The Ethnobotany and Chemistry of South African Meliaceae: A Review
Abstract
1. Introduction
2. The Ethnobotany and Chemistry of South African Meliaceae
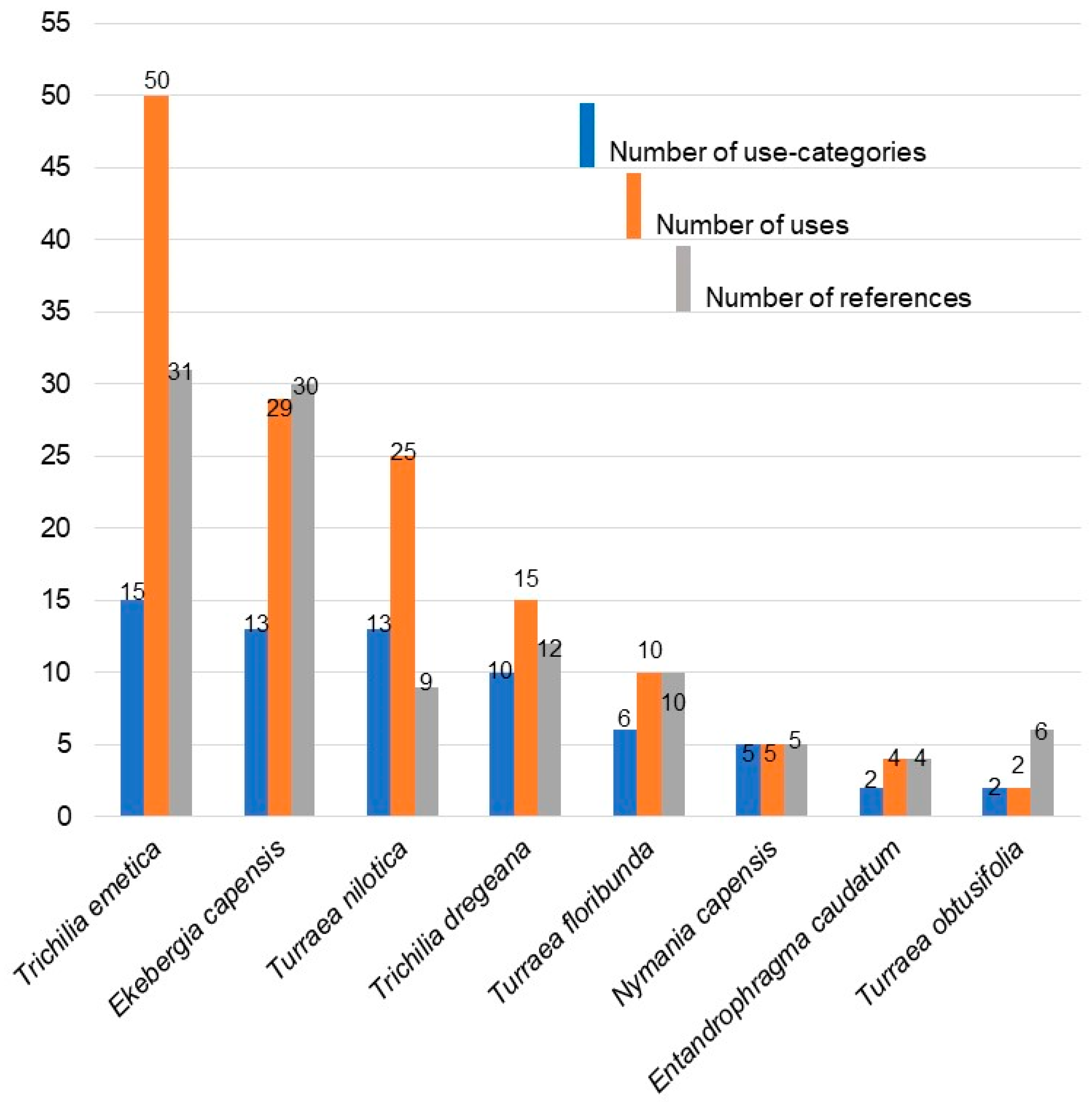
2.1. Ethnobotanical Uses
| Taxa | Local Names | Traditional Use | References | ||
|---|---|---|---|---|---|
| Medicinal Use | Part Use | Preparation and Administration | |||
| Ekebergia capensis Sparrm. | Esseboom, essenhout (A); dogplum, cape ash (E); munyonga, mmidibidi (NS); umNyamatsi (S); mumbafwe (T); nyamaru (Ts); mudouma, muṱobvuma, muzhouzhou (V); umgwenyezinja (X); imanaya, isimanaye, mahlunzidintaba, ronyamati, simanaya, umathunzi wentaba, umathunzini, umgwenyana wezinja, umnyamathi, umthoma, usimanaye, uvungu, (Z) | Analgesic | |||
| Headache | Root | Powdered, charred pulverized roots are sniffed | [12,41,42] | ||
| Leaf | NR | [43] | |||
| Malaria | Root and leaf | Extracts from maceration of crushed roots and leaves are drunk | [44] | ||
| Bark | inner bark is boiled and drunk | [45] | |||
| Anthelmintic | |||||
| Worms | Bark and leaf | Bark powder is added to leaf decoction and drunk | [6,46] | ||
| Antimicrobial | |||||
| Anthrax | Leaf | Crushed leaf is boiled and drunk | [47,48] | ||
| Venereal diseases | Bark and root | Freshly collected bark and roots are boiled in water and the extract is drunk three times daily | [49] | ||
| Cardio-vascular | |||||
| Blood purifier and blood pressure | Leaf and inner bark | Leaf or inner bark is boiled and drunk | [13,45,47] | ||
| Heart ailment | Bark | NR | [13] | ||
| Cytological | |||||
| Cancers | Fruits | Fruits are crushed, sieved, and drunk | [50] | ||
| Dermatological | |||||
| Abscess, scabies, and acne | Bark | Infusion or maceration of the bark powder is applied | [6,39,51,52] | ||
| Scabies | Root and leaf | NR | [12,41] | ||
| Abscess and boil | Bark | Crushed bark added to flour and water poultices is applied | [13,51] | ||
| Pimples | Bark | Crushed bark in hot water infusion is drunk and used as a wash | [13,51] | ||
| Skin ailments | Leaf | NR | [37] | ||
| Gastro-Intestinal | |||||
| Bloody stool | Bark | Bark is macerated with bark of Diospyros lycioides Desf. and extract is drunk | [53] | ||
| Emetic and heartburn | Bark and root | Bark or root decoctions are taken as emetics | [6,43,54] | ||
| Gastritis, dysentery, and heartburn | Bark and root | One teaspoon of bark and root powder in a half cup of hot water is taken as tea | [6,12,41,44,55] | ||
| Purgative | Leaf | A cup of leaf infusion is drunk | [13] | ||
| Stomachache | Fruits | Fruits are masticated and swallowed | [50] | ||
