Pollinaria Reconfiguration Mechanism of Widespread Euro-Mediterranean Orchids: The Effects of Increasing Air Temperature
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Pollinaria Reconfiguration Time
4.3. Air Temperature Effects
4.4. Pollinator Observation
4.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arditti, J. Fundamentals of Orchids Biology; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Matsuki, Y.; Tateno, R.; Shibata, M.; Isag, Y. Pollination efficiencies of flower-visiting insects as determined by direct genetic analysis of pollen origin. Am. J. Bot. 2008, 95, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Hesse, M. Types of pollen dispersal units in orchids, and their consequences for germination and fertilization. Ann. Bot. 2002, 89, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jersáková, J.; Johnson, S.D.; Kindlmann, P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 2006, 81, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P.; Schlüter, P.M. Floral isolation, specialized pollination, and pollinator behavior in orchids. Annu. Rev. Entomol. 2009, 54, 425–446. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, G.; Gargano, D.; Noce, M.; Musacchio, A. Reproductive biology and pollination limitation of Serapias vomeracea (Burm.) Briq. (Orchidaceae). Plant Species Biol. 2005, 20, 33–39. [Google Scholar] [CrossRef]
- Peter, C.I.; Johnson, S.D. Doing the twist, a test of Darwin’s cross-pollination hypothesis for pollinarium reconfiguration. Biol. Lett. 2006, 2, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Borba, E.L.; Semir, J. Temporal variation in pollinarium size after its removal in species of Bulbophyllum: A different mechanism preventing self-pollination in Orchidaceae. Plant Syst. Evol. 1999, 217, 197–204. [Google Scholar] [CrossRef]
- Catling, P.M.; Catling, V.R. Anther-cap retention inTipularia discolor. Lindleyana 1991, 6, 113–116. [Google Scholar]
- Xiaohua, J.; Dezhu, L.; Zongxin, R.; Xiaoguo, X. A generalized deceptive pollination system of Doritis pulcherrima (Aeridinae: Orchidaceae) with non-reconfigured pollinaria. BMC Plant Biol. 2012, 12, 67. [Google Scholar] [CrossRef] [Green Version]
- Darwin, C. On the Various Contrivances by Which British and Foreign Orchids Are Fertilised by Insects, and on the Good Effects of Intercrossing; John Murray: London, UK, 1877. [Google Scholar]
- Fritz, A.L. Deceit pollination of Orchis spitzelii (Orchidaceae) on the Island of Gotland in the Baltic: A suboptimal system. Nord. J. Bot. 1990, 9, 577–587. [Google Scholar] [CrossRef]
- Johnson, S.D.; Edwards, T.J. The structure and function of orchid pollinaria. Plant Syst. Evol. 2000, 222, 243–269. [Google Scholar] [CrossRef]
- Kullenberg, B. Studies in Ophrys pollination. Zool. Bidr. Fran Upps. 1961, 34, 1–340. [Google Scholar]
