. J. Exp. Bot. 2005, 56, 1269–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, Y.; Mitani, N.; Yamaji, N.; Ma, J.F. HvLsi1 Is a Silicon Influx Transporter in Barley. Plant J. 2009, 57, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Prychid, C.J.; Rudall, P.J.; Gregory, M. Systematics and Biology of Silica Bodies in Monocotyledons. Bot. Rev. 2003, 69, 377–440. [Google Scholar] [CrossRef]
- Frick, D.A.; Remus, R.; Sommer, M.; Augustin, J.; Kaczorek, D.; Von Blanckenburg, F. Silicon Uptake and Isotope Fractionation Dynamics by Crop Species. Biogeosciences 2020, 17, 6475–6490. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Interaction between Calcium and Silicon in Water-Cultured Rice Plants. Plant Soil 1993, 148, 107–113. [Google Scholar] [CrossRef]
- Drew, M.C.; Biddulph, O. Effect of Metabolic Inhibitors and Temperature on Uptake and Translocation of 45 Ca and 42 K by Intact Bean Plants. Plant Physiol. 1971, 48, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Fleck, A.T.; Schulze, S.; Hinrichs, M.; Specht, A.; Waßmann, F.; Schreiber, L.; Schenk, M.K. Silicon Promotes Exodermal Casparian Band Formation in Si-Accumulating and Si-Excluding Species by Forming Phenol Complexes. PLoS ONE 2015, 10, e0138555. [Google Scholar] [CrossRef]
- Dishon, M.; Zohar, O.; Sivan, U. Effect of Cation Size and Charge on the Interaction between Silica Surfaces in 1:1, 2:1, and 3:1 Aqueous Electrolytes. Langmuir 2011, 27, 12977–12984. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Zheng, W.; Gao, Z.; Yin, C.; Li, T.; Liang, Y. Silicon Alleviates Salt Stress-Induced Potassium Deficiency by Promoting Potassium Uptake and Translocation in Rice (Oryza sativa L.). J. Plant Physiol. 2021, 258–259, 153379. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous Silicon (Si) Increases Antioxidant Enzyme Activity and Reduces Lipid Peroxidation in Roots of Salt-Stressed Barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y. Effects of Silicon on Enzyme Activity and Sodium, Potassium and Calcium Concentration in Barley under Salt Stress. Plant Soil 1999, 209, 217–224. [Google Scholar] [CrossRef]
- Carrasco-Gil, S.; Rodríguez-Menéndez, S.; Fernández, B.; Pereiro, R.; de la Fuente, V.; Hernandez-Apaolaza, L. Silicon Induced Fe Deficiency Affects Fe, Mn, Cu and Zn Distribution in Rice (Oryza Sativa L.) Growth in Calcareous Conditions. Plant Physiol. Biochem. PPB 2018, 125, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Apaolaza, L.; Escribano, L.; Zamarreño, Á.M.; García-Mina, J.M.; Cano, C.; Carrasco-Gil, S. Root Silicon Addition Induces Fe Deficiency in Cucumber Plants, but Facilitates Their Recovery After Fe Resupply. A Comparison With Si Foliar Sprays. Front. Plant Sci. 2020, 11, 1851. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The Controversies of Silicon’s Role in Plant Biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Inal, A.; Bagci, E.G.; Pilbeam, D.J. Silicon-Mediated Changes of Some Physiological and Enzymatic Parameters Symptomatic for Oxidative Stress in Spinach and Tomato Grown in Sodic-B Toxic Soil. Plant Soil 2007, 290, 103–114. [Google Scholar] [CrossRef]
- Soylemezoglu, G.; Demir, K.; Inal, A.; Gunes, A. Effect of Silicon on Antioxidant and Stomatal Response of Two Grapevine (Vitis Vinifera L.) Rootstocks Grown in Boron Toxic, Saline and Boron Toxic-Saline Soil. Sci. Hortic. 2009, 123, 240–246. [Google Scholar] [CrossRef]