| Gynaecological and Obstetrics; Genital system | |||||
| Dystocia | Leaf and twig | Leafy twigs mixed with Indigofera oubanguiensis Tisser. are taken orally and used as a wash to treat dystocia | [30,56] | ||
| Infertility | Bark | NR | [13] | ||
| Nervous system | |||||
| Epilepsy | Bark | Bark infusion is drunk | [57] | ||
| Stress relief | NR | NR | [14] | ||
| Respiratory system | |||||
| Chest complaints and coughs | Bark and root | Bark or root decoctions are taken orally | [54] | ||
| Chronic cough | Leaf | NR | [43] | ||
| Cough and respiratory complaints | Bark | Bark decoction with root of Euclea natalensis A.DC. is drunk | [54] | ||
| Trauma | |||||
| Snakebite | Root and leaf | Extracts from maceration of crushed roots and leaves are drunk | [44] | ||
| Ethnoveterinary | |||||
| Tuberculosis | Bark | Crushed bark is boiled and administered orally | [48,58] | ||
| Abortion | Galls | Galls on plant are boiled and administered orally | [59] | ||
| Magic | |||||
| Protection | Bark | Bark is used to protect chiefs against witchcraft | [13,60] | ||
| Love | Bark | Bark decoction is drunk as love charm emetics | [13,60] | ||
| Ekebergia pterophylla (C.DC.) Hofmeyr | Rotsessenhout (A); rock ash (E); maGwedla (S) | No Ethnomedicinal Records | |||
| Entandrophragma caudatum (Sprague) Sprague | Bergmahonie (A); mountain mahogany, wooden-banana (E); mophumêna (Ts); munzhounzhou (V) | Analgesic | |||
| Malaria | Bark | NR | [61] | ||
| Antimicrobial | |||||
| Gonorrhoea | Root | Root decoction is drunk | [62] | ||
| Genital warts | Fruit | Burnt fruit peels mixed with Vaseline are applied topically | [62] | ||
| Nymania capensis (Thunb.) Lindb. | Kankerbos, kiepkiepies, klapperbos, klapperbossie, lanternbos, oumeidsbos, oumeidebos, stuipebos, stuipebossie, stinkbossie, ystervarkbos (A); chinese lantern tree, kipkippers, klapper (E) | Ear, Nose, and Throat | |||
| Influenza | Leaf | Leaf infusion is taken | [63] | ||
| Gastro-Intestinal | |||||
| Stomach complaints and nausea | Root | Root decoction is taken | [64] | ||
| Nervous system | |||||
| Convulsion | Leaf | Leaf decoction | [12,53,63] | ||
| Trauma | |||||
| Wound healing | Root | Roasted, pulverized roots are sprinkled on the affected part and can also be mixed with fat into an ointment and applied as a salve | [64] | ||
| Urinary system | |||||
| Kidney problem | Root | Root decoction is taken | [64] | ||
| Pseudobersama mossam bicensis (Sim) Verdc. | Valswitessenhout (A); umopho (Z) | No Ethnomedicinal Records | |||
