Arabidopsis Growth-Promotion and Root Architecture Responses to the Beneficial Rhizobacterium Phyllobacterium brassicacearum Strain STM196 Are Independent of the Nitrate Assimilatory Pathway
Abstract
:1. Introduction
2. Results
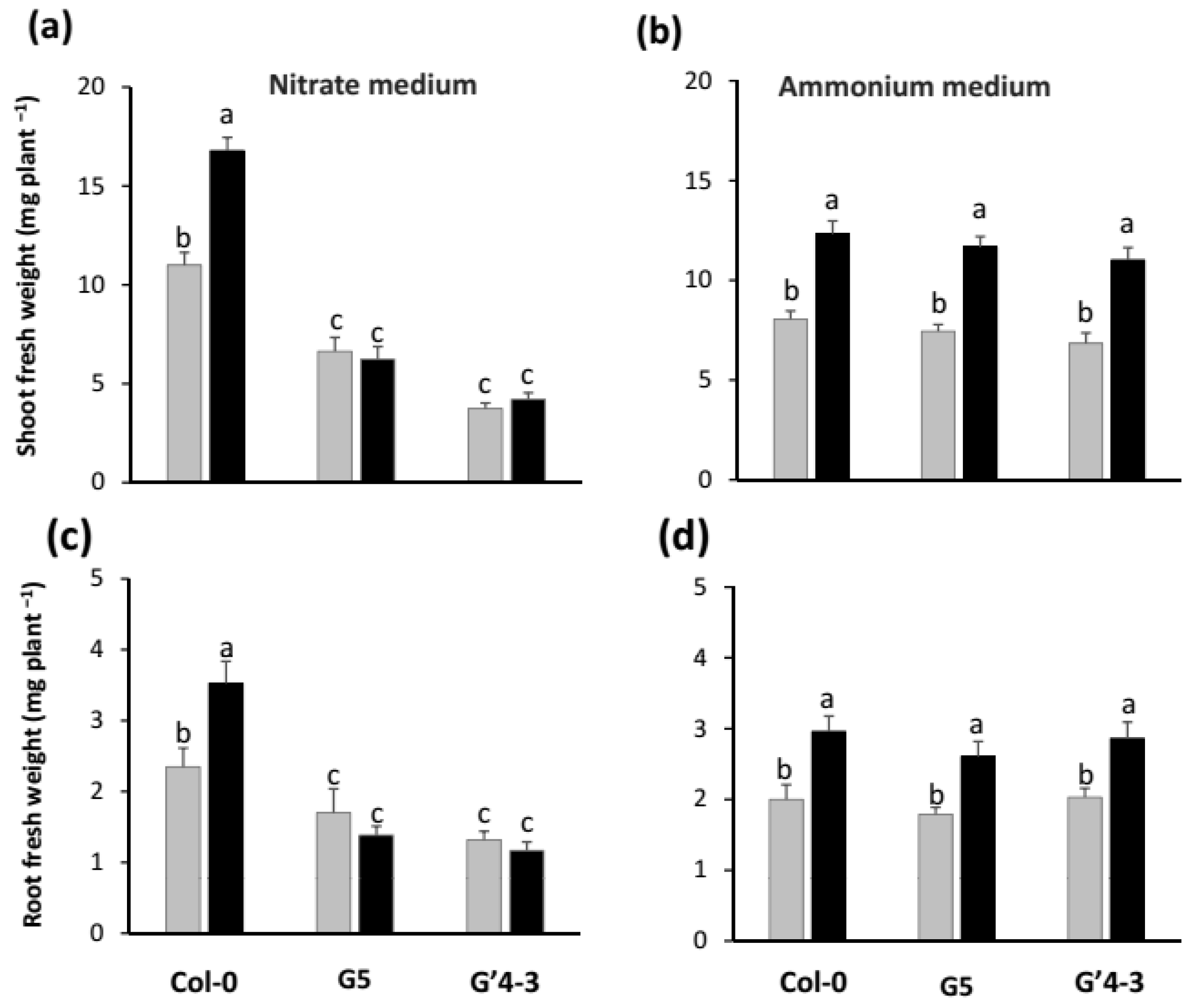
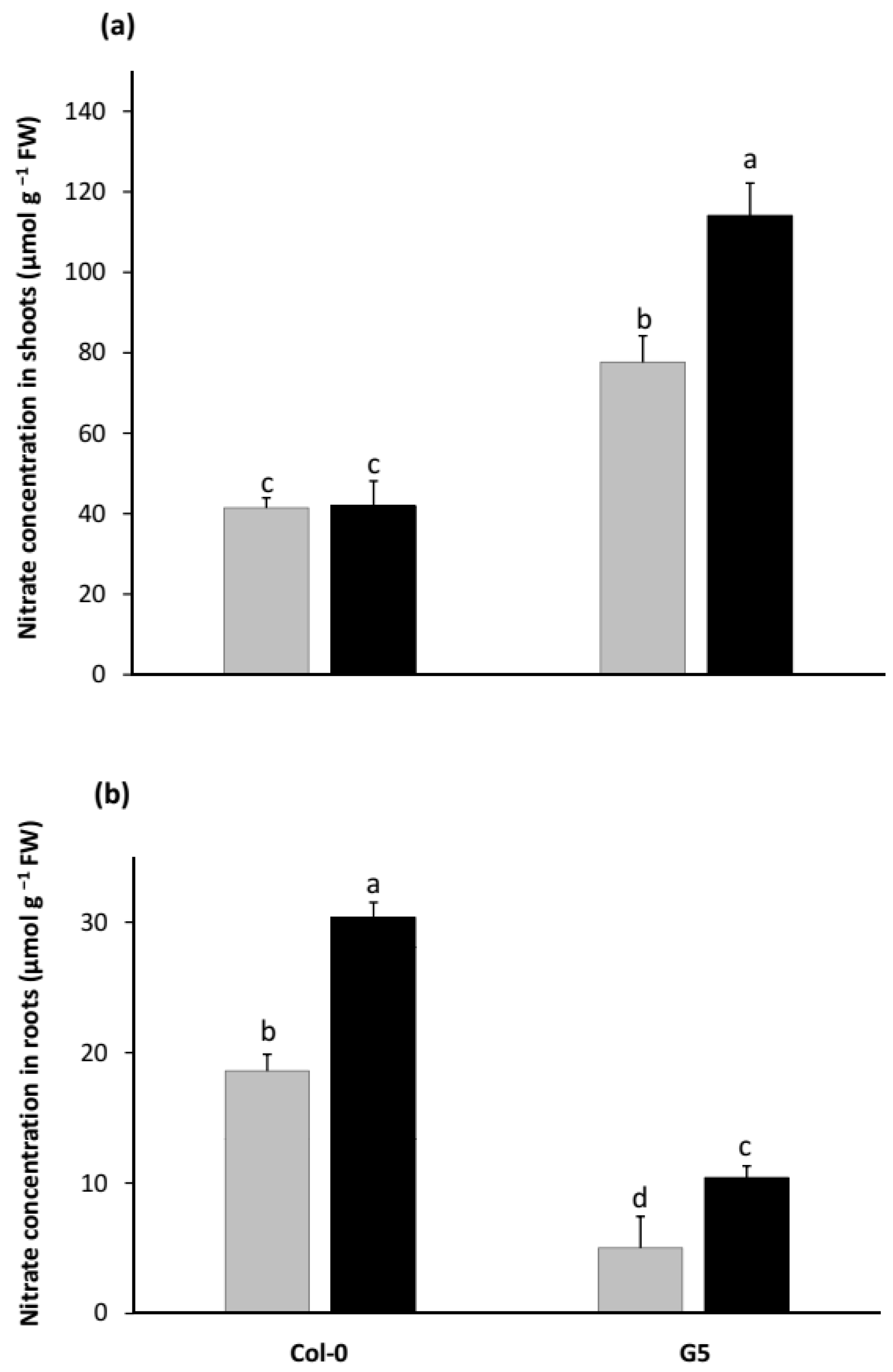
2.1. Growth Promotion by STM196 Is Independent of NRA and Nitrate Accumulation
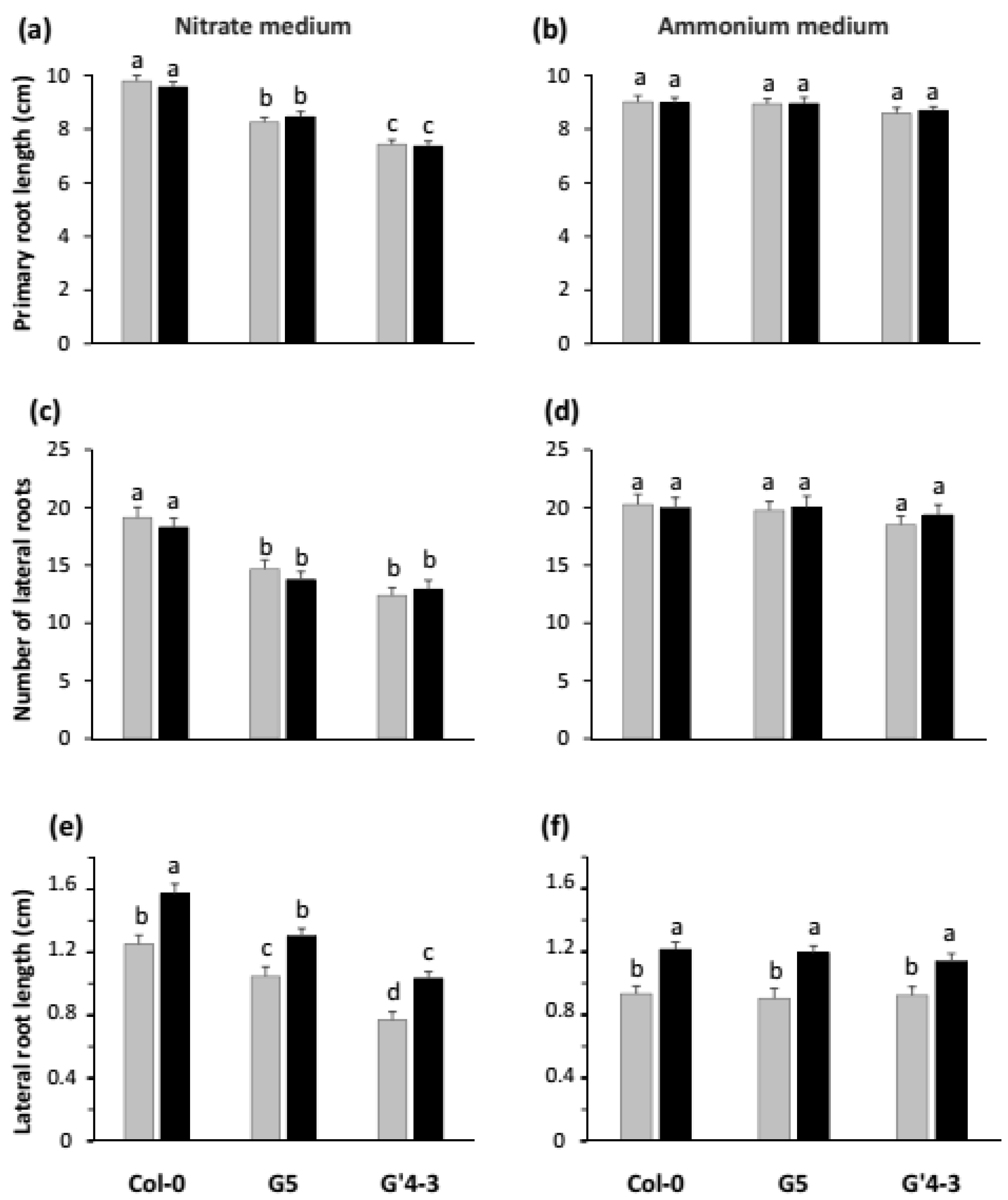
2.2. nia Mutations and STM196 Inoculation Have Antagonistic Additive Effects on Root System Architecture
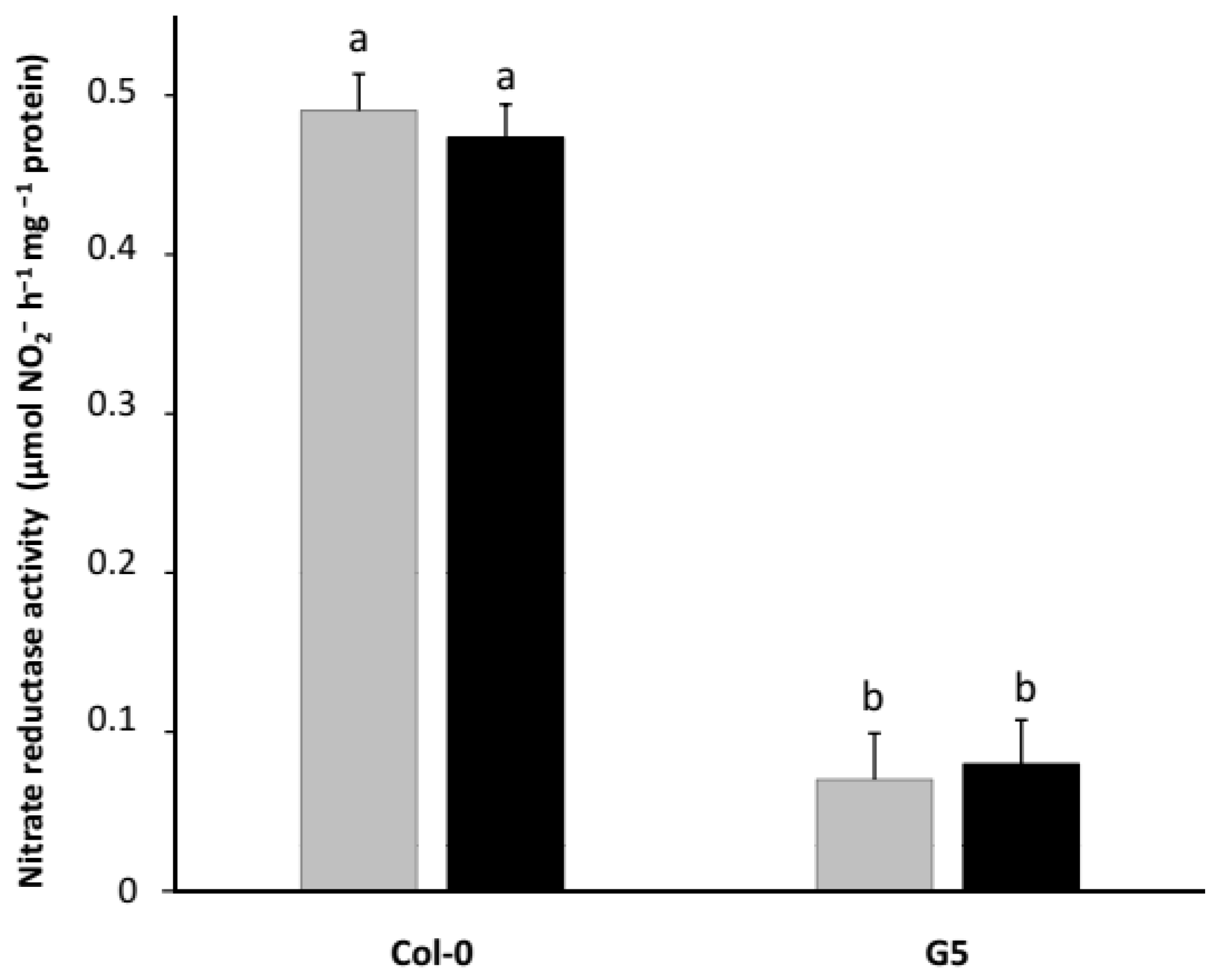
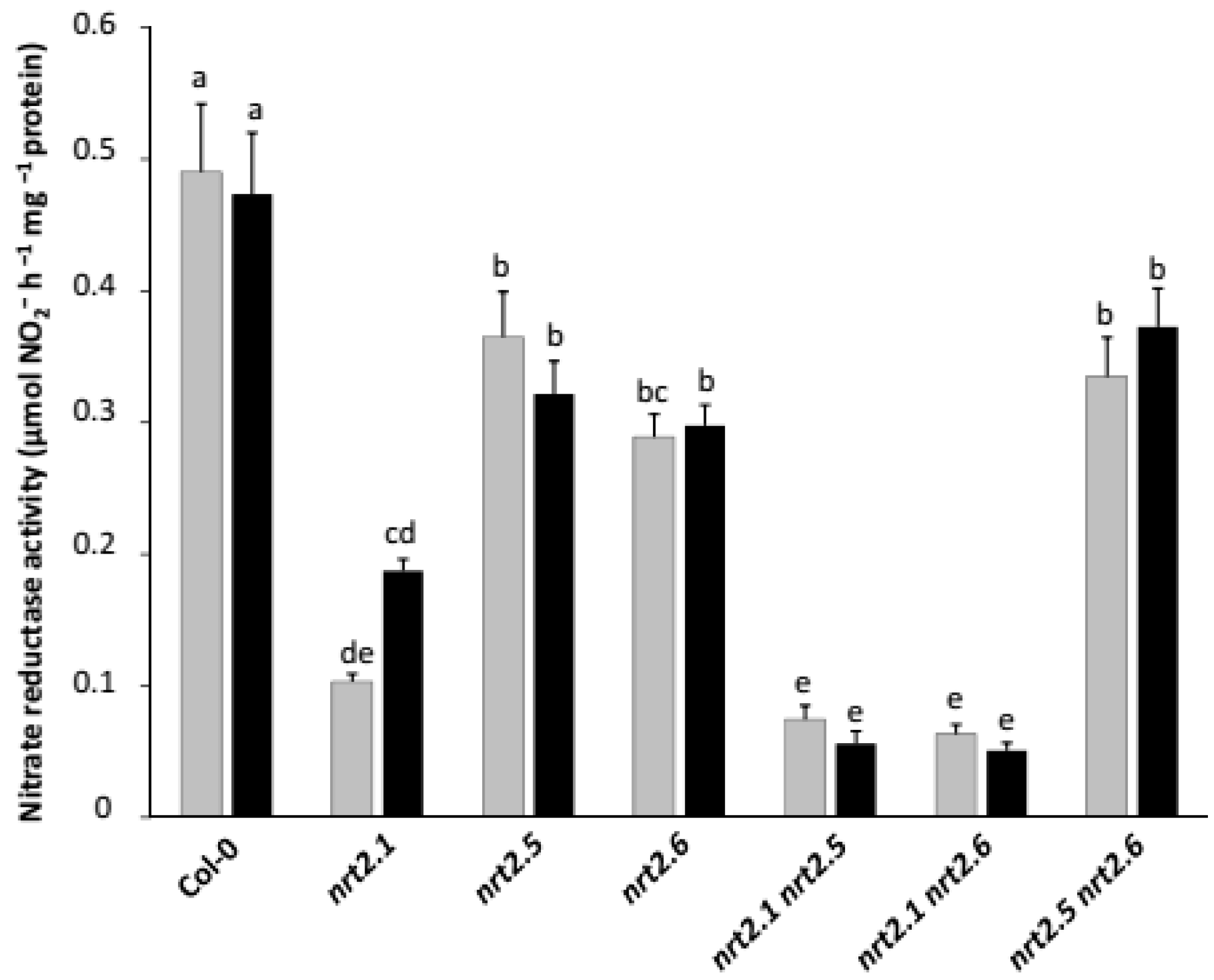
2.3. Inoculation with STM196 Have No or Very Low Effect on Nitrate Reductase Activity in Nrt2 Mutants
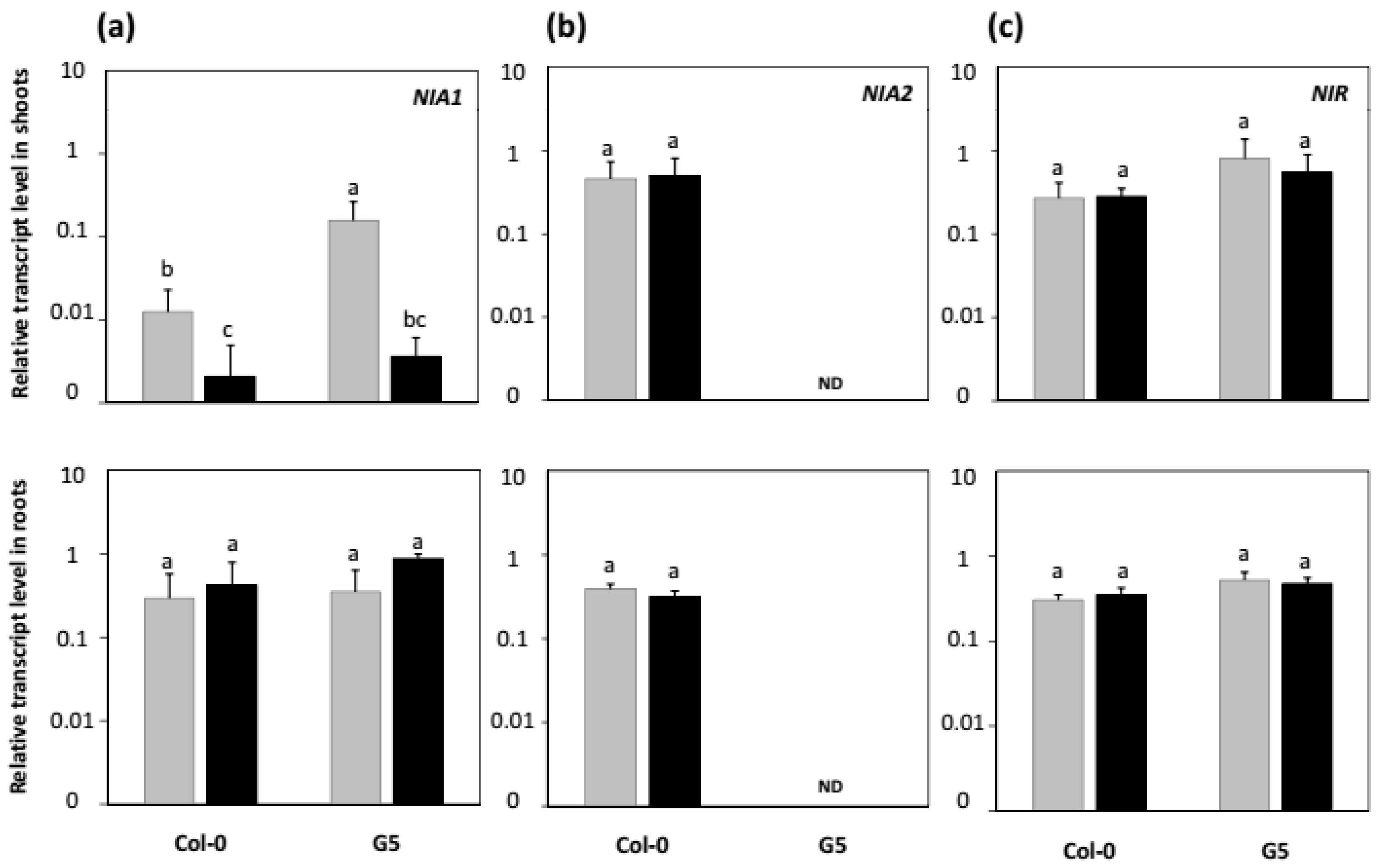
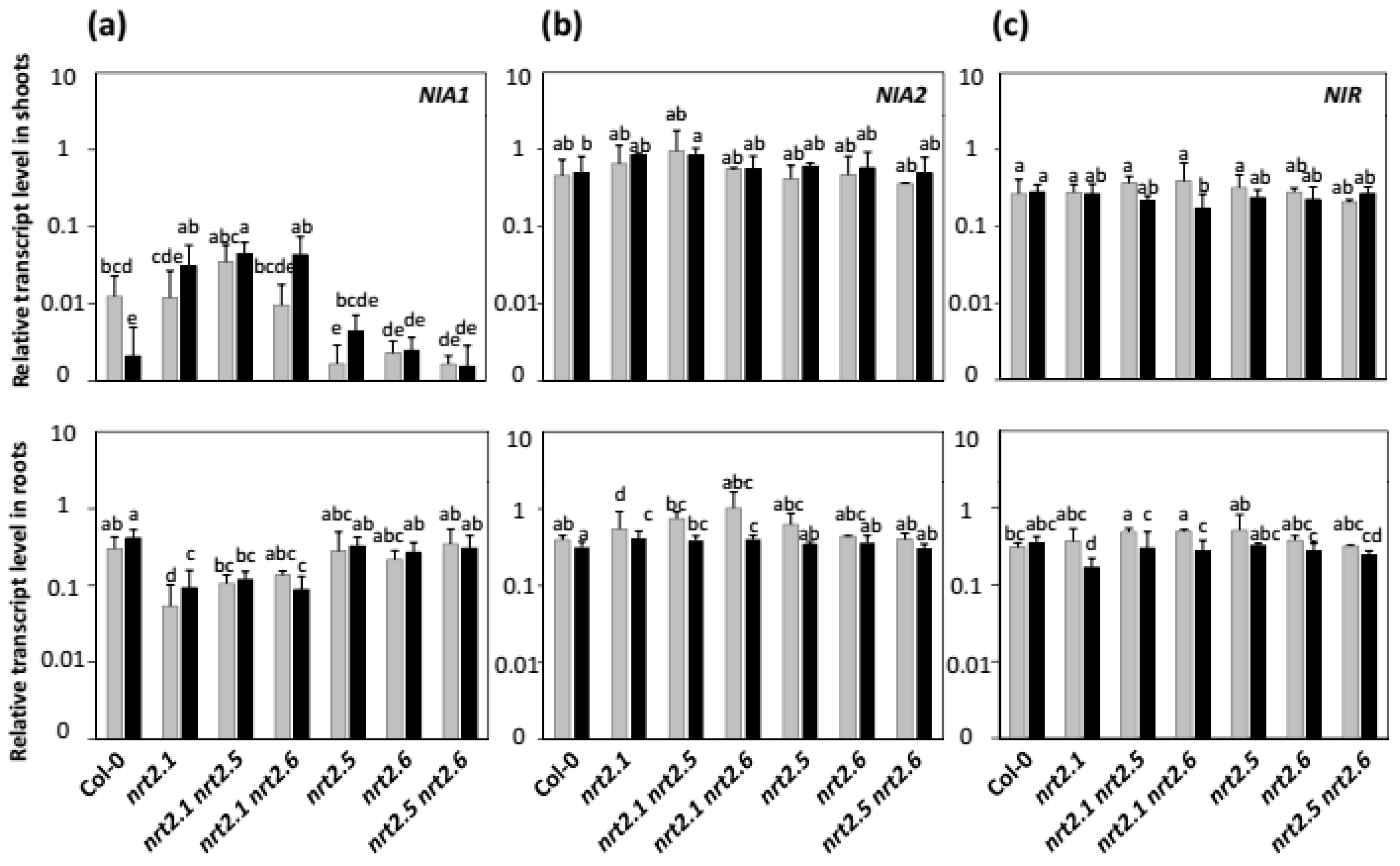
2.4. Effects of nia2 Mutation and Inoculation with STM196 on Nitrate Reductase and Nitrite Reductase Genes Expression Levels
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Plant Growth Conditions and Inoculation
4.3. Root Architecture Analysis
4.4. Measurements of Fresh Weight and Endogenous Nitrate Content
4.5. Measurements of Nitrate Reductase Activity
4.6. Analysis of Transcript Levels by Quantitative Real-Time PCR
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drogue, B.; Doré, H.; Borland, S.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Which specificity in cooperation between phytostimulating rhizobacteria and plants? Res. Microbiol. 2012, 163, 500–510. