Hydrogen Sulfide Enhances Plant Tolerance to Waterlogging Stress
Abstract
:1. Introduction
2. The Role of H2S in Response to Hypoxia Stress Induced by Waterlogging in Plants
2.1. Multiple Factors Affect Tolerance to Waterlogging Stress
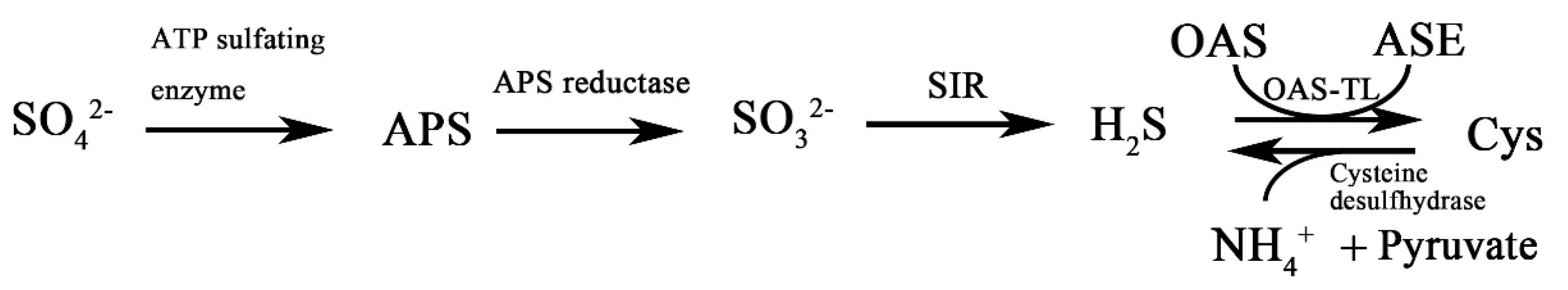
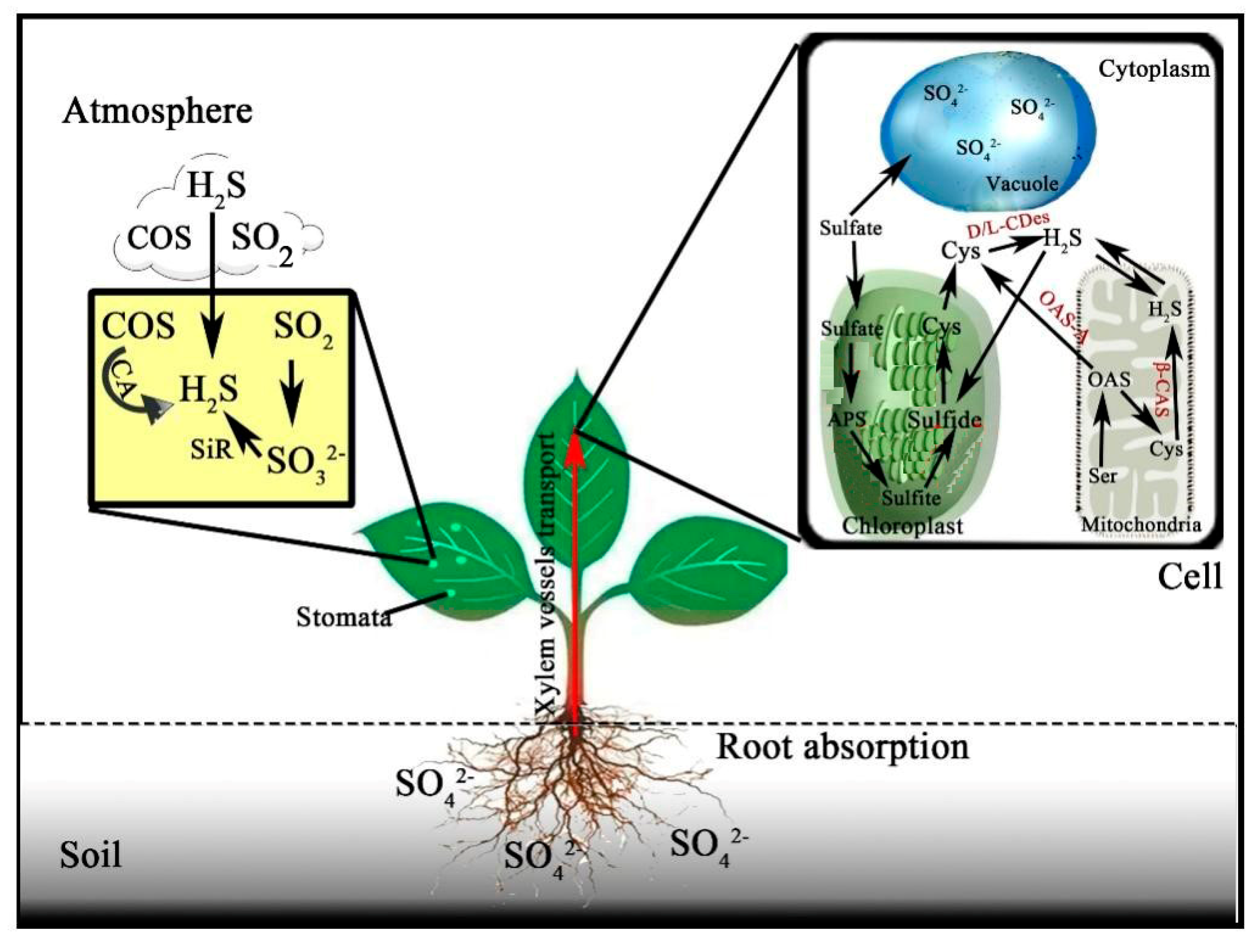
2.2. Synthesis of H2S in Plants
2.3. Intermediate Metabolite of H2S in Plants Promotes Stress Tolerance
3. Adventitious Root Formation, Photosynthesis Efficiency Improvement, Cell Death Alleviation Promoted by H2S against Waterlogging Stress
3.1. H2S Enhances the Occurrence of Adventitious Roots
3.2. H2S Elevates the Photosynthetic Efficiency of Plants
3.3. H2S Alleviates Plant Cell Death
4. How H2S Enhances the Hypoxia Tolerance of Plants during Waterlogging
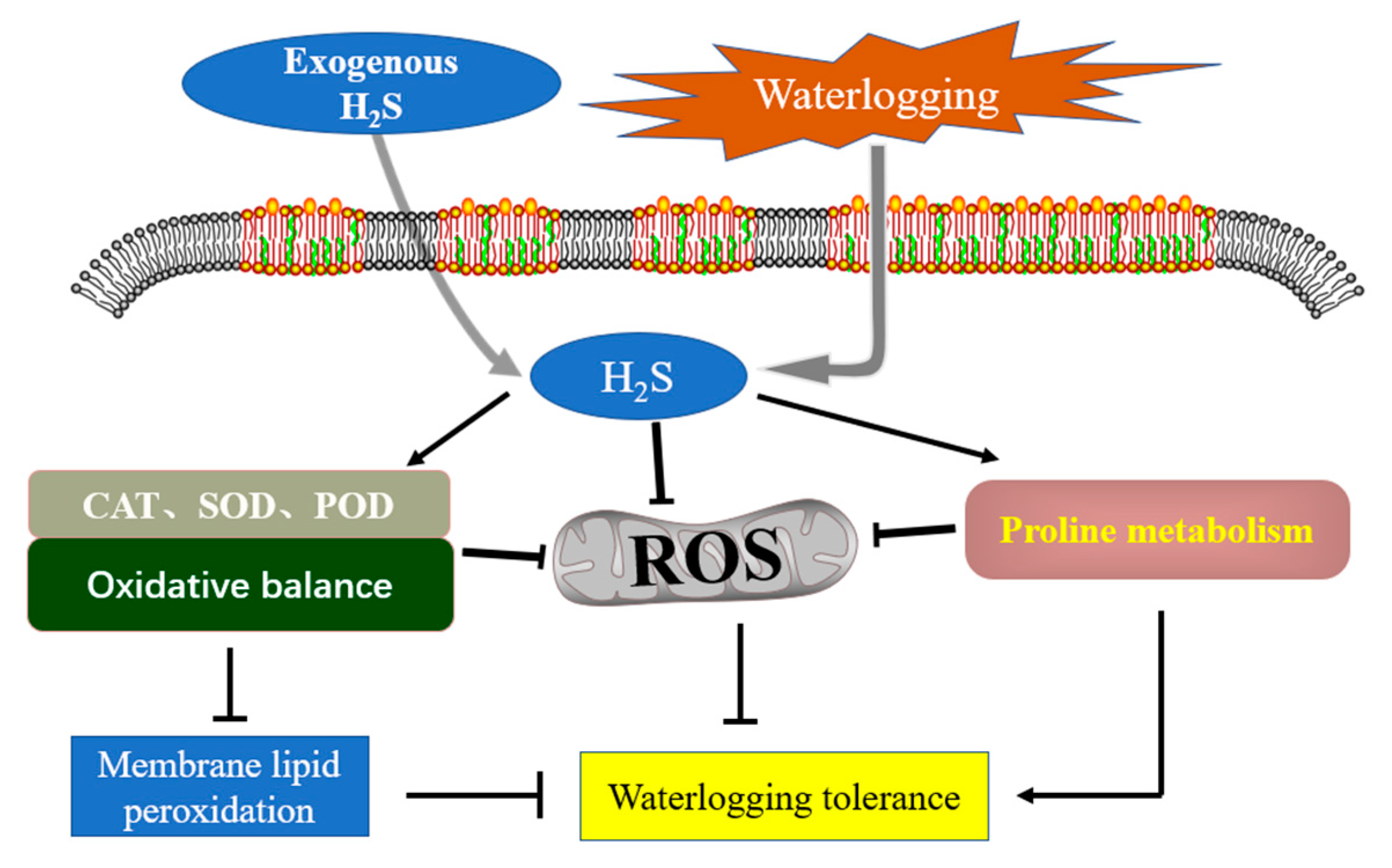
4.1. H2S Enhances the Activity of Antioxidant System to Gain Waterlogging Tolerance
4.2. The Crosstalk between H2S and Hormones Improves the Hypoxia Tolerance
4.3. H2S Affects Respiratory Metabolism to Improve Hypoxia Stress Tolerance
4.4. Sulfur-Sulfhydrylation of Proteins by H2S Strengthens Hypoxia Tolerance
4.5. H2S Associating with Ca2+ Elevates Hypoxia Tolerance
4.6. H2S Involves in Regulating Gene Expression to Improve Tolerance to Waterlogging Stress
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| H2S | Hydrogen sulfide |
| NO | Nitric oxide |
| CO | Carbon monoxide |
| L/D-CDes | L/D-cysteine desulfhydrases |
| D-CDes | D-cysteine desulfhydrase |
| APS | 5’-adenylylsulfate |
| SIR | Sulfite reductase |
| OAS | O-acetyl serine |
| OAS-TL | O-acetyl-L-serine (mercaptan) lyase |
| ASE | Acetate |
| Cys | Cysteine |
| GSH | Glutathione |
| SRPs | Sulfur-rich proteins |
| OAS-A1 | O-acetylserine(thiol)lyase isoform a1 |
| DES1 | Desulfhydrase 1 |
| COS | Carbonyl sulfide |
| IAA | Indoleacetic acid |
| ROS | Reactive oxygen species |
| CAT | Catalase |
| POD | Peroxidase |
| SOD | Superoxide dismutase |
| GSH/GSSG | Glutathione/oxidized glutathione |
| AsA | Ascorbic acid |
| DHA | Dehydroascorbate |
| Gly I | Glyoxalase I |
| Gly II | Glyoxalase II |
| Gly | Glycine |
| MG | Methylglyoxal |
| SA | Salicylic acid |
| ABA | Abscisic acid |
| JA | Jasmonic acid |
| MDH | Malic dehydrogenase |
| PFK | Phosphofructokinase |
| G-6-PDH | Glucose-6-phosphate dehydrogenase |
| APX | Ascorbate peroxidase |
| CaM | Calmodulin |
| EGTA | Ethylene glycol diethyl ether diamine tetraacetic acid |
References
- Peng, Y.J.; Nanduri, J.; Raghuraman, G.; Souvannakitti, D.; Gadalla, M.M.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. USA 2010, 107, 10719–10724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hu, S.-L.; Zhang, Z.-J.; Hu, L.-Y.; Jiang, C.-X.; Wei, Z.-J.; Liu, J.; Wang, H.-L.; Jiang, S.-T. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol. Technol. 2011, 60, 251–257. [Google Scholar] [CrossRef]
