Glucosinolate Induction and Resistance to the Cabbage Moth, Mamestra brassicae, Differs among Kale Genotypes with High and Low Content of Sinigrin and Glucobrassicin
Abstract
1. Introduction
2. Results
2.1. Glucosinolate Content in Kale Genotypes
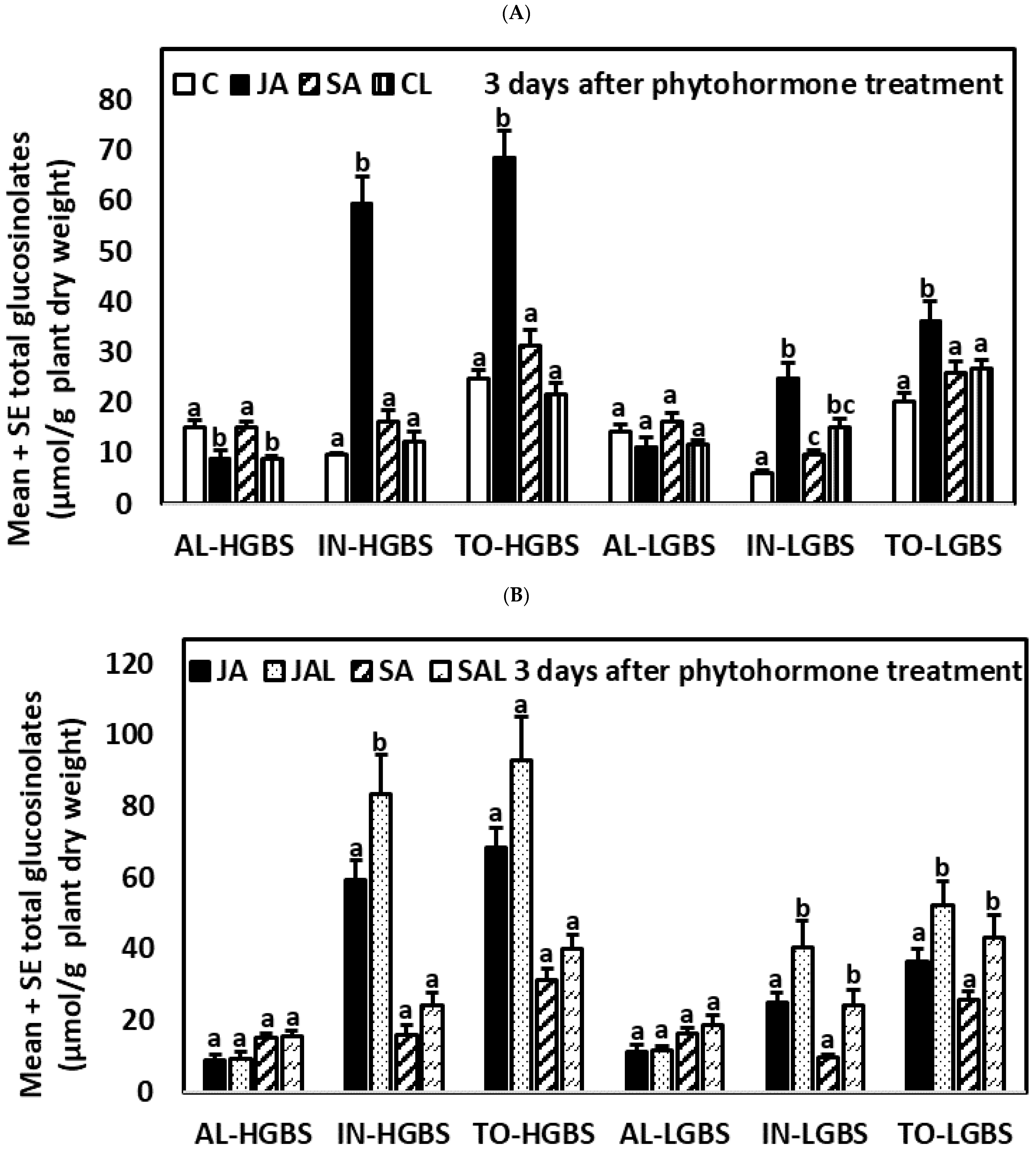
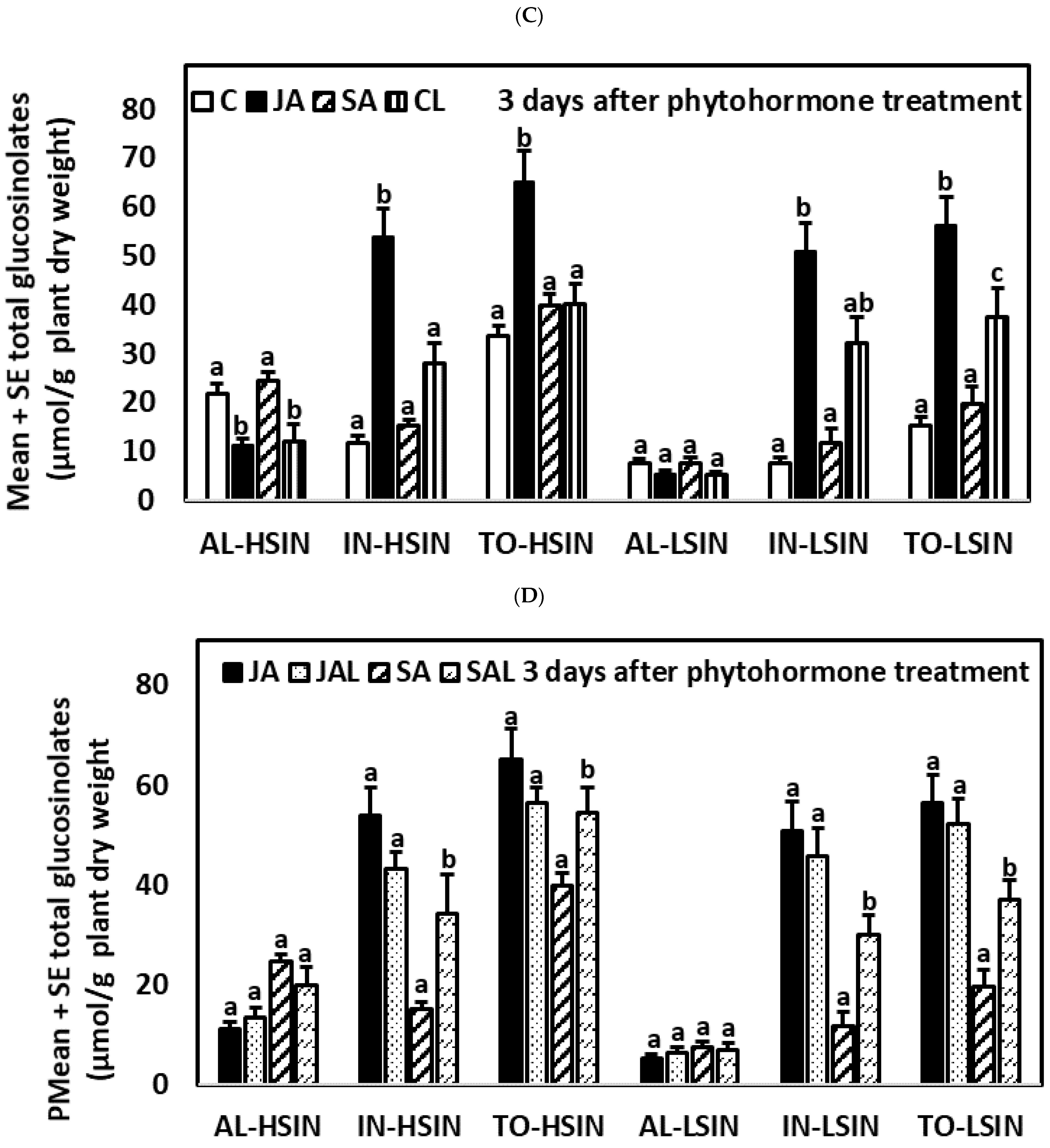
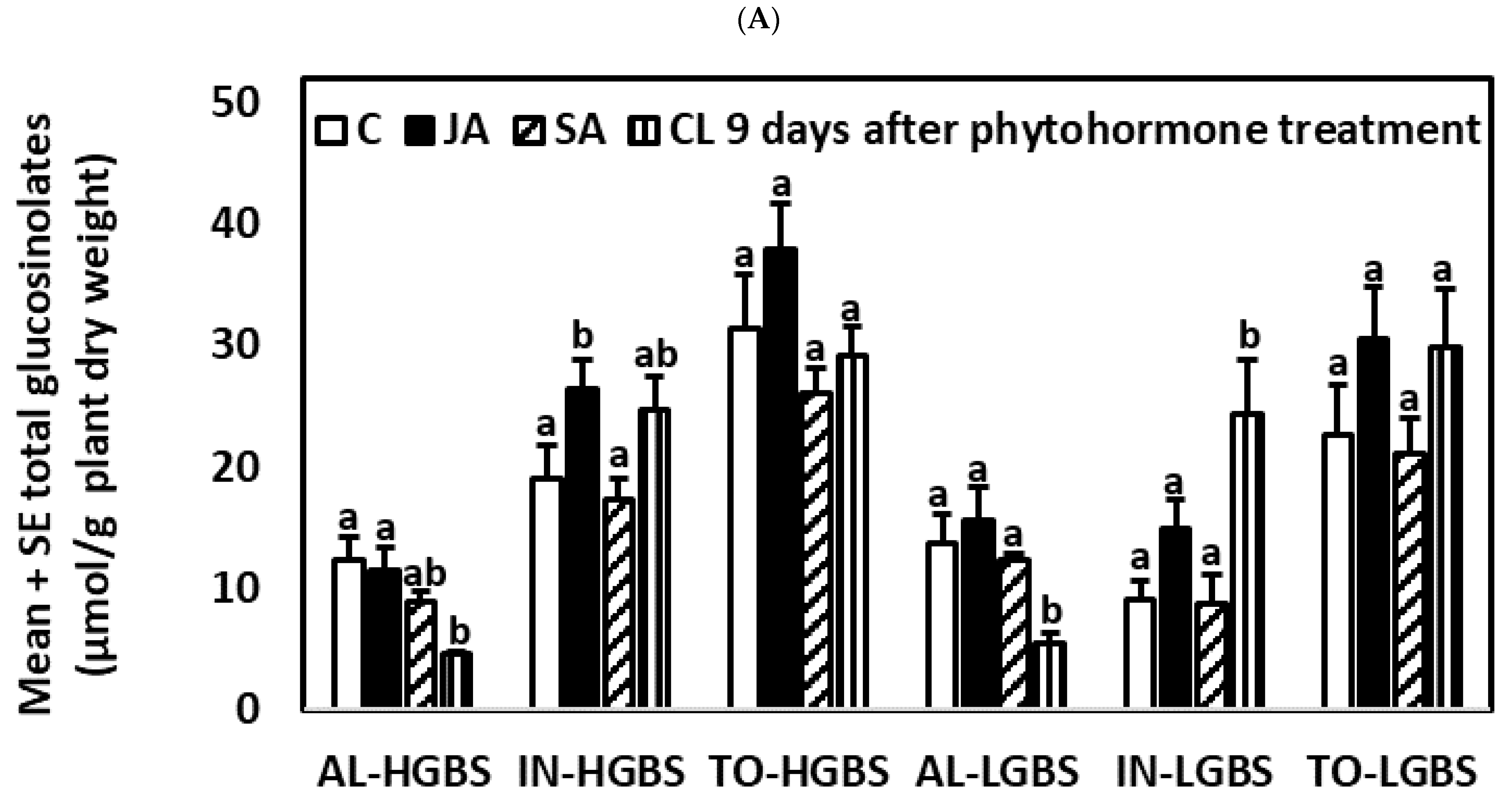
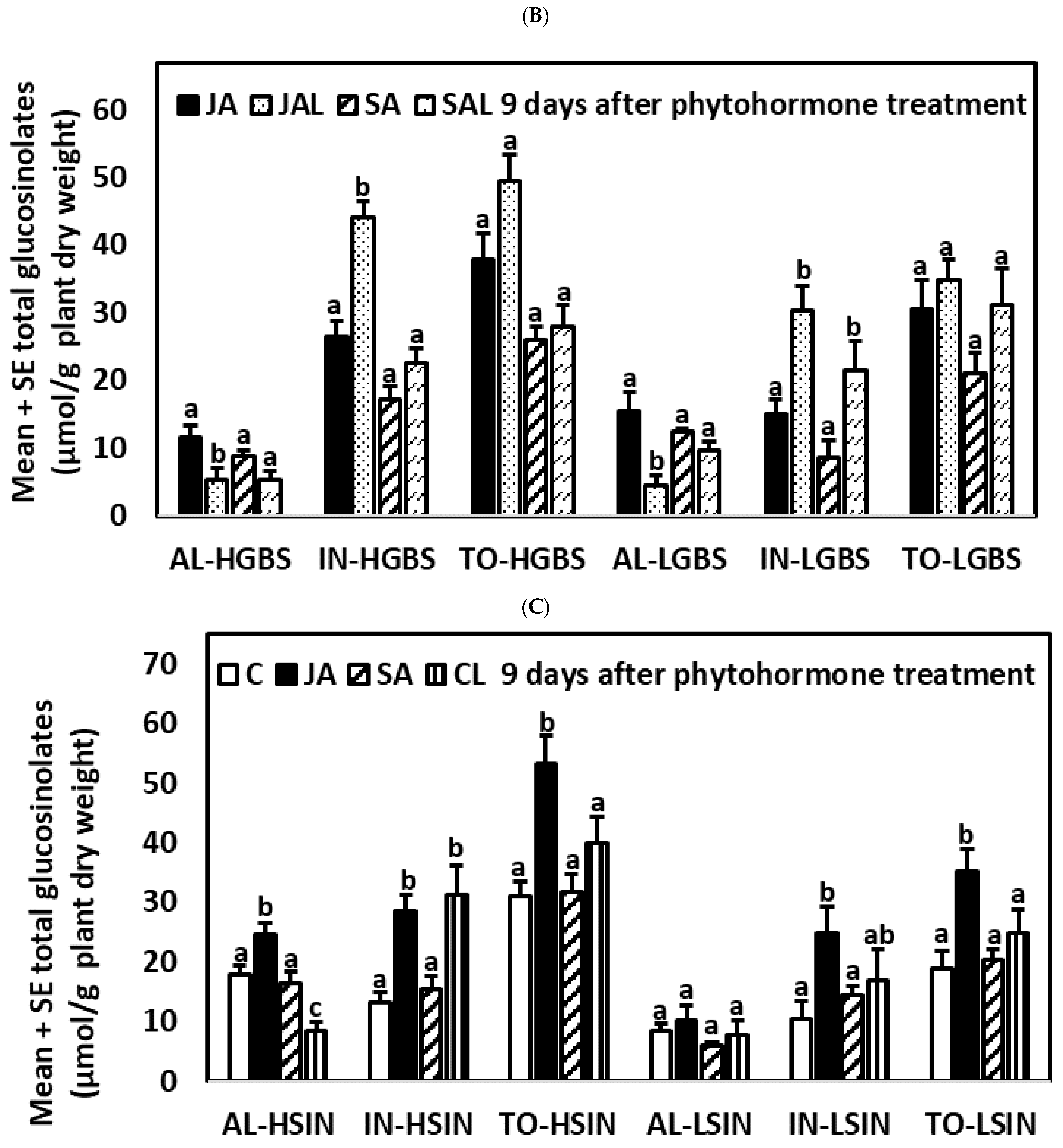
2.2. Glucosinolate Induction over Time after Phytohormone and Herbivory Treatments
2.2.1. Control Plants
2.2.2. Plants Treated with JA
2.2.3. Plants Treated with SA
2.2.4. Control Plants with Larval Herbivory (CL Treatment)
2.2.5. Plants Treated with JA and Larval Herbivory (JAL Treatment)
2.2.6. Plants Treated with SA and Larval Herbivory (SAL Treatment)
2.3. Differences in Glucosinolate Induction among Treatments and Kale Genotypes: Effect of Phytohormone and Herbivory Treatments
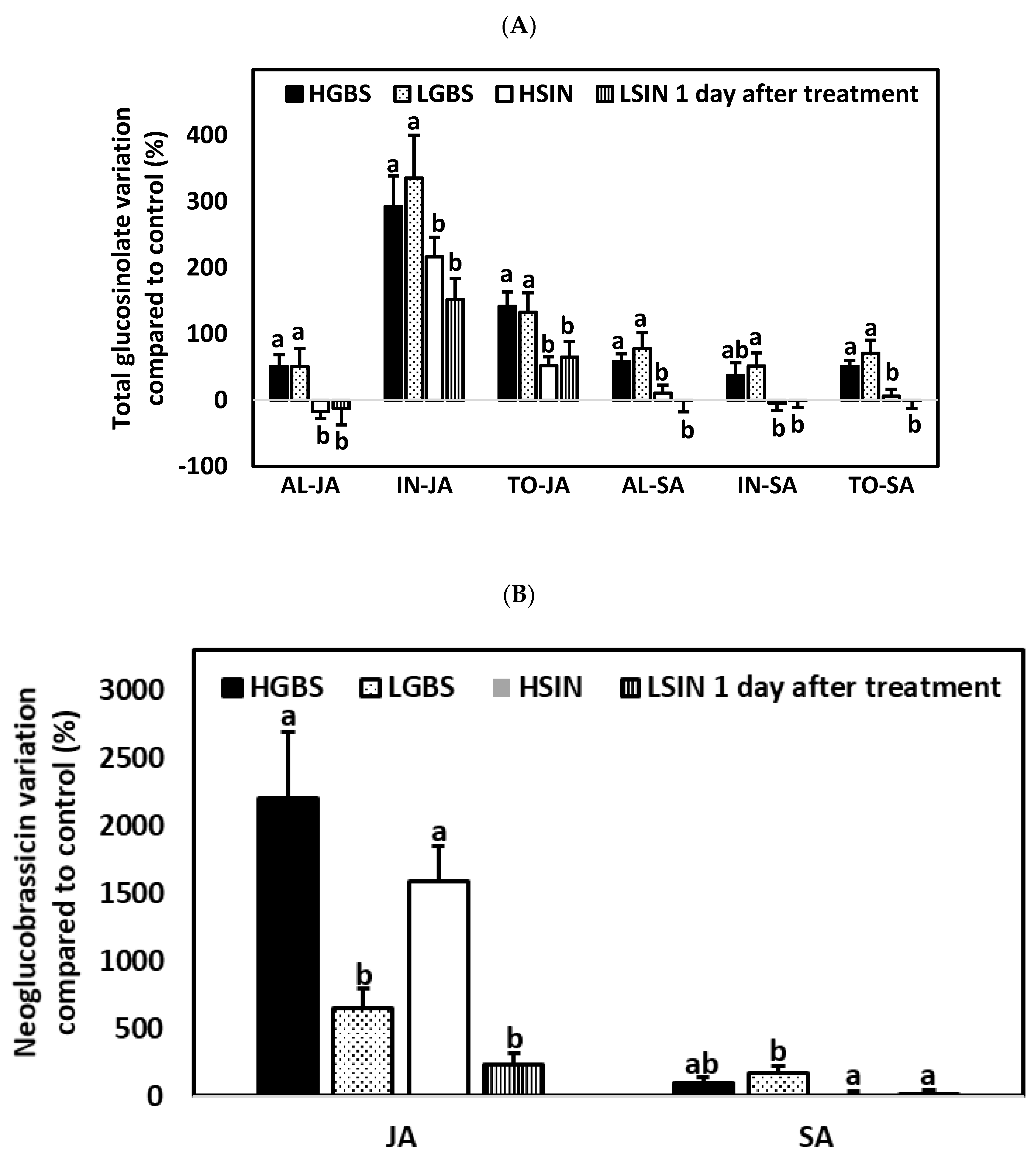
2.3.1. One Day after the Application of Phytohormones
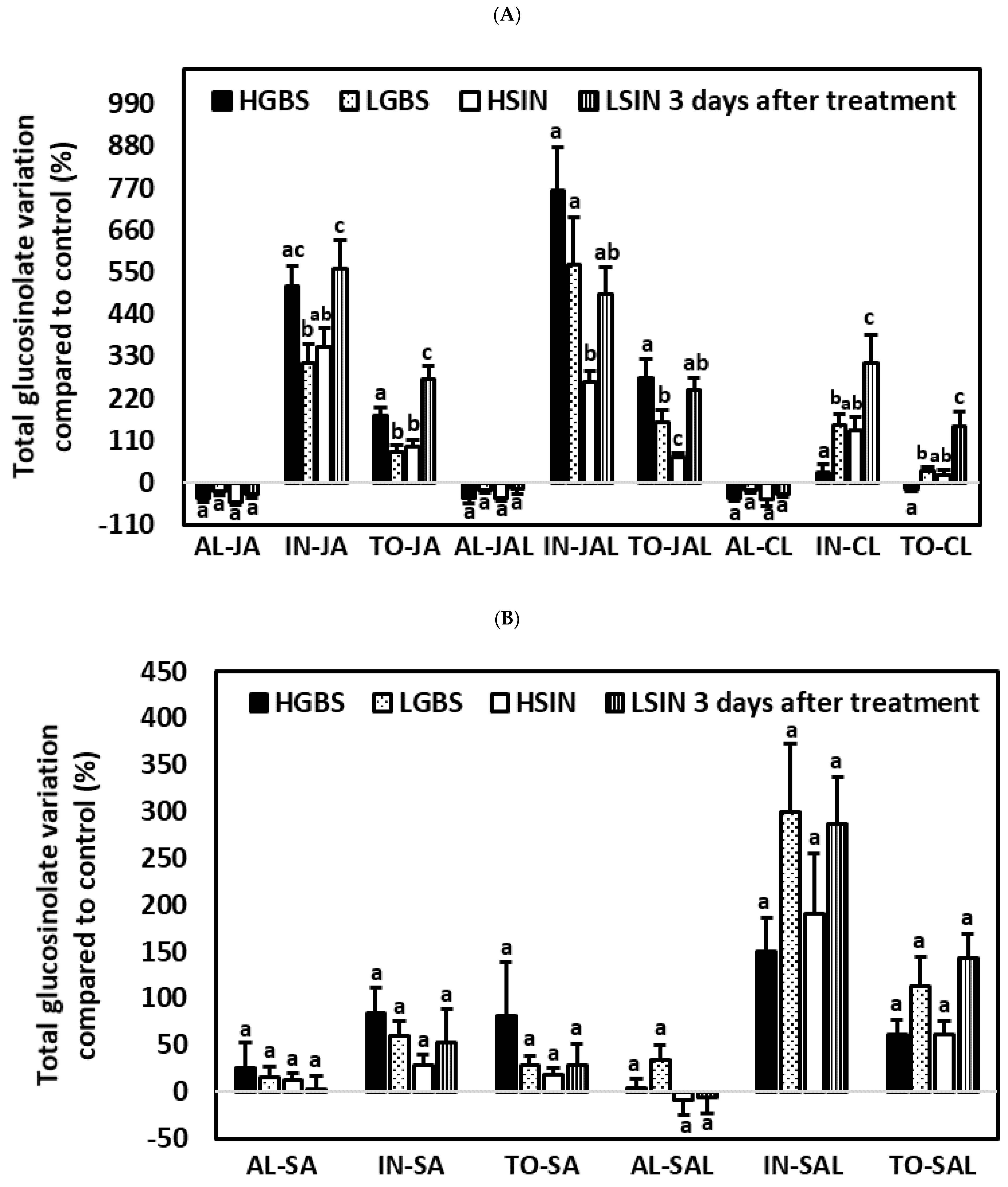
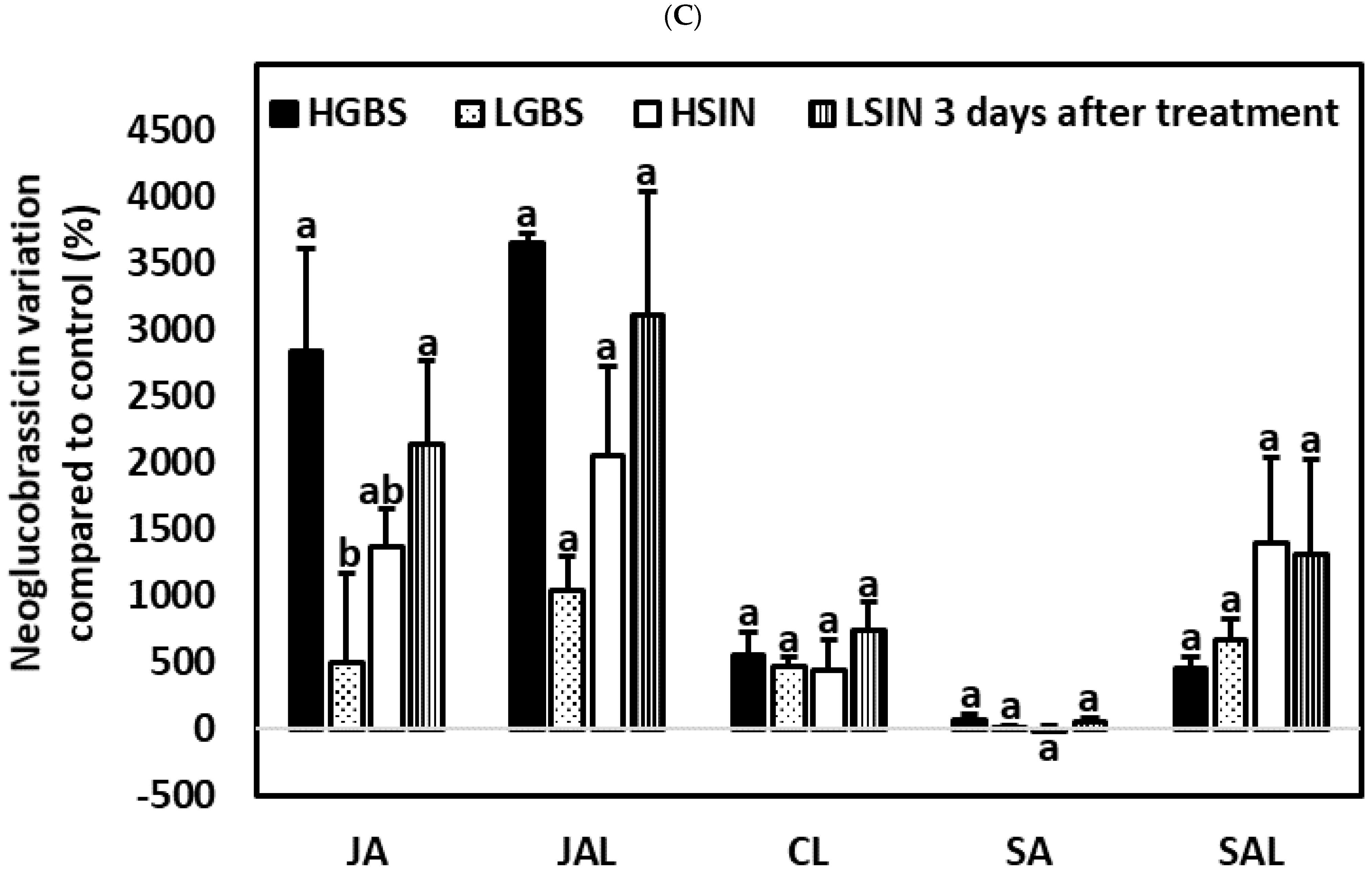
2.3.2. Three Days after the Application of Phytohormones
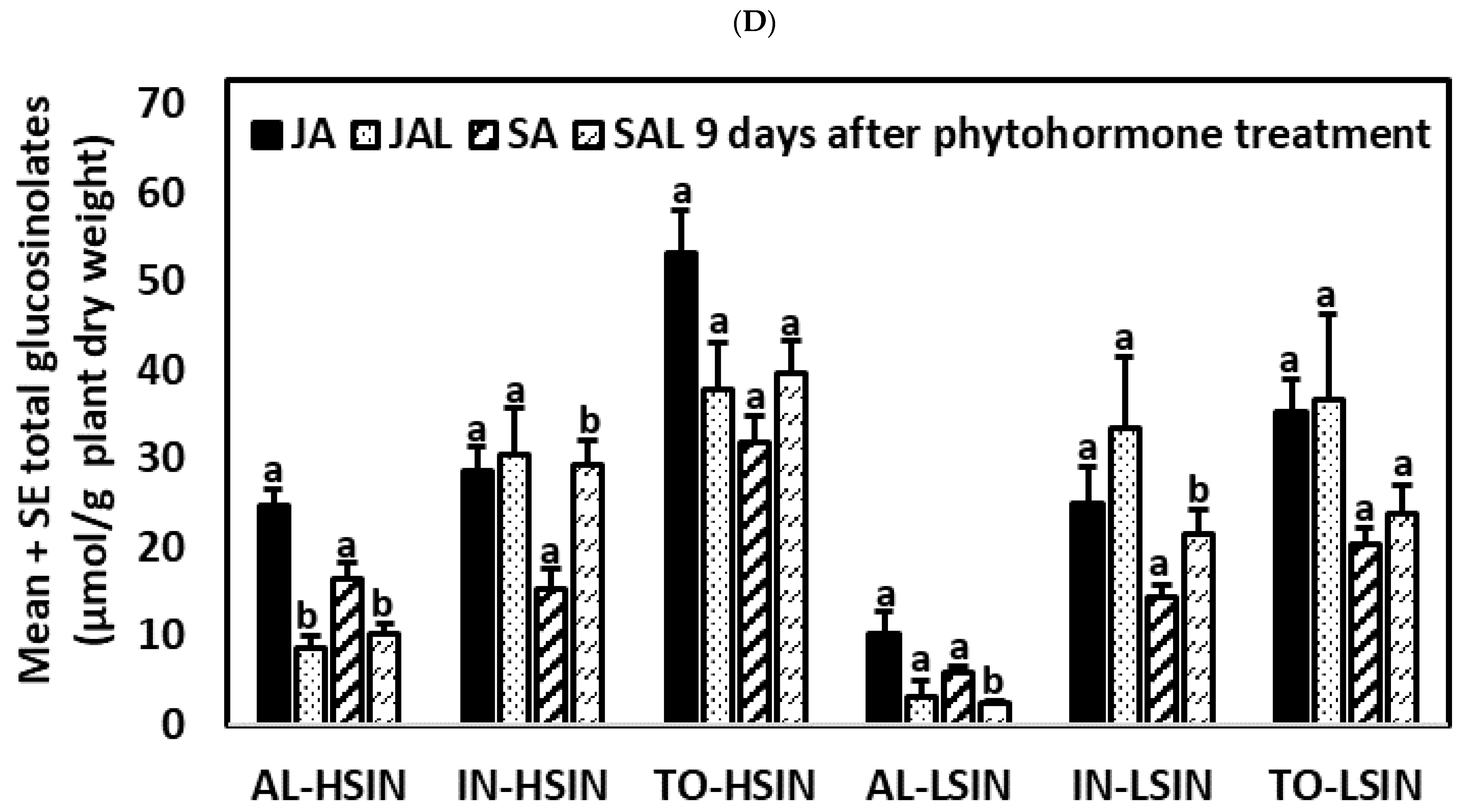
2.3.3. Nine Days after the Application of Phytohormones
2.4. Differences in Glucosinolate Induction among Treatments and Kale Genotypes: Glucosinolate Differences among Genotypes
2.4.1. One Day after the Application of Phytohormones
2.4.2. Three Days after the Application of Phytohormones
2.4.3. Nine Days after the Application of Phytohormones
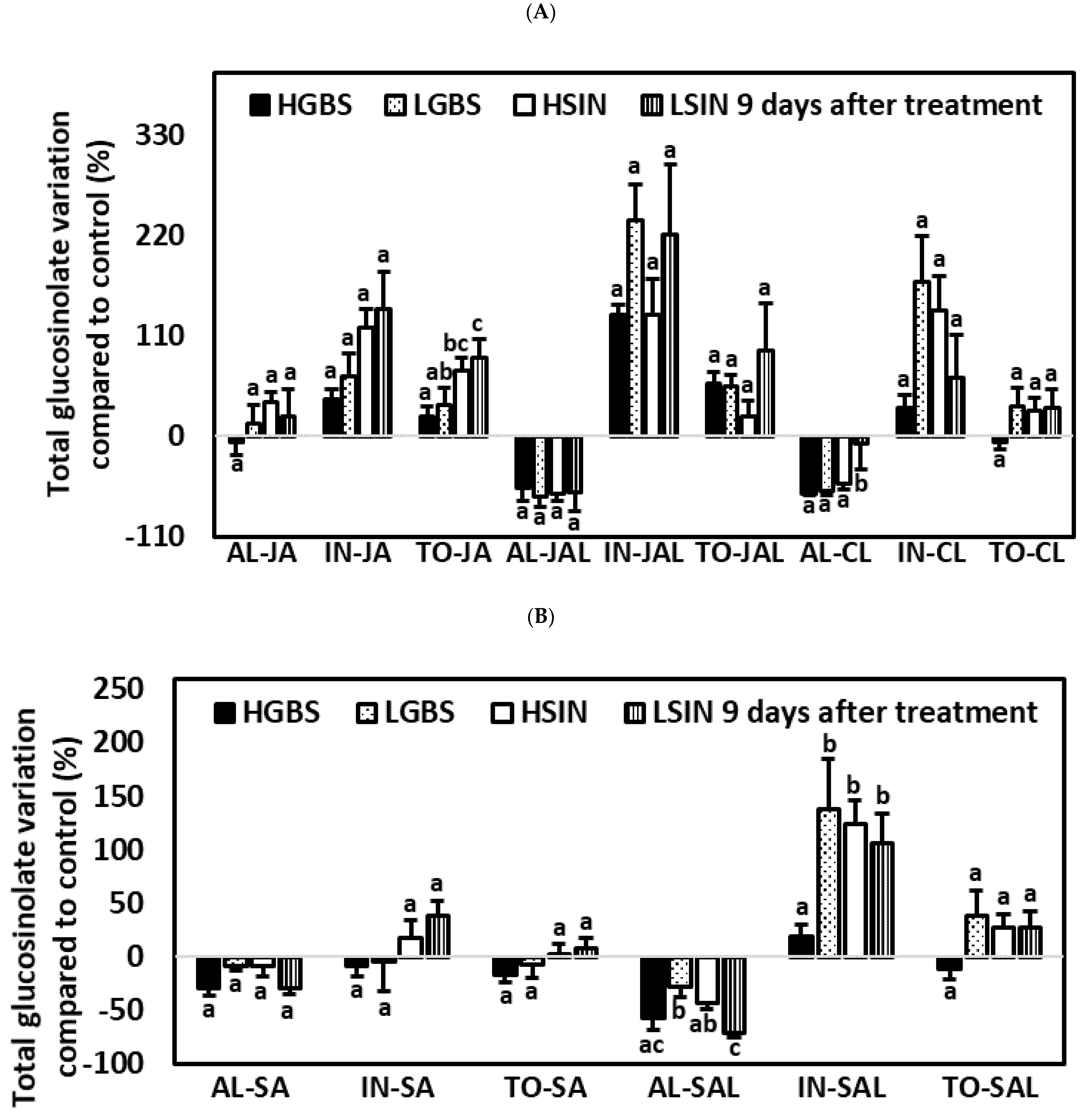
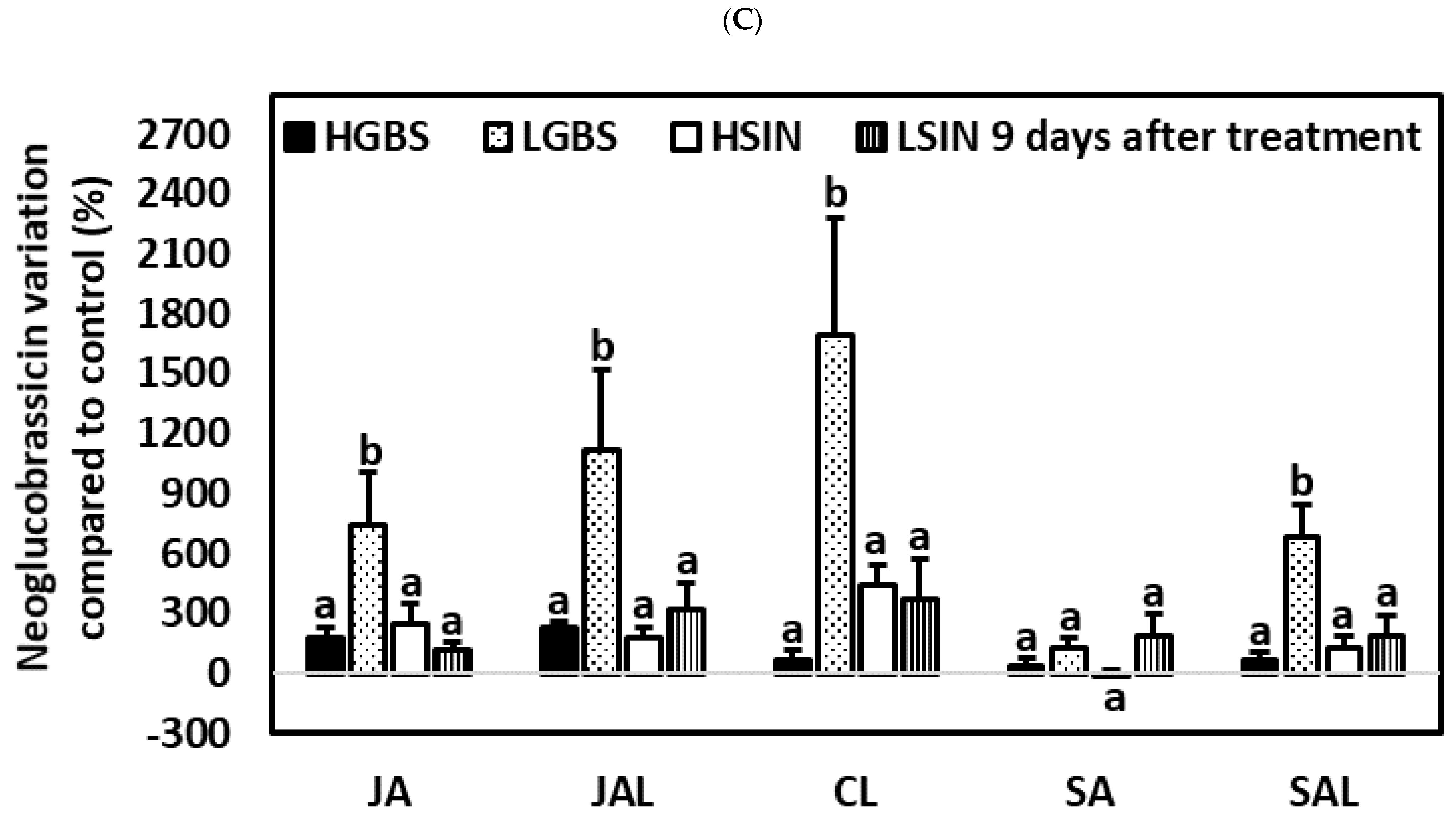
2.5. Differences in Glucosinolate Induction among Treatments and Kale Genotypes: Percent Glucosinolate Variation among Genotypes
2.5.1. One Day after the Application of Phytohormones
2.5.2. Three Days after the Application of Phytohormones
2.5.3. Nine Days after the Application of Phytohormones
2.6. Correlation between Induced Aliphatic and Indolic Glucosinolates
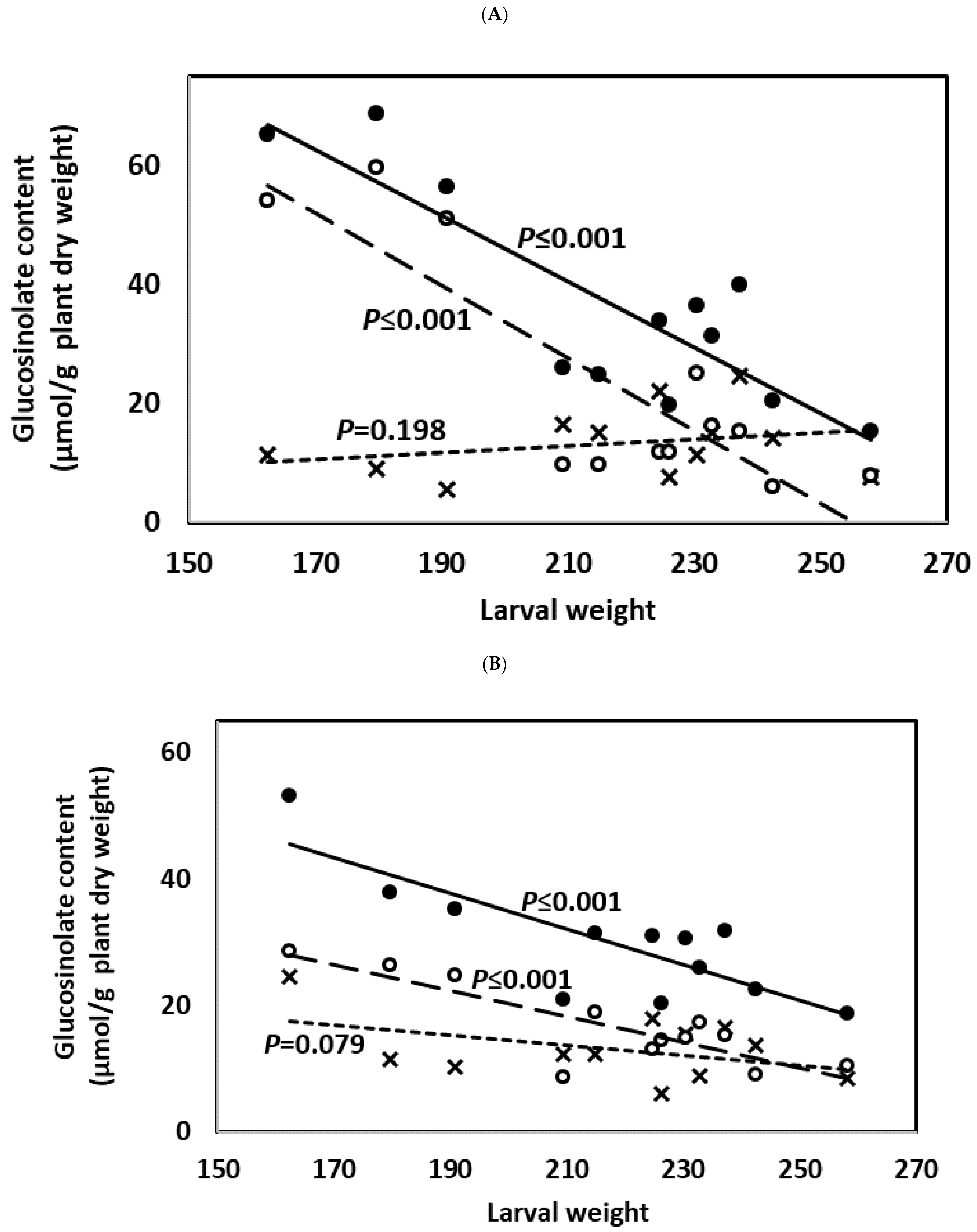
2.7. Herbivory and Larval Weight Gain Experiments
3. Discussion
4. Materials and Methods
4.1. Plants and Insects Used in the Experiments
4.2. Application of Phytohormones on Plants
4.3. Interaction between Phytohormone and Herbivore Induction
4.4. Analysis of Glucosinolates in Plants
4.5. Herbivory and Larval Weight Gain Experiments
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mithen, R. Glucosinolates—Biochemistry, genetics and biological activity. Plant Growth Regul. 2001, 34, 91–103. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Agerbirk, N.; Olsen, C.E.; Boeve, J.L.; Schaffner, U.; Brakefield, P.M. Sequestration of host plant glucosinolates in the defensive hemolymph of the sawfly Athalia rosae. J. Chem. Ecol. 2001, 27, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Beran, F.; Pauchet, Y.; Kunert, G.; Reichelt, M.; Wielsch, N.; Vogel, H.; Reinecke, A.; Svatoš, A.; Mewis, I.; Schmid, D.; et al. Phyllotreta Striolata Flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system. Proc. Natl. Acad. Sci. USA 2014, 111, 7349–7354. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, V.; Gershenzon, J.; Vassão, D.G. Chapter Eight—Insect detoxification of glucosinolates and their hydrolysis products. In Advances in Botanical Research; Kopriva, S., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 80, pp. 199–245. [Google Scholar]