- Sletvold, N.; Grindeland, J.M.; Ågren, J. Vegetation context influences the strength and targets of pollinator-mediated selection in a deceptive orchid. Ecology 2013, 94, 1236–1242. [Google Scholar] [CrossRef] [Green Version]
- Ongaro, S.; Martellos, S.; Bacaro, G.; De Agostini, A.; Cogoni, A.; Cortis, P. Distributional pattern of Sardinian orchids under a climate change scenario. Community Ecol. 2018, 19, 223–232. [Google Scholar] [CrossRef]
- Pellegrino, G.; Mahmoudi, M.; Palermo, A.M. Pollen viability of Euro-Mediterranean orchids under different storage conditions: The possible effects of climate change. Plant Biol. 2021, 23, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.I.; Johnson, S.D. Generalized food deception: Colour signals and efficient pollen transfer in bee-pollinated species of Eulophia (Orchidaceae). Bot. J. Linn. 2013, 171, 713–729. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, L.A. Anthecology of Orchis mascula (Orchidaceae). Nord. J. Bot. 1983, 3, 157–179. [Google Scholar] [CrossRef]
- Molnár, A.; Tökölyi, J.; Végvári, Z.; Sramkó, G.; Sulyok, J.; Barta, Z. Pollination mode predicts phenological response to climate change in terrestrial orchids: A case study from central Europe. J. Ecol. 2012, 100, 1141–1152. [Google Scholar] [CrossRef]
