Genome-Wide Identification and Analysis of the MADS-Box Gene Family in American Beautyberry (Callicarpa americana)
Abstract
:1. Introduction
2. Results
2.1. Identification of MADS-Box Genes and Their Distribution in C. americana Genome
2.2. Phylogenetic Analysis of MADS-Box Genes in C. americana
2.3. Conservative Motif Distribution and Gene Structure Analysis of C. americana MADS-Box Genes
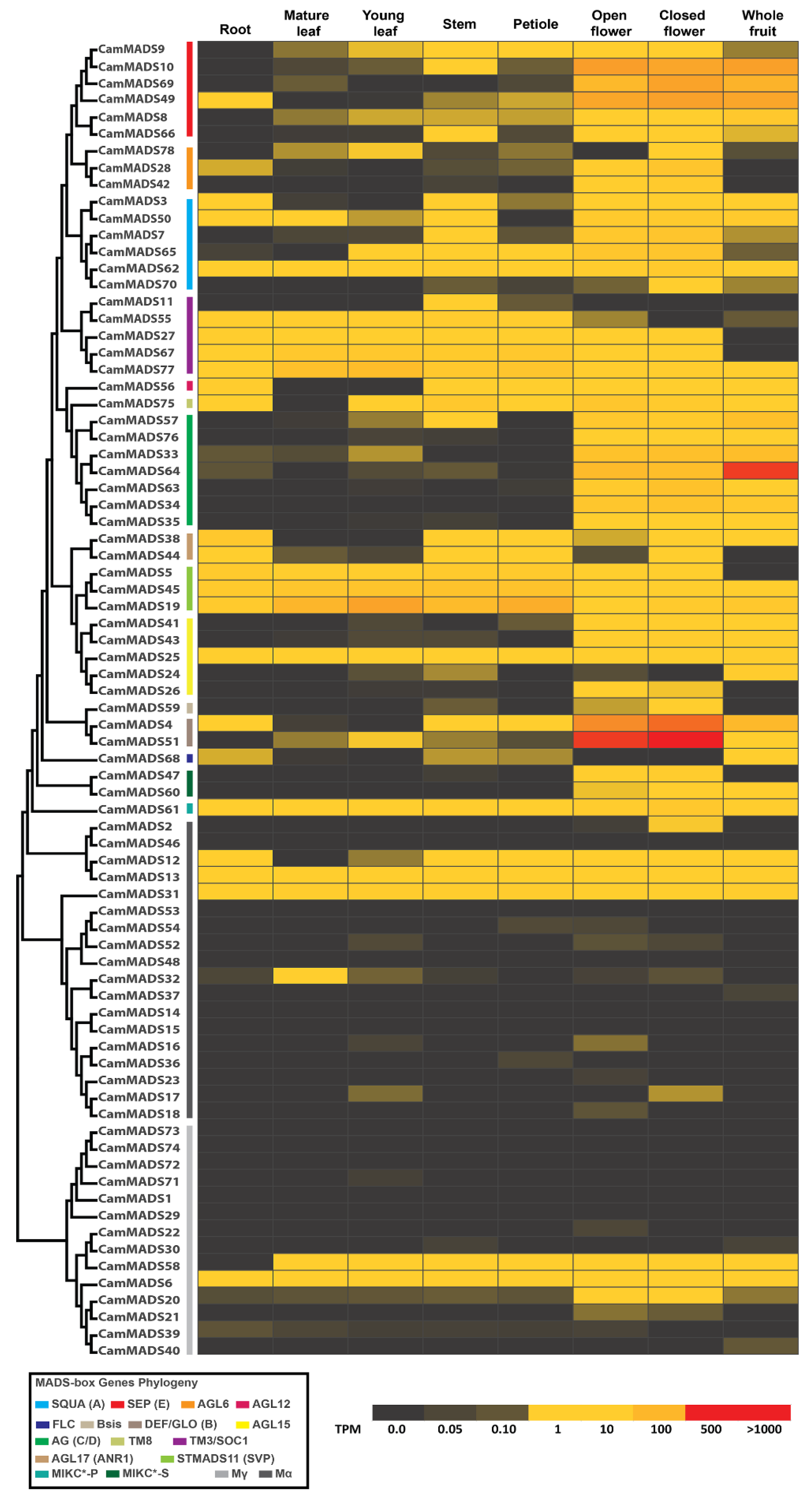
2.4. Expression of C. americana MADS-Box Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Identification and Sequence Analysis of MADS-Box Genes
5.2. Assigning the Location of MADS-Box Genes to the C. americana Genome
5.3. Alignment and Phylogenetic Analysis of MADS-Box Genes
5.4. Gene Structure and Conserved Motif Analysis
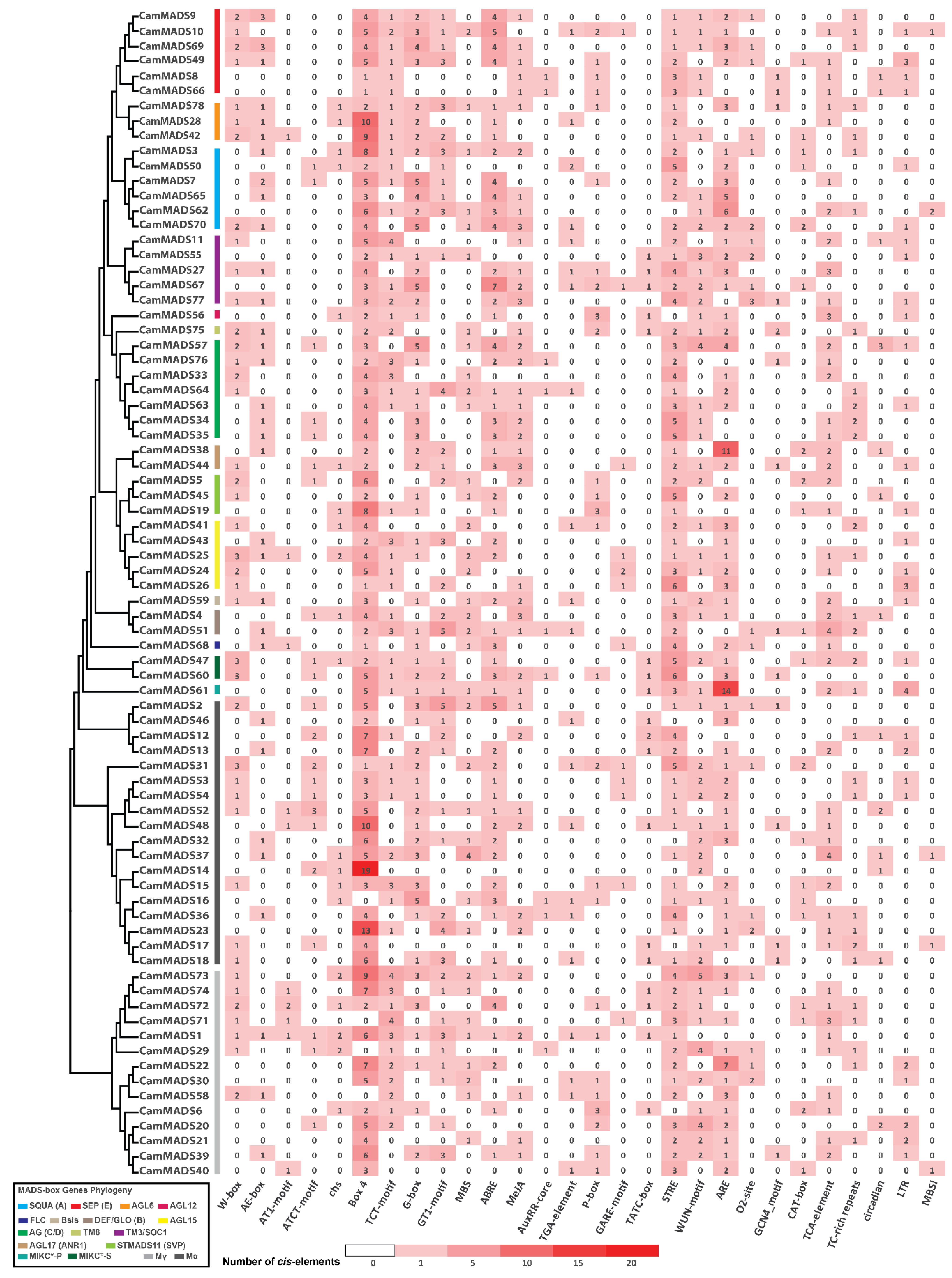
5.5. Expression Profiling of MADS-Box Genes and Cis-Acting Regulatory Element Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Learning from our elders: Folk Remedy Yields Mosquito-Thwarting Compound. Agricultural Research Magazine, 6 February 2006.
- Cantrell, C.L.; Klun, J.A.; Bryson, C.T.; Kobaisy, M.; Duke, S.O. Isolation and identification of mosquito bite deterrent terpenoids from leaves of American (Callicarpa americana) and Japanese (Callicarpa japonica) beautyberry. J. Agric. Food Chem. 2005, 15, 5948–5953. [Google Scholar] [CrossRef]
- Carroll, J.F.; Cantrell, C.L.; Klun, J.A.; Kramer, M. Repellency of two terpenoid compounds isolated from Callicarpa americana (Lamiaceae) against Ixodes scapularis and Amblyomma americanum ticks. Exp. Appl. Acarol. 2007, 41, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P.; Godden, G.T.; Lanier, E.; Bhat, W.W.; Kinser, T.J.; Vaillancourt, B.; Wang, H.; Wood, J.C.; Jiang, M.; Soltis, P.S.; et al. Generation of a chromosome-scale genome assembly of the insect-repellent terpenoid-producing Lamiaceae species, Callicarpa americana. GigaScience 2020, 9, giaa093. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Günter, T. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenetics Evol. 2003, 29, 464–489. [Google Scholar] [CrossRef]
- Theissen, G.; Becker, A.; Di Rosa, A.; Kanno, A.; Kim, J.T.; Münster, T.; Winter, K.-U.; Saedler, H. A short history of MADS-box genes in plants. Plant Mol. Biol. 2000, 42, 115–149. [Google Scholar] [CrossRef]
- Ng, M.; Yanofsky, M.F. Function and evolution of the plant MADS-box gene family. Nat. Rev. Genet. 2001, 2, 186–195. [Google Scholar] [CrossRef]
- Castelán-Muñoz, N.; Herrera, J.; Cajero-Sánchez, W.; Arrizubieta, M.; Trejo, C.; Garcia-Ponce, B.; de la Paz Sánchez, M.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. MADS-box genes are key components of genetic regulatory networks involved in abiotic stress and plastic developmental responses in plants. Front. Plant Sci. 2019, 10, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.Y.; Hu, Z.; Hu, J.; Zhu, Z.; Yu, X.; Cui, B.; Chen, G. Tomato (Solanum lycopersicum) MADS-box transcription factor SlMBP8 regulates drought, salt tolerance and stress-related genes. Plant Growth Regul. 2017, 83, 55–68. [Google Scholar]
