Whole-Genome Resequencing of Near-Isogenic Lines Reveals a Genomic Region Associated with High Trans-Lycopene Contents in Watermelon
Abstract
:1. Introduction
2. Results
2.1. Lycopene Contents Analysis
2.2. WGRS
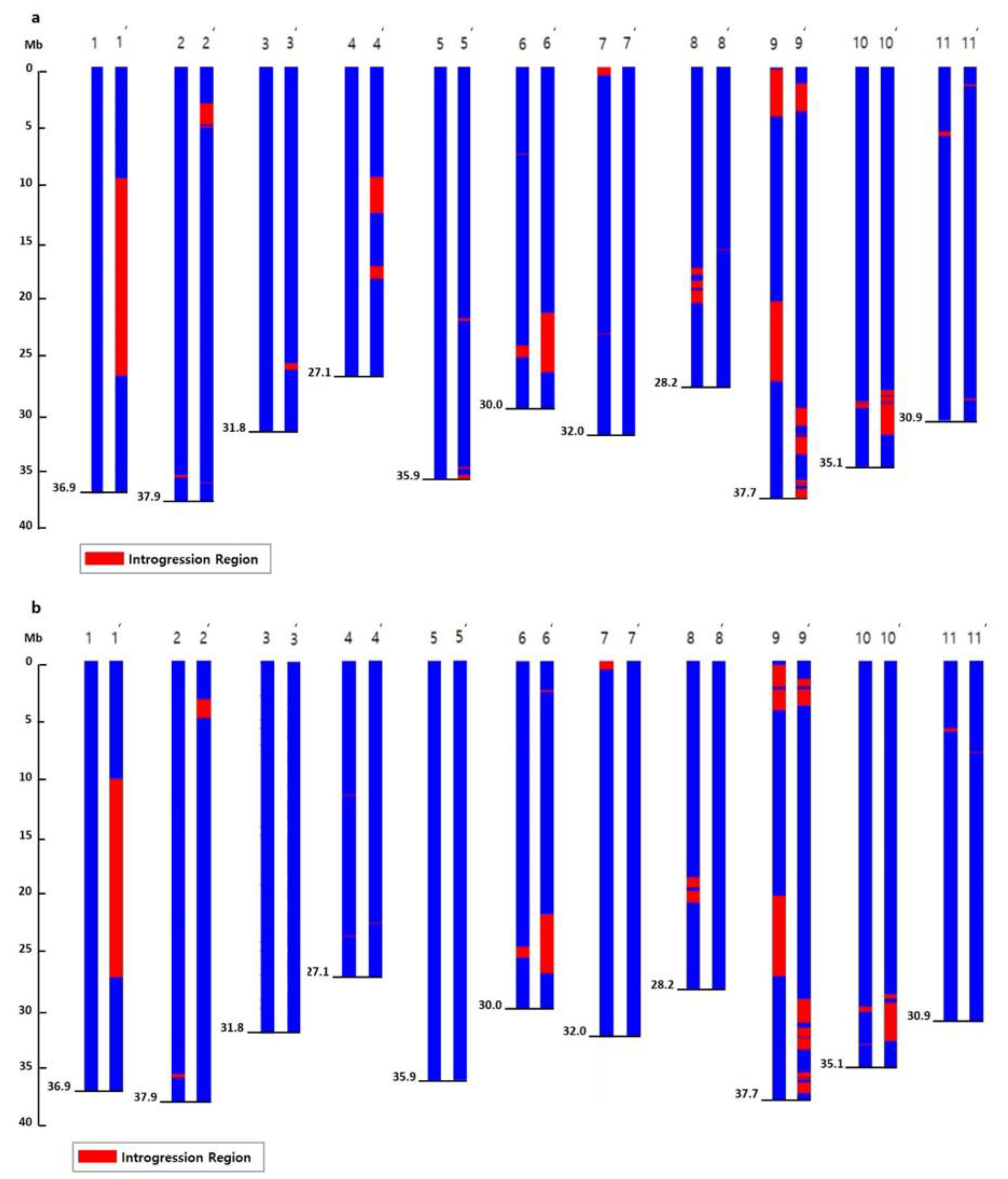
2.3. Detection of SNPs and Introgressed Regions in NILs
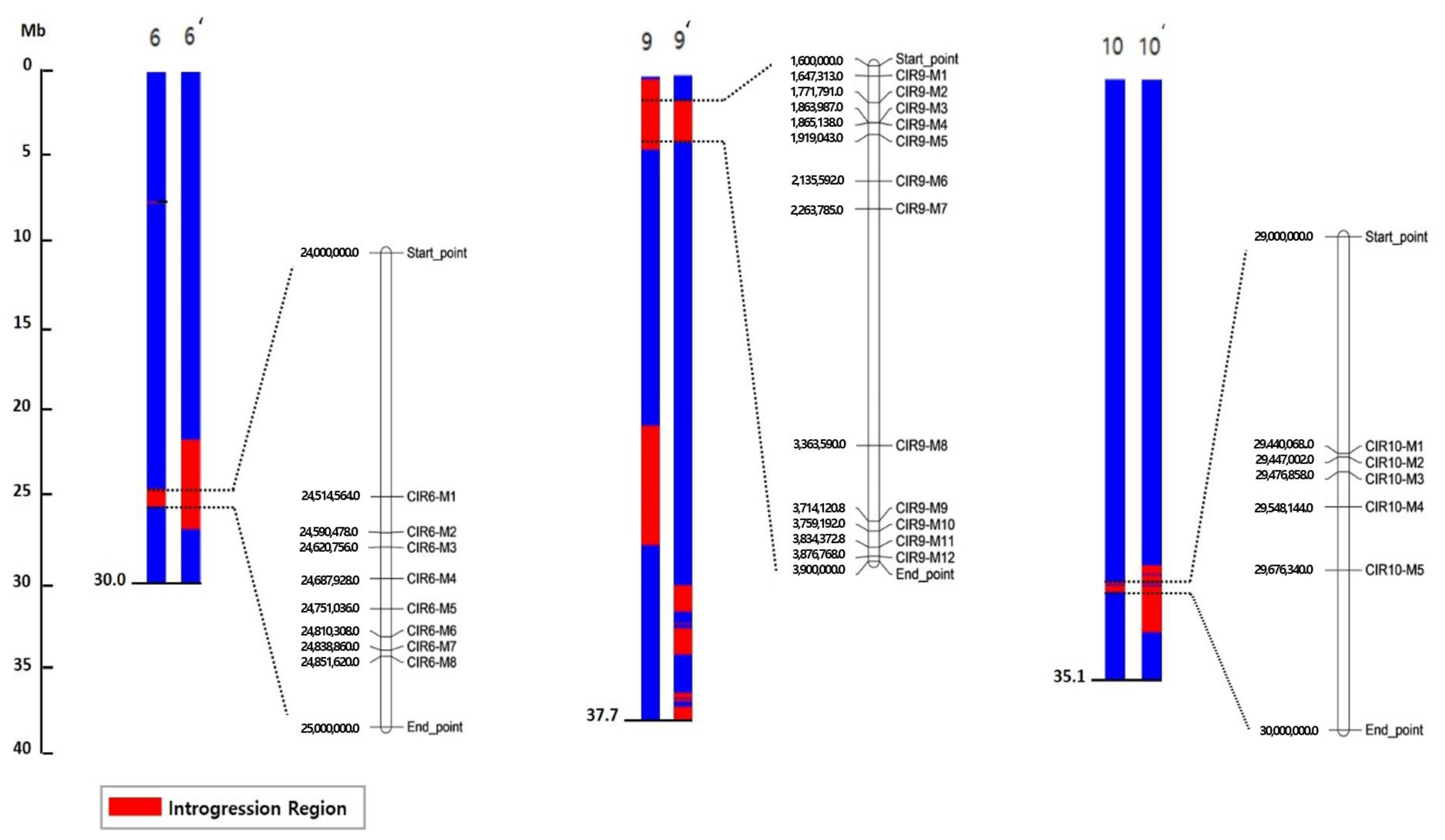
2.4. Detection of Common Introgressed Regions (CIRs) and SNPs
2.5. Development of CAPS Markers Associated with HL Contents
2.6. Gene Annotation Analysis
3. Discussion
4. Materials and Methods
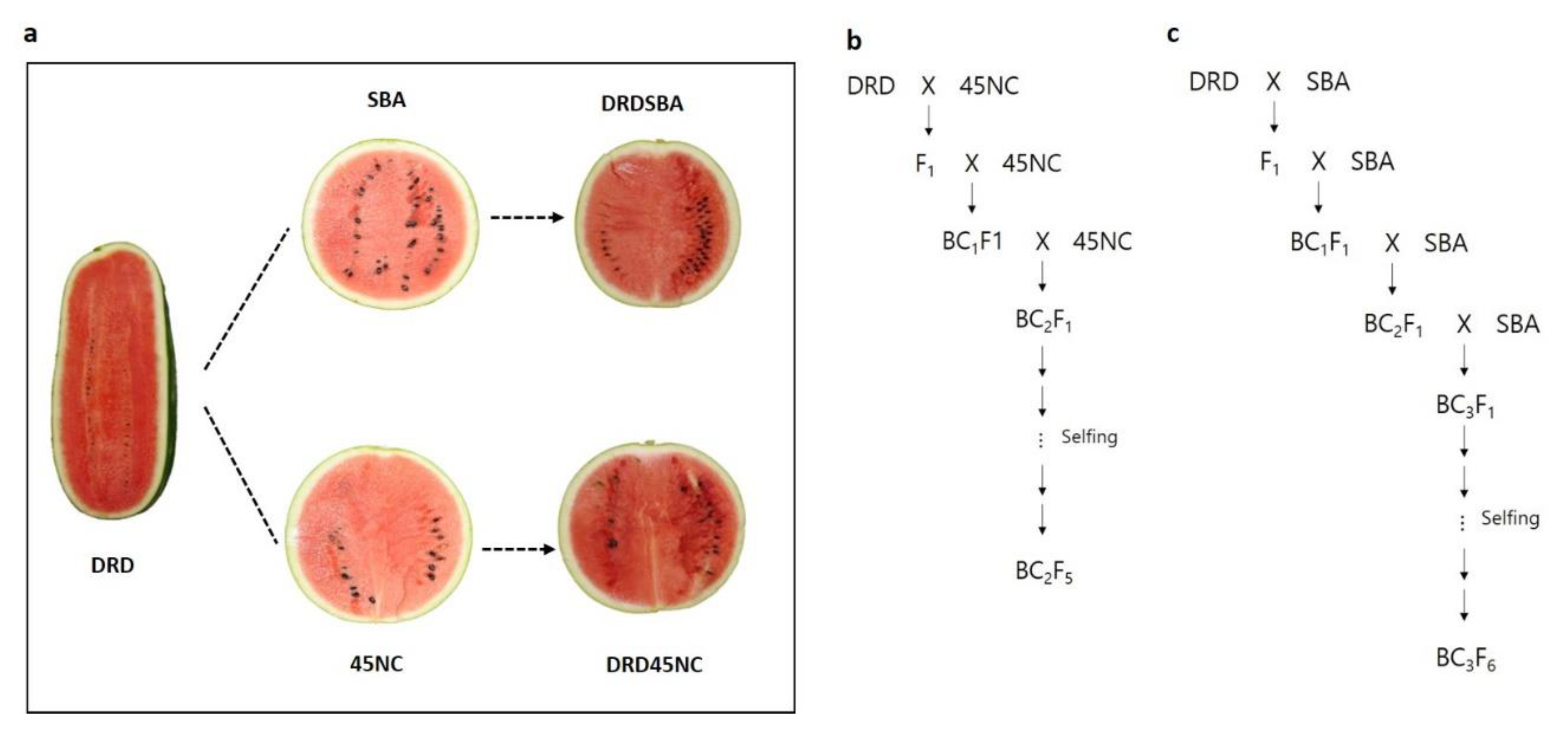
4.1. Plant Materials
4.2. Planting and Harvesting
4.3. Lycopene Contents Analysis
4.4. WGRS Based on NGS
4.5. Detection of Introgressed Regions
4.6. Development of Molecular Markers Associated with HL Contents
4.7. Gene Annotation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akashi, K.; Mifune, Y.; Morita, K.; Ishitsuka, S.; Tsujimoto, H.; Ishihara, T. Spatial accumulation pattern of citrulline and other nutrients in immature and mature watermelon fruits. J. Sci. Food Agric. 2017, 97, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Cho, M.-C.; Lee, J.G. Genotypic variation in carotenoid, ascorbic acid, total phenolic, and flavonoid contents, and antioxidant activity in selected tomato breeding lines. Hortic. Environ. Biotechnol. 2016, 57, 440–452. [Google Scholar] [CrossRef]
- Mohanty, N.K.; Saxena, S.; Singh, U.P.; Goyal, N.K.; Arora, R.P. Lycopene as a chemopreventive agent in the treatment of high-grade prostate intraepithelial neoplasia. Urol. Oncol. 2005, 23, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lv, P.; Gu, H. Studies on carotenoids in watermelon flesh. Agric. Sci. 2013, 4, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, H.; Dai, Z.; Liu, X.; Liu, Y.; Deng, X.; Chen, F.; Xu, J. Volatile chemical and carotenoid profiles in watermelons [Citrullus vulgaris (Thunb.) Schrad (Cucurbitaceae)] with different flesh colors. Food Sci. Biotechnol. 2012, 21, 531–541. [Google Scholar] [CrossRef]
- Bang, H.; Kim, S.; Leskovar, D.; King, S. Genotype analysis of fruit color using a molecular marker in watermelon [Citrullus lanatus (Thunb.) Matsum & Nakai]. HortScience 2005, 40, 1114. [Google Scholar] [CrossRef]
- Tadmor, Y.; King, S.; Levi, A.; Davis, A.; Meir, A.; Wasserman, B.; Hirschberg, J.; Lewinsohn, E. Comparative fruit colouration in watermelon and tomato. Food Res. Int. 2005, 38, 837–841. [Google Scholar] [CrossRef]