- Zalucki, M.P.; Zalucki, J.M.; Perkins, L.E.; Schramm, K.; Vassão, D.G.; Gershenzon, J.; Heckel, D.G. A generalist herbivore copes with specialized plant defence: The effects of induction and feeding by Helicoverpa armigera (Lepidoptera: Noctuidae) larvae on intact Arabidopsis thaliana (Brassicales) plants. J. Chem. Ecol. 2017, 43, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Badenes-Pérez, F.R.; Gershenzon, J.; Heckel, D.G. Plant glucosinolate content increases susceptibility to diamondback moth (Lepidoptera: Plutellidae) regardless of its diet. J. Pest Sci. 2020, 93, 491–506. [Google Scholar] [CrossRef]
- Beekwilder, J.; van Leeuwen, W.; van Dam, N.M.; Bertossi, M.; Grandi, V.; Mizzi, L.; Soloviev, M.; Szabados, L.; Molthoff, J.W.; Schipper, B.; et al. The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. PLoS ONE 2008, 3, e2068. [Google Scholar] [CrossRef]
- Gols, R.; Bukovinszky, T.; van Dam, N.; Dicke, M.; Bullock, J.; Harvey, J. Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. J. Chem. Ecol. 2008, 34, 132–143. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Sotelo, T.; Velasco, P.; Cartea, M.E. Antibiotic properties of the glucosinolates of Brassica oleracea var. acephala similarly affect generalist and specialist larvae of two lepidopteran pests. J. Pest Sci. 2016, 89, 195–206. [Google Scholar]
- Müller, C.; Schulz, M.; Pagnotta, E.; Ugolini, L.; Yang, T.; Matthes, A.; Lazzeri, L.; Agerbirk, N. The role of the glucosinolate-myrosinase system in mediating greater resistance of Barbarea verna than B. vulgaris to Mamestra brassicae larvae. J. Chem. Ecol. 2018, 44, 1190–1205. [Google Scholar] [CrossRef]
- Jeschke, V.; Kearney, E.E.; Schramm, K.; Kunert, G.; Shekhov, A.; Gershenzon, J.; Vassão, D.G. How glucosinolates affect generalist lepidopteran larvae: Growth, development and glucosinolate metabolism. Front. Plant Sci. 2017, 8, 1995. [Google Scholar] [CrossRef] [PubMed]
- Gols, R.; Wagenaar, R.; Bukovinszky, T.; Dam, N.M.; van Dicke, M.; Bullock, J.M.; Harvey, J.A. Genetic variation in defense chemistry in wild cabbage affects herbivores and their endoparasitoids. Ecology 2008, 89, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Poelman, E.H.; van Dam, N.; van Loon, J.J.A.; Vet, L.E.M.; Dicke, M. Chemical diversity in Brassica oleracea affects biodiversity of insect herbivores. Ecology 2009, 90, 1863–1877. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant Immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.J.; Thaler, J.S.; Thomma, B.P.H.J. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Badenes-Pérez, F.R.; Broekgaarden, C.; Zheng, S.