- Schwarz-Sommer, Z.; Sommer, H. Genetic Control of Flower Development by Homeotic Genes in Antirrhinum majus. Science 1990, 250, 931–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Theissen, G. Development of floral organ identity: Stories from the MADS house. Curr. Opin. Plant Biol. 2001, 4, 75–85. [Google Scholar] [CrossRef]
- Honma, T.; Goto, K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 2001, 409, 525–529. [Google Scholar] [CrossRef]
- Causier, B.; Schwarz-Sommer, Z.; Davies, B. Floral organ identity: 20 years of ABCs. Semin. Cell Dev. Biol. 2010, 21, 73–79. [Google Scholar] [CrossRef]
- Immink, R.G.H.; Tonaco, I.A.N.; De Folter, S.; Shchennikova, A.; Van Dijk, A.D.J.; Busscher-Lange, J.; Borst, J.W.; Angenent, G.C. SEPALLATA3: The ‘glue’ for MADS box transcription factor complex formation. Genome Biol. 2009, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhindi, T.; Zhang, Z.; Ruelens, P.; Coenen, H.; DeGroote, H.; Iraci, N.; Geuten, K. Protein interaction evolution from promiscuity to specificity with reduced flexibility in an increasingly complex network. Sci. Rep. 2017, 7, 1–15. [Google Scholar]
- Alvarez-Buylla, E.R.; Pelaz, S.; Liljegren, S.J.; Gold, S.E.; Burgeff, C.; Ditta, G.S.; de Pouplana, L.R.; Martínez-Castilla, L.; Yanofsky, M.F. An ancestral MADS-box gene duplication occurred before the divergence of plants and animals. Proc. Natl. Acad. Sci. USA 2000, 97, 5328–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofuji, R.; Sumikawa, N.; Yamasaki, M.; Kondo, K.; Ueda, K.; Ito, M.; Hasebe, M. Evolution and divergence of the MADS-box gene family based on genome-wide expression analyses. Mol. Biol. Evol. 2003, 20, 1963–1977. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Laura, F.; Thomas, J. The K domain mediates heterodimerization of the Arabidopsis floral organ identity proteins, APETALA3 and PISTILLATA. Plant J. 2003, 33, 47–59. [Google Scholar] [CrossRef]
- Kaufmann, K.; Rainer, M.; Günter, T. MIKC-type MADS-domain proteins: Structural modularity, protein interactions and network evolution in land plants. Gene 2005, 347, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Immink, R.G.H.; Kerstin, K.; Gerco, C.A. The ‘ABC’ of MADS domain protein behaviour and interactions. Acad. Press Semin. Cell Dev. Biol. 2010, 21, 87–93. [Google Scholar] [CrossRef]
- Kwantes, M.; Daniela, L.; Wim, V. How MIKC* MADS-box genes originated and evidence for their conserved function throughout the evolution of vascular plant gametophytes. Mol. Biol. Evol. 2012, 29, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, L.; Guenter, T. A hitchhiker’s guide to the MADS world of plants. Genome Biol. 2010, 11, 214. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.P.; Kinghorn, A.D. Biologically active natural products of the genus Callicarpa. Curr. Bioactive Compd. 2008, 4, 15–32. [Google Scholar] [CrossRef] [PubMed]
- HMMR v3.3.2. Available online: http://hmmer.org/2021 (accessed on 15 June 2021).