- Grassi, S.; Piro, G.; Lee, J.; Zheng, Y.; Fei, Z.; Dalessandro, G.; Giovannoni, J.; Lenucci, M. Comparative genomics reveals candidate carotenoid pathway regulators of ripening watermelon fruit. BMC Genom. 2013, 14, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusmini, G.; Wehner, T. Qualitative inheritance of rind pattern and flesh color in watermelon. J. Hered. 2006, 97, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.; Lee, J.; Kweon, S.; Cho, Y.; Choi, Y.; Lee, S.J.; Park, Y. Analysis of flesh color-related carotenoids and development of a CRTISO gene-based DNA marker for prolycopene accumulation in watermelon. Hortic. Environ. Biotechnol. 2019, 60, 399–410. [Google Scholar] [CrossRef]
- Branham, S.; Vexler, L.; Meir, A.; Tzuri, G.; Frieman, Z.; Levi, A.; Wechter, W.; Tadmor, Y.; Gur, A. Genetic mapping of a major codominant QTL associated with β-carotene accumulation in watermelon. Mol. Breed. 2017, 37, 1–13. [Google Scholar] [CrossRef]
- Bang, H.; Kim, S.; Leskovar, D.; King, S. Development of a codominant CAPS marker for allelic selection between canary yellow and red watermelon based on SNP in lycopene β-cyclase (LCYB) gene. Mol. Breed. 2007, 20, 63–72. [Google Scholar] [CrossRef]
- Glover, K.; Wang, D.; Arelli, P.; Carlson, S.; Cianzio, S.; Diers, B.W. Near isogenic lines confirm a soybean cyst nematode resistance gene from PI 88788 on linkage group J. Crop. Sci. 2004, 44, 936–941. [Google Scholar] [CrossRef]
- Maughan, J.; Maroof, S.; Buss, G.; Huestis, G. Amplified fragment length polymorphism (AFLP) in soybean: Species diversity, inheritance, and near-isogenic line analysis. Theor. Appl. Genet. 1996, 93, 392–401. [Google Scholar] [CrossRef]
- Samsampour, D.; Zanjani, B.; Jk, P.; Singh, A.; Charpe, A.; Gupta, S.; Prabhu, K. Identification of molecular markers linked to adult plant leaf rust resistance gene Lr48 in wheat and detection of Lr48 in the thatcher near-isogenic line with gene Lr25. Euphytica 2010, 174, 337–342. [Google Scholar] [CrossRef]