-J.; David, A.; Boland, W.; Dicke, M. Plant-mediated facilitation between a leaf-feeding and a phloem-feeding insect in a brassicaceous plant: From insect performance to gene transcription. Funct. Ecol. 2012, 26, 156–166. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Biotic elicitors effectively increase the glucosinolates content in Brassicaceae sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.; Krumbein, A.; Knorr, D.; Smetanska, I. Enhanced glucosinolates in root exudates of Brassica rapa ssp. rapa mediated by salicylic acid and methyl jasmonate. J. Agric. Food Chem. 2011, 59, 1400–1405. [Google Scholar]
- Thiruvengadam, M.; Kim, S.H.; Chung, I.M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Reichelt, M.; Gershenzon, J.; Heckel, D.G. Interaction of glucosinolate content of Arabidopsis thaliana mutant lines and feeding and oviposition by generalist and specialist lepidopterans. Phytochemistry 2013, 86, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Rohr, F.; Ulrichs, C.; Schreiner, M.; Zrenner, R.; Mewis, I. Responses of Arabidopsis thaliana plant lines differing in hydroxylation of aliphatic glucosinolate side chains to feeding of a generalist and specialist caterpillar. Plant Physiol. Biochem. 2012, 55, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; van Dam, N.M.; Winge, P.; Trælnes, M.; Heydarova, A.; Rohloff, J.; Langaas, M.; Bones, A.M. Plant defence responses in oilseed rape MINELESS plants after attack by the cabbage moth Mamestra brassicae. J. Exp. Bot. 2015, 66, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.; Gershenzon, J. Herbivore induction of the glucosinolate–myrosinase defense system: Major trends, biochemical bases and ecological significance. Phytochem. Rev. 2009, 8, 149–170. [Google Scholar] [CrossRef]
- Sotelo, T.; Velasco, P.; Soengas, P.; Rodríguez, V.M.; Cartea, M.E. Modification of leaf glucosinolate contents in Brassica oleracea by divergent selection and effect on expression of genes controlling glucosinolate pathway. Front. Plant Sci. 2016, 7, 1012. [Google Scholar] [CrossRef] [PubMed]
- Sontowski, R.; Gorringe, N.J.; Pencs, S.; Schedl, A.; Touw, A.J.; van Dam, N.M. Same difference? Low and high glucosinolate Brassica rapa varieties show similar responses upon feeding by two specialist root herbivores. Front. Plant Sci. 2019, 10, 1451. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.-E.; Robin, A.; Yang, K.; Park, J.-I.; Hwang, B.; Nou, I.-S. Exogenous methyl jasmonate and salicylic acid induce subspecies-specific patterns of glucosinolate accumulation and gene expression in Brassica oleracea L. Molecules 2016, 21, 1417. [Google Scholar] [CrossRef]
- Kim, M.; Chiu, Y.-C.; Kim, N.; Park, H.; Lee, C.; Juvik, J.; Ku, K.-M. Cultivar-specific changes in primary and secondary metabolites in pak choi (Brassica rapa, chinensis group) by methyl jasmonate. Int. J. Mol. Sci. 2017, 18, 1004. [Google Scholar] [CrossRef]
- Bodnaryk, R.P. Potent effect of jasmonates on indole glucosinolates in oilseed rape and mustard. Phytochemistry 1994, 35, 301–305. [Google Scholar] [CrossRef]
