Persicaria lapathifolia Essential Oil: Chemical Constituents, Antioxidant Activity, and Allelopathic Effect on the Weed Echinochloa colona
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of P. lapathifolia EO
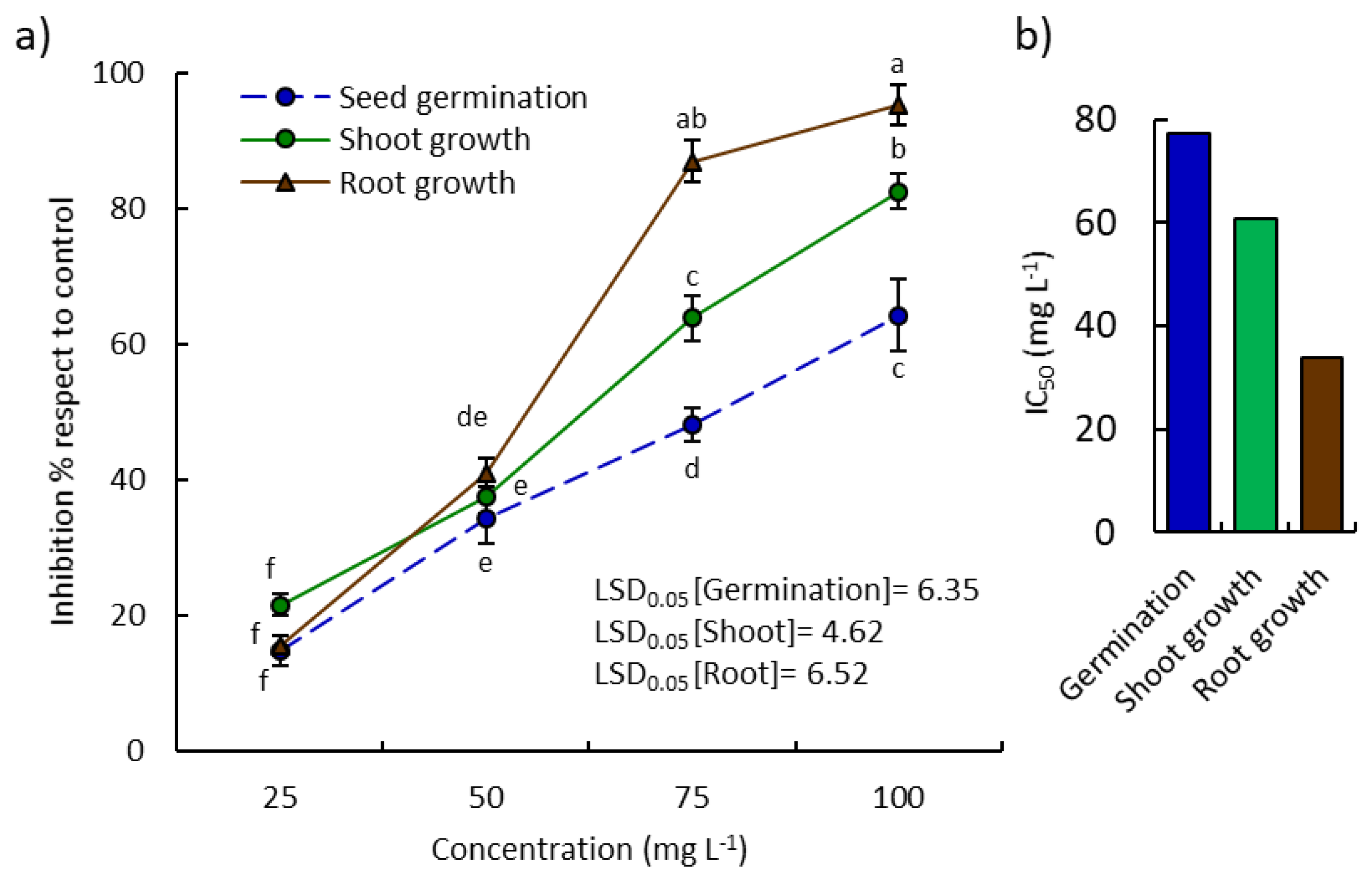
2.2. Allelopathic Activity of P. lapathifolia EO
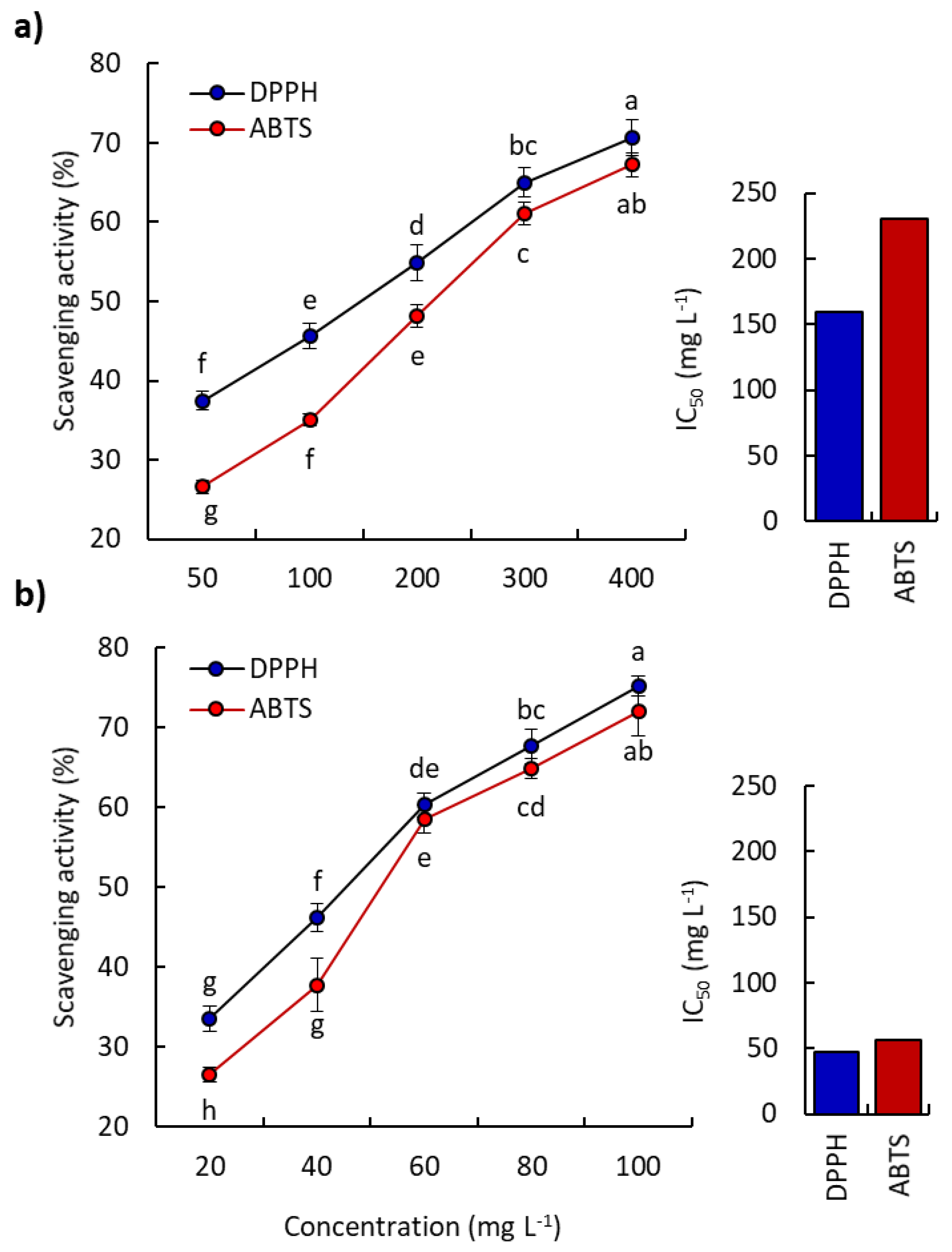
2.3. Antioxidant Activity of P. lapathifolia EO
3. Materials and Methods
3.1. Plant Materials Collected and Preparation
3.2. Extraction of EO, GC-MS Analysis, and Identification of Chemical Constituents
3.3. Allelopathic Bioassay
3.4. Antioxidant Activity Estimation
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Essa, A.F.; El-Hawary, S.S.; Abd-ElGawad, A.M.; Kubacy, T.M.; El-Khrisy, E.E.-D.A.; Elshamy, A.; Younis, I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, A.M.; Ahmed, R.F.; Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Elshamy, A.I.; Nassar, M.I. Chemical profiles, anticancer, and anti-aging activities of essential oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants 2021, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Fayed, E.M.; Abd-EIGawad, A.M.; Elshamy, A.I.; El-Halawany, E.S.F.; EI-Amier, Y.A. Essential oil of Deverra tortuosa aerial parts: Detailed chemical profile, allelopathic, antimicrobial, and antioxidant activities. Chem. Biodivers. 2021, 18, e2000914. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.; Abd-ElGawad, A.; El Gendy, A.E.-N.; Abd El Aty, A.; Mohamed, T.; Kassem, H.; Aldosri, F.; Elshamy, A.; Hegazy, M.-E.F. Phytotoxic and antimicrobial activities of Teucrium polium and Thymus decussatus essential oils extracted using hydrodistillation and microwave-assisted techniques. Plants 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; Al-Rowaily, S.L.; Ragab, T.I.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crops Prod. 2020, 148, 112272. [Google Scholar] [CrossRef]
- Ali, H.; Al-Khalifa, A.R.; Aouf, A.; Boukhebti, H.; Farouk, A. Effect of nanoencapsulation on volatile constituents, and antioxidant and anticancer activities of Algerian Origanum glandulosum Desf. essential oil. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, W.; Livinalli, N.; Baldasso, C.; Tessaro, I. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef]