- Li, Z.G.; Min, X.; Zhou, Z.H. Hydrogen Sulfide: A signal molecule in plant cross-adaptation. Front. Plant Sci. 2016, 7, 1621. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004, 26, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T. Hydrogen sulfide and environmental stresses. Environ. Exp. Bot. 2019, 161, 50–56. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant. Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- Shkolnik-Inbar, D.; Bar-Zvi, D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010, 22, 3560–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L, cv. Micro-tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Chen, J.; Kuang, L.; Wang, N.; Zhang, G.; Jiang, L.; Wu, D. Effects of waterlogging stress on early seedling development and transcriptomic responses in Brassica napus. Mol. Breed. 2020, 40, 1–14. [Google Scholar] [CrossRef]
- Mendiondo, G.M.; Gibbs, D.J.; Szurman-Zubrzycka, M.; Korn, A.; Marquez, J.; Szarejko, I.; Maluszynski, M.; King, J.; Axcell, B.; Smart, K.; et al. Enhanced waterlogging tolerance in barley by manipulation of expression of the N-end rule pathway E3 ligase PROTEOLYSIS6. Plant Biotechnol. J. 2016, 14, 40–50. [Google Scholar] [CrossRef]
- Zaman, M.S.U.; Malik, A.I.; Erskine, W.; Kaur, P. Changes in gene expression during germination reveal pea genotypes with either “quiescence” or “escape” mechanisms of waterlogging tolerance. Plant Cell Environ. 2019, 42, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Gu, M.; Cong, Y.; Zou, C.-s.; Zhang, X.-k.; Wang, H.-z. Combining ability and genetic effects of germination traits of brassica napus L. under Waterlogging Stress Condition. Agric. Sci. China 2010, 9, 951–957. [Google Scholar] [CrossRef]
- Zou, X.-L.; Zeng, L.; Lu, G.-Y.; Cheng, Y.; Xu, J.-S.; Zhang, X.-K. Comparison of transcriptomes undergoing waterlogging at the seedling stage between tolerant and sensitive varieties of Brassica napus L. J. Integr. Agric. 2015, 14, 1723–1734. [Google Scholar] [CrossRef]
- Xuan, L.; Li, J.; Wang, X.; Wang, C. Crosstalk between hydrogen sulfide and other signal molecules regulates plant growth and development. Int. J. Mol. Sci. 2020, 21, 4593. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yuan, G.; Zhang, Q.; Xuan, L.; Li, J.; Zhou, L.; Shi, H.; Wang, X.; Wang, C. Transcriptome and metabolome analyses reveal the pivotal role of hydrogen sulfide in promoting submergence tolerance in Arabidopsis. Environ. Exp. Bot. 2021, 183, 104365. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y.; Zhai, F.; Zhang, J.; Zhang, F.; Yuan, X.; Xie, Y. Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol. Biochem. 2020, 155, 213–220. [Google Scholar] [CrossRef]
- Li, L.H.; Yi, H.L.; Xiu-Ping, L.; Qi, H.X. Sulfur dioxide enhance drought tolerance of wheat seedlings through H2S signaling. Ecotoxicol. Environ. Saf. 2021, 207, 111248. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shang, Y.T.; Wang, W.H.; Chen, X.Y.; He, E.M.; Zheng, H.L.; Shangguan, Z. Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front. Plant Sci. 2016, 7, 1173. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.Y.; Fu, X.; Zhang, X.W.; Liu, F.J.; Bi, H.G.; Ai, X.Z. Hydrogen sulfide is required for salicylic acid-induced chilling tolerance of cucumber seedlings. Protoplasma 2020, 257, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, Y.; Yang, W.; Chang, G.; Li, P.; Wei, J.; Yuan, X.; Huang, J.; Hu, X. The hydrogen sulfide signal enhances seed germination tolerance to high temperatures by retaining nuclear COP1 for HY5 degradation. Plant Sci. 2019, 285, 34–43. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, Y.; Ye, X.Y.; Li, Z.G. Signaling molecule hydrogen sulfide improves seed germination and seedling growth of maize (Zea mays L.) under high temperature by inducing antioxidant system and osmolyte biosynthesis. Front. Plant Sci. 2018, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G. Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal. Behav. 2015, 10, e1051278. [Google Scholar] [CrossRef] [Green Version]