- Du, L.; Halkier, B.A. Isolation of a microsomal enzyme system involved in glucosinolate biosynthesis from seedlings of Tropaeolum majus L. Plant Physiol. 1996, 111, 831–837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zang, Y.; Ge, J.; Huang, L.; Gao, F.; Lv, X.; Zheng, W.; Hong, S.; Zhu, Z. Leaf and root glucosinolate profiles of Chinese cabbage (Brassica rapa ssp. pekinensis) as a systemic response to methyl jasmonate and salicylic acid elicitation. J. Zhejiang Univ. Sci. B 2015, 16, 696–708. [Google Scholar] [CrossRef]
- Augustine, R.; Bisht, N.C. Biotic elicitors and mechanical damage modulate glucosinolate accumulation by co-ordinated interplay of glucosinolate biosynthesis regulators in polyploid Brassica juncea. Phytochemistry 2015, 117, 43–50. [Google Scholar] [CrossRef]
- Francisco, M.; Cartea, M.E.; Soengas, P.; Velasco, P. Effect of genotype and environmental conditions on health-promoting compounds in Brassica rapa. J. Agric. Food Chem. 2011, 59, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.; Hanschen, F.S.; Schreiner, M.; Glatt, H.; Zrenner, R. Induced production of 1-methoxy-indol-3-ylmethyl glucosinolate by jasmonic acid and methyl jasmonate in sprouts and leaves of pak choi (Brassica rapa ssp. chinensis). Int. J. Mol. Sci. 2013, 14, 14996–15016. [Google Scholar] [CrossRef] [PubMed]
- Ode, P.J.; Harvey, J.A.; Reichelt, M.; Gershenzon, J.; Gols, R. Differential induction of plant chemical defenses by parasitized and unparasitized herbivores: Consequences for reciprocal, multitrophic interactions. Oikos 2016, 125, 1398–1407. [Google Scholar] [CrossRef]
- Madloo, P.; Lema, M.; Francisco, M.; Soengas, P. Role of major glucosinolates in the defense of kale against Sclerotinia sclerotiorum and Xanthomonas campestris pv. campestris. Phytopathology 2019, 109, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Piślewska-Bednarek, M.; Svatoš, A.; Schneider, B.; Doubský, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Chassin, E.; Bilat, J.; Glauser, G.; Reymond, P. Trade-off between constitutive and inducible resistance against herbivores is only partially explained by gene expression and glucosinolate production. J. Exp. Bot. 2015, 66, 2527–2534. [Google Scholar] [CrossRef]
- Cipollini, D.; Enright, S.; Traw, M.B.; Bergelson, J. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol. Ecol. 2004, 13, 1643–1653. [Google Scholar] [CrossRef]
- Mewis, I.; Tokuhisa, J.G.; Schultz, J.C.; Appel, H.M.; Ulrichs, C.; Gershenzon, J. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry 2006, 67, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, N.; Reichelt, M.; van de Mortel, J.E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J.J.A.; Dicke, M.; Pineda, A. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schedl, A.; Sontowski, R.; Driscoll, B.T.; van Dam, N.M.; Bede, J.C. Distinct Arabidopsis responses to two generalist caterpillar species differing in host breadth. PhytoFrontiers 2021, 1, 21–39. [Google Scholar] [CrossRef]