- Blum, U. Plant–plant allelopathic interactions. In Plant-Plant Allelopathic Interactions; Springer: Dordrecht, The Netherlands, 2011; pp. 1–7. [Google Scholar]
- Serra, S.N.; Shanmuganathan, R.; Becker, C. Allelopathy in rice: A story of momilactones, kin recognition, and weed management. J. Exp. Bot. 2021, 72, 4022–4037. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Mazzoleni, S.; Abd-ElGawad, A.M. Microbiota modulation of allelopathy depends on litter chemistry: Mitigation or exacerbation? Sci. Total Environ. 2021, 776, 145942. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Amier, Y.A.; El Gendy, A.E.-N.G.; Al-Barati, S.A.; Dar, B.A.; Al-Rowaily, S.L.; Assaeed, A.M. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020, 13, 4237–4245. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Assaeed, A.M.; Elgamal, A.M.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Dar, B.A.; Mohamed, T.K.; Elshamy, A.I. Essential oil of Calotropis procera: Comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules 2020, 25, 5203. [Google Scholar] [CrossRef] [PubMed]
- Aziman, N.; Abdullah, N.; Bujang, A.; Mohd Noor, Z.; Abdul Aziz, A.; Ahmad, R. Phytochemicals of ethanolic extract and essential oil of Persicaria hydropiper and their potential as antibacterial agents for food packaging polylactic acid film. J. Food Saf. 2021, 41, e12864. [Google Scholar] [CrossRef]
- Sankarikutty, B.; Narayanan, C.S. Essential oils isolation and production. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 2185–2189. [Google Scholar]
- Boulos, L. Flora of Egypt; All Hadara Publishing: Cairo, Egypt, 1999; Volume 1. [Google Scholar]
- Tackholm, V. Students’ Flora of Egypt, 2nd ed.; Cairo University Press: Cairo, Egypt, 1974. [Google Scholar]
- Heywood, V.H.; Brummitt, R.; Culham, A.; Seberg, O. Flowering Plant Families of the World; Kew Publishing: London, UK, 2007; Volume 88. [Google Scholar]