- Letunic, I.; Supriya, K.; Peer, B. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.A.; Cerutti, L.; Hulo, N.; Gattiker, A.; Falquet, L.; Pagni, M.; Bairoch, A.; Bucher, P. PROSITE: A documented database using patterns and profiles as motif descriptors. Brief Bioinform. 2002, 3, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, V.; Kosarev, P.; Seledsov, I.; Vorobyev, D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Jin, J.; Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2020, 48, D1104–D1113. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramzow, L.; Lisa, W.; Günter, T. MADS goes genomic in conifers: Towards determining the ancestral set of MADS-box genes in seed plants. Ann. Bot. 2017, 114, 1407–1429. [Google Scholar] [CrossRef] [Green Version]
- Parenicová, L.; de Folter, S.; Kieffer, M.; Horner, D.S.; Favalli, C.; Busscher, J.; Cook, H.E.; Ingram, R.M.; Kater, M.M.; Davies, B.; et al. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: New openings to the MADS world. Plant Cell 2003, 15, 1538–1551. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, J.; Hu, Z.; Guo, X.; Tian, S.; Chen, G. Genome-Wide analysis of the MADS-Box transcription factor family in Solanum lycopersicum. Int. J. Mol. Sci. 2019, 20, 2961. [Google Scholar] [CrossRef] [Green Version]
- Xin, W.; Wang, L.; Yu, J.; Zhang, Y.; Li, D.; Zhang, X. Genome-wide identification and analysis of the MADS-box gene family in sesame. Gene 2015, 569, 66–76. [Google Scholar]
- Simonini, S.; Roig-Villanova, I.; Gregis, V.; Colombo, B.; Colombo, L.; Kater, M.M. Basic pentacysteine proteins mediate MADS domain complex binding to the DNA for tissue-specific expression of target genes in Arabidopsis. Plant Cell 2012, 24, 4163–4172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, T.; Katsani, K.R.; Verrijzer, C.P. GAGA can mediate enhancer function in trans by linking two separate DNA molecules. EMBO J. 2002, 21, 1775–1781. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.K.; Chacko, A.R.; Gandhimathi, A.; Ghosh, P.; Harini, K.; Joseph, A.P.; Joshi, A.G.; Karpe, S.D.; Kaushik, N.; Kuravadi, N.; et al. Genome sequencing of herb Tulsi (Ocimum tenuiflorum) unravels key genes behind its strong medicinal properties. BMC Plant Biol. 2015, 15, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Y.; Yu, D.; Wang, D.; Guo, D.; Guo, C. Genome-wide survey and expression analysis of the MADS-box gene family in soybean. Mol. Biol. Rep. 2013, 40, 3901–3911. [Google Scholar] [CrossRef]