- Kos, M.; Houshyani, B.; Wietsma, R.; Kabouw, P.; Vet, L.E.M.; van Loon, J.J.A.; Dicke, M. Effects of glucosinolates on a generalist and specialist leaf-chewing herbivore and an associated parasitoid. Phytochemistry 2012, 77, 162–170. [Google Scholar] [CrossRef][Green Version]
- Velasco, P.; Cartea, M.E.; González, C.; Vilar, M.; Ordás, A. Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Gershenzon, J.; Heckel, D.G. Insect attraction versus plant defense: Young leaves high in glucosinolates stimulate oviposition by a specialist herbivore despite poor larval survival due to high saponin content. PLoS ONE 2014, 9, e95766. [Google Scholar]
- Badenes-Pérez, F.R.; Shelton, A.M. Pest management and other agricultural practices among farmers growing cruciferous crops in the central and western highlands of Kenya and the western Himalayas of India. Int. J. Pest Manag. 2006, 52, 303–315. [Google Scholar] [CrossRef]
- Cartea, M.E.; Padilla, G.; Vilar, M.; Velasco, P. Incidence of the major Brassica pests in northwestern Spain. J. Econ. Entomol. 2009, 102, 767–773. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Velasco, P.; Soengas, P.; Cartea, M.E. Bottom-up and top-down herbivore regulation mediated by glucosinolates in Brassica oleracea var. acephala. Oecologia 2014, 174, 893–907. [Google Scholar] [CrossRef]
- Zalucki, J.M.; Heckel, D.G.; Wang, P.; Kuwar, S.; Vassão, D.G.; Perkins, L.; Zalucki, M.P. A generalist feeding on Brassicaceae: It does not get any better with selection. Plants 2021, 10, 954. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E.; Bibby, B.M.; Frandsen, H.O.; Brown, L.D.; Nielsen, J.K.; Renwick, J.A.A. A saponin correlated with variable resistance of Barbarea vulgaris to the diamondback moth Plutella xylostella. J. Chem. Ecol. 2003, 29, 1417–1433. [Google Scholar] [CrossRef] [PubMed]
- van Leur, H.; Vet, L.E.M.; van der Puten, W.H.; van Dam, N.M. Barbarea vulgaris glucosinolate phenotypes differentially affect performance and preference of two different species of lepidopteran herbivores. J. Chem. Ecol. 2008, 34, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Badenes-Pérez, F.R.; López-Pérez, J.A. Resistance and susceptibility to powdery mildew, root-knot nematode, and western flower thrips in two types of winter cress (Brassicaceae). Crop Prot. 2018, 110, 41–47. [Google Scholar] [CrossRef]
- Christensen, S.; Enge, S.; Jensen, K.R.; Müller, C.; Kiær, L.P.; Agerbirk, N.; Heimes, C.; Hauser, T.P. Different herbivore responses to two co-occurring chemotypes of the wild crucifer Barbarea vulgaris. Arthropod-Plant Interact. 2018, 13, 19–30. [Google Scholar] [CrossRef]
- Mosleh Arany, A.; de Jong, T.; Kim, H.; van Dam, N.; Choi, Y.; Verpoorte, R.; van der Meijden, E. Glucosinolates and other metabolites in the leaves of Arabidopsis thaliana from natural populations and their effects on a generalist and a specialist herbivore. Chemoecology 2008, 18, 65–71. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Reichelt, M.; Heckel, D.G. Can sulfur fertilisation increase the effectiveness of trap crops for diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae)? Pest Manag. Sci. 2010, 66, 832–838. [Google Scholar]