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Alawiye, T.T.; Babalola, O.O. Bacterial diversity and community structure in typical plant rhizosphere. Diversity 2019, 11, 179. [Google Scholar] [CrossRef] [Green Version]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moenne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dye, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [Green Version]
- Verbon, E.H.; Liberman, L.M. Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Boddey, R.M.; Urquiaga, S.; Alves, B.J.R.; Reis, V. Endophytic nitrogen fixation in sugarcane: Present knowledge and future applications. Plant Soil 2003, 252, 139–149. [Google Scholar] [CrossRef]
- Rodriguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, N.; Vassileva, M.; Nikolaeva, I. Simultaneous P-solubilizing and biocontrol activity of microorganisms: Potentials and future trends. Appl. Microbiol. Biotechnol. 2006, 71, 137–144. [Google Scholar] [CrossRef]
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Sun, Y.; Xie, X.; Kim, M.-S.; Dowd, S.E.; Paré, P.W. A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 2009, 58, 568–577. [Google Scholar] [CrossRef]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Trinh, C.S.; Lee, W.J.; Jeong, C.Y.; Truong, H.A.; Chung, N.; Kang, C.S.; Lee, H. Bacillus subtilis strain L1 promotes nitrate reductase activity in Arabidopsis and elicits enhanced growth performance in Arabidopsis, lettuce, and wheat. J. Plant Res. 2020, 133, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Okamoto, M.; Crawford, N.M.; Siddiqi, M.Y.; Glass, A.D.M. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007, 143, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantelin, S.; Desbrosses, G.; Larcher, M.; Tranbarger, T.J.; Cleyet-Marel, J.-C.; Touraine, B. Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp. Planta 2006, 223, 591–603. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [Green Version]