- Muehlbauer, G.; Specht, J.; Staswick, P.; Graef, G.; Thomas-Compton, M. Application of the near-isogenic line gene mapping technique to isozyme markers. Crop. Sci. 1989, 29, 1548–1553. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, S.; Lu, X.; He, N.; Zhu, H.; Dou, J.; Wenge, L. Comparative transcriptome analysis reveals key genes potentially related to soluble sugar and organic acid accumulation in watermelon. PLoS ONE 2018, 13, e0190096:1–e0190096:22. [Google Scholar] [CrossRef]
- Han, B.K.; Rhee, S.J.; Jang, Y.J.; Sim, T.Y.; Kim, Y.J.; Park, T.S.; Lee, G. Identification of a causal pathogen of watermelon powdery mildew in Korea and development of a genetic linkage marker for resistance in watermelon (Citrullus lanatus). Hortic. Sci. Technol. 2016, 34, 912–923. [Google Scholar] [CrossRef]
- Varshney, R.; Hoisington, D.; Nayak, S.; Graner, A. Molecular plant breeding: Methodology and achievements. Plant. Genome 2009, 513, 283–304. [Google Scholar] [CrossRef] [Green Version]
- Voelkerding, K.; Dames, S.; Durtschi, J. Next generation sequencing for clinical diagnostics-principles and application to targeted resequencing for hypertrophic cardiomyopathy: A paper from the 2009 william beaumont hospital symposium on molecular pathology. J. Mol. Diagn. 2010, 12, 539–551. [Google Scholar] [CrossRef]
- Kang, B.; Zhao, W.E.; Hou, Y.; Tian, P. Expression of carotenogenic genes during development and ripening of watermelon fruit. Sci. Hortic. 2010, 124, 368–375. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Xu, J.; Zhang, M.; Hou, Q.; Zhu, L.; Huang, Y.; Xiong, A.-S. Morphological observation, RNA-Seq quantification, and expression profiling: Novel insight into grafting-responsive carotenoid biosynthesis in watermelon grafted onto pumpkin rootstock. Acta Biochim. Biophys. Sin. 2016, 49, 216–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, P.; Li, N.; Liu, H.; Gu, H.; Zhao, W.E. Changes in carotenoid profiles and in the expression pattern of the genes in carotenoid metabolisms during fruit development and ripening in four watermelon cultivars. Food Chem. 2014, 174, 52–59. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Z.; Gerendas, J.; Zimmermann, N. Glucosinolates in chinese Brassica campestris vegetables: Chinese cabbage, purple cai-tai, choysum, pakchoi, and turnip. HortScience 2008, 43, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Saleh, B.K.; Omer, A.; Teweldemedhin, B. Medicinal uses and health benefits of chili pepper (Capsicum spp.): A review. MOJ Food Process. Technol. 2018, 6, 325–328. [Google Scholar] [CrossRef]