- Robbirt, K.M.; Roberts, D.L.; Hutchings, M.J.; Davy, A.J. Potential disruption of pollination in a sexually deceptive orchid by climatic change. Curr. Biol. 2014, 24, 2845–2849. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, M.J.; Robbirt, K.M.; Roberts, D.L.; Davy, A.J. Vulnerability of a specialized pollination mechanism to climate change revealed by a 356-year analysis. Bot. J. Linn. 2018, 186, 498–509. [Google Scholar] [CrossRef]
- Vaknin, Y.; Disikowitch, D. Effects of short-term storage on germinability of pistachio pollen. Plant Breed. 2008, 119, 347–350. [Google Scholar] [CrossRef]
- Bellusci, F.; Musacchio, A.; Stabile, R.; Pellegrino, G. Differences in pollen viability in relation to different deceptive pollination strategies in Mediterranean orchids. Ann. Bot. 2010, 106, 769–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.M.; Ye, X.Q.; Jia, X.P.; Liang, L.J. Pollen germination in vitro and pollen tube growth of Jasminum sambac Aiton. Acta Agric. Bor. Sin. 2014, 29, 172–178. [Google Scholar]
- Descamps, C.; Jambrek, A.; Quinet, M.; Jacquermart, A.L. Warm temperatures reduce flower attractiveness and bumblebee foraging. Insects 2021, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Kühsel, S.; Blüthgen, N. High diversity stabilizes the thermal resilience of pollinator communities in intensively managed grasslands. Nat. Commun. 2015, 6, 7989. [Google Scholar] [CrossRef]