- Spaepen, S.; Dobbelaere, S.; Croonenborghs, A.; Vanderleyden, J. Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 2008, 312, 15–23. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Galimsyanova, N.; Kuzmina, L.; Vysotskaya, L.; Sidorova, L.; Gabbasova, I.; Melentiev, A.I.; Kudoyarova, G.R. Effect of seed bacterization with plant growth-promoting bacteria on wheat productivity and phosphorus mobility in the rhizosphere. Plant Soil Environ. 2019, 65, 313–319. [Google Scholar] [CrossRef]
- Zhang, H.M.; Rong, H.L.; Pilbeam, D. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 2329–2338. [Google Scholar] [CrossRef] [Green Version]
- Contesto, C.; Milesi, S.; Mantelin, S.; Zancarini, A.; Desbrosses, G.; Varoquaux, F.; Bellini, C.; Kowalczyk, M.; Touraine, B. The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 2010, 232, 1455–1470. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.; Hasnain, S. Rhizobacterial potential to alter auxin content and growth of Vigna radiata (L.). World J. Microbiol. Biotechnol. 2010, 26, 1379–1384. [Google Scholar] [CrossRef]
- Imsande, J.; Touraine, B. N demand and the regulation of nitrate uptake. Plant Physiol. 1994, 105, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Lappartient, A.G.; Vidmar, J.J.; Leustek, T.; Glass, A.D.M.; Touraine, B. Inter-organ signaling in plants: Regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound. Plant J. 1999, 18, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, I.G. Short and long-term effects of a change in the spatial distribution of nitrate in the root zone on N uptake, growth and root development of young lettuce plants. Plant Cell Environ. 1991, 14, 21–33. [Google Scholar] [CrossRef]
- Mantelin, S.; Touraine, B. Plant growth-promoting bacteria and nitrate availability: Impacts on root development and nitrate uptake. J. Exp. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, H.; Nalin, R.; Bally, R.; Cleyet-Marel, J.-C. Isolation and identification of the most efficient plant growth-promoting bacteria associated with canola (Brassica napus). Biol. Fertil. Soils 2001, 33, 152–156. [Google Scholar] [CrossRef]
- Kechid, M.; Desbrosses, G.; Rokhsi, W.; Varoquaux, F.; Djekoun, A.; Touraine, B. The NRT2.5 and NRT2.6 genes are involved in growth promotion of Arabidopsis by the plant growth-promoting rhizobacterium (PGPR) strain Phyllobacterium brassicacearum STM196. New Phytol. 2013, 198, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.-R.; Lauerer, M.; Schulze, E.-D.; Caboche, M.; Stitt, M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997, 11, 671–691. [Google Scholar] [CrossRef] [Green Version]
- Kotur, Z.; Mackenzie, N.; Ramesh, S.; Tyerman, S.D.; Kaiser, B.N.; Glass, A.D.M. Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1. New Phytol. 2012, 194, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.-B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2014, 80, 230–241. [Google Scholar] [CrossRef]