| Trichilia dregeana Sond. | Bos Rooi-essenhout, bosrooiessenhout (A); cape mahogany, forest mahogany, forest natal mahogany, white mahogany (E); mutshikili, mutuhu, muuhu (V); umhlakele, umkhuhlu, umkhuhlwa (X), ixolo, umathunzi, umathunzini, umkhuhlu (Z) | Analgesic | |||
| Back pain | Bark | A teaspoon of pulverised bark boiled in a cup of milk is allowed to cool and strained, then half a cup of the extract is taken as an enema in the early morning | [12,13] | ||
| Fever | Root | Root decoction is taken orally | [12,65] | ||
| Toothache | NR | NR | [14] | ||
| Antimicrobial | |||||
| Gonorrhoea and Syphilis | Leaf | Handful of leaves is boiled with a handful of Albizia adianthifolia leaves in 2 L water and half a cup of the decoction is taken daily | [27,43,53,66] | ||
| Leprosy | NR | NR | [65] | ||
| Cardio-Vascular | |||||
| Blood purifier | Bark | Bark is taken as an enema for men | [43] | ||
| Dermatological | |||||
| Bruises and eczema | Leaf or fruit | Leaf or fruit poultice is applied topically | [65] | ||
| Gastro-Intestinal | |||||
| Bloody diarrhoea | Bark | Bark decoction is taken daily | [53,65] | ||
| Purgative and stomach complaints | Bark or root | Bark infusion is taken as an enema or root decoction is taken orally | [13,65,67] | ||
| Gynaecological and Obstetrics; Genital system | |||||
| Abortifacient | Bark | Bark infusion or decoction is taken orally or used as an enema | [65,67] | ||
| Musculo-Skeletal | |||||
| Lumbago | NR | NR | [65] | ||
| Rheumatism | Seed | Oil from seed is used for massage | [65] | ||
| Trauma | |||||
| Fractures | Seed | Oil from seed is rubbed into scarifications made on fractured limb | [65] | ||
| Urinary system | |||||
| Kidney problem | Bark | Bark maceration is taken as an enema | [13,43,53] | ||
| Ethnoveterinary | |||||
| Fishing poison | Bark | NR | [8,65,67] | ||
| Trichilia emetica Vahl | Rooiessenhout (A); natal-mahogany (E); mamba (NS); umkuhlu (Si); ankulu, nkulu (T); mutshikili, mutuhu (V); umkhuhlu (X); umathunzini (Z) | Analgesic | |||
| Back pain | Bark or leaf | Bark or leaf maceration is taken as an enema | [12,13,68] | ||
| Dental care | Twig, trunk, wood, root or flower | Twig, trunk, wood or root is chewed and the crushed flowers is used as a toothpaste | [69] | ||
| Headache | Leaf | Leaf infusion is used to wash the head | [69] | ||
| Malaria | Leaf, bark, and root | Leaf decoction mixed with lemon is drunk or used as a bath for 3–7 days. Bark decoction mixed with honey is also taken orally. Root, stem, and leaf decoction is taken 2–3 times daily for 3 days. Root maceration of T. emetica, Pseudocedrala kotschii (Schweinf.) Harms and Nauclea latifolia Sm. mixed with honey can also be drunk for 10 days | [38] | ||