- Abdel-Farid, I.B.; Jahangir, M.; Mustafa, N.R.; Dam, N.M.; van Hondel, C.A.M.J.J.; van den Kim, H.K.; Choi, Y.H.; Verpoorte, R. Glucosinolate profiling of Brassica rapa cultivars after infection by Leptosphaeria maculans and Fusarium oxysporum. Biochem. Syst. Ecol. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Laila, R.; Abuyusuf, M.; Park, J.-I.; Nou, I.-S. Leptosphaeria maculans alters glucosinolate accumulation and expression of aliphatic and indolic glucosinolate biosynthesis genes in blackleg disease-resistant and -susceptible cabbage lines at the seedling stage. Front. Plant Sci. 2020, 11, 1134. [Google Scholar] [CrossRef]
- Andini, S.; Dekker, P.; Gruppen, H.; Araya-Cloutier, C.; Vincken, J.-P. Modulation of glucosinolate composition in Brassicaceae seeds by germination and fungal elicitation. J. Agric. Food Chem. 2019, 67, 12770–12779. [Google Scholar] [CrossRef] [PubMed]
- Abuyusuf, M.; Robin, A.H.K.; Kim, H.-T.; Islam, M.R.; Park, J.-I.; Nou, I.-S. Altered glucosinolate profiles and expression of glucosinolate biosynthesis genes in ringspot-resistant and susceptible cabbage lines. Int. J. Mol. Sci. 2018, 19, 2833. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Vrieling, K.; Kim, H.K.; Mulder, P.P.J.; Klinkhamer, P.G.L. Application of methyl jasmonate and salicylic acid lead to contrasting effects on the plant’s metabolome and herbivory. Plant Sci. 2021, 303, 110784. [Google Scholar] [CrossRef] [PubMed]
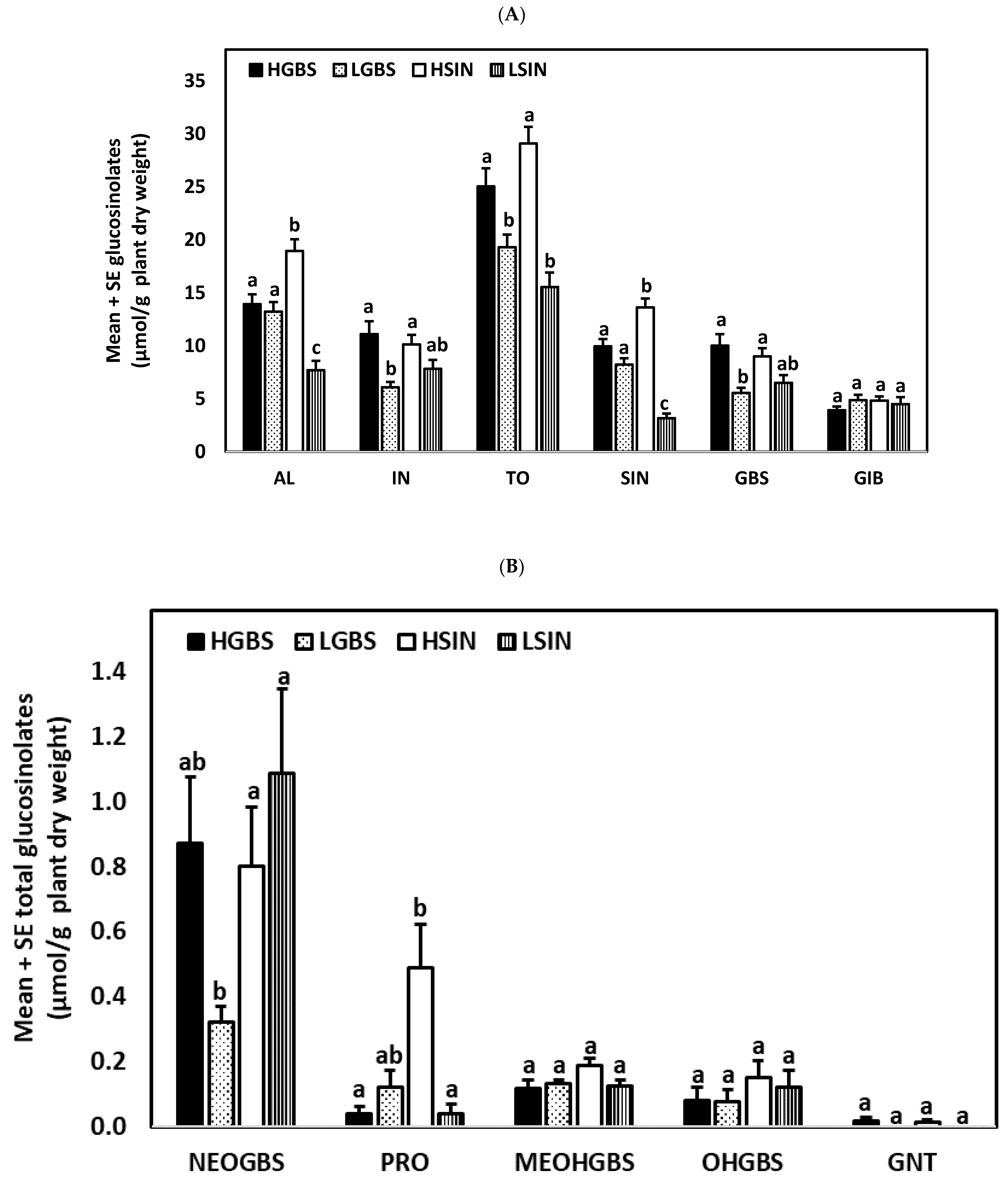
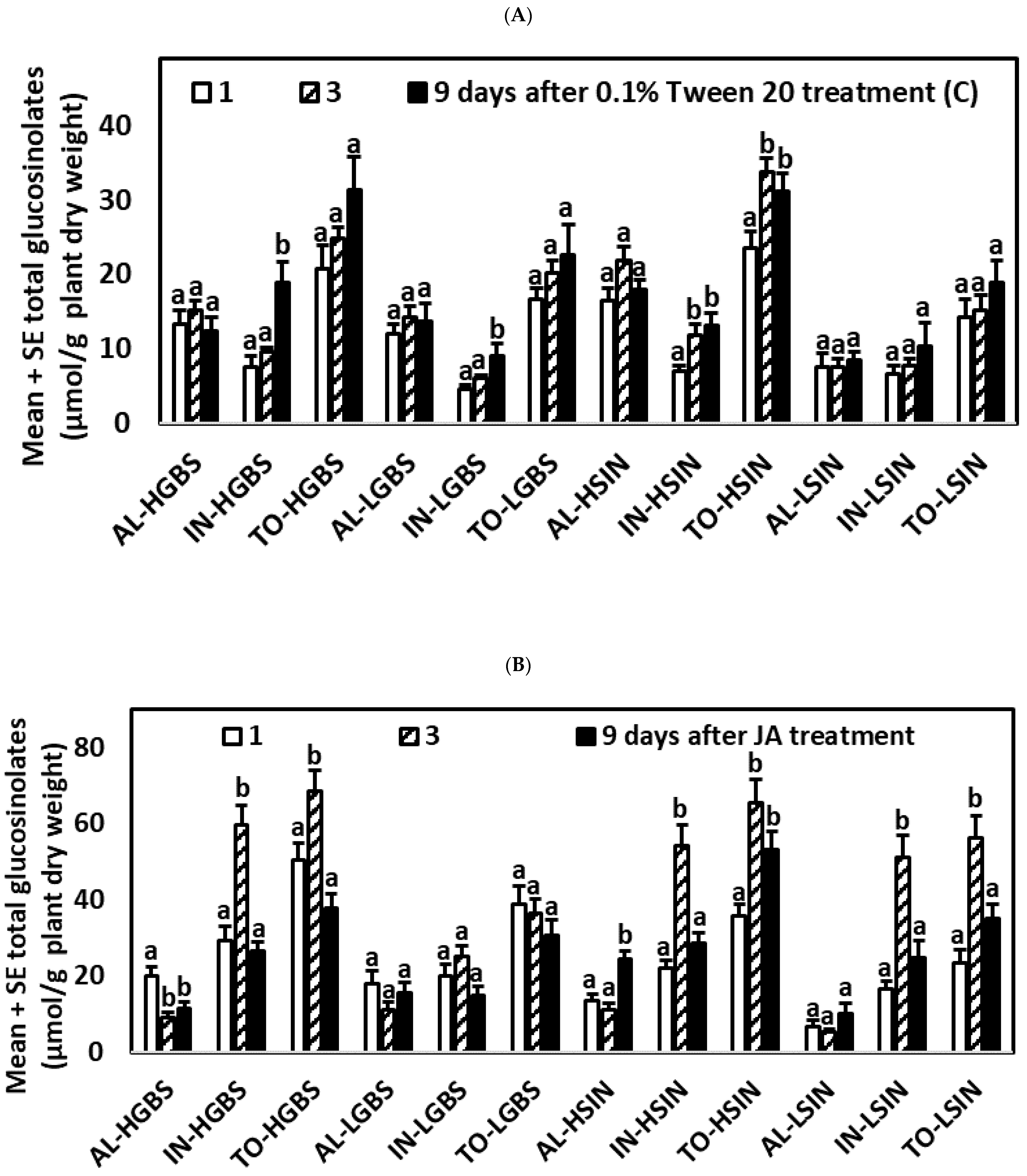
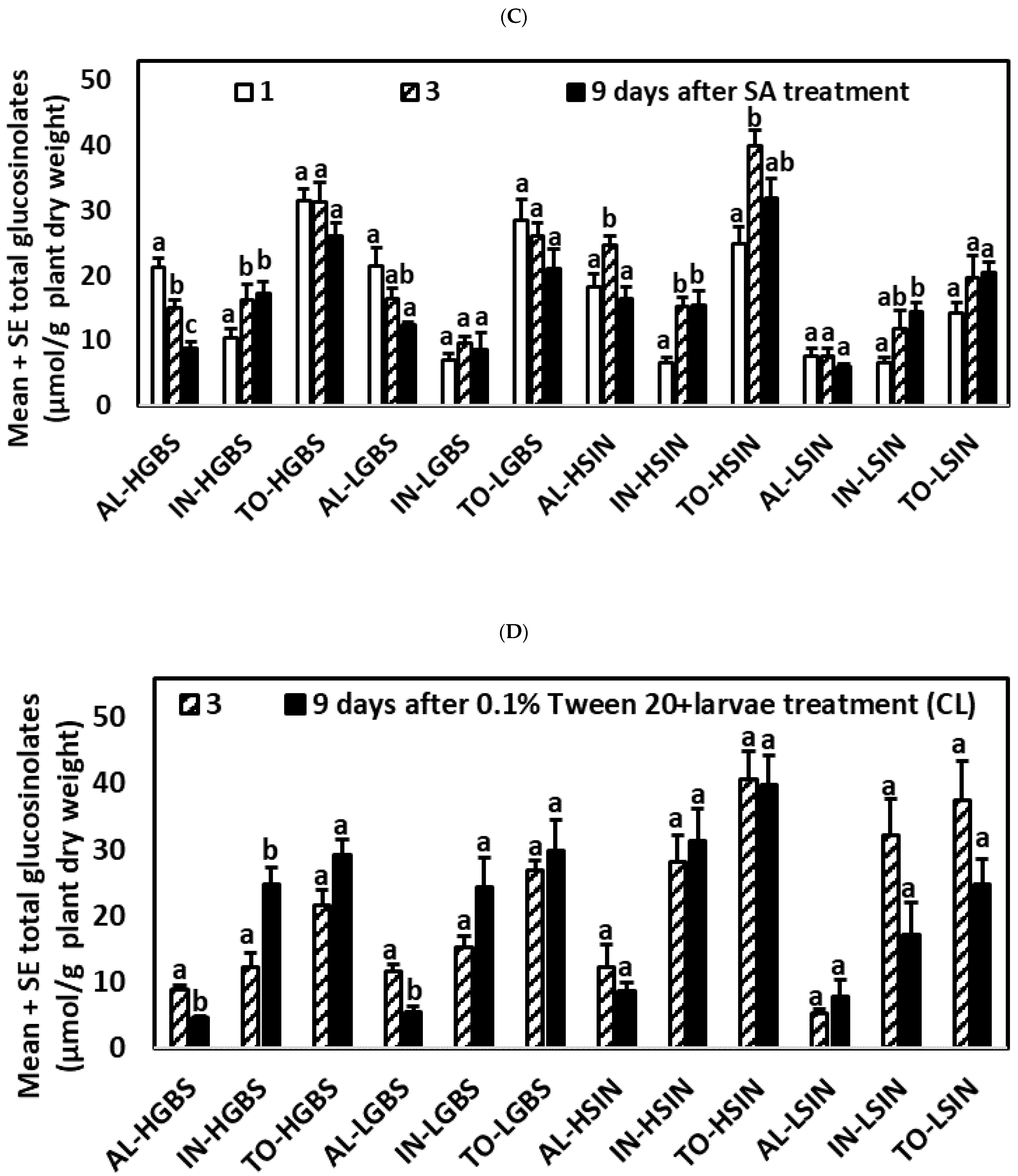

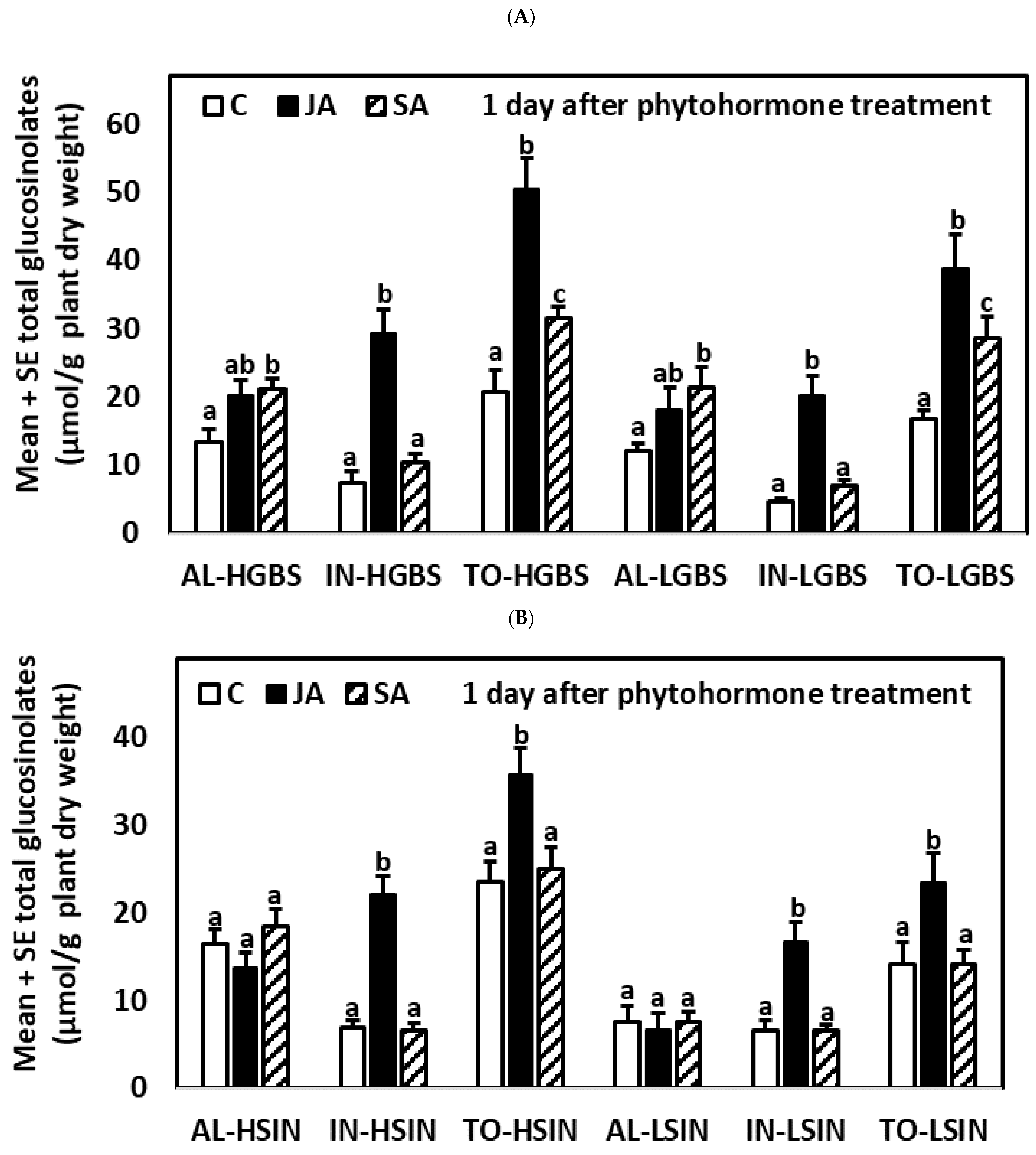


| Days after Treat. | Tr. | Genotype | GIB | SIN | GBS | NEO | AL | IN | TO |
|---|---|---|---|---|---|---|---|---|---|
| 1 | JA | HGBS | 69.3 ± 30.7 a | 43.4 ± 17.5 a | 195.7 ± 41.1 a | 2198.0 ± 492.8 a | 51.1 ± 17.7 a | 292.3 ± 46.4 a | 142.0 ± 21.7 a |
| LGBS | 60.8 ± 26.2 a | 48.5 ± 29.8 a | 320.9 ± 70.0 a | 655.2 ± 139.4 b | 50.8 ± 27.4 a | 335.7 ± 64.5 a | 132.7 ± 29.5 a | ||
| HSIN | 24.2 ± 18.6 a | −29.1 ± 10.1 a | 171.8 ± 30.7 a | 1584.8 ± 260.9 a | −17.2 ± 10.7 b | 216.6 ± 29.5 ab | 52.1 ± 13.3 b | ||
| LSIN | −46.4 ± 6.9 b | 37.4 ± 58.4 a | 147.6 ± 36.6 a | 230.6 ± 87.7 b | −12.6 ± 24.8 b | 151.9 ± 32.1 b | 65.0 ± 24.1 b | ||
| SA | HGBS | 97.2 ± 25.1 a | 38.5 ± 17.8 a | 29.3 ± 21.1 a | 94.5 ± 46.1 ab | 58.9 ± 11.3 a | 37.7 ± 19.0 ab | 51.3 ± 8.6 a | |
| LGBS | 82.6 ± 21.7 a | 77.1 ± 33.7 a | 47.5 ± 20.3 a | 171.8 ± 51.5 b | 78.2 ± 23.6 a | 51.6 ± 19.5 a | 70.9 ± 19.7 a | ||
| HSIN | 10.0 ± 12.2 b | 13.3 ± 16.7 a | −6.5 ± 11.8 a | 5.7 ± 25.5 a | 11.0 ± 12.1 b | −5.0 ± 34.4 b | 6.2 ± 10.6 b | ||
| LSIN | −18.4 ± 13.5 b | 38.5 ± 28.3 a | 5.5 ± 12.2 a | −9.7 ± 34.7 a | −0.7 ± 16.8 b | −0.3 ± 10.6 b | −0.5 ± 12.1 b | ||
| 3 | JA | HGBS | −32.3 ± 11.7 a | −43.7 ± 10.1 a | 355.3 ± 40.1 a | 2837.5 ± 777.7 a | −40.4 ± 9.8 a | 514.4 ± 54.0 ac | 176.2 ± 21.9 a |
| LGBS | −26.6 ± 14.2 a | −19.1 ± 15.3 a | 300.4 ± 50.5 a | 498.6 ± 222.2 b | −20.1 ± 13.1 a | 313.6 ± 49.4 b | 79.7 ± 19.1 b | ||