- Dechorgnat, J.; Patrit, O.; Krapp, A.; Fagard, M.; Daniel-Vedele, F. Characterization of the Nrt2.6 gene in Arabidopsis thaliana: A link with plant response to biotic and abiotic stress. PLoS ONE 2012, 7, e42491. [Google Scholar] [CrossRef] [Green Version]
- Parniske, M. Intracellular accomodation of microbes by plants: A common developmental program for symbiosis and disease? Curr. Opin. Plant Biol. 2000, 3, 320–328. [Google Scholar] [CrossRef]
- Wang, E.; Schornack, S.; Marsh, J.F.; Gobbato, E.; Schwessinger, B.; Eastmond, P.; Schultze, M.; Kamoun, S.; Oldroyd, G.E. A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr. Biol. 2012, 22, 2242–2246. [Google Scholar] [CrossRef] [Green Version]
- Rey, T.; Nars, A.; Bonhomme, M.; Bottin, A.; Huguet, S.; Balzergue, S.; Jardinaud, M.F.; Bono, J.J.; Cullimore, J.; Dumas, B.; et al. NFP, a LysM protein controlling Nod factor perception, also intervenes in Medicago truncatula resistance to pathogens. New Phytol. 2013, 198, 875–886. [Google Scholar] [CrossRef]
- Cheeseman, J.M.; Tankou, S.K. Nitrate reductase and growth of Arabidopsis thaliana in solution culture. Plant Soil 2004, 266, 143–152. [Google Scholar] [CrossRef]
- Krapp, A.; Berthomé, R.; Orsel, M.; Mercey-Boutet, S.; Yu, A.; Castaings, L.; Elftieh, S.; Major, H.; Renou, J.-P.; Daniel-Vedele, F. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol. 2011, 157, 1255–1282. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.Q.; Crawford, N.M. Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes Nia1 and Nia2. Mol. Gen. Genet. 1993, 239, 289–297. [Google Scholar] [CrossRef]