- Bulbul, L.; Sushanta, S.M.; Uddin, M.J.; Tanni, S. Phytochemical and pharmacological evaluations of Polygonum lapathifolium stem extract for anthelmintic and antiemetic activity. Int. Curr. Pharm. J. 2013, 2, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Hailemariam, A.; Feyera, M.; Deyou, T.; Abdissa, N. Antimicrobial Chalcones from the Seeds of Persicaria lapathifolia. Biochem. Pharmacol. 2018, 7, 2167-0501. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Kuroki, S.; Tokuda, H.; Nishino, H. Cancer chemopreventive activity of phenylpropanoid esters of sucrose, vanicoside B and lapathoside A, from Polygonum lapathifolium. Cancer Lett. 2001, 173, 133–138. [Google Scholar] [CrossRef]
- Kubínová, R.; Pořízková, R.; Bartl, T.; Navrátilová, A.; Čížek, A.; Valentová, M. Biological activities of polyphenols from Polygonum lapathifolium. B. Latinoam. Caribe Plantas Med. Aromát. 2014, 13, 506–516. [Google Scholar]
- Park, S.-H.; Oh, S.R.; Jung, K.Y.; Lee, I.S.; Ahn, K.S.; Kim, J.H.; Kim, Y.S.; Lee, J.J.; Lee, H.-K. Acylated flavonol glycosides with anti-complement activity from Persicaria lapathifolia. Chem. Pharm. Bull. 1999, 47, 1484–1486. [Google Scholar] [CrossRef] [Green Version]
- Smolarz, H.D. Flavonoids from Polygonum lapathifolium ssp. tomentosum. Pharm. Biol. 2002, 40, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Peerzada, A.M.; Bajwa, A.A.; Ali, H.H.; Chauhan, B.S. Biology, impact, and management of Echinochloa colona (L.) Link. Crop Prot. 2016, 83, 56–66. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, P.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1991. [Google Scholar]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Houdková, M.; Ventura, K.; Bajerová, P. Chemical composition and determination of the antibacterial activity of essential oils in liquid and vapor phases extracted from two different southeast Asian herbs—Houttuynia cordata (saururaceae) and Persicaria odorata (polygonaceae). Molecules 2020, 25, 2432. [Google Scholar] [CrossRef]
- Almarie, A.; Mamat, A.; Wahab, Z.; Rukunudin, I. Chemical composition and phytotoxicity of essential oils isolated from Malaysian plants. Allelopathy J. 2016, 37, 55–70. [Google Scholar]
- Hunter, M.V.; Brophy, J.J.; Ralph, B.J.; Bienvenu, F.E. Composition of Polygonum odoratum Lour. from southern Australia. J. Essent. Oil Res. 1997, 9, 603–604. [Google Scholar] [CrossRef]