- Walsh, R.P.; Michaels, H.J. When it pays to cheat: Examining how generalized food deception increases male and female fitness in a terrestrial orchid. PLoS ONE 2017, 12, e0171286. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.D.; Peter, C.I.; Angren, J. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. R. Soc. 2004, 271, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Peter, C.I.; Johnson, S.D. Reproductive biology of Acrolophia cochlearis (Orchidaceae): Estimating rates of cross-pollination in epidendroid orchids. Ann. Bot. 2009, 104, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Schatz, B.; Genoud, D.; Escudié, P.; Geniez, P.; Wunsch, K.G.; Joffard, N. Is Ophrys pollination more opportunistic than previously thought? Insights from different field methods of pollinator observation. Bot. Lett. 2021, 128, 333–347. [Google Scholar] [CrossRef]
- Sletvold, N.; Grindeland, J.M.; Zu, P.; Ågren, J. Strong inbreeding depression and local outbreeding depression in the rewarding orchid Gymnadenia conopsea. Conserv. Genet. 2012, 13, 1305–1315. [Google Scholar] [CrossRef]
- Ostrowiecka, B.; Tałałaj, I.; Brzosko, E.; Jermakowicz, E.; Mirski, P.; Kostro-Ambroziak, A.; Mielczarek, K.; Lasoń, A.; Kupryjanowicz, J.; Kotowicz, J.; et al. Pollinators and visitors of the generalized food-deceptive orchid Dactylorhiza majalis in North-Eastern Poland. Biologia 2019, 74, 1247–1257. [Google Scholar] [CrossRef] [Green Version]