- Kagale, S.; Koh, C.; Nixon, J.; Bollina, V.; Clarke, W.E.; Tuteja, R.; Spillane, C.; Robinson, S.J.; Links, M.; Clarke, C.; et al. The emerging biofuel crop Camelina sativa retains a highly undifferentiated hexaploid genome structure. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veron, A.S.; Kaufmann, K.; Bornberg-Bauer, E. Evidence of interaction network evolution by whole-genome duplications: A case study in MADS-Box proteins. Mol. Biol. Evol. 2007, 24, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Vekemans, D.; Proost, S.; Vanneste, K.; Coenen, H.; Viaene, T.; Ruelens, P.; Maere, S.; de Peer, Y.V.; Geuten, K. Gamma paleohexaploidy in the stem lineage of core eudicots: Significance for MADS-box gene and species diversification. Mol. Biol. Evol. 2012, 29, 3793–3806. [Google Scholar] [CrossRef] [Green Version]
- Ohno, S. Evolution by Gene Duplication; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Godden, G.T.; Taliesin, J.K.; Pamela, S.S.; Douglas, E.S. Phylotranscriptomic analyses reveal asymmetrical gene duplication dynamics and signatures of ancient polyploidy in mints. Genome Biol. Evol. 2019, 11, 3393–3408. [Google Scholar] [CrossRef]
- Lemons, D.; William, M. Genomic evolution of Hox gene clusters. Science 2006, 313, 1918–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theißen, G.; Rümpler, F.; Gramzow, L.A. Array of MADS-box genes: Facilitator for rapid adaptation? Trends Plant Sci. 2018, 23, 563–576. [Google Scholar] [CrossRef]
- Bemer, M.; Heijmans, K.; Airoldi, C.; Davies, B.; Angenent, G.C. An atlas of type I MADS box gene expression during female gametophyte and seed development in Arabidopsis. Plant Physiol. 2010, 154, 287–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coenen, H.; Viaene, T.; Vandenbussche, M.; Geuten, K. TM8 represses developmental timing in Nicotiana benthamiana and has functionally diversified in angiosperms. BMC Plant Biol. 2018, 18, 1–16. [Google Scholar] [CrossRef]
- Tani, E.; Polidoros, A.; Flemetakis, E.; Stedel, C.; Kalloniati, C.; Demetriou, K.; Katinakis, P.; Tsaftaris, A.S. Characterization and expression analysis of AGAMOUS-like, SEEDSTICK-like, and SEPALLATA-like MADS-box genes in peach (Prunus persica) fruit. Plant Physiol. Biochem. 2009, 47, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Giménez, E.; Dominguez, E.; Pineda, B.; Heredia, A.; Moreno, V.; Lozano, R.; Angosto, T. Transcriptional activity of the MADS box ARLEQUIN/TOMATO AGAMOUS-LIKE1 gene is required for cuticle development of tomato fruit. Plant Physiol. 2015, 168, 1036–1048. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.R.; Roy, S.; Nag, A.; Singh, S.K.; Sengupta, D.N. Characterization of an AGAMOUS-like MADS box protein, a probable constituent of flowering and fruit ripening regulatory system in banana. PLoS ONE 2012, 7, e44361. [Google Scholar] [CrossRef]