- Da-Silva, C.J.; Mollica, D.C.F.; Vicente, M.H.; Peres, L.E.P.; Modolo, L.V. NO, hydrogen sulfide does not come first during tomato response to high salinity. Nitric Oxide 2018, 76, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostami, F.; Nasibi, F.; Manouchehri Kalantari, K. Alleviation of UV-B radiation damages by sodium hydrosulfide (H2S donor) pre-treatment in Borage seedlings. J. Plant Interact. 2019, 14, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Duan, X.; Wang, Y.; Zhu, K.; Zhang, J.; Wang, R.; Hu, H.; Qi, F.; Pan, J.; Yan, Y.; et al. Methane protects against polyethylene glycol-induced osmotic stress in maize by improving sugar and ascorbic acid metabolism. Sci. Rep. 2017, 7, 46185. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhou, Y.; Li, H.; Liu, R.; Wang, W.; Wu, W.; Yang, N.; Wang, S. Osmotic stress-triggered stomatal closure requires Phospholipase Dδ and hydrogen sulfide in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2021, 534, 914–920. [Google Scholar] [CrossRef]
- Li, Z.G. Analysis of some enzymes activities of hydrogen sulfide metabolism in plants. Methods Enzymol. 2015, 555, 253–269. [Google Scholar]
- He, H.; Li, Y.; He, L.F. The central role of hydrogen sulfide in plant responses to toxic metal stress. Ecotoxicol. Environ. Saf. 2018, 157, 403–408. [Google Scholar] [CrossRef]
- Fang, H.; Liu, Z.; Jin, Z.; Zhang, L.; Liu, D.; Pei, Y. An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ. Pollut. 2016, 213, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Jing, T.; Liu, Z.; Zhang, L.; Jin, Z.; Pei, Y. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, Z.; Cui, W.; Zhang, Y.; Hu, H.; Yu, X.; Wang, Q.; Shen, W. Methane alleviates alfalfa cadmium toxicity via decreasing cadmium accumulation and reestablishing glutathione homeostasis. Ecotoxicol. Environ. Saf. 2018, 147, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kong, L.; Wang, Y.; Su, J.; Shen, W. Methane control of cadmium tolerance in alfalfa roots requires hydrogen sulfide. Environ. Pollut. 2021, 284, 117123. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Sehar, Z.; Anjum, N.A.; Masood, A.; Khan, N.A. The outcomes of the functional interplay of nitric oxide and hydrogen sulfide in metal stress tolerance in plants. Plant Physiol. Biochem. 2020, 155, 523–534. [Google Scholar] [CrossRef]
- Luo, S.; Calderón-Urrea, A.; Yu, J.; Liao, W.; Xie, J.; Lv, J.; Feng, Z.; Tang, Z. The role of hydrogen sulfide in plant alleviates heavy metal stress. Plant Soil 2020, 449, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, H.Z.; Zheng, M.Y.; Xian, M.; Qi, Z.Q.; Li, Y.Q.; Hu, L.B.; Chen, J.; Yang, L.F. Selenium inhibits root elongation by repressing the generation of endogenous hydrogen sulfide in Brassica rapa. PLoS ONE 2014, 9, e110904. [Google Scholar] [CrossRef]
