The Citrus Mutant Jedae-unshiu Induced by Gamma Irradiation Exhibits a Unique Fruit Shape and Increased Flavonoid Content
Abstract
:1. Introduction
2. Results and Discussion
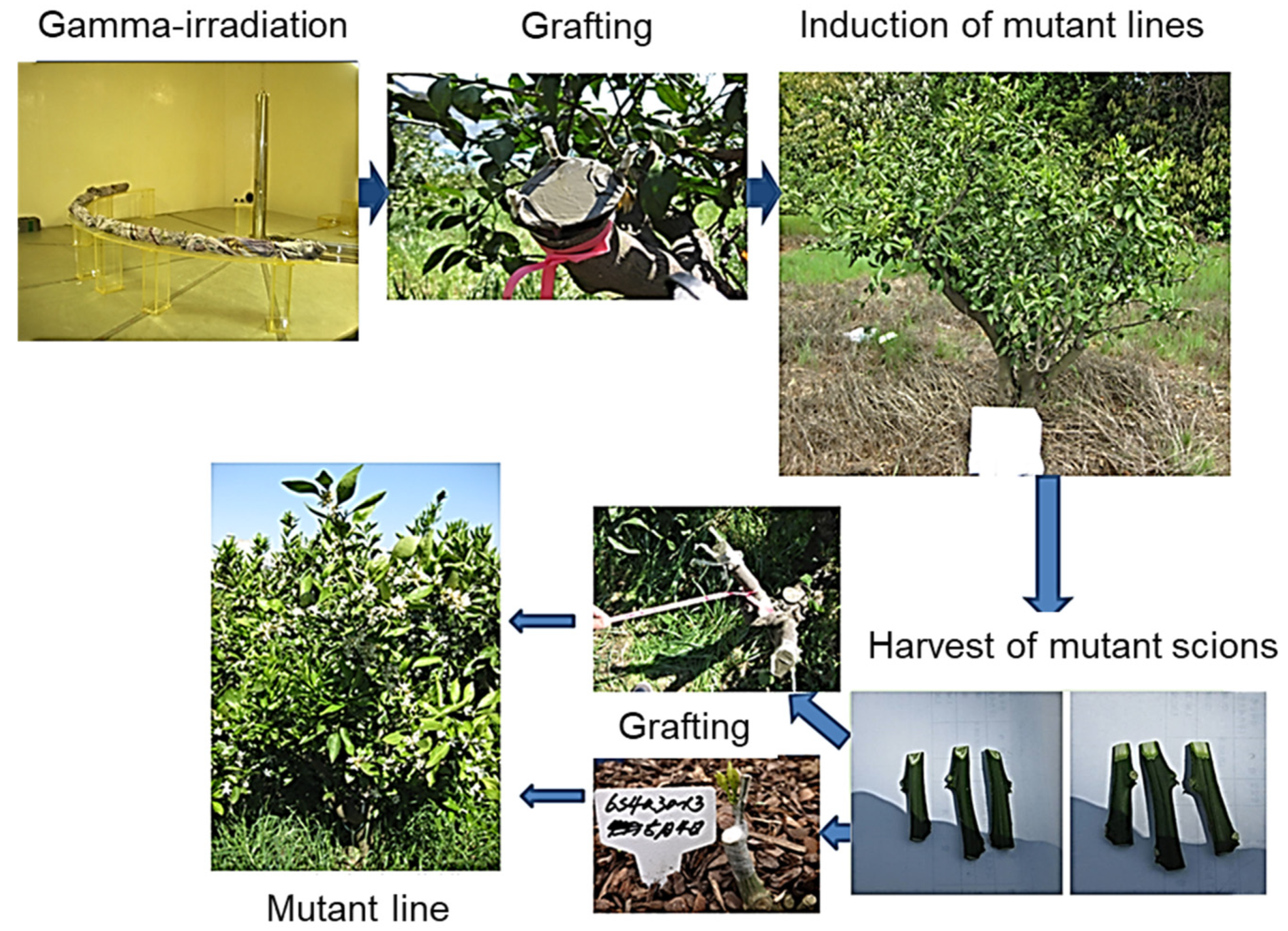
2.1. Selection of Mutant Lines Produced by Gamma Irradiation
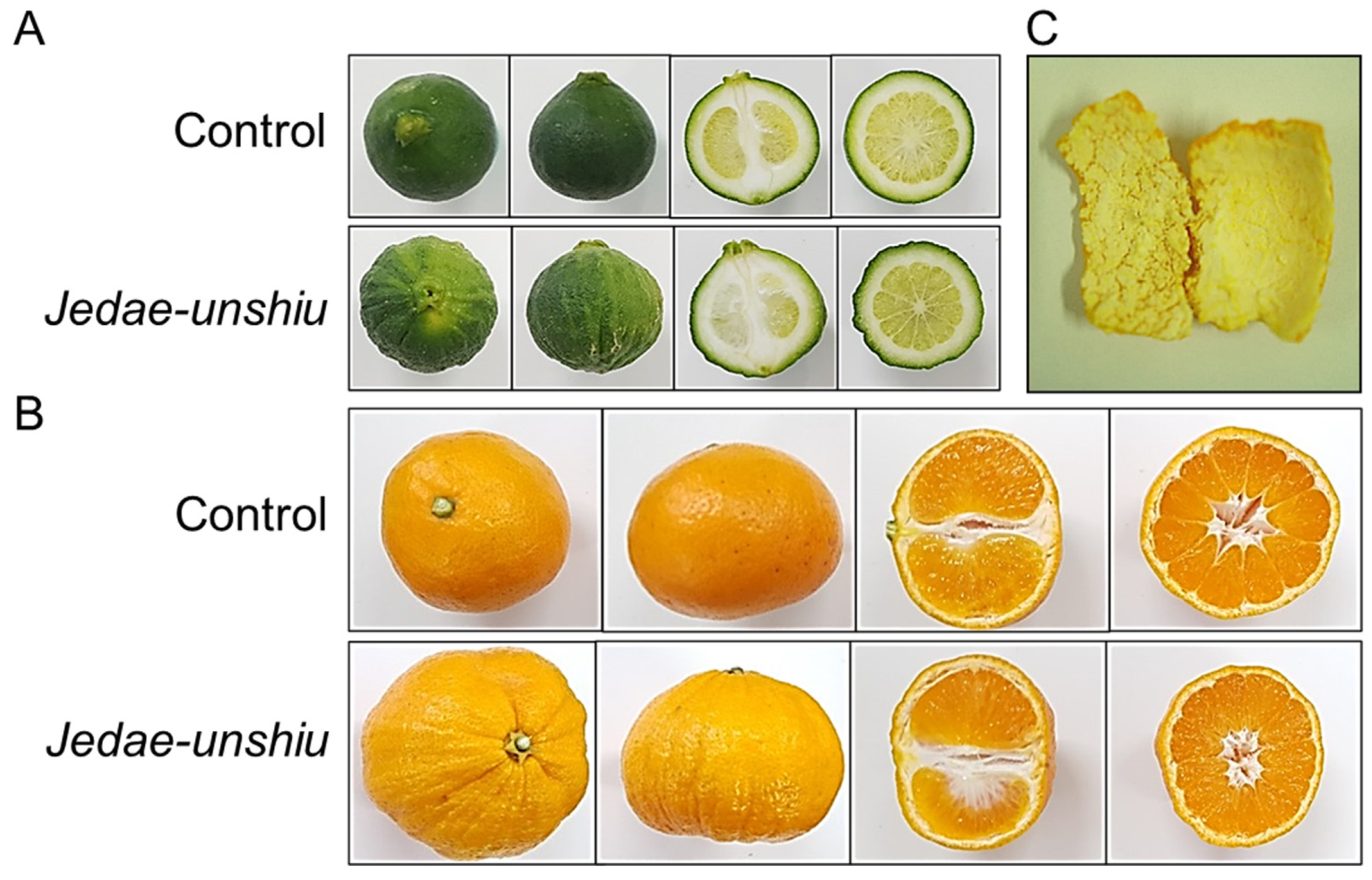
2.2. Comparison of External Morphological Traits between Jedae-unshiu and Control Fruits
2.3. Comparison of Flavonoid Compounds in Jedae-unshiu and Control Fruits
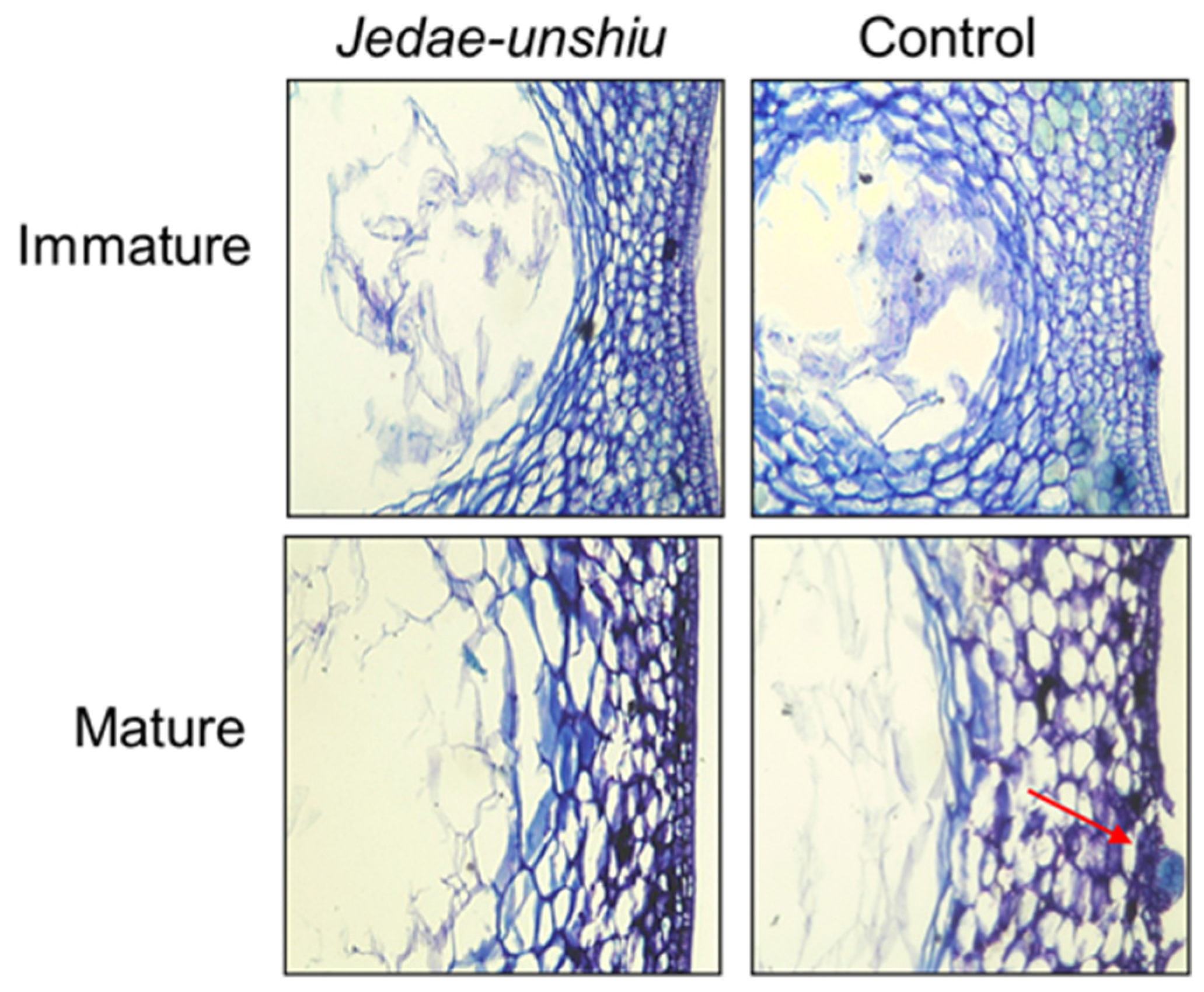
2.4. Cell-Level Microscopic Observations of Peels from Jedae-unshiu and Control Fruits
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Analysis of Fruit Traits
3.4. Detection of Flavonoid Components by High-Performance Liquid Chromatography (HPLC) Analysis
3.5. Microscopic Observation of Citrus Peels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, A.; Souza, E.H.; Souza, F.V.; Fadini, M.; Girardi, E.A.; Soares Filho, W.S. Genetic variation of Citrus and related genera with ornamental potential. Euphytica 2015, 205, 503–520. [Google Scholar] [CrossRef]
- Lamport, D.J.; Pal, D.; Macready, A.L.; Barbosa-Boucas, S.; Fletcher, J.M.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br. J. Nutr. 2016, 116, 2160–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budiarto, R.; Poerwanto, R.; Santosa, E.; Efendi, D. The Potentials of Limau (Citrus amblycarpa Hassk. Ochse) as A Functional Food and Ornamental Mini Tree based on Metabolomic and Morphological Approaches. J. Trop. Crop Sci. 2017, 4, 49–57. [Google Scholar] [CrossRef]