- Wilkinson, J.Q.; Crawford, N.M. Identification of the Arabidopsis CHL3 gene as the nitrate reductase structural gene NIA2. Plant Cell 1991, 3, 461–471. [Google Scholar]
- Scheible, W.R.; Gonzalez Fontes, A.; Lauerer, M.; Mueller Roeber, B.; Caboche, M.; Stitt, M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 1997, 9, 783–798. [Google Scholar] [CrossRef]
- Helali, S.M.R.; Nebli, H.; Kaddour, R.; Mahmoudi, H.; Lachaâl, M.; Ouerghi, Z. Influence of nitrate-ammonium ratio on growth and nutrition of Arabidopsis thaliana. Plant Soil 2010, 336, 65–74. [Google Scholar] [CrossRef]
- Contesto, C.; Desbrosses, G.; Lefoulon, C.; Béna, G.; Borel, F.; Galland, M.; Gamet, L.; Varoquaux, F.; Touraine, B. Effects of rhizobacterial ACC deaminase activity on Arabidopsis indicate that ethylene mediates local root responses to plant growth-promoting rhizobacteria. Plant Sci. 2008, 175, 178–189. [Google Scholar] [CrossRef]
- Galland, M.; Gamet, L.; Varoquaux, F.; Touraine, B.; Touraine, B.; Desbrosses, G. The ethylene pathway contributes to root hair elongation induced by the beneficial bacteria Phyllobacterium brassicacearum STM196. Plant Sci. 2012, 190, 74–81. [Google Scholar] [CrossRef]
- Cerezo, M.; Tillard, P.; Filleur, S.; Munos, S.; Daniel-Vedele, F.; Gojon, A. Major alterations of the regulation of root NO3− uptake was associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol. 2001, 127, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Weston, D.J.; Rogers, A.; Tschaplinski, T.J.; Gunter, L.E.; Jawdy, S.A.; Engle, N.L.; Heady, L.E.; Tuskan, G.A.; Wullschleger, S.D. Scaling nitrogen and carbon interactions: What are the consequences of biological buffering? Ecol. Evol. 2015, 5, 2839–2850. [Google Scholar] [CrossRef]