- Kropf, M.; Renner, S. Pollinator-mediated selfing in two deceptive orchids and a review of pollinium tracking studies addressing geitonogamy. Oecologia 2008, 155, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Niiniaho, J. The Role of Geitonogamy in the Reproduction Success of a Nectarless Dactylorhiza maculata (Orchidaceae). Master’s Thesis, University of Jyväskylä, Jyväskylä, Finland, 18 September 2011. [Google Scholar]
- Caloiero, T.; Callegari, G.; Cantasano, N.; Coletta, V.; Pellicone, G.; Veltri, A. Bioclimatic analysis in a region of southern Italy (Calabria). Plant Biosyst. 2016, 150, 1282–1295. [Google Scholar] [CrossRef]
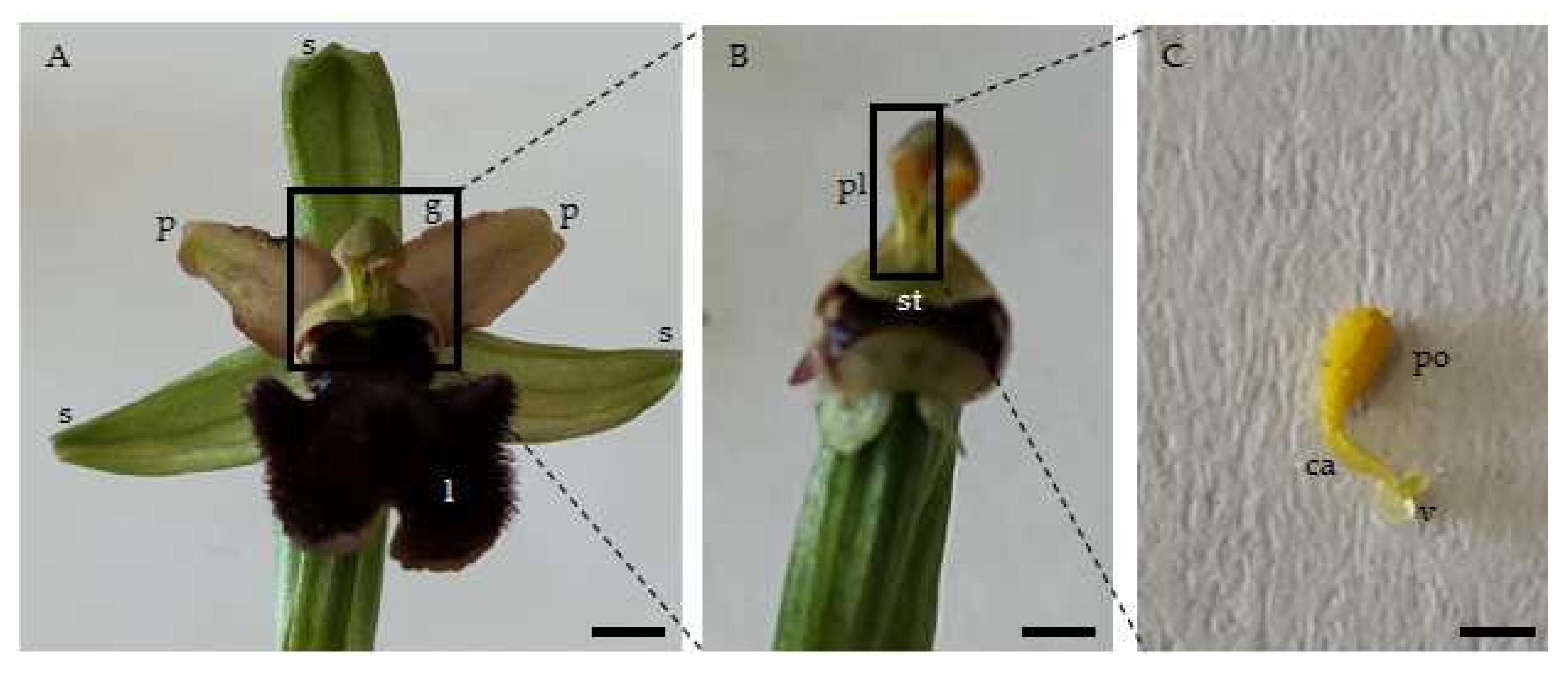
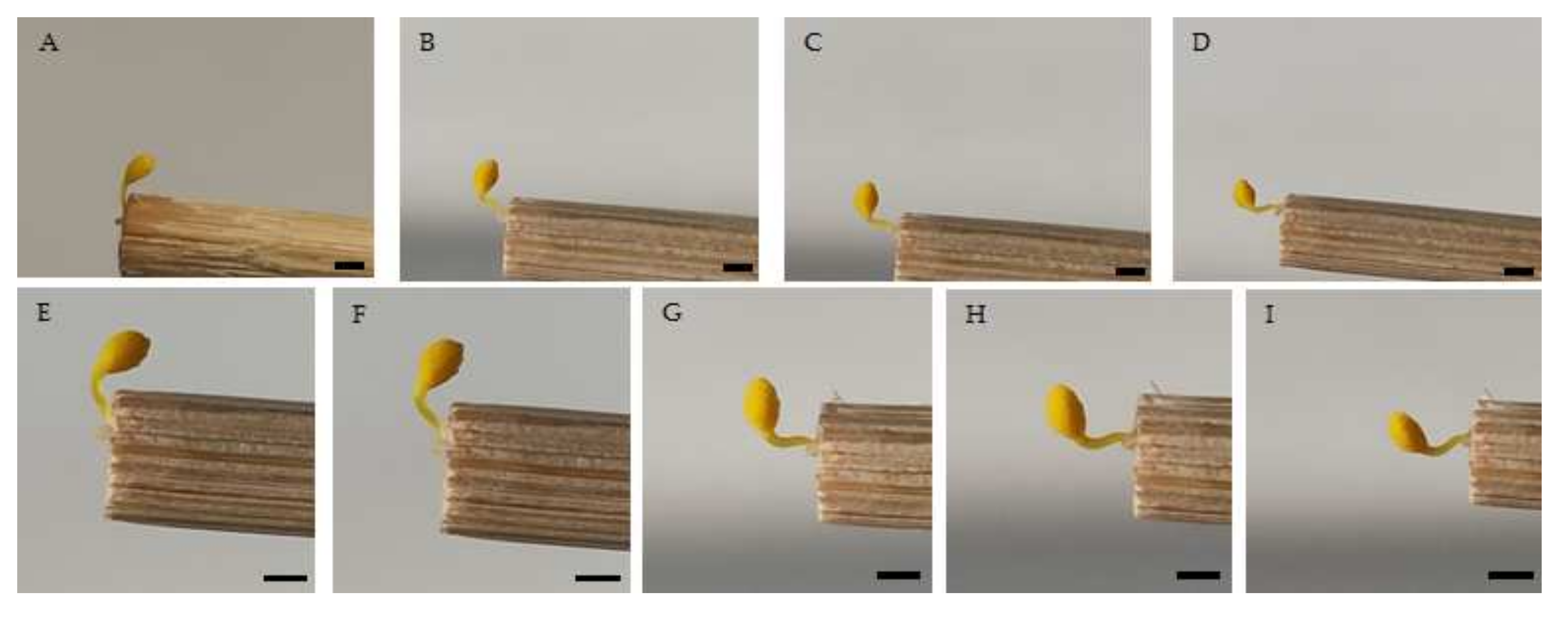
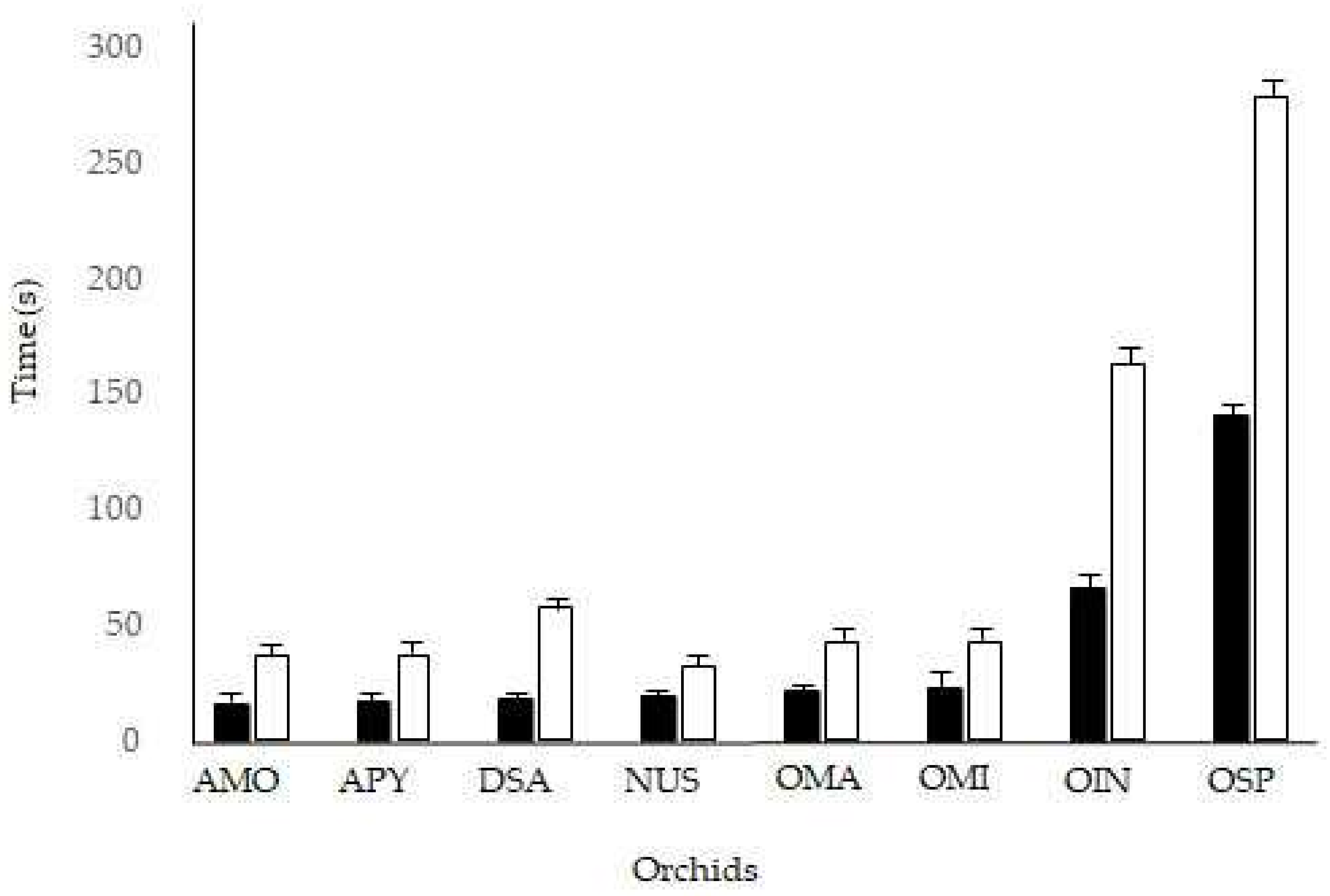
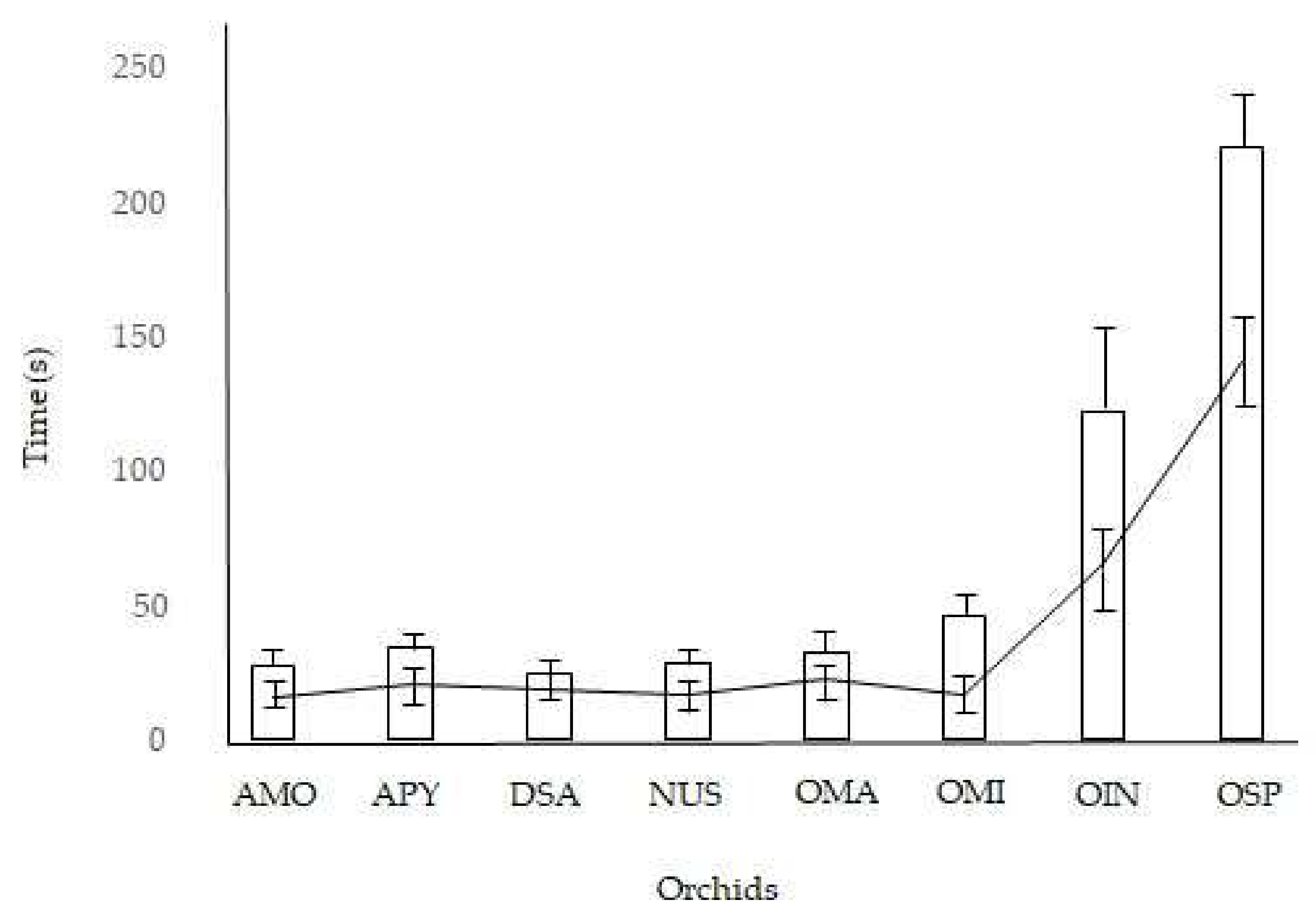
| Taxon | Reconfiguration Time (s) n = 160 | Pollinator Visit Time (s) n = 80 | ||||
|---|---|---|---|---|---|---|
| min | max | mean ± SE | min | max | Mean ± SE | |
| Anacamptis morio | 35 | 38 | 36.5 ± 1.2 | 12 | 20 | 15.6 ± 2.6 |
| Anacamptis pyramidalis | 40 | 45 | 43.2 ± 1.7 | 16 | 28 | 21.2 ± 3.7 |
| Dactylorhiza sambucina | 30 | 35 | 32.5 ± 1.9 | 15 | 22 | 18.9 ± 2.6 |
| Neotinea ustulata | 35 | 40 | 37.3 ± 2.1 | 12 | 22 | 17.0 ± 3.0 |
| Ophrys sphegodes | 150 | 172 | 163.3 ± 7.4 | 50 | 82 | 65.6 ± 9.8 |
| Ophrys insectifera | 250 | 310 | 279.1 ± 19.6 | 120 | 158 | 141.1 ± 10.6 |
| Orchis mascula | 40 | 46 | 43.0 ± 2.5 | 18 | 28 | 22.6 ± 3.5 |
| Orchis militaris | 55 | 62 | 57.8 ± 2.4 | 12 | 24 | 17.5 ± 3.7 |