- Sottile, F.; Signore, M.B.D.; Barone, E. Ornacitrus: Citrus plants (Citrus spp.) as ornamentals. Folia Hort. 2019, 31, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tian, Z.; Chai, Y.; Yang, H.; Zhang, M.; Yang, C.; Xu, R.; Zhu, F.; Zeng, Y.; Deng, X.; et al. Cytological and proteomic evidence reveals the involvement of mitochondria in hypoxia-induced quality degradation in postharvest citrus fruit. Food Chem. 2019, 375, 131833. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Handayani, E.; Wakana, A.; Sato, M.; Miyamoto, M.; Miyazaki, R.; Zhou, X.; Sakai, K.; Mizunoe, Y.; Shigyo, M.; et al. Distribution and evolution of Citrus accessions with S3 and/or S11 alleles for self-incompatibility with an emphasis on sweet orange [Citrus sinensis (L.) Osbeck; SfS3 or SfS3sm]. Genet. Resour. Crop Evol. 2020, 67, 2101–2117. [Google Scholar] [CrossRef]
- Raveh, E.; Goldenberg, L.; Porat, R.; Carmi, N.; Gentile, A.; La Malfa, S. Conventional breeding of cultivated Citrus varieties. In The Citrus Genome. Compendium of Plant Genomes; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020; pp. 33–48. [Google Scholar]
- Ollitrault, P.; Ahmed, D.; Costantino, G.; Evrard, J.C.; Cardi, C.; Mournet, P.; Perdereau, A.; Froelicher, Y. Segregation distortion for male parents in high density genetic maps from reciprocal crosses between two self-incompatible cultivars confirms a gametophytic system for self-incompatibility in citrus. Agreculture 2021, 11, 379. [Google Scholar] [CrossRef]