- Yordanova, R. Antioxidative enzymes in barley plants subjected to soil flooding. Environ. Exp. Bot. 2004, 51, 93–101. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Sasidharan, R. Ethylene- and oxygen signalling-drive plant survival during flooding. Plant Biol. 2013, 15, 426–435. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Licausi, F.; van Dongen, J.T.; Giuntoli, B.; Novi, G.; Santaniello, A.; Geigenberger, P.; Perata, P. HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J. 2010, 62, 302–315. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.; van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C.; Perata, P. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 2017, 40, 473–482. [Google Scholar] [CrossRef]
- Valliyodan, B.; van Toai, T.T.; Alves, J.D.; de Fatima, P.G.P.; Lee, J.D.; Fritschi, F.B.; Rahman, M.A.; Islam, R.; Shannon, J.G.; Nguyen, H.T. Expression of root-related transcription factors associated with flooding tolerance of soybean (Glycine max). Int. J. Mol. Sci. 2014, 15, 17622–17643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruperti, B.; Botton, A.; Populin, F.; Eccher, G.; Brilli, M.; Quaggiotti, S.; Trevisan, S.; Cainelli, N.; Guarracino, P.; Schievano, E.; et al. Flooding responses on grapevine: A physiological, transcriptional, and metabolic perspective. Front. Plant Sci. 2019, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Loreti, E.; Perata, P. The Many Facets of Hypoxia in Plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef]
- Lothier, J.; Diab, H.; Cukier, C.; Limami, A.M.; Tcherkez, G. Metabolic responses to waterlogging differ between roots and shoots and reflect phloem transport alteration in Medicago truncatula. Plants 2020, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Mustroph, A. Plant oxygen sensing is mediated by the N-end rule pathway: A milestone in plant anaerobiosis. Plant Cell 2011, 23, 4173–4183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.; Perata, P.; van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef]
- Hwang, S.T.; Li, H.; Alavilli, H.; Lee, B.H.; Choi, D. Molecular and physiological characterization of AtHIGD1 in Arabidopsis. Biochem. Biophys. Res. Commun. 2017, 487, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Ou, S.L.; Yang, C.Y. The seedlings of different japonica rice varieties exhibit differ physiological properties to modulate plant survival rates under submergence stress. Plants 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.F.; Capelli, N.; Folzer, H.; Bourgeade, P.; Badot, P.M. Sensing and signalling during plant flooding. Plant Physiol. Biochem. 2004, 42, 273–282. [Google Scholar] [CrossRef]
- Van Veen, H.; Vashisht, D.; Akman, M.; Girke, T.; Mustroph, A.; Reinen, E.; Hartman, S.; Kooiker, M.; van Tienderen, P.; Schranz, M.E.; et al. Transcriptomes of eight Arabidopsis thaliana accessions reveal core conserved, genotype- and organ-specific responses to flooding stress. Plant Physiol. 2016, 172, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Wilson, I.W.; Yang, J.; Buerstenbinder, K.; Llewellyn, D.; Dennis, E.S.; Sauter, M.; Dolferus, R. Arabidopsis RAP2.2: An ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 2010, 153, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parveen, M.; Asaeda, T.; Rashid, M.H. Biochemical adaptations of four submerged macrophytes under combined exposure to hypoxia and hydrogen sulphide. PLoS ONE 2017, 12, e0182691. [Google Scholar] [CrossRef] [Green Version]