- Kumar, R.; Subramanian, V.; Nadanasabapathi, S. Health benefits of quercetin. Def. Life Sci. J. 2017, 2, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions? A global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef]
- Jan, A.T.; Kamli, M.; Murtaza, I.; Singh, J.; Ali, A.; Haq, Q. Dietary flavonoid quercetin and associated health benefits—An overview. Food Rev. Int. 2010, 26, 302–317. [Google Scholar] [CrossRef]
- Perkins, P.; Davis, A. In search of high lycopene watermelon. Rep. Cucurbit Genet. Coop. 2004, 27, 51–53. [Google Scholar]
- Wang, C.; Qiao, A.; Fang, X.; Sun, L.; Gao, P.; Davis, A.; Liu, S.; Luan, F. Fine mapping of lycopene content and flesh color related gene and development of molecular marker–assisted selection for flesh color in watermelon (Citrullus lanatus). Front. Plant. Sci. 2019, 10, 1240. [Google Scholar] [CrossRef]
- Edwards, A.; Vinyard, B.; Wiley, E.; Brown, E.; Collins, J.; Perkins, P.; Baker, R.; Clevidence, B. Consumption of watermelon juice increases plasma concentrations of lycopene and β-carotene in humans. J. Nutr. 2003, 133, 1043–1050. [Google Scholar] [CrossRef]
- Via, F.; Gomes, S.; Costa, P.; Campos, M.; Tonon, R.; Couri, S.; Cabral, L. Watermelon juice pretreatment with microfiltration process for obtaining lycopene. Food Sci. Technol. Int. 2013, 48, 601–608. [Google Scholar] [CrossRef]
- Maoto, M.; Beswa, D.; Jideani, A. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Sreelakshmi, A.; Vidhya, D. Preparation of nutrient rich jam using watermelon (Citrullus Lanatus) and hibiscus extract. IJPRSE 2020, 1, 75–78. [Google Scholar]
- Perkins, P.; Collins, J. Flesh quality and lycopene stability of fresh-cut watermelon. Postharvest Biol. Technol. 2004, 31, 159–166. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Azulay, Y.; Meir, A.; Zamir, D.; Tadmor, Y. Carotenoid pigmentation affects the volatile composition of tomato and watermelon fruits, as revealed by comparative genetic analyses. J. Agric. Food Chem. 2005, 53, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Wehner, T.; Naegele, R.; Perkins, P. Heritability and genetic variance components associated with citrulline, arginine, and lycopene content in diverse watermelon cultigens. HortScience 2017, 52, 936–940. [Google Scholar] [CrossRef] [Green Version]