- Mariana, B.D.; Arisah, H.; Yenni, Y.; Selvawajayant, M. Seedless fruit pummelo induced by Gamma Ray irradiation: Fruit morphological characters and stability evaluation. Biodiversitas 2018, 19, 706–711. [Google Scholar] [CrossRef]
- Mahmoud, G.A.; El-Tobgy, K.M.K.; Abo-El-Seoud, M. Application of combined biocides and gamma radiation for keeping good quality stored grapefruits. Archiv. Phytopath. Plant Protec. 2010, 43, 712–721. [Google Scholar] [CrossRef]
- Starrantino, A.; Russo, F.; Donini, B.; Spina, P. Lemon mutants obtained by gamma irradiation of the nucellus cultured in vitro. Proc. Int. Soc. Citric. 1988, 2, 231–235. [Google Scholar]
- Gulsen, O.; Uzun, A.; Pala, H.; Canilhos, E.; Kafa, G. Development of seedless and Mal seco tolerant mutant lemons through budwood irradiation. Sci. Hort. 2007, 112, 184–190. [Google Scholar] [CrossRef]
- Kim, I.J.; Kim, O.R.; Kim, H.W.; Lee, S.H.; Kim, K.; Lee, H.Y. Status of citrus mutation breeding with gamma ray irradiation. J. Subtrop. Agric. Biotechnol. 2008, 24, 37–42. [Google Scholar]
- Kim, I.J.; Song, S.Y.; Lee, H.Y. Putative citrus mutant induced by gamma ray irradiation. J. Asian Agric. Biotechnol. 2009, 25, 1–4. [Google Scholar]