- Hartman, S.; Liu, Z.; van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; van Dongen, N.; Bosman, F.; Bassel, G.W.; et al. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Vantoai, T.; Moy, L.P.; Bock, G.; Linford, L.D.; Quackenbush, J. Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol. 2005, 137, 1115–1129. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda-Garcia, E.B.; Pulido-Barajas, J.F.; Huerta-Heredia, A.A.; Pena-Castro, J.M.; Liu, R.; Barrera-Figueroa, B.E. Differential expression of maize and teosinte microRNAs under submergence, drought, and alternated stress. Plants 2020, 9, 1367. [Google Scholar] [CrossRef]
- Buraschi, F.B.; Mollard, F.P.O.; Grimoldi, A.A.; Striker, G.G. Eco-physiological traits related to recovery from complete submergence in the model legume Lotus japonicus. Plants 2020, 9, 538. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Q.; Zhang, Y.; Yang, N.; Wu, G.; Li, Q.; Wang, W. Alleviation of osmotic stress by H2S is related to regulated PLDalpha1 and suppressed ROS in Arabidopsis thaliana. J. Plant Res. 2020, 133, 393–407. [Google Scholar] [CrossRef]
- Chen, H.J.; Ngowi, E.E.; Qian, L.; Li, T.; Qin, Y.Z.; Zhou, J.J.; Li, K.; Ji, X.Y.; Wu, D.D. Role of hydrogen sulfide in the endocrine system. Front. Endocrinol. 2021, 12, 704620. [Google Scholar] [CrossRef]
- Alvarez, C.; Garcia, I.; Moreno, I.; Perez-Perez, M.E.; Crespo, J.L.; Romero, L.C.; Gotor, C. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell 2012, 24, 4621–4634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, H.; Ogura, M.P.; Kingjoe, K.A.; Cohen, M.F. d-Cysteine-induced rapid root abscission in the water fern azolla pinnata: Implications for the linkage between d-amino acid and reactive sulfur species (RSS) in plant environmental responses. Antioxidants 2019, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Romero, L.C.; Garcia, I.; Gotor, C. L-Cysteine desulfhydrase 1 modulates the generation of the signaling molecule sulfide in plant cytosol. Plant Signal. Behav. 2013, 8, e24007. [Google Scholar] [CrossRef] [Green Version]
- Mei, Y.; Zhao, Y.; Jin, X.; Wang, R.; Xu, N.; Hu, J.; Huang, L.; Guan, R.; Shen, W. L-Cysteine desulfhydrase-dependent hydrogen sulfide is required for methane-induced lateral root formation. Plant Mol. Biol. 2019, 99, 283–298. [Google Scholar] [CrossRef]
- Shan, C.-J.; Zhang, S.-L.; Li, D.-F.; Zhao, Y.-Z.; Tian, X.-L.; Zhao, X.-L.; Wu, Y.-X.; Wei, X.-Y.; Liu, R.-Q. Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiol. Plant. 2011, 33, 2533–2540. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; Lai, D.; Sun, Y.; Samma, M.K.; Zhang, J.; Shen, W. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J. Plant Physiol. 