| HSIN | −42.8 ± 8.9 a | −49.7 ± 8.1 a | 274.2 ± 55.4 a | 1363.6 ± 292.9 ab | −48.6 ± 7.0 a | 357.1 ± 46.6 ab | 93.6 ± 18.2 b | ||
| LSIN | −13.6 ± 14.6 a | −49.1 ± 13.6 a | 376.1 ± 71.3 a | 2145.0 ± 625.1 a | −27.9 ± 10.5 a | 558.1 ± 75.1 c | 269.9 ± 37.0 c | ||
| JAL | HGBS | −3.1 ± 15.7 a | −54.2 ± 14.5 a | 568.9 ± 86.5 a | 3655.5 ± 709.6 a | −39.2 ± 14.6 a | 763.2 ± 113.1 a | 273.8 ± 50.0 a | |
| LGBS | −4.0 ± 18.3 a | −27.1 ± 18.7 a | 545.6 ± 132.7 a | 1039.6 ± 257.5 a | −18.0 ± 8.8 a | 570.8 ± 124.4 a | 158.1 ± 33.2 b | ||
| HSIN | −37.3 ± 19.1 a | −37.1 ± 13.4 a | 118.2 ± 62.5 b | 2051.9 ± 669.5 a | −38.8 ± 9.0 a | 265.3 ± 27.9 b | 67.7 ± 8.4 c | ||
| LSIN | −12.7 ± 24.0 a | −44.4 ± 13.1 a | 201.3 ± 74.2 b | 3106.3 ± 933.5 a | −13.7 ± 16.7 a | 492.8 ± 71.3 ab | 243.8 ± 32.1 ab | ||
| SA | HGBS | −10.7 ± 12.4 a | 16.8 ± 15.3 a | 71.4 ± 26.5 a | 64.0 ± 45.9 a | 26.1 ± 27.4 a | 83.9 ± 27.7 a | 82.3 ± 56.1 a | |
| LGBS | 10.9 ± 17.2 a | 16.3 ± 14.7 a | 55.2 ± 17.0 a | 10.4 ± 19.6 a | 15.2 ± 11.4 a | 60.1 ± 15.8 a | 28.6 ± 10.0 a | ||
| HSIN | 24.0 ± 14.3 a | 10.5 ± 10.3 a | 27.1 ± 10.3 a | −3.8 ± 30.4 a | 12.5 ± 7.0 a | 29.0 ± 11.3 a | 18.2 ± 7.2 a | ||
| LSIN | −8.9 ± 20.7 a | 8.5 ± 15.8 a | 50.4 ± 35.1 a | 49.1 ± 36.7 a | 2.1 ± 15.2 a | 52.9 ± 35.9 a | 28.7 ± 23.3 a | ||
| SAL | HGBS | −3.9 ± 17.5 a | 7.0 ± 12.2 a | 129.1 ± 38.0 a | 458.4 ± 75.9 a | 3.8 ± 11.1 a | 150.7 ± 36.1 a | 60.9 ± 16.9 a | |
| LGBS | 34.2 ± 10.4 a | 33.2 ± 26.2 a | 276.3 ± 76.6 a | 662.8 ± 168.0 a | 33.6 ± 17.0 a | 300.2 ± 72.8 a | 113.3 ± 31.5 a | ||
| HSIN | 22.0 ± 14.9 a | −16.2 ± 20.1 a | 90.3 ± 26.0 a | 1397.6 ± 642.7 a | −8.6 ± 16.2 a | 190.9 ± 64.9 a | 61.3 ± 14.9 a | ||
| LSIN | 2.7 ± 15.1 a | −14.9 ± 32.8 a | 173.1 ± 84.3 a | 1314.8 ± 718.6 a | −5.4 ± 17.3 a | 286.9 ± 50.6 a | 143.8 ± 26.1 a | ||
| CL | HGBS | −9.7 ± 7.5 a | −53.8 ± 6.2 a | −11.6 ± 15.3 a | 558.6 ± 164.9 a | −40.9 ± 3.8 a | 25.9 ± 22.3 a | −12.9 ± 9.0 a | |
| LGBS | 24.6 ± 6.6 a | −16.2 ± 10.2 a | 130.0 ± 27.0 b | 467.1 ± 73.6 a | −18.3 ± 7.0 a | 151.3 ± 27.6 b | 32.4 ± 7.9 b | ||
| HSIN | −47.8 ± 14.5 a | −42.2 ± 17.9 a | 113.4 ± 23.4 b | 439.3 ± 224.6 a | −44.4 ± 16.0 a | 137.3 ± 34.7 ab | 20.6 ± 12.3 ab | ||
| LSIN | −23.8 ± 13.5 a | −35.7 ± 10.9 a | 268.7 ± 69.1 c | 740.9 ± 216.6 a | −28.9 ± 6.6 a | 314.6 ± 71.6 c | 146.1 ± 39.2 c | ||
| 9 | JA | HGBS | 55.1 ± 34.2 a | −28.9 ± 21.1 a | 21.5 ± 13.8 a | 177.1 ± 51.3 a | −7.0 ± 14.4 a | 39.6 ± 12.2 a | 21.2 ± 11.9 a |
| LGBS | 63.9 ± 45.7 a | −6.7 ± 19.6 a | 42.6 ± 30.9 a | 746.9 ± 256.8 b | 14.2 ± 20.0 a | 65.7 ± 25.5 a | 34.7 ± 18.8 ab | ||
| HSIN | 25.1 ± 14.9 a | 45.4 ± 16.7 a | 94.8 ± 23.0 a | 252.1 ± 103.1 a | 37.1 ± 11.1 a | 118.5 ± 20.9 a | 71.4 ± 15.0 bc | ||
| LSIN | 28.0 ± 35.2 a | 10.8 ± 32.9 a | 142.8 ± 44.8 a | 116.4 ± 38.4 a | 22.2 ± 29.7 a | 138.8 ± 41.3 a | 86.7 ± 19.7 c | ||
| JAL | HGBS | −42.8 ± 30.5 a | −61.7 ± 9.7 a | 122.3 ± 15.3 a | 226.7 ± 36.0 a | −57.1 ± 14.1 a | 132.9 ± 12.2 a | 57.7 ± 12.5 a | |