- Kim, S.M.; Jo, Y.D.; Chun, J.I.; Kim, J.B.; Kang, J.H. Chronic Gamma Irradiation Changes Phenotype and Gene Expression Partially Transmitted to Next-Generation Tomato Seedlings. Agronomy 2021, 11, 1638. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, W.J.; Kim, S.H.; Lee, K.S.; Jo, H.J.; Kim, E.Y.; Kim, S.H.; Kang, S.Y.; Lee, J.H.; Ha, B.K. Characterization of genetic variation and antioxidant properties in strawberry (Fragaria x ananassa Duch.) mutant genotypes. Genet. Resour. Crop Evol. 2020, 67, 1457–1471. [Google Scholar] [CrossRef]
- Nogata, Y.; Salamoto, K.; Shiratsushi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oufedjikh, H.; Mahrouz, M.; Lacroix, M.; Amiot, M.J.; Taccini, M. The influence of gamma irradiation on flavonoïds content during storage of irradiated clementina. Radiat. Phys. Chem. 1998, 52, 107–112. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Jaafar, H.; Ibrahim, R.; Rahmat, A.; Aziz, M.A.; Philip, E. Effects of acute gamma irradiation on physiological traits and flavonoid accumulation of Centella asiatica. Molecules 2011, 16, 4994–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topuz, A.; Ozdemir, F. Influences of gamma irradiation and storage on the capsaicinoids of sun-dried and dehydrated paprika. Food Chem. 2004, 86, 509–515. [Google Scholar] [CrossRef]
- Senevirathne, M.; Kim, S.H.; Kim, Y.D.; Oh, C.K.; Oh, M.C.; Ahn, C.B.; Je, J.Y.; Lee, W.W.; Jeon, Y.J. Effect of far-infrared radiation drying of citrus press-cakes on free radical scavenging and antioxidant activities. J. Food Eng. 2010, 97, 168–176. [Google Scholar] [CrossRef]