- Kotur, Z.; Glass, A.D.M. A 150 kDa plasma membrane complex of AtNRT2.5 and AtNAR2.1 is the major contributor to constitutive high-affinity nitrate influx in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 1490–1502. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric oxide: A multitasked signaling gas in plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef] [Green Version]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 16. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [Green Version]
- Mantelin, S.; Fisher-Le Saux, M.; Zakhia, F.; Béna, G.; Bonneau, S.; Jeder, H.; de Lajudie, P.; Cleyet-Marel, J.-C. Emended description of the genus Phyllobacterium and description of four novel species associated with plant roots: Phyllobacterium bourgognense sp. nov., Phyllobacterium ifriqiyense sp. nov., Phyllobacterium leguminum sp. nov. and Phyllobacterium brassicacearum sp. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 827–839. [Google Scholar]
- Henricksen, A.; Semer-Olsen, A.R. Automatic methods for determining nitrate and nitrite in water and soil extracts. Analyst 1970, 95, 514–518. [Google Scholar] [CrossRef]
- Robin, P. Etude de quelques conditions d’extraction de la nitrate réductase des racines et des feuilles de plantules de maïs. Physiol. Veg. 1979, 17, 45–54. [Google Scholar]
- Mengel, K.; Robin, P.; Salsac, L. Nitrate reductase activity in shoots and roots of maize seedlings as affected by the form of nitrogen nutrition and the pH of the nutrient solution. Plant Physiol. 1983, 71, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Bradford, N.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kechid, M.; Desbrosses, G.; Gamet, L.; Castaings, L.; Varoquaux, F.; Djekoun, A.; Touraine, B. Arabidopsis Growth-Promotion and Root Architecture Responses to the Beneficial Rhizobacterium Phyllobacterium brassicacearum Strain STM196 Are Independent of the Nitrate Assimilatory Pathway. Plants 2022, 11, 128. https://doi.org/10.3390/plants11010128
Kechid M, Desbrosses G, Gamet L, Castaings L, Varoquaux F, Djekoun A, Touraine B. Arabidopsis Growth-Promotion and Root Architecture Responses to the Beneficial Rhizobacterium Phyllobacterium brassicacearum Strain STM196 Are Independent of the Nitrate Assimilatory Pathway. Plants. 2022; 11(1):128. https://doi.org/10.3390/plants11010128
Chicago/Turabian StyleKechid, Maya, Guilhem Desbrosses, Lydia Gamet, Loren Castaings, Fabrice Varoquaux, Abdelhamid Djekoun, and Bruno Touraine. 2022. "Arabidopsis Growth-Promotion and Root Architecture Responses to the Beneficial Rhizobacterium Phyllobacterium brassicacearum Strain STM196 Are Independent of the Nitrate Assimilatory Pathway" Plants 11, no. 1: 128. https://doi.org/10.3390/plants11010128
APA StyleKechid, M., Desbrosses, G., Gamet, L., Castaings, L., Varoquaux, F., Djekoun, A., & Touraine, B. (2022). Arabidopsis Growth-Promotion and Root Architecture Responses to the Beneficial Rhizobacterium Phyllobacterium brassicacearum Strain STM196 Are Independent of the Nitrate Assimilatory Pathway. Plants, 11(1), 128. https://doi.org/10.3390/plants11010128