- Perkins, P.; Collins, J.; Pair, S.; Roberts, W. Lycopene content differs among red-fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 81, 983–987. [Google Scholar] [CrossRef]
- Li, N.; Shang, J.; Wang, J.; Zhou, D.; Li, N.; Ma, S. Discovery of the genomic region and candidate genes of the scarlet red flesh color (Yscr) locus in watermelon (Citrullus lanatus L.). Front. Plant. Sci. 2020, 11, 116. [Google Scholar] [CrossRef]
- Collard, B.; Cruz, C.; McNally, K.; Virk, P.; Mackill, D. Rice molecular breeding laboratories in the genomics era: Current status and future considerations. Int. J. Plant. Genom. 2008, 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pumphrey, M.; Bernardo, R.; Anderson, J.A. Validating the QTL for fusarium head blight resistance in near-isogenic wheat lines developed from breeding populations. Crop. Sci. 2007, 47, 200–206. [Google Scholar] [CrossRef]
- Fletcher, R.; Mullen, J.; Yoder, S.; Bauerle, W.; Reuning, G.; Sen, S.; Meyer, E.; Juenger, T.; McKay, J. Development of a next-generation NIL library in arabidopsis thaliana for dissecting complex traits. BMC Genom. 2013, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Yang, T.-J.; Park, Y.-H.; Lee, Y.-J.; Kang, S.-C.; Kim, Y.-K.; Cho, J.-L. Development of near isogenic lines with various seed sizes and study on seed size-related characteristics in watermelon. Korean J. Breed. Sci. 2009, 41, 403–411. [Google Scholar]
- Jiang, H.; Tian, H.; Yan, C.; Jia, L.; Wang, Y.; Wang, M.; Jiang, C.J.; Li, Y.; Jiang, J.; Fang, L.; et al. RNA-seq analysis of watermelon (Citrullus lanatus) to identify genes involved in fruit cracking. Sci Hortic 2019, 248, 248–255. [Google Scholar] [CrossRef]
- Dou, J.-L.; Yuan, P.-L.; Zhao, S.-J.; He, N.; Zhu, H.-J.; Gao, L.; Ji, W.-l.; Lu, X.-Q.; Liu, W.-G. Effect of ploidy level on expression of lycopene biosynthesis genes and accumulation of phytohormones during watermelon (Citrullus lanatus) fruit development and ripening. J. Integr Agric. 2017, 16, 1956–1967. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Park, Y.-O.; Kim, S.-C.; Lee, Y.-J.; Kang, J.-S.; Choi, Y.-W.; Son, B.-G.; Park, Y.-H. Evaluation of genetic diversity among persimmon cultivars (Diospyros kaki Thunb.) using microsatellite markers. J. Life Sci. 2010, 20, 632–638. [Google Scholar] [CrossRef]