2014, 171, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, D.; Penna, C.; Merlino, A.; del Soldato, P.; Wink, D.A.; Pagliaro, P. Physiological and pharmacological features of the novel gasotransmitter: Hydrogen sulfide. Biochim. Biophys. Acta 2009, 1787, 864–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Li, Y.; He, L.F. Role of nitric oxide and hydrogen sulfide in plant aluminum tolerance. Biometals 2019, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Gotor, C.; Garcia, I.; Crespo, J.L.; Romero, L.C. Sulfide as a signaling molecule in autophagy. Autophagy 2013, 9, 609–611. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, J.; Liu, J.; Liu, T.; Xue, S. Hydrogen sulfide (H2S) signaling in plant development and stress responses. Abiotech 2021, 2, 1–32. [Google Scholar] [CrossRef]
- Ma, Q.; Hill, P.W.; Chadwick, D.R.; Wu, L.; Jones, D.L. Competition for S-containing amino acids between rhizosphere microorganisms and plant roots: The role of cysteine in plant S acquisition. Biol. Fertil. Soils 2021, 57, 825–836. [Google Scholar] [CrossRef]
- Khan, M.N.; Al Zuaibr, F.M.; Al-Huqail, A.A.; Siddiqui, M.H.; Ali, H.M.; Al-Muwayhi, M.A.; Al-Haque, H.N. Hydrogen sulfide-mediated activation of o-acetylserine (Thiol) lyase and l/d-cysteine desulfhydrase enhance dehydration tolerance in eruca sativa mill. Int. J. Mol. Sci. 2018, 19, 3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, S.; van Dongen, N.; Renneberg, D.; Welschen-Evertman, R.A.M.; Kociemba, J.; Sasidharan, R.; Voesenek, L. Ethylene differentially modulates hypoxia responses and tolerance across solanum species. Plants 2020, 9, 1022. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Lombardi, L.; Pencik, A.; Novak, O.; Weits, D.A.; Loreti, E.; Perata, P.; Giuntoli, B.; Licausi, F. Jasmonate signalling contributes to primary root inhibition upon oxygen deficiency in Arabidopsis thaliana. Plants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Salvatierra, A.; Toro, G.; Mateluna, P.; Opazo, I.; Ortiz, M.; Pimentel, P. Keep calm and survive: Adaptation strategies to energy crisis in fruit trees under root hypoxia. Plants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Li, M.-Y.; Cui, W.-T.; Lu, W.; Shen, W.-B. Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J. Plant Growth Regul. 2012, 31, 519–528. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Qi, F.; Cui, W.; Xie, Y.; Shen, W. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J. Plant Physiol. 2014, 171, 1–8. [Google Scholar] [CrossRef]
- Fang, T.; Cao, Z.; Li, J.; Shen, W.; Huang, L. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol. Biochem. 2014, 76, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Chen, H.; Shen, W.; Shen, W.; Huang, L. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, F.H.; Wang, W.H.; Zheng, C.J.; Lin, G.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ye, Y.-K.; Wang, S.-H.; Luo, J.-P.; Tang, J.; Ma, D.-F. Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. J. Plant Growth Regul. 2009, 58, 243–250. [Google Scholar] [CrossRef]
- Hu, H.; Liu, D.; Li, P.; Shen, W. Hydrogen sulfide delays leaf yellowing of stored water spinach (Ipomoea aquatica) during dark-induced senescence by delaying chlorophyll breakdown, maintaining energy status and increasing antioxidative capacity. Postharvest Biol. Technol. 2015, 108, 8–20. [Google Scholar] [CrossRef]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Tripathi, D.K.; Roychoudhury, A. Hydrogen sulphide trapeze: Environmental stress amelioration and phytohormone crosstalk. Plant Physiol. Biochem. 2018, 132, 46–53. [Google Scholar] [CrossRef]