- Dũng, N.X.; Van Hac, L.; Leclercq, P.A. Volatile constituents of the aerial parts of Vietnamese Polygonum odoratum L. J. Essent. Oil Res. 1995, 7, 339–340. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Costa, A.V.; Tomaz, M.A.; Rodrigues, W.N.; Fialho Silva, W.P.; Moreira Valente, V.M. Characterization of the Essential Oil of Mastic Tree from Different Biomes and its Phytotoxic Potential on Cobbler’s Pegs. J. Essent. Oil-Bear. Plants 2016, 19, 972–979. [Google Scholar] [CrossRef]
- Bali, A.S.; Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K. Chemical characterization and phytotoxicity of foliar volatiles and essential oil of Callistemon viminalis. J. Essent. Oil-Bear. Plants 2017, 20, 535–545. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crops Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Silva, E.R.; Overbeck, G.E.; Soares, G.L.G. Phytotoxicity of volatiles from fresh and dry leaves of two Asteraceae shrubs: Evaluation of seasonal effects. S. Afr. J. Bot. 2014, 93, 14–18. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; An, M.; Wu, H.; Li Liu, D.; Stanton, R. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silverleaf nightshade (Solanum elaeagnifolium Cav.) in Australia. Plant Growth Regul. 2012, 68, 231–237. [Google Scholar] [CrossRef]
- Benyelles, B.; Allali, H.; Dib, M.E.A.; Djabou, N.; Paolini, J.; Costa, J. Chemical composition variability of essential oils of Daucus gracilis Steinh. from Algeria. Chem. Biodivers. 2017, 14, e1600490. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cervantes, M.; Pérez-Alonso, M.J.; Blanco-Salas, J.; Soria, A.C.; Ruiz-Téllez, T. Analysis of the essential oils of Chamaemelum fuscatum (Brot.) Vasc. from Spain as a contribution to reinforce its ethnobotanical use. Forests 2019, 10, 539. [Google Scholar] [CrossRef] [Green Version]
- Elshamy, A.; Abd-ElGawad, A.M.; El-Amier, Y.A.; El Gendy, A.; Al-Rowaily, S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
- Assaeed, A.; Elshamy, A.; El Gendy, A.E.-N.; Dar, B.; Al-Rowaily, S.; Abd-ElGawad, A. Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensis exhibited antioxidant activity and allelopathic effect on weeds. Agronomy 2020, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- El-Shora, H.M.; Abd El-Gawad, A.M. Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresenius Environ. Bull. 2015, 24, 386–393. [Google Scholar]