- Cox, M.P.; Peterson, D.A.; Biggs, P.J. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinform. 2010, 11, 485. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Subgroup, G.P.D.P. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-E.; Oh, S.-K.; Lee, J.-H.; Lee, B.-M.; Jo, S.-H. Genome-wide SNP calling using next generation sequencing data in tomato. Mol. Cells 2014, 37, 36. [Google Scholar] [CrossRef] [PubMed]
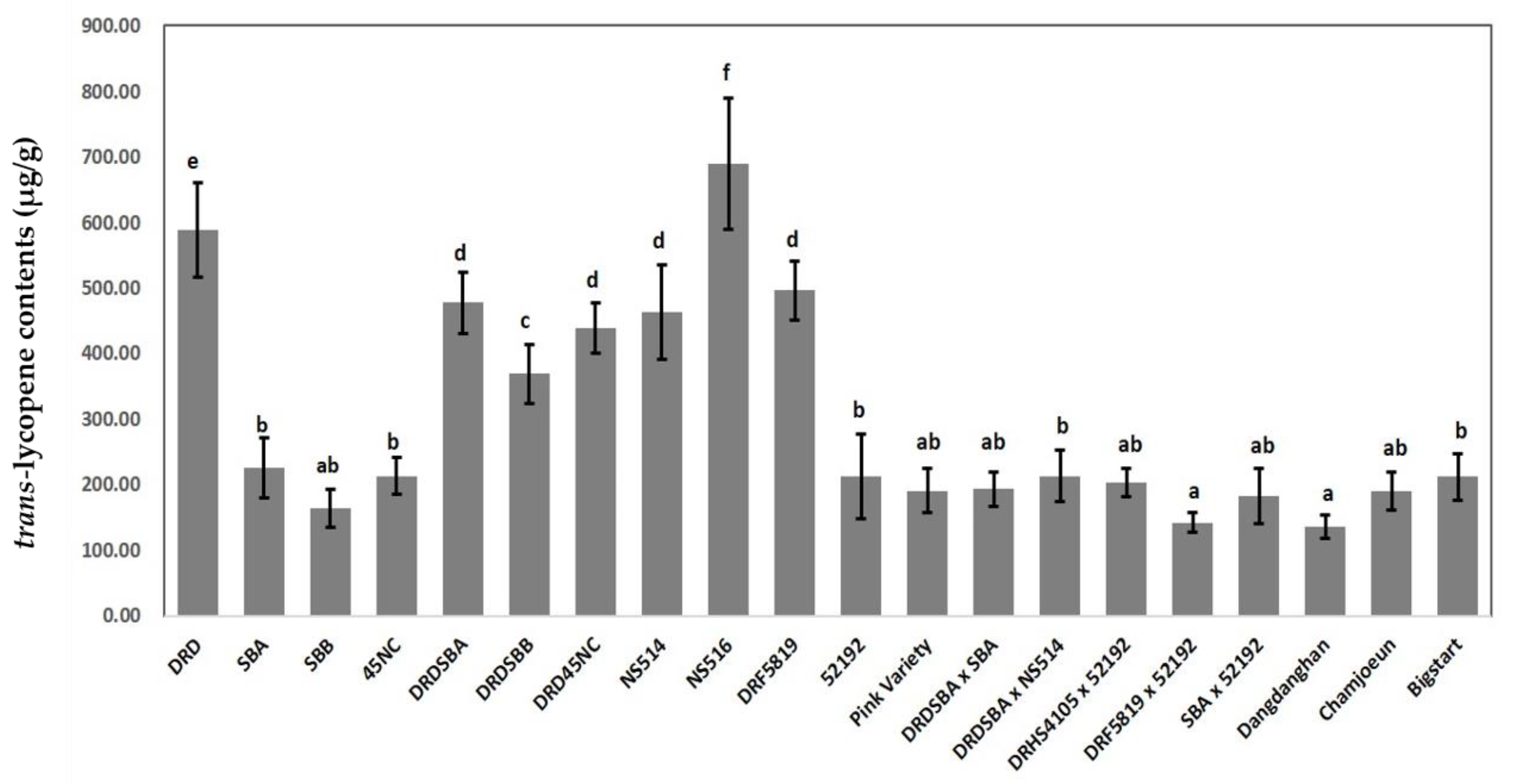

| EN. | Cultivar | Generation | Flesh Color | Lycopene Contents (µg/g) a |
|---|---|---|---|---|
| 1 | DRD | Inbred | Scarlet red | 589.45 ± 71.88e |
| 2 | SBA | Inbred | Coral red | 225.84 ± 45.06b |
| 3 | SBB | Inbred | Coral red | 164.04 ± 28.53ab |
| 4 | 45NC | Inbred | Coral red | 213.47 ± 28.25b |
| 5 | DRDSBA | NIL (BC3F6) | Scarlet red | 477.69 ± 46.92d |
| 6 | DRDSBB | NIL (BC3F9) | Scarlet red | 369.60 ± 44.79c |
| 7 | DRD45NC | NIL (BC2F5) | Scarlet red | 439.04 ± 38.78d |
| 8 | NS514 | Inbred | Scarlet red | 463.13 ± 72.55d |
| 9 | NS516 | Inbred | Scarlet red | 689.17 ± 100.29f |
| 10 | DRF5819 | Inbred | Scarlet red | 496.16 ± 44.69d |
| 11 | 52192 | Inbred | Coral red | 213.16 ± 64.66b |
| 12 | Pink Variety | Inbred | Coral red | 191.05 ± 33.83ab |
| 13 | DRDSBA × SBA | Experimental F1 | Coral red | 193.43 ± 25.46ab |
| 14 | DRDSBA × NS514 | Experimental F1 | Coral red | 213.73 ± 39.57b |
| 15 | DRHS4105 × 52192 | Experimental F1 | Coral red | 203.17 ± 20.92ab |
| 16 | DRF5819 × 52192 | Experimental F1 | Coral red | 142.64 ± 14.76a |
| 17 | SBA × 52192 | Experimental F1 | Coral red | 189.62 ± 46.04ab |
| 18 | Dangdanghan | Commercial F1 | Coral red | 135.77 ± 18.07a |
| 19 | Chamjoeun | Commercial F1 | Coral red | 189.79 ± 29.12ab |
| 20 | Bigstart | Commercial F1 | Coral red | 211.99 ± 35.02b |
| Samples | No. of Reads | Total Length (bp) | Avg. Length | Trimmed/Raw(%) | Genome CoverAge (×) a |
|---|---|---|---|---|---|
| DRD | 58,036,656 | 5,382,805,154 | 92.75 | 79.24 | 24.76 |
| 58,036,656 | 5,140,713,545 | 88.58 | 75.68 | ||
| SBA | 67,875,107 | 6,288,288,055 | 92.64 | 79.72 | 29.03 |
| 67,875,107 | 6,049,639,852 | 89.13 | 76.70 | ||
| 45NC | 58,499,614 | 5,428,904,346 | 92.80 | 81.46 | 25.02 |
| 58,499,614 | 5,206,666,818 | 89.00 | 78.12 | ||
| DRDSBA | 78,305,938 | 7,244,678,852 | 92.52 | 78.89 | 33.40 |
| 78,305,938 | 6,951,305,936 | 88.77 | 75.69 | ||
| DRD45NC | 57,130,281 | 5,298,242,278 | 92.74 | 81.88 | 24.44 |