| Anthelmintic | |||||
| Teniasis | Bark | Crushed bark mixed with root of Securidaca longependonculata Fresen. is taken orally for 3 days | [38] | ||
| Worm | Bark or root | Bark or root decoction is taken daily for 3 days | [53,70] | ||
| Antimicrobial | |||||
| Dysentery | Bark or leaf | Bark or leaf maceration is taken as an enema | [12,13,71] | ||
| Gonorrhoea and syphilis | Bark and leaf | Bark and leaf decoction is drunk | [43,62] | ||
| Leprosy | Root | Root maceration is drunk or used as a bath | [38,42,71] | ||
| Cardio-vascular | |||||
| Blood and digestive tract cleanser | Bark | Bark infusion is applied as an enema | [13,43] | ||
| Blood pressure | Leaf | A teaspoon of 1 h decoction of T. emetica leaf, Aloe. marlothii leaf, and Hyphaene coriaceae root is taken orally three times daily | [27] | ||
| Dermatological | |||||
| Burns and bruises | Leaf | Hot leaf infusion is applied to the affected part | [69] | ||
| Dermatitis | Leaf and bark | Leaf decoction is used in a steam bath or crushed leaves are applied on the affected part while the powdered bark can also be used for cleansing | [38,72] | ||
| Eczema | Fruit or leaf | Fruit or leaf poultice is applied topically | [12,13,73,74] | ||
| Ear, Nose, and Throat | |||||
| Colds and bronchial inflammation | Root | Root decoction is taken orally | [37] | ||
| Gastro-Intestinal | |||||
| Abdominal pain | Leaf or root | Crushed leaf or root decoction is used as a bath and taken orally with salt and lemon twice daily | [38] | ||
| Constipation | Bark | 50 g of chopped bark is boiled with 50 g of chopped bark of Spirostachys africana Sond. in 5 L of water and taken orally | [68] | ||
| Diarrhoea | Bark | Bark infusion is administered anally twice a day | [27] | ||
| Digestive infections | Root | Root decoction mixed with coffee is taken orally for 3 days or decoction mixed with Cassia sieberiana DC. root and honey drunk in the morning for 5 days | [38] | ||
| Emetic | Bark or root | Bark maceration or pulverized bark in hot water is taken, root extract can also be taken orally | [12,13,37,46,72,75,76,77] | ||
| Flatulence | Root | Powdered root infusion added to Acacia nilotica seed powder is taken | [38] | ||
| Hemorrhoids | Root | Crushed root bark mixed with black pepper and crushed fruit of Xylopia aethiopica (Dunal) A. Rich. is taken daily, while the powdered root in salt water is used as an enema | [38] | ||