- El-Shora, H.M.; El-Gawad, A.M.A. Response of Cicer arietinum to allelopathic effect of Portulaca oleracea root extract. Phyton-Ann. Rei Bot. 2015, 55, 215–232. [Google Scholar]
- Reigosa, M.J.; Sánchez-Moreiras, A.; González, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci. 1999, 18, 577–608. [Google Scholar] [CrossRef]
- Kim, J. Phytotoxic and antimicrobial activities and chemical analysis of leaf essential oil from Agastache rugosa. J. Plant Biol. 2008, 51, 276–283. [Google Scholar] [CrossRef]
- Amri, I.; Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest Sci. 2012, 85, 199–207. [Google Scholar] [CrossRef]
- Chopra, N.; Tewari, G.; Tewari, L.M.; Upreti, B.; Pandey, N. Allelopathic effect of Echinochloa colona L. and Cyperus iria L. Weed extracts on the seed germination and seedling growth of rice and soyabean. Adv. Agric. 2017, 2017, 5748524. [Google Scholar] [CrossRef] [Green Version]
- Hegab, M.; Abdelgawad, H.; Abdelhamed, M.; Hammouda, O.; Pandey, R.; Kumar, V.; Zinta, G. Effects of tricin isolated from jungle rice (Echinochloa colona L.) on amylase activity and oxidative stress in wild oat (Avena fatua L.). Allelopathy J. 2013, 31, 345–354. [Google Scholar]
- Khaliq, A.; Matloob, A.; Cheema, Z.A.; Farooq, M. Allelopathic activity of crop residue incorporation alone or mixed against rice and its associated grass weed jungle rice (Echinochloa colona [L.] Link. Chil. J. Agric. Res. 2011, 71, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.J.; Basri, M.; Ee, G.C.L.; Omar, D. Phytoinhibitory activities and extraction optimization of potent invasive plants as eco-friendly weed suppressant against Echinochloa colona (L.) Link. Ind. Crops Prod. 2017, 100, 19–34. [Google Scholar] [CrossRef]
- Cook, T.; Storrie, A.; Moylan, P.; Adams, B. Field testing of glyphosate-resistant awnless barnyard grass (Echinochloa colona) in northern NSW. In Proceedings of the Proceedings of the 16th Australian Weeds Conference, Brisbane, QL, Australia, 2008; pp. 20–23.