| 57,130,281 | 5,090,776,571 | 89.11 | 78.68 |
| CIR | Location | No. of Genes | SNP | ||||
|---|---|---|---|---|---|---|---|
| Chr. | Mb | Promoter a | Exon | Intron | Total | ||
| CIR6 | 6 | 24.06–24.99 | 1031 | 105 | 42 | 111 | 909 |
| CIR9 | 9 | 1.60–3.96 | 3171 | 160 | 61 | 200 | 960 |
| CIR10 | 10 | 29.38–29.97 | 401 | 14 | 5 | 44 | 138 |
| Marker ID | Cultivars a | R2 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||
| CIR6-M1 | hh | ll | ll | ll | hh | hh | hh | hh | hh | hh | ll | ll | hl | hl | ll | hl | ll | ll | ll | ll | 0.722 |
| CIR6-M2 | hh | ll | ll | ll | hh | hh | hh | hh | hh | hh | hh | ll | hl | hl | hl | hh | hl | ll | ll | ll | 0.461 |
| CIR6-M3 | hh | ll | ll | ll | hh | hh | hh | hh | hh | hh | hh | ll | hl | hl | hl | hh | hl | ll | ll | ll | 0.461 |
| CIR6-M4 | hh | ll | ll | ll | hh | hh | hh | hh | hh | hh | ll | ll | hl | hl | ll | hl | ll | ll | ll | ll | 0.722 |
| CIR6-M5 | hh | ll | ll | ll | hh | hh | hh | hh | hh | hh | hh | ll | hl | hl | hl | hh | hl | ll | ll | ll | 0.461 |
| CIR6-M6 | hh | ll | ll | ll | hh | hh | hh | hh | hh | hh | hh | ll | hl | hl | hl | hh | hl | ll | ll | ll | 0.461 |
| CIR6-M7 | hh | ll | ll | ll | hh | hh | hh | hh | hh | hh | hh | ll | hl | hl | hl | hh | hl | ll | hl | ll | 0.446 |
| CIR6-M8 | hh | ll | ll | ll | hh | hh | hh | hh | hh | hh | hh | ll | hl | ll | hl | hh | ll | ll | hl | ll | 0.472 |
| CIR9-M1 | hh | ll | ll | ll | hh | ll | hh | hh | hh | hh | hh | ll | hl | hh | hl | hh | hl | ll | hl | ll | 0.316 |
| CIR9-M2 | hh | ll | ll | ll | hh | ll | hh | hh | hh | hh | ll | ll | hl | hh | hl | hl | hh | hl | m | hh | 0.295 |
| CIR9-M3 | hh | ll | ll | ll | hh | ll | hh | hh | hh | ll | ll | ll | hl | hh | ll | ll | ll | hh | hl | ll | 0.284 |
| CIR9-M4 | hh | ll | ll | ll | hh | ll | hh | hh | hh | ll | ll | ll | hl | hh | ll | ll | ll | ll | ll | ll | 0.480 |
| CIR9-M5 | hh | ll | ll | ll | hh | ll | hh | hh | hh | hh | ll | ll | hl | hh | hl | hl | ll | hl | hl | hl | 0.449 |
| CIR9-M6 | hh | ll | ll | ll | hh | ll | hh | hh | hh | ll | ll | ll | hl | hh | hl | ll | ll | hl | hl | hh | 0.268 |
| CIR9-M7 | hh | ll | ll | ll | hl | ll | hh | hh | hh | ll | ll | ll | hl | hh | hl | ll | ll | hl | hl | hh | 0.226 |
| CIR9-M8 | hh | ll | ll | ll | hh | ll | hh | hh | hh | hh | hh | ll | hl | hh | hh | hh | hl | hl | hh | hh | 0.149 |