| Gastric ulcer | Bark | Crushed bark mixed with salt and ginger is added to porridge and taken twice daily | [38] | ||
| Hernia | Root | Crushed root is added to porridge and taken orally | [38] | ||
| Inflamed anus | Bark | Pulverized bark puffed into the anus | [30] | ||
| Jaundice | Root | Root decoction is taken daily for 3 days, chopped roots are also mixed with honey and used as a bath | [38,70] | ||
| Laxative | Bark | Bark is mixed with eggs and taken orally to clean the stomach | [78] | ||
| Purgative | Bark | Bark infusion or decoction is used as an enema or taken orally | [53,67,76] | ||
| Stomach complaints | Bark | Bark infusion or decoction is taken orally or administered as an enema | [43,53,54,67] | ||
| Gynaecological and Obstetrics; Genital system | |||||
| Abortifacient | Bark | Bark infusion is taken | [67,71] | ||
| Bark and root | One handful of crushed bark and root in a litre of milk and Coca Cola mixture is boiled for 15 min and taken orally | [27] | |||
| Breast pain | Leaf | Leaf decoction is used to bath | [38] | ||
| Dysmenorrhoea | Leaf | Leaf decoction added to Tamarindus indica L. or lemon is taken orally for 7 days | [38,79] | ||
| Fertility | Leaf | Leaf decoction with Combretum molle R. Br. ex G. Don is taken orally | [38] | ||
| Bark | Crushed bark mixed with the same amount of Gymnosporia senegalensisa root is boiled and decoction is used as an enema | [27] | |||
| Labour pain | Leaf | Hot leaf decoction is used to massage the belly of a woman during labour to ease pain until she delivers | [27] | ||
| Sterility | Root | Powdered root with ginger and salt is taken as a porridge | [38] | ||
| Musculo-Skeletal | |||||
| Lumbago | Bark or leaf | Bark or leaf maceration is taken as an enema | [12,30] | ||
| Paralyses | Root | Crushed root is mixed with porridge and taken orally | [38] | ||
| Rheumatism | Seed | Oil from boiled seeds is taken orally and rubbed topically | [8,12,13,46,75] | ||
| Opthalmic | |||||
| Eye infection | Leaf and bark | Leaf and bark decoction is used for eye cleansing for 2 days | [38] | ||
| Respiratory system | |||||
| Cardiac problems | Leaf | Leaf decoction is taken orally | [80] | ||
| Chest pain | Leaf | Leaf decoction is used in a steam bath or rubbed on the chest | [38] | ||
| Cough | Bark and root | Bark and root decoction is drunk | [81] | ||
| Pneumonia | Root or leaf | Root or leaf decoction is taken orally or used as a bath for 12 days | [37,38] | ||
| Trauma | |||||
| Fracture | Seed | Oil from the seed is rubbed into incisions on broken limbs and baked pulverized root of Sideroxylon inerme L. is applied | [8,12,13,82] | ||