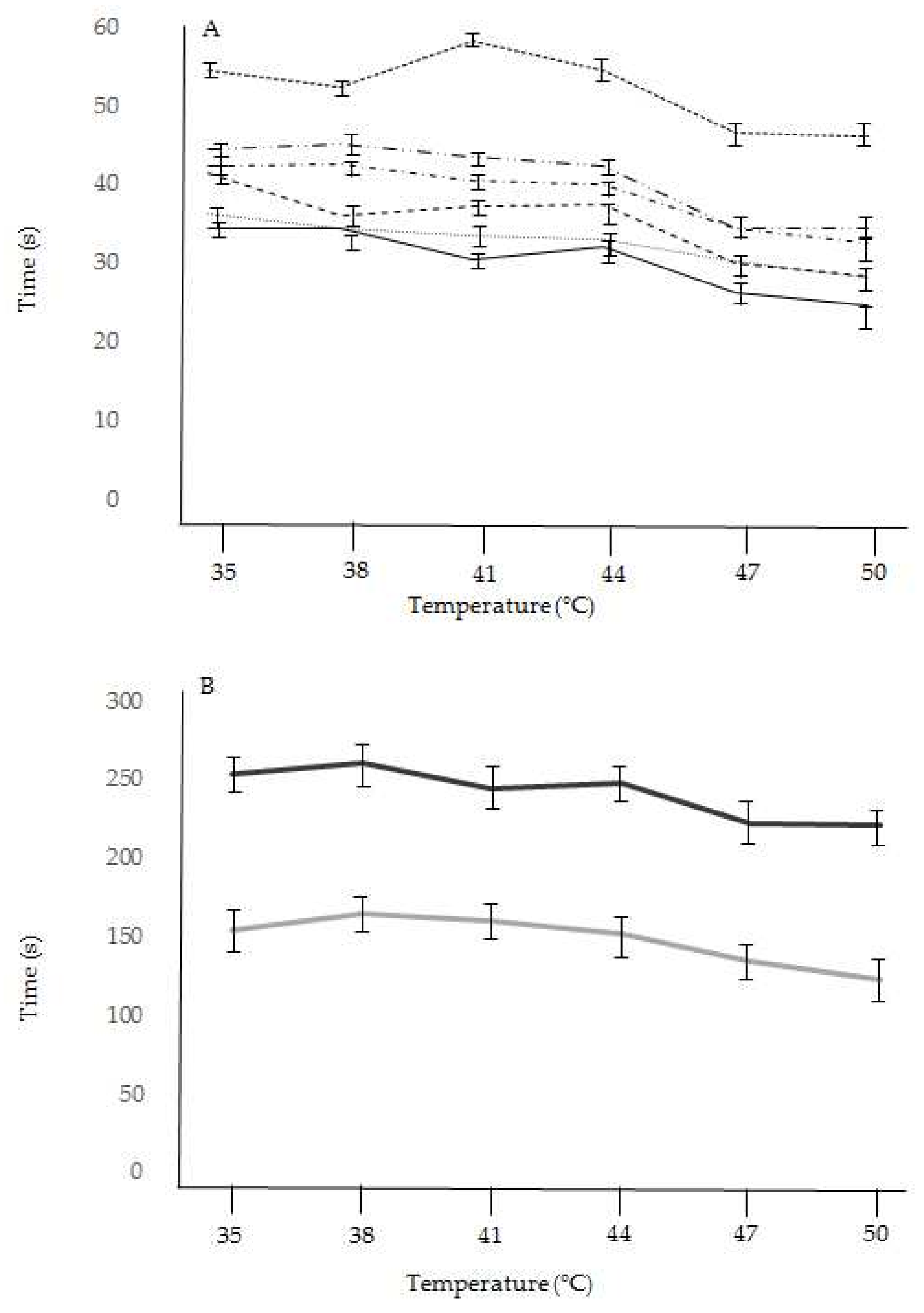
| Taxon (n = 240) | Temperature | |||||
|---|---|---|---|---|---|---|
| 35 °C | 38 °C | 41 °C | 44 °C | 47 °C | 50 °C | |
| Anacamptis morio | 36.2 ± 1.2 | 34.4 ± 1.1 | 33.5 ± 1.3 | 33.0 ± 1.2 | 30.1 ± 1.2 | 28.2 ± 1.4 |
| Anacamptis pyramidalis | 44.5 ± 1.6 | 45.2 ± 1.6 | 43.5 ± 1.7 | 42.3 ± 1.5 | 34.4 ± 1.6 | 34.3 ± 1.5 |
| Dactylorhiza sambucina | 34.5 ± 1.7 | 34.3 ± 1.5 | 30.5 ± 1.4 | 32.0 ± 1.6 | 26.3 ± 1.7 | 24.5 ± 1.3 |
| Neotinea ustulata | 41.5 ± 2.2 | 36.0 ± 1.9 | 37.3 ± 1.8 | 37.5 ± 1.8 | 30.0 ± 1.6 | 28.3 ± 1.4 |
| Ophrys sphegodes | 154.5 ± 5.2 | 164.3 ± 6.2 | 160.5 ± 5.5 | 152.0 ± 4.2 | 134.3 ± 4.8 | 122.5 ± 4.2 |
| Ophrys insectifera | 254.0 ± 11.2 | 260.5 ± 10.6 | 244.2 ± 10.2 | 248.3 ± 11.4 | 222.5 ± 10.9 | 220.5 ± 11.2 |
| Orchis mascula | 42.3 ± 2.2 | 42.5 ± 2.3 | 40.5 ± 2.1 | 40.0 ± 1.9 | 34.5 ± 2.2 | 32.5 ± 1.9 |
| Orchis militaris | 54.5 ± 2.3 | 52.3 ± 2.4 | 58.2 ± 2.1 | 54.5 ± 2.2 | 46.5 ± 2.1 | 46.2 ± 2.0 |
| Species | Sampling Location * | Pollination Syndrome | Predominant Pollinators |
|---|---|---|---|
| Anacamptis morio | Mangone | Food deception | Bumblebees |
| Cupone | |||
| Anacamptis pyramidalis | Acquaformosa | Food deception | Butterflies |
| Cassano | |||
| Dactylorhiza sambucina | Carlo Magno | Food deception | Bumblebees |
| Botte Donato | |||
| Neotinea ustulata | Petrosa | Food deception | Tachinid flies |