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. Flavour Fragr. J. 2010, 25, 219–312. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]

| No | Rt a | Relative Conc. (%) b | Compound Name | Type | Identification c | |
|---|---|---|---|---|---|---|
| KIObserved d | KIPublished e | |||||
| 1 | 3.23 | 1.74 ± 0.02 | α-Pinene | MH | 932 | 935 |
| 2 | 4.14 | 1.97 ± 0.05 | Sabinene | MH | 969 | 966 |
| 3 | 5.43 | 8.04 ± 0.06 | 2E-Hexenoic acid | Others | 1007 | 1009 |
| 4 | 13.84 | 3.09 ± 0.05 | Limonene | MH | 1024 | 1028 |
| 5 | 14.54 | 10.43 ± 0.14 | 2-Methyl butyl isovalerate | OM | 1104 | 1104 |
| 6 | 18.26 | 1.98 ± 0.03 | Hydrocinnamyl alcohol | Others | 1227 | 1232 |
| 7 | 20.12 | 3.51 ± 0.06 | γ-Nonalactone | Others | 1361 | 1367 |
| 8 | 21.67 | 1.29 ± 0.02 | trans-Caryophyllene | SH | 1408 | 1403 |
| 9 | 22.64 | 22.61 ± 0.16 | n-Dodecanal | Others | 1408 | 1415 |
| 10 | 23.07 | 11.29 ± 0.13 | α-Humulene | SH | 1452 | 1459 |
| 11 | 32.46 | 2.69 ± 0.04 | 2-Ethyl chromone | Others | 1614 | 1618 |
| 12 | 32.84 | 3.78 ± 0.04 | Phytane | DH | 1810 | 1818 |
| 13 | 36.88 | 1.75 ± 0.02 | Hexahydrofarnesyl acetone | Car | 1845 | 1847 |
| 14 | 38.16 | 3.19 ± 0.03 | Carissone | OS | 1927 | 1934 |
| 15 | 39.42 | 8.97 ± 0.08 | 2,4-Dimethylicosane | Others | 2080 | 2087 |
| 16 | 40.02 | 1.08 ± 0.04 | Abienol | OD | 2150 | 2156 |
| 17 | 42.56 | 2.27 ± 0.06 | n-Docosane | Others | 2200 | 2202 |
| 18 | 46.02 | 1.18 ± 0.03 | n-Tricosane | Others | 2300 | 2300 |
| 19 | 46.2 | 2.72 ± 0.05 | n-Nonacosane | Others | 2900 | 2905 |
| 20 | 49.22 | 3.01 ± 0.04 | n-Hentriacontane | Others | 3100 | 3101 |
| 21 | 51.14 | 1.71 ± 0.03 | n-Dotriacontane | Others | 3200 | 3203 |
| 6.80 ± 0.07 | Monoterpene Hydrocarbons (MH) | |||||
| 10.43 ± 0.14 | Oxygenated Monoterpenes (OM) | |||||
| 12.58 ± 0.12 | Sesquiterpene Hydrocarbons (SH) | |||||
| 3.19 ± 0.03 | Oxygenated Sesquiterpenes (OS) | |||||
| 3.78 ± 0.04 | Diterpene Hydrocarbons (DH) | |||||
| 1.08 ± 0.04 | Oxygenated Diterpenes (OD) | |||||
| 1.75 ± 0.02 | Carotenoid derived compounds (Car) | |||||
| 58.69 ± 0.13 | Other compounds (Others) | |||||
| 98.3 | Total | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; Bonanomi, G.; Al-Rashed, S.A.; Elshamy, A.I. Persicaria lapathifolia Essential Oil: Chemical Constituents, Antioxidant Activity, and Allelopathic Effect on the Weed Echinochloa colona. Plants 2021, 10, 1798. https://doi.org/10.3390/plants10091798
Abd-ElGawad AM, Bonanomi G, Al-Rashed SA, Elshamy AI. Persicaria lapathifolia Essential Oil: Chemical Constituents, Antioxidant Activity, and Allelopathic Effect on the Weed Echinochloa colona. Plants. 2021; 10(9):1798. https://doi.org/10.3390/plants10091798
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Giuliano Bonanomi, Sarah A. Al-Rashed, and Abdelsamed I. Elshamy. 2021. "Persicaria lapathifolia Essential Oil: Chemical Constituents, Antioxidant Activity, and Allelopathic Effect on the Weed Echinochloa colona" Plants 10, no. 9: 1798. https://doi.org/10.3390/plants10091798
APA StyleAbd-ElGawad, A. M., Bonanomi, G., Al-Rashed, S. A., & Elshamy, A. I. (2021). Persicaria lapathifolia Essential Oil: Chemical Constituents, Antioxidant Activity, and Allelopathic Effect on the Weed Echinochloa colona. Plants, 10(9), 1798. https://doi.org/10.3390/plants10091798