| Year | Horizontal (mm) | Vertical (mm) | Weight (g) | Peel Thickness (mm) | Hardness (G) | |
|---|---|---|---|---|---|---|
| Control | 2018 | 52.13 ± 2.36 | 70.18 ± 2.26 | 129.36 ± 11.16 | 2.18 ± 0.37 | 679.25 ± 130.02 |
| 2019 | 51.75 ± 6.72 | 61.03 ± 7.94 | 97.46 ± 25.92 | 2.08 ± 0.31 | 615.90 ± 347.39 | |
| 2020 | 52.65 ± 1.66 | 61.48 ± 7.80 | 104.40 ± 7.99 | 2.65 ± 0.23 | 834.87 ± 62.47 | |
| Average | 51.73 ± 0.96 | 63.58 ± 4.40 | 105.69 ± 16.63 | 2.30 ± 0.30 | 710.01 ± 112.68 | |
| Jedae-unshiu | 2018 | 61.32 ± 11.18 | 68.80 ± 9.13 | 126.55 ± 28.56 | 3.45 ± 0.58 | 924.89 ± 379.10 |
| 2019 | 53.62 ± 5.94 | 64.75 ± 5.44 | 109.60 ± 19.72 | 2.15 ± 0.43 | 984.00 ± 308.50 | |
| 2020 | 48.46 ± 2.79 | 61.63 ± 3.72 | 90.22 ± 11.37 | 3.01 ± 0.85 | 1140.62 ± 117.11 | |
| Average | 53.06 ± 5.99 | 64.71 ± 3.02 | 105.60 ± 16.15 | 3.05 ± 0.65 * | 1016.50 ± 111.48 * | |
| Year | Sugar (Brix) | Acidity (wt%) | Hunter Color Value | |||
|---|---|---|---|---|---|---|
| L | a | b | ||||
| Control | 2018 | 9.29 ± 0.43 | 0.65 ± 0.08 | 61.60 ± 1.65 | 25.92 ± 1.42 | 37.16 ± 1.12 |
| 2019 | 7.35 ± 0.56 | 0.84 ± 0.09 | 59.23 ± 3.26 | 18.54 ± 2.82 | 35.56 ± 1.95 | |
| 2020 | 7.54 ± 0.79 | 0.85 ± 0.07 | 62.38 ± 1.92 | 24.79 ± 1.17 | 38.05 ± 0.58 | |
| Average | 8.28 ± 0.98 | 0.76 ± 0.10 | 62.445 ± 3.06 | 23.65 ± 3.44 | 44.30 ± 14.80 | |
| Jedae-unshiu | 2018 | 7.26 ± 0.44 | 0.68 ± 0.08 | 59.76 ± 3.46 | 22.84 ± 2.68 | 36.27 ± 2.16 |