- Xu, C.X.; Ma, Y.P.; Liu, Y.L. Effects of Silicon (Si) on Growth, Quality and Ionic Homeostasis of Aloe under Salt Stress. S. Afr. J. Bot. 2015, 98, 26–36. [Google Scholar] [CrossRef]
- Buchelt, A.C.; Teixeira, G.C.M.; Oliveira, K.S.; Rocha, A.M.S.; de Mello Prado, R.; Caione, G. Silicon Contribution Via Nutrient Solution in Forage Plants to Mitigate Nitrogen, Potassium, Calcium, Magnesium, and Sulfur Deficiency. J. Soil Sci. Plant Nutr. 2020, 20, 1532–1548. [Google Scholar] [CrossRef]
- Maillard, A.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.C.; Hosseini, S.A. Silicon Transcriptionally Regulates Sulfur and ABA Metabolism and Delays Leaf Senescence in Barley under Combined Sulfur Deficiency and Osmotic Stress. Environ. Exp. Bot. 2018, 155, 394–410. [Google Scholar] [CrossRef]
- Réthoré, E.; Ali, N.; Yvin, J.C.; Hosseini, S.A. Silicon Regulates Source to Sink Metabolic Homeostasis and Promotes Growth of Rice Plants Under Sulfur Deficiency. Int. J. Mol. Sci. 2020, 21, 3677. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Kühl, M.; Klimant, I.; Reising, H. Measurement of Chlorophyll Fluorescence within Leaves Using a Modified PAM Fluorometer with a Fiber-Optic Microprobe. Photosynth. Res. 1996, 47, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Monni, S.; Uhlig, C.; Hansen, E.; Magel, E. Ecophysiological Responses of Empetrum Nigrum to Heavy Metal Pollution. Environ. Pollut. 2001, 112, 121–129. [Google Scholar] [CrossRef]
- Packard, T.T. The Measurement of Respiratory Electron-Transport Activity Tn Marine Phytoplankton. J. Mar. Res. 1971, 29, 235–243. [Google Scholar]
- Kenner, R.A.; Ahmed, S.I. Measurements of Electron Transport Activities in Marine Phytoplankton. Mar. Biol. 1975, 33, 119–127. [Google Scholar] [CrossRef]
- Nečemer, M.; Kump, P.; Ščančar, J.; Jaćimović, R.; Simčič, J.; Pelicon, P.; Budnar, M.; Jeran, Z.; Pongrac, P.; Regvar, M.; et al. Original Research Article. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1240–1247. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrič Čermelj, A.; Fideršek, E.; Golob, A.; Kacjan Maršić, N.; Vogel Mikuš, K.; Germ, M. Different Concentrations of Potassium Silicate in Nutrient Solution Affects Selected Growth Characteristics and Mineral Composition of Barley (Hordeum vulgare L.). Plants 2022, 11, 1405. https://doi.org/10.3390/plants11111405
Mavrič Čermelj A, Fideršek E, Golob A, Kacjan Maršić N, Vogel Mikuš K, Germ M. Different Concentrations of Potassium Silicate in Nutrient Solution Affects Selected Growth Characteristics and Mineral Composition of Barley (Hordeum vulgare L.). Plants. 2022; 11(11):1405. https://doi.org/10.3390/plants11111405
Chicago/Turabian StyleMavrič Čermelj, Anja, Eva Fideršek, Aleksandra Golob, Nina Kacjan Maršić, Katarina Vogel Mikuš, and Mateja Germ. 2022. "Different Concentrations of Potassium Silicate in Nutrient Solution Affects Selected Growth Characteristics and Mineral Composition of Barley (Hordeum vulgare L.)" Plants 11, no. 11: 1405. https://doi.org/10.3390/plants11111405
APA StyleMavrič Čermelj, A., Fideršek, E., Golob, A., Kacjan Maršić, N., Vogel Mikuš, K., & Germ, M. (2022). Different Concentrations of Potassium Silicate in Nutrient Solution Affects Selected Growth Characteristics and Mineral Composition of Barley (Hordeum vulgare L.). Plants, 11(11), 1405. https://doi.org/10.3390/plants11111405