- Gil-Monreal, M.; Royuela, M.; Zabalza, A. Hypoxic treatment decreases the physiological action of the herbicide imazamox on pisum sativum roots. Plants 2020, 9, 981. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, W.; Shi, J.; Zhang, W.; Shen, Y.; Du, H.; Wu, S. Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. J. Sci. Food Agric. 2014, 94, 2699–2704. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, L.; Jiao, C.; Su, M.; Yang, T.; Zhou, L.; Peng, R.; Wang, R.; Wang, C. Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol. Biochem. 2013, 70, 278–286. [Google Scholar] [CrossRef]
- Peng, R.; Bian, Z.; Zhou, L.; Cheng, W.; Hai, N.; Yang, C.; Yang, T.; Wang, X.; Wang, C. Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep. 2016, 35, 2325–2340. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in arop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, C. Regulation of plant reactive oxygen species (ROS) in stress responses: Learning from AtRBOHD. Plant Cell Rep. 2016, 35, 995–1007. [Google Scholar] [CrossRef]
- Filomeni, G.; Desideri, E.; Cardaci, S.; Rotilio, G.; Ciriolo, M.R. Under the ROS: Thiol network is the principal suspect for autophagy commitment. Autophagy 2010, 6, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Ma, H.; Zeng, L.; Cheng, Y.; Lu, G.; Xu, J.; Zhang, X.; Zou, X. The effect of waterlogging on yield and seed quality at the early flowering stage in Brassica napus L. Field Crops Res. 2015, 180, 238–245. [Google Scholar] [CrossRef]
- Lin, X.; Yang, R.; Dou, Y.; Zhang, W.; Du, H.; Zhu, L.; Chen, J. Transcriptome analysis reveals delaying of the ripening and cell-wall degradation of kiwifruit by hydrogen sulfide. J. Sci. Food Agric. 2020, 100, 2280–2287. [Google Scholar] [CrossRef]
- Hancock, J.T.; Whiteman, M. Hydrogen sulfide signaling: Interactions with nitric oxide and reactive oxygen species. Ann. N. Y. Acad. Sci. 2016, 1365, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bratt, A.; Rosenwasser, S.; Meyer, A.; Fluhr, R. Organelle redox autonomy during environmental stress. Plant Cell Environ. 2016, 39, 1909–1919. [Google Scholar] [CrossRef]
- Penella, C.; Calatayud, A.; Melgar, J.C. Ascorbic acid alleviates water stress in young peach trees and improves their performance after rewatering. Front. Plant Sci. 2017, 8, 1627. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Saegusa, D.; Fujita, M.; Tran, L.S. Hydrogen sulfide regulates salt tolerance in rice by maintaining Na(+)/K(+) balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front. Plant Sci. 2015, 6, 1055. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Zhu, H.; Zhang, H.; Sun, J.; Zhou, J.; Deng, C.; Zhang, Y.; Zhao, R.; Zhou, X.; Lu, C.; et al. Hydrogen sulfide mediates K(+) and Na(+) homeostasis in the roots of salt-resistant and salt-sensitive poplar species subjected to NaCl stress. Front. Plant Sci. 2018, 9, 1366. [Google Scholar] [CrossRef] [Green Version]
- Steffens, B.; Sauter, M. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell 2009, 21, 184–196. [Google Scholar] [CrossRef] [Green Version]