| LGBS | −61.0 ± 11.0 a | −69.4 ± 10.4 a | 211.9 ± 42.7 a | 1122.6 ± 394.2 b | −66.9 ± 10.3 a | 236.7 ± 39.0 a | 54.0 ± 13.4 a | ||
| HSIN | −36.8 ± 21.7 a | −73.5 ± 10.6 a | 130.2 ± 47.4 a | 179.1 ± 54.1 a | −63.9 ± 7.9 a | 132.7 ± 40.0 a | 21.4 ± 38.0 a | ||
| LSIN | −79.1 ± 4.0 a | −44.3 ± 38.7 a | 207.8 ± 68.4 a | 316.0 ± 130.4 a | −62.3 ± 20.0 a | 221.2 ± 76.8 a | 94.5 ± 51.2 a | ||
| SA | HGBS | −17.6 ± 17.0 a | −32.6 ± 6.5 ac | −17.8 ± 15.6 a | 40.7 ± 33.2 a | −29.0 ± 7.5 a | −8.9 ± 9.7 a | −16.9 ± 6.3 a | |
| LGBS | −6.2 ± 19.6 a | −14.7 ± 5.3 ab | −12.7 ± 30.1 a | 128.8 ± 48.3 a | −9.1 ± 3.8 a | −4.3 ± 28.0 a | −7.2 ± 13.1 a | ||
| HSIN | −31.2 ± 12.8 a | 1.7 ± 10.0 b | 21.2 ± 17.9 a | −8.1 ± 23.5 a | −8.3 ± 10.0 a | 17.2 ± 17.0 a | 2.4 ± 9.7 a | ||
| LSIN | −13.2 ± 16.9 a | −45.7 ± 12.6 c | 9.4 ± 17.7 a | 189.6 ± 110.3 a | −28.9 ± 6.0 a | 38.4 ± 13.8 a | 8.3 ± 9.0 a | ||
| SAL | HGBS | −34.6 ± 14.5 ab | −64.5 ± 11.7 a | 13.6 ± 11.5 a | 68.6 ± 42.5 a | −59.2 ± 10.4 ac | 19.4 ± 10.9 a | −10.9 ± 10.2 a | |
| LGBS | −12.5 ± 11.8 a | −35.7 ± 11.2 bc | 121.8 ± 44.2 a | 685.7 ± 162.8 b | −28.5 ± 9.1 b | 138.2 ± 47.0 b | 37.9 ± 23.5 a | ||
| HSIN | −57.4 ± 13.9 b | −40.6 ± 4.9 ab | 124.7 ± 24.3 a | 124.3 ± 62.2 a | −42.9 ± 6.3 ab | 123.9 ± 21.7 b | 27.4 ± 11.8 a | ||
| LSIN | −68.8 ± 7.9 b | −75.4 ± 6.0 c | 91.1 ± 28.6 a | 193.3 ± 93.5 a | −71.4 ± 3.0 c | 10.6 ± 27.3 ab | 26.8 ± 16.0 a | ||
| CL | HGBS | −70.1 ± 7.3 a | −80.7 ± 1.0 a | 24.3 ± 14.3 a | 71.5 ± 46.4 a | −63.6 ± 2.2 a | 30.2 ± 14.4 a | −6.9 ± 7.9 a | |
| LGBS | −40.4 ± 11.1 a | −83.3 ± 3.3 a | 117.6 ± 42.6 a | 1697.2 ± 577.7 b | −59.8 ± 5.7 a | 170.0 ± 50.0 a | 31.7 ± 20.9 a | ||
| HSIN | −59.2 ± 8.0 a | −78.2 ± 3.2 a | 97.5 ± 33.0 a | 437.9 ± 103.4 a | −52.3 ± 7.0 a | 138.7 ± 37.2 a | 28.2 ± 14.5 a | ||
| LSIN | −1.0 ± 20.4 b | −67.8 ± 17.1 a | 2.6 ± 53.8 a | 370.9 ± 206.5 a | −7.8 ± 29.0 b | 63.3 ± 48.1 a | 31.5 ± 35.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badenes-Pérez, F.R.; Cartea, M.E. Glucosinolate Induction and Resistance to the Cabbage Moth, Mamestra brassicae, Differs among Kale Genotypes with High and Low Content of Sinigrin and Glucobrassicin. Plants 2021, 10, 1951. https://doi.org/10.3390/plants10091951
Badenes-Pérez FR, Cartea ME. Glucosinolate Induction and Resistance to the Cabbage Moth, Mamestra brassicae, Differs among Kale Genotypes with High and Low Content of Sinigrin and Glucobrassicin. Plants. 2021; 10(9):1951. https://doi.org/10.3390/plants10091951
Chicago/Turabian StyleBadenes-Pérez, Francisco Rubén, and María Elena Cartea. 2021. "Glucosinolate Induction and Resistance to the Cabbage Moth, Mamestra brassicae, Differs among Kale Genotypes with High and Low Content of Sinigrin and Glucobrassicin" Plants 10, no. 9: 1951. https://doi.org/10.3390/plants10091951
APA StyleBadenes-Pérez, F. R., & Cartea, M. E. (2021). Glucosinolate Induction and Resistance to the Cabbage Moth, Mamestra brassicae, Differs among Kale Genotypes with High and Low Content of Sinigrin and Glucobrassicin. Plants, 10(9), 1951. https://doi.org/10.3390/plants10091951