- Song, Y.H.; Yoo, C.M.; Hong, A.P.; Kim, S.H.; Jeong, H.J.; Shin, S.Y.; Kim, H.J.; Yun, D.-J.; Lim, C.O.; Bahk, J.D.; et al. DNA-binding study identifies C-box and hybrid C/G-box or C/A-box motifs as high-affinity binding sites for STF1 and LONG HYPOCOTYL5 proteins. Plant Physiol. 2008, 146, 1862–1877. [Google Scholar] [CrossRef] [Green Version]
- Petrella, R.; Caselli, F.; Roig-Villanova, I.; Vignati, V.; Chiara, M.; Ezquer, I.; Tadini, L.; Kater, M.M.; Gregis, V. BPC transcription factors and a Polycomb Group protein confine the expression of the ovule identity gene SEEDSTICK in Arabidopsis. Plant J. 2020, 102, 582–599. [Google Scholar] [CrossRef]
- Schauer, S.E.; Schlüter, P.M.; Baskar, R.; Gheyselinck, J.; Bolaños, A.; Curtis, M.D.; Grossniklaus, U. Intronic regulatory elements determine the divergent expression patterns of AGAMOUS-LIKE6 subfamily members in Arabidopsis. Plant J. 2009, 59, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, E.S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Gasteiger, E.; Christine, H.; Alexandre, G.; Marc, R.W.; Ron, D.A.; Amos, B. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.T.; William, R.T.; Janet, M.T. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K.; Battistuzzi, F.U. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Sneddon, T.P.; Li, P.; Edmunds, S.C. GigaDB: Announcing the GigaScience database. GigaScience 2012, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Microsoft Corporation. Microsoft Excel. 2018. Available online: https://office.microsoft.com/excel (accessed on 15 June 2021).
- Magali, L.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; de Peer, Y.V.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar]
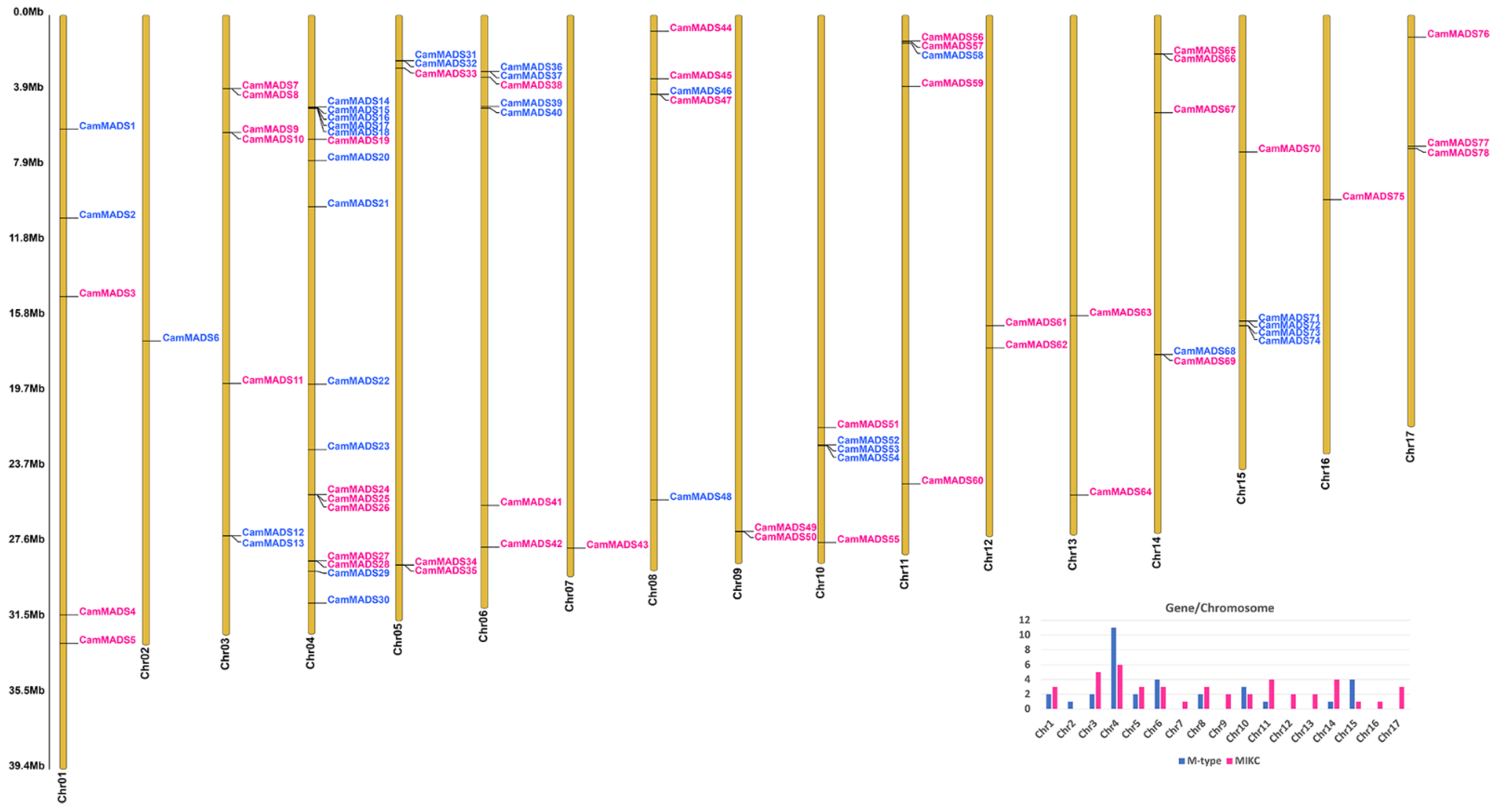

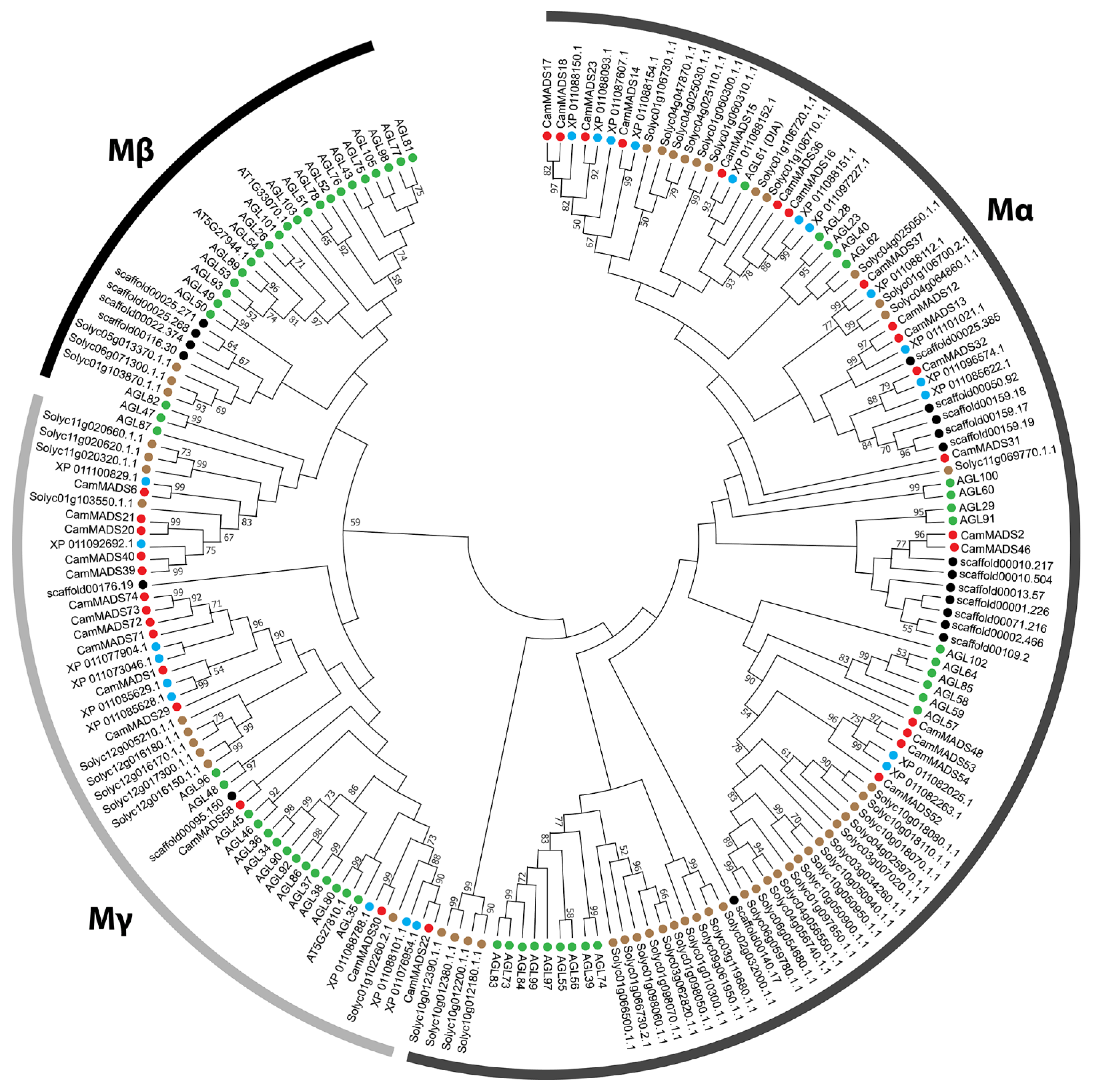
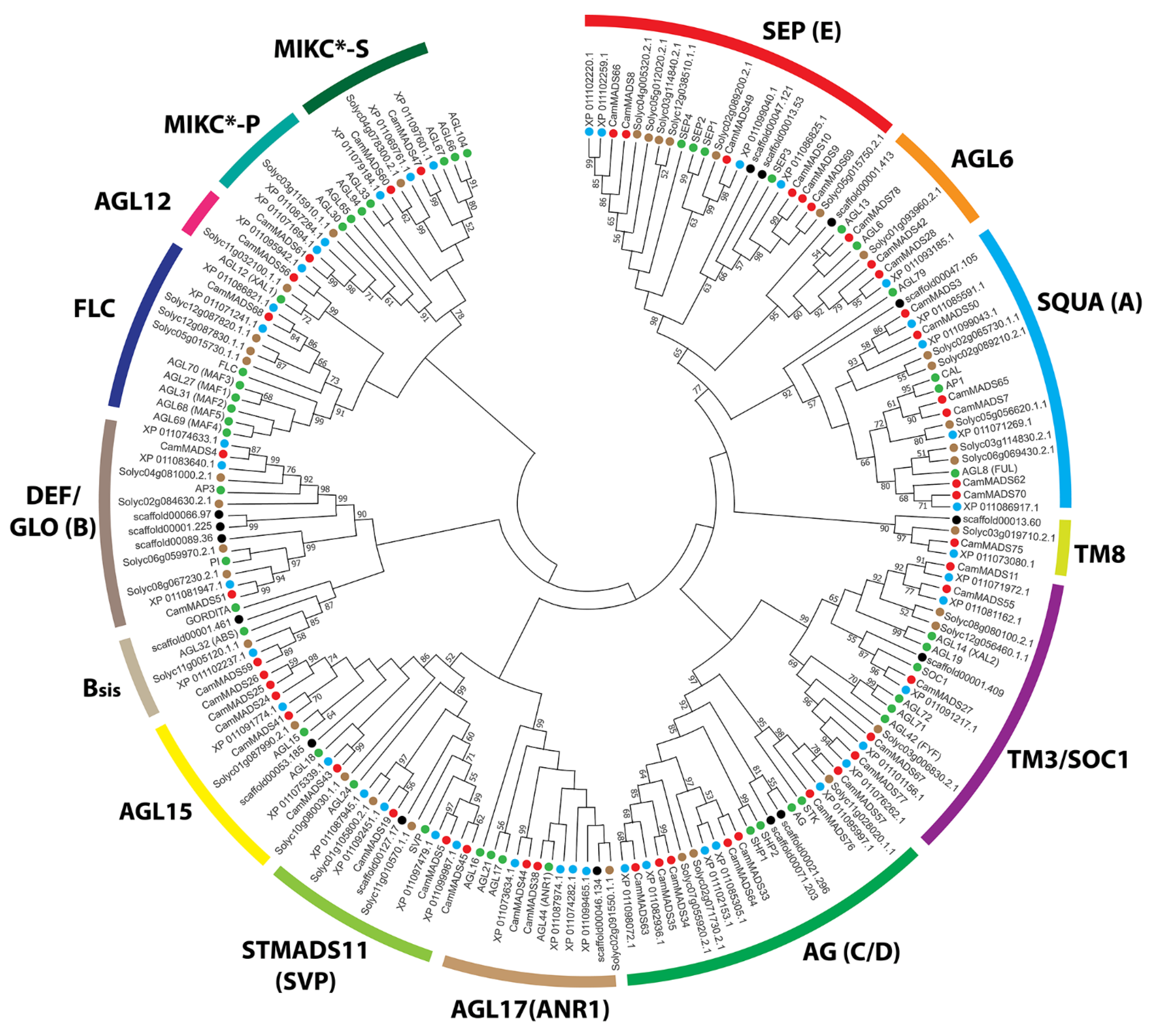
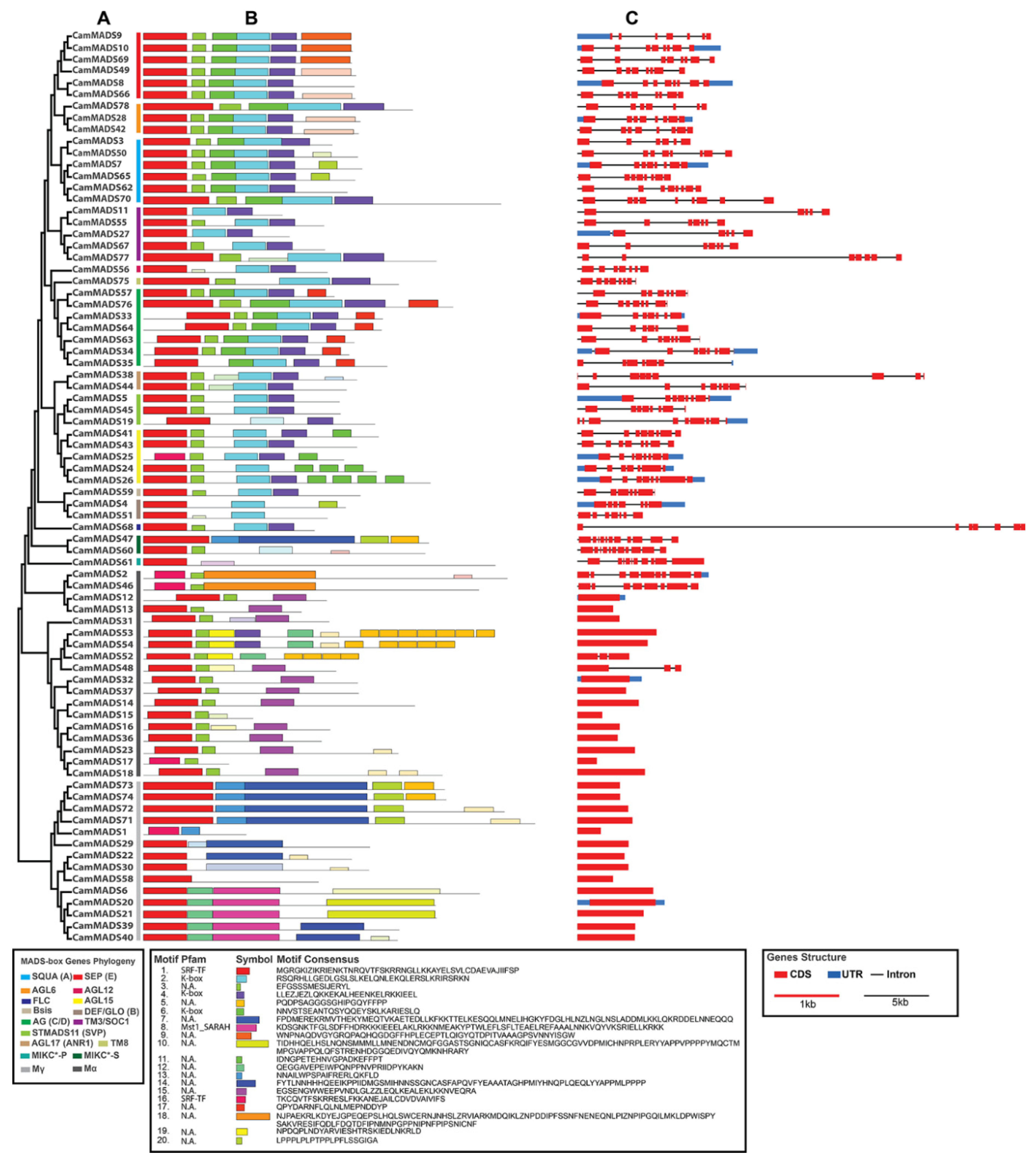
| Gene Name | Gene ID | Chr | Exons | Length (aa) | PI | MW (Da) | Group | Ortholog | E-Value | Subgroup |
|---|---|---|---|---|---|---|---|---|---|---|
| CamMADS1 | Calam.01G080200.1 | 01 | 1 | 119 | 8.58 | 14,045.36 | M-type | - | - | γ |
| CamMADS2 | Calam.01G122600.1 | 01 | 9 | 421 | 4.96 | 48,179.99 | M-type | - | - | α |
| CamMADS3 | Calam.01G172300.3 * | 01 | 6 | 203 | 10.1 | 23,879.46 | MIKCc | - | - | SQUA (A) |
| CamMADS4 | Calam.01G249900.1 | 01 | 7 | 234 | 9.5 | 27,268.13 | MIKCc | AP3 | 10−90 | DEF/GLO (B) |
| CamMADS5 | Calam.01G262900.1 | 01 | 8 | 227 | 5.92 | 25,470.73 | MIKCc | SVP | 10−109 | STMADS11 (SVP) |