| CIR9-M9 | hh | ll | ll | ll | hh | ll | hh | hh | hl | hh | hl | ll | ll | hh | hl | hh | hl | ll | hl | ll | 0.258 |
| CIR9-M10 | hh | ll | ll | ll | hh | ll | hh | hh | hh | ll | hh | ll | hl | hh | hl | hl | hl | ll | ll | ll | 0.269 |
| CIR9-M11 | hh | ll | ll | ll | hh | ll | hh | hh | hh | hh | hh | ll | hl | hh | hl | hh | hl | ll | hl | ll | 0.316 |
| CIR9-M12 | hh | ll | ll | ll | hh | ll | hh | hh | hh | hh | m | ll | hl | hh | hl | hh | hl | ll | hl | ll | 0.356 |
| CIR9-M13 | hh | ll | ll | ll | hh | ll | hh | hh | hh | hh | hh | ll | hl | hh | hl | hh | hl | ll | hl | ll | 0.316 |
| CIR10-M1 | hh | ll | ll | ll | hh | ll | hh | hh | hh | ll | ll | ll | hl | hh | ll | ll | ll | ll | hh | ll | 0.356 |
| CIR10-M2 | hh | ll | ll | ll | hh | ll | hh | hh | hh | ll | hh | ll | hl | hh | hl | hl | hl | ll | hh | ll | 0.193 |
| CIR10-M3 | hh | ll | ll | ll | d | ll | ll | d | d | ll | ll | ll | d | d | ll | ll | ll | ll | d | ll | - |
| CIR10-M4 | hh | ll | ll | ll | d | ll | d | d | d | ll | ll | ll | d | d | ll | ll | ll | ll | d | ll | - |
| CIR10-M5 | hh | ll | ll | ll | hh | ll | hh | hh | hh | ll | ll | ll | hl | hh | ll | ll | hl | m | hh | m | 0.329 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Park, G.; Choi, Y.; Park, S.; Kim, H.; Lee, O.; Kim, T.; Park, Y. Whole-Genome Resequencing of Near-Isogenic Lines Reveals a Genomic Region Associated with High Trans-Lycopene Contents in Watermelon. Plants 2022, 11, 8. https://doi.org/10.3390/plants11010008
Lee S, Park G, Choi Y, Park S, Kim H, Lee O, Kim T, Park Y. Whole-Genome Resequencing of Near-Isogenic Lines Reveals a Genomic Region Associated with High Trans-Lycopene Contents in Watermelon. Plants. 2022; 11(1):8. https://doi.org/10.3390/plants11010008
Chicago/Turabian StyleLee, Siyoung, Girim Park, Yunseo Choi, Seoyeon Park, Hoytaek Kim, Oakjin Lee, Taebok Kim, and Younghoon Park. 2022. "Whole-Genome Resequencing of Near-Isogenic Lines Reveals a Genomic Region Associated with High Trans-Lycopene Contents in Watermelon" Plants 11, no. 1: 8. https://doi.org/10.3390/plants11010008
APA StyleLee, S., Park, G., Choi, Y., Park, S., Kim, H., Lee, O., Kim, T., & Park, Y. (2022). Whole-Genome Resequencing of Near-Isogenic Lines Reveals a Genomic Region Associated with High Trans-Lycopene Contents in Watermelon. Plants, 11(1), 8. https://doi.org/10.3390/plants11010008