| Stiffness or sprains | Bark | Bark extract is applied topically | [69] | ||
| Wound | Seed | Oil from the seed is applied to prevent infection from maggots | [37] | ||
| Urinary system | |||||
| Kidney problem | Bark | Bark decoction is administered as an enema | [43] | ||
| Renal ailments | Bark | Bark decoction is taken | [13] | ||
| Ethnoveterinary | |||||
| Fishing poison | Bark | NR | [67] | ||
| Magic | |||||
| Burial rituals | Leaf | Leaves are worn during burial rituals | [54] | ||
| Turraea floribunda Hochst. | Kanferfoelieboom (A); honeysuckle-tree, wild honeysuckle-tree (E); umdlozana, inkunzane (Si); umhlatholana, umlahlana (X); umadlozane, umlulama, ubhukulo (Z) | Dermatological | |||
| Abscesses | Root | Root decoction is taken orally | [30] | ||
| Gastro-Intestinal | |||||
| Ascites | Root | Root maceration is taken orally | [12,13] | ||
| Emetic | Bark | Bark extract is taken orally | [8,37] | ||
| Purgative | Bark and root | Bark and root decoction are taken orally | [37] | ||
| Musculo-Skeletal | |||||
| Rheumatism | Root | Root maceration is taken orally | [8,12,13,75,76] | ||
| Respiratory system | |||||
| Cardiac problems | Root | Root maceration is taken orally | [12,13,47,75,76] | ||
| Cough | Root | Root decoction is taken orally | [37] | ||
| Urinary system | |||||
| Urethral infection | Bark | Bark decoction is taken orally 1-3 times daily | [83] | ||
| Magic | |||||
| To induce a state of trance | Bark | Bark infusion is taken orally | [30,75,76] | ||
| Protection from bad dreams | Bark | NR | [76] | ||
| Turraea nilotica Kotschy and Peyr. | Bushveld honeysuckle-tree, lowveld honeysuckle-tree, miombo honeysuckle-tree, small mahogany (E) isidlamvundala (N); chipindura, chirambagavakava, chitsvimbovarisa, chitunguru, mudyakuwe, mukondanyoka, muzaramhanga (Sh) | Analgesic | |||
| Headache | Root or leaf | Root decoction or leaf infusion is taken orally | [46,67] | ||
| Toothache | Root | Root decoction is used as a mouthwash | [76,84] | ||
| Anthelmintic | |||||
| Ascariasis | Root | Root decoction is taken orally | [30] | ||
| Antimicrobial | |||||
| Gonorrhoea | Root | Root decoction is taken orally | [81] | ||
| Venereal diseases | Root | Root infusion is taken orally | [8,67] | ||
| Dermatological | |||||
| Abscesses | Root | Root decoction is taken orally and applied as a compress | [30] | ||
| Gastro-Intestinal | |||||
| Abdominal pain | Leaf or root | Leaf decoction or root infusion is taken orally | [8,67,85] | ||
| Constipation | Root | Root bark decoction is taken orally | [8,81] | ||