| CamMADS6 | Calam.02G117500.1 | 02 | 1 | 389 | 7.99 | 44,905.13 | M-type | - | - | γ |
| CamMADS7 | Calam.03G039300.1 | 03 | 8 | 253 | 8.45 | 29,036.21 | MIKCc | CAL | 10−107 | SQUA (A) |
| CamMADS8 | Calam.03G039400.1 | 03 | 8 | 244 | 9.05 | 28,027.89 | MIKCc | SEP4 | 10−89 | SEP (E) |
| CamMADS9 | Calam.03G072400.1 * | 03 | 8 | 242 | 8.99 | 27,364.05 | MIKCc | SEP3 | 10−125 | FLC |
| CamMADS10 | Calam.03G072500.1 | 03 | 8 | 242 | 8.99 | 27,574.33 | MIKCc | SEP3 | 10−127 | SEP (E) |
| CamMADS11 | Calam.03G115700.1 * | 03 | 5 | 161 | 9.3 | 18,451.68 | MIKCc | AGL14 | 10−46 | TM3/SOC1 |
| CamMADS12 | Calam.03G148300.1 | 03 | 1 | 212 | 6.94 | 23,923 | M-type | - | - | α |
| CamMADS13 | Calam.03G148400.1 | 03 | 1 | 183 | 4.96 | 20,172.29 | M-type | - | - | α |
| CamMADS14 | Calam.04G062300.1 | 04 | 1 | 314 | 9.53 | 34,355.29 | M-type | - | - | α |
| CamMADS15 | Calam.04G062400.1 | 04 | 1 | 127 | 10.03 | 14,061.52 | M-type | - | - | α |
| CamMADS16 | Calam.04G062600.1 * | 04 | 1 | 216 | 9.11 | 24,304.77 | M-type | - | - | α |
| CamMADS17 | Calam.04G062900.1 | 04 | 1 | 99 | 8.96 | 10,888.37 | M-type | - | - | α |
| CamMADS18 | Calam.04G063300.1 | 04 | 1 | 346 | 5.17 | 37,570.27 | M-type | - | - | α |
| CamMADS19 | Calam.04G078200.1 | 04 | 9 | 268 | 9.32 | 30,383.48 | MIKCc | AGL24 | 10−48 | STMADS11 (SVP) |
| CamMADS20 | Calam.04G086600.1 | 04 | 1 | 338 | 8.83 | 38,982.02 | M-type | - | - | γ |
| CamMADS21 | Calam.04G092200.1 | 04 | 1 | 339 | 8.99 | 39,032.72 | M-type | - | - | γ |
| CamMADS22 | Calam.04G128000.1 | 04 | 1 | 241 | 9.27 | 26,959 | M-type | - | - | γ |
| CamMADS23 | Calam.04G142100.1 | 04 | 1 | 295 | 9.57 | 32,333.33 | M-type | - | - | α |
| CamMADS24 | Calam.04G164100.1 | 04 | 7 | 270 | 5.19 | 30,133.31 | MIKCc | AGL15 | 10−39 | AGL15 |
| CamMADS25 | Calam.04G164200.1 | 04 | 8 | 232 | 5.01 | 26,355.97 | MIKCc | AGL15 | 10−29 | AGL15 |
| CamMADS26 | Calam.04G164300.1 | 04 | 8 | 332 | 5.37 | 37,578.7 | MIKCc | AGL15 | 10−45 | AGL15 |
| CamMADS27 | Calam.04G209300.1 * | 04 | 5 | 169 | 9.39 | 19,122.03 | MIKCc | SOC1 | 10−59 | TM3/SOC1 |
| CamMADS28 | Calam.04G209500.1 | 04 | 8 | 251 | 8.91 | 28,497.25 | MIKCc | AGL6 | 10−103 | AGL6 |
| CamMADS29 | Calam.04G216300.1 | 04 | 1 | 262 | 7.04 | 30,161.8 | M-type | - | - | γ |
| CamMADS30 | Calam.04G237100.1 | 04 | 1 | 261 | 5.74 | 28,960.63 | M-type | - | - | γ |
| CamMADS31 | Calam.05G032500.1 | 05 | 1 | 215 | 8.88 | 24,307.89 | M-type | - | - | α |
| CamMADS32 | Calam.05G032800.1 | 05 | 1 | 248 | 5.35 | 28,277.95 | M-type | - | - | α |
| CamMADS33 | Calam.05G039300.2 | 05 | 7 | 277 | 9.26 | 31,920.44 | MIKCc | AG | 10−117 | AG (C/D) |