| Firmo | |||
| Ophrys insectifera | Cassano | Sexual deception | Wasps |
| Petrosa | |||
| Ophrys sphegodes | Piano Monello | Sexual deception | Sand bees |
| Piano Lago | |||
| Orchis mascula | Cupone | Food deception | Cuckoo bumblebees |
| Cecita | and solitary bees | ||
| Orchis militaris | Rogliano | Food deception | Cuckoo bumblebees |
| Frascineto | and solitary bees |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzino, M.; Palermo, A.M.; Pellegrino, G. Pollinaria Reconfiguration Mechanism of Widespread Euro-Mediterranean Orchids: The Effects of Increasing Air Temperature. Plants 2022, 11, 1327. https://doi.org/10.3390/plants11101327
Lanzino M, Palermo AM, Pellegrino G. Pollinaria Reconfiguration Mechanism of Widespread Euro-Mediterranean Orchids: The Effects of Increasing Air Temperature. Plants. 2022; 11(10):1327. https://doi.org/10.3390/plants11101327
Chicago/Turabian StyleLanzino, Micaela, Anna Maria Palermo, and Giuseppe Pellegrino. 2022. "Pollinaria Reconfiguration Mechanism of Widespread Euro-Mediterranean Orchids: The Effects of Increasing Air Temperature" Plants 11, no. 10: 1327. https://doi.org/10.3390/plants11101327
APA StyleLanzino, M., Palermo, A. M., & Pellegrino, G. (2022). Pollinaria Reconfiguration Mechanism of Widespread Euro-Mediterranean Orchids: The Effects of Increasing Air Temperature. Plants, 11(10), 1327. https://doi.org/10.3390/plants11101327