| Diarrhoea | Leaf or root | Leaf or root decoction is taken orally or pulverized root is added to porridge | [8,67,85] | ||
| Indigestion | Root | Root decoction is taken orally | [37] | ||
| Schistosomiasis or hernia | Root | Root infusion mixed with honey is taken orally | [81] | ||
| Gynaecological and Obstetrics; Genital system | |||||
| Aphrodisiac | Root | Powdered root mixed with beer or porridge is taken | [67] | ||
| Dysmenorrhoea | Root | Powdered root mixed with porridge is taken | [8,67] | ||
| Prevent abortion | Root | Root infusion is taken orally | [67] | ||
| Sterility | Root | Root decoction is taken orally | [81] | ||
| Nervous system | |||||
| Dizziness | Leaf | Leaf infusion is taken orally | [67] | ||
| Epilepsy | Root | Powdered root mixed with porridge is taken | [8,67] | ||
| Opthalmic | |||||
| Eye problems | Leaf | Leaf paste is applied to the eyelids | [67] | ||
| Respiratory system | |||||
| Dyspnea | Root | Powdered root mixed with porridge is taken orally | [67] | ||
| Pneumonia | Root | Powdered root is rubbed into scarification on the painful area, root infusion is taken orally, and smoke from the burnt roots is inhaled | [8,67] | ||
| Trauma | |||||
| Snakebite antidote | Root | Burnt root ashes are applied to the bite | [67] | ||
| Wound | Root | Root scrapings are applied topically | [30] | ||
| Urinary system | |||||
| Dysuria or rectal prolapse | Root | Root bark decoction is taken orally | [67] | ||
| Ethnoveterinary | |||||
| Anthelminthic for dogs | Root | Root infusion is administered orally | [67] | ||
| Magic | |||||
| To calm the insane | Leaf | Leaf infusion is administered orally and smoke from burnt leaves is inhaled | [67] | ||
| Turraea obtusifolia Hochst. | Kleinkanferfoelieboom (A); small honeysuckle tree, lesser honeysuckle tree, (E); amzulu, ikhambi-lomsinga, ikunzi, inkunzi, inkunzi-embomvana, umhlatholana, uswazi (Z) | Gastro-Intestinal | |||
| Stomach and intestinal complaints | Leaf, bark and root | Hot water maceration of leaf, bark or root is given as an enema and also mixed with porridge to be taken orally | [13,54,75,76,86] | ||
| Ethnoveterinary | |||||
| Wounds in livestock | Leaf | Crushed leaves are applied topically on the affected part | [87] | ||
| Turraea pulchella (Harms) T.D.Penn. | No Ethnobotanical Record | ||||
| Turraea streyi F. White and Styles | No Ethnobotanical Record | ||||
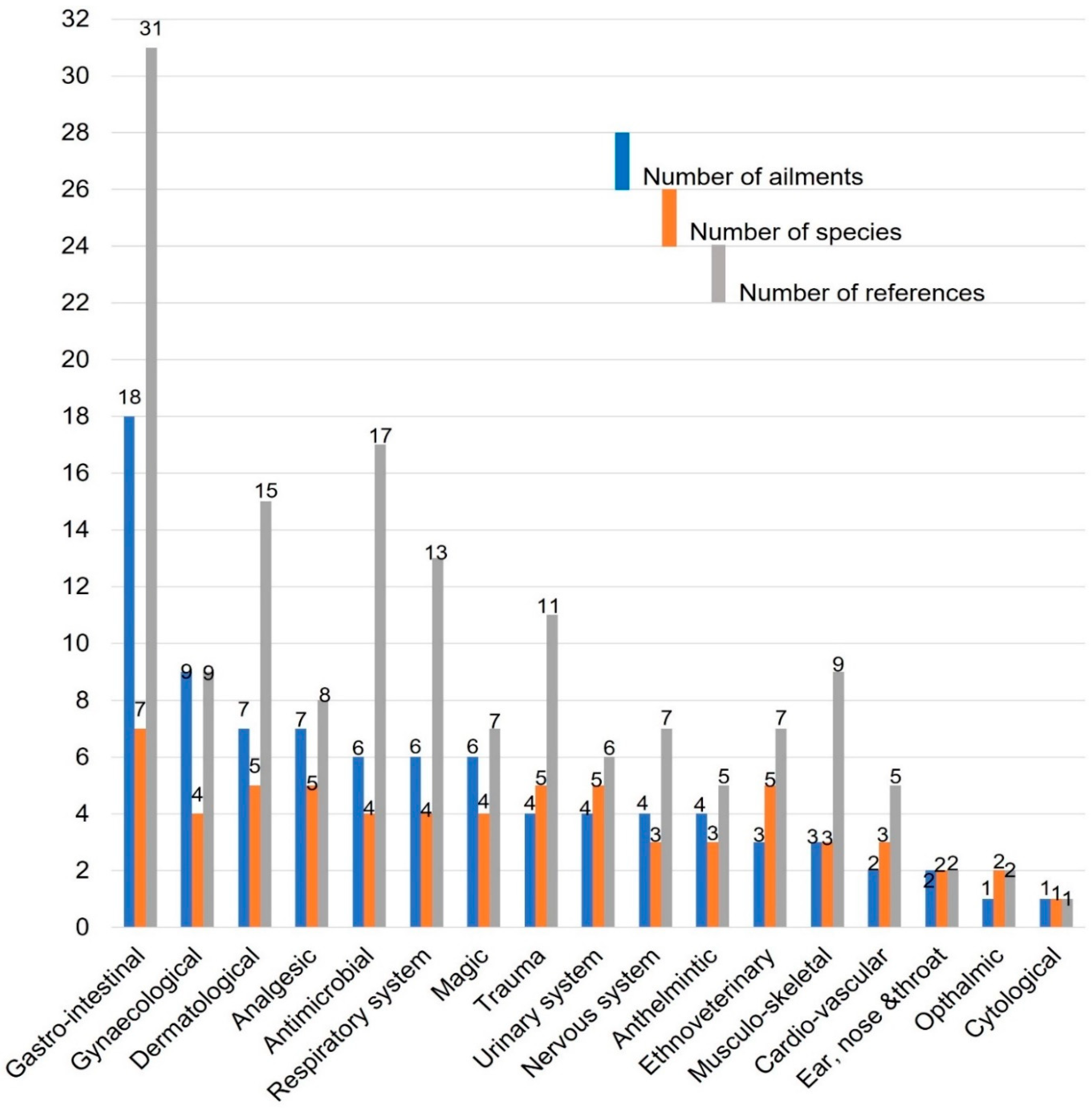
2.1.1. Categories of Medicinal Uses
2.1.2. Categories of Uses
2.1.3. Plant Parts Used
2.1.4. Mode of Herbal Preparation
2.1.5. Other Uses