| CamMADS34 | Calam.05G230800.1 | 05 | 7 | 238 | 9.69 | 27,566.42 | MIKCc | SHP1 | 10−111 | AG (C/D) |
| CamMADS35 | Calam.05G230900.2 * | 05 | 9 | 282 | 9.41 | 33,043.92 | MIKCc | SHP1 | 10−89 | AG (C/D) |
| CamMADS36 | Calam.06G036400.1 | 06 | 1 | 206 | 7.05 | 23,534.14 | M-type | - | - | α |
| CamMADS37 | Calam.06G036500.1 | 06 | 1 | 249 | 5.18 | 27,366.98 | M-type | - | - | α |
| CamMADS38 | Calam.06G039300.2 * | 06 | 9 | 247 | 9.44 | 28,010.07 | MIKCc | ANR | 10−80 | AGL17 (ANR1) |
| CamMADS39 | Calam.06G055800.1 | 06 | 1 | 296 | 7.78 | 34,434.83 | M-type | - | - | γ |
| CamMADS40 | Calam.06G056500.1 | 06 | 1 | 294 | 7.72 | 33,540.55 | M-type | - | - | γ |
| CamMADS41 | Calam.06G225200.2 | 06 | 8 | 272 | 9.01 | 30,558.2 | MIKCc | AGL15 | 10−58 | AGL15 |
| CamMADS42 | Calam.06G255900.1 | 06 | 8 | 249 | 8.6 | 28,570.49 | MIKCc | AGL6 | 10−101 | AGL6 |
| CamMADS43 | Calam.07G213100.1 | 07 | 8 | 247 | 8.25 | 27,924.98 | MIKCc | AGL18 | 10−54 | AGL15 |
| CamMADS44 | Calam.08G005200.1 | 08 | 7 | 235 | 9.13 | 27,057.88 | MIKCc | AGL21 | 10−106 | AGL17 (ANR1) |
| CamMADS45 | Calam.08G031600.1 | 08 | 8 | 228 | 6.56 | 25,831.27 | MIKCc | SVP | 10−111 | STMADS11 (SVP) |
| CamMADS46 | Calam.08G041400.1 * | 08 | 8 | 388 | 5.08 | 44,294.51 | M-type | - | - | α |
| CamMADS47 | Calam.08G041600.1 | 08 | 11 | 329 | 5.57 | 36,829.16 | MIKC* | AGL104 | 10−60 | S |
| CamMADS48 | Calam.08G166600.1 | 08 | 3 | 223 | 9.53 | 25,926.95 | M-type | - | - | α |
| CamMADS49 | Calam.09G141700.1 | 09 | 8 | 246 | 8.19 | 28,172.93 | MIKCc | SEP2 | 10−120 | SEP (E) |
| CamMADS50 | Calam.09G141800.1 | 09 | 8 | 248 | 9.31 | 28,813.63 | MIKCc | AP1 | 10−64 | SQUA (A) |
| CamMADS51 | Calam.10G107400.1 | 10 | 7 | 213 | 7.82 | 25,002.75 | MIKCc | PI | 10−82 | DEF/GLO (B) |
| CamMADS52 | Calam.10G116200.1 | 10 | 3 | 249 | 4.97 | 26,032.55 | M-type | - | - | α |
| CamMADS53 | Calam.10G116300.1 | 10 | 1 | 406 | 5.46 | 43,692.96 | M-type | - | - | α |
| CamMADS54 | Calam.10G116700.1 | 10 | 1 | 360 | 5.41 | 39,162.3 | M-type | - | - | α |
| CamMADS55 | Calam.10G171700.1 | 10 | 7 | 209 | 9.08 | 23,889.61 | MIKCc | AGL19 | 10−68 | TM3/SOC1 |
| CamMADS56 | Calam.11G009500.1 | 11 | 7 | 213 | 8.76 | 24,464.63 | MIKCc | AGL12 | 10−65 | AGL12 |
| CamMADS57 | Calam.11G010000.1 | 11 | 7 | 221 | 9.55 | 25,595.38 | MIKCc | STK | 10−100 | AG (C/D) |
| CamMADS58 | Calam.11G011000.1 * | 11 | 1 | 182 | 9.38 | 20,612.54 | M-type | - | - | γ |
| CamMADS59 | Calam.11G041000.1 | 11 | 6 | 251 | 6.84 | 29,406.47 | MIKCc | ABS | 10−51 | Bsis |
| CamMADS60 | Calam.11G131900.1 | 11 | 11 | 326 | 5.5 | 37,186.12 | MIKC* | AGL66 | 10−54 | S |
| CamMADS61 | Calam.12G155600.11 | 12 | 10 | 370 | 5.76 | 46,664.87 | MIKC* | AGL65 | 10−80 | P |