| 2019 | 7.76 ± 0.61 | 0.78 ± 0.06 | 59.67 ± 3.64 | 18.51 ± 3.26 | 36.03 ± 2.68 | |
| 2020 | 7.28 ± 0.68 | 0.85 ± 0.10 | 64.45 ± 2.98 | 22.19 ± 2.65 | 35.07 ± 2.75 | |
| Average | 7.67 ± 0.54 | 0.74 ± 0.09 | 62.92 ± 3.95 | 21.92 ± 2.41 | 43.71 ± 15.85 | |
| HD | NRT | ||
|---|---|---|---|
| Peel | Control | 463.1 ± 1.11 | 60.8 ± 1.51 |
| Jedae-unshiu | 546.6 ± 3.38 * | 78.5 ± 0.61 * | |
| Flesh | Control | 178.8 ± 0.43 | 92.4 ± 0.59 |
| Jedae-unshiu | 276.3 ± 2.07 * | 132.2 ± 1.53 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eun, C.-H.; Kim, I.-J. The Citrus Mutant Jedae-unshiu Induced by Gamma Irradiation Exhibits a Unique Fruit Shape and Increased Flavonoid Content. Plants 2022, 11, 1337. https://doi.org/10.3390/plants11101337
Eun C-H, Kim I-J. The Citrus Mutant Jedae-unshiu Induced by Gamma Irradiation Exhibits a Unique Fruit Shape and Increased Flavonoid Content. Plants. 2022; 11(10):1337. https://doi.org/10.3390/plants11101337
Chicago/Turabian StyleEun, Chang-Ho, and In-Jung Kim. 2022. "The Citrus Mutant Jedae-unshiu Induced by Gamma Irradiation Exhibits a Unique Fruit Shape and Increased Flavonoid Content" Plants 11, no. 10: 1337. https://doi.org/10.3390/plants11101337
APA StyleEun, C.-H., & Kim, I.-J. (2022). The Citrus Mutant Jedae-unshiu Induced by Gamma Irradiation Exhibits a Unique Fruit Shape and Increased Flavonoid Content. Plants, 11(10), 1337. https://doi.org/10.3390/plants11101337