- Yemelyanov, V.V.; Chirkova, T.V.; Shishova, M.F.; Lindberg, S.M. Potassium efflux and cytosol acidification as primary anoxia-induced events in wheat and rice seedlings. Plants 2020, 9, 1216. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen polysulfide (H2Sn) signaling along with hydrogen sulfide (H2S) and nitric oxide (NO). J. Neural Transm. 2016, 123, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Luo, Q.; Wang, R.; Xu, J. Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway. Sci. Rep. 2017, 7, 868. [Google Scholar] [CrossRef]
- Lai, D.; Mao, Y.; Zhou, H.; Li, F.; Wu, M.; Zhang, J.; He, Z.; Cui, W.; Xie, Y. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K(+) loss in seedlings of Medicago sativa. Plant Sci. 2014, 225, 117–129. [Google Scholar] [CrossRef]
- Li, Z.G.; Xie, L.R.; Li, X.J. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J. Plant Physiol. 2015, 177, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, D.J.; Lee, S.C.; Isa, N.M.; Gramuglia, S.; Fukao, T.; Bassel, G.W.; Correia, C.S.; Corbineau, F.; Theodoulou, F.L.; Bailey-Serres, J.; et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011, 479, 415–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.Y.; Hu, S.L.; Wu, J.; Li, Y.H.; Zheng, J.L.; Wei, Z.J.; Liu, J.; Wang, H.L.; Liu, Y.S.; Zhang, H. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. 2012, 60, 8684–8693. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen sulfide signaling in plants: Emerging roles of protein persulfidation. Front. Plant Sci. 2018, 9, 1369. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, P.; Gu, Z.; Tao, Y.; Shen, C.; Zhou, Y.; Han, Y.; Yang, R. Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress. Food Chem. X 2019, 2, 100023. [Google Scholar] [CrossRef]
- Liu, J.; Niu, Y.; Zhang, J.; Zhou, Y.; Ma, Z.; Huang, X. Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: Recent advances. Plant Cell Tissue Organ Cult. 2017, 132, 413–424. [Google Scholar] [CrossRef]
- Fan, G.; Jian, D.; Sun, M.; Zhan, Y.; Sun, F. Endogenous and exogenous calcium involved in the betulin production from submerged culture of phellinus linteus induced by hydrogen sulfide. Appl. Biochem. Biotechnol. 2016, 178, 594–603. [Google Scholar] [CrossRef]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca(2+) trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef]
- Jia, H.; Hu, Y.; Fan, T.; Li, J. Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci. Rep. 2015, 5, 8251. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, D.; Xu, K.; Jin, L.; Peng, R. Hydrogen Sulfide Enhances Plant Tolerance to Waterlogging Stress. Plants 2021, 10, 1928. https://doi.org/10.3390/plants10091928
Li Y, Sun D, Xu K, Jin L, Peng R. Hydrogen Sulfide Enhances Plant Tolerance to Waterlogging Stress. Plants. 2021; 10(9):1928. https://doi.org/10.3390/plants10091928
Chicago/Turabian StyleLi, Yaoqi, Da Sun, Ke Xu, Libo Jin, and Renyi Peng. 2021. "Hydrogen Sulfide Enhances Plant Tolerance to Waterlogging Stress" Plants 10, no. 9: 1928. https://doi.org/10.3390/plants10091928
APA StyleLi, Y., Sun, D., Xu, K., Jin, L., & Peng, R. (2021). Hydrogen Sulfide Enhances Plant Tolerance to Waterlogging Stress. Plants, 10(9), 1928. https://doi.org/10.3390/plants10091928