| CamMADS62 | Calam.12G166700.1 | 12 | 8 | 236 | 9.11 | 27,139.8 | MIKCc | FUL | 10−82 | SQUA (A) |
| CamMADS63 | Calam.13G078400.1 | 13 | 7 | 244 | 9.17 | 28,250.83 | MIKCc | SHP1 | 10−105 | AG (C/D) |
| CamMADS64 | Calam.13G161700.1 * | 13 | 6 | 229 | 9.13 | 26,437.96 | MIKCc | AG | 10−107 | AG (C/D) |
| CamMADS65 | Calam.14G029500.1 | 14 | 8 | 245 | 7.65 | 28,377.37 | MIKCc | AP1 | 10−117 | SQUA (A) |
| CamMADS66 | Calam.14G029600.1 | 14 | 8 | 245 | 9.18 | 27,993.7 | MIKCc | SEP4 | 10−82 | SEP (E) |
| CamMADS67 | Calam.14G070200.1 | 14 | 7 | 210 | 9.32 | 24,190.64 | MIKCc | FYF | 10−80 | TM3/SOC1 |
| CamMADS68 | Calam.14G138300.1 * | 14 | 7 | 198 | 7.63 | 22,305.52 | MIKCc | FLC | 10−34 | FLC |
| CamMADS69 | Calam.14G138400.2 | 14 | 8 | 241 | 8.81 | 27,314.03 | MIKCc | SEP3 | 10−126 | SEP (E) |
| CamMADS70 | Calam.15G063200.4 | 15 | 9 | 300 | 7.65 | 31,373.45 | MIKCc | FUL | 10−94 | SQUA (A) |
| CamMADS71 | Calam.15G133600.1 | 15 | 1 | 282 | 8.46 | 31,931.5 | M-type | - | - | γ |
| CamMADS72 | Calam.15G133700.1 | 15 | 1 | 260 | 6.37 | 29,363.63 | M-type | - | - | γ |
| CamMADS73 | Calam.15G135000.1 | 15 | 1 | 217 | 9.38 | 24,754.44 | M-type | - | - | γ |
| CamMADS74 | Calam.15G135100.1 | 15 | 1 | 218 | 9.52 | 24,769.46 | M-type | - | - | γ |
| CamMADS75 | Calam.16G114400.2 | 16 | 7 | 195 | 9.56 | 22,684.08 | MIKCc | TM8 | 10−85 | TM8 |
| CamMADS76 | Calam.17G013600.1 | 17 | 7 | 223 | 9.57 | 25,780.54 | MIKCc | STK | 10−89 | AG (C/D) |
| CamMADS77 | Calam.17G075300.1 * | 17 | 7 | 211 | 9.28 | 24,291.01 | MIKCc | AGL71 | 10−59 | TM3/SOC1 |
| CamMADS78 | Calam.17G076100.1 | 17 | 7 | 194 | 9.64 | 22,181.55 | MIKCc | AGL13 | 10−48 | AGL6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhindi, T.; Al-Abdallat, A.M. Genome-Wide Identification and Analysis of the MADS-Box Gene Family in American Beautyberry (Callicarpa americana). Plants 2021, 10, 1805. https://doi.org/10.3390/plants10091805
Alhindi T, Al-Abdallat AM. Genome-Wide Identification and Analysis of the MADS-Box Gene Family in American Beautyberry (Callicarpa americana). Plants. 2021; 10(9):1805. https://doi.org/10.3390/plants10091805
Chicago/Turabian StyleAlhindi, Tareq, and Ayed M. Al-Abdallat. 2021. "Genome-Wide Identification and Analysis of the MADS-Box Gene Family in American Beautyberry (Callicarpa americana)" Plants 10, no. 9: 1805. https://doi.org/10.3390/plants10091805
APA StyleAlhindi, T., & Al-Abdallat, A. M. (2021). Genome-Wide Identification and Analysis of the MADS-Box Gene Family in American Beautyberry (Callicarpa americana). Plants, 10(9), 1805. https://doi.org/10.3390/plants10091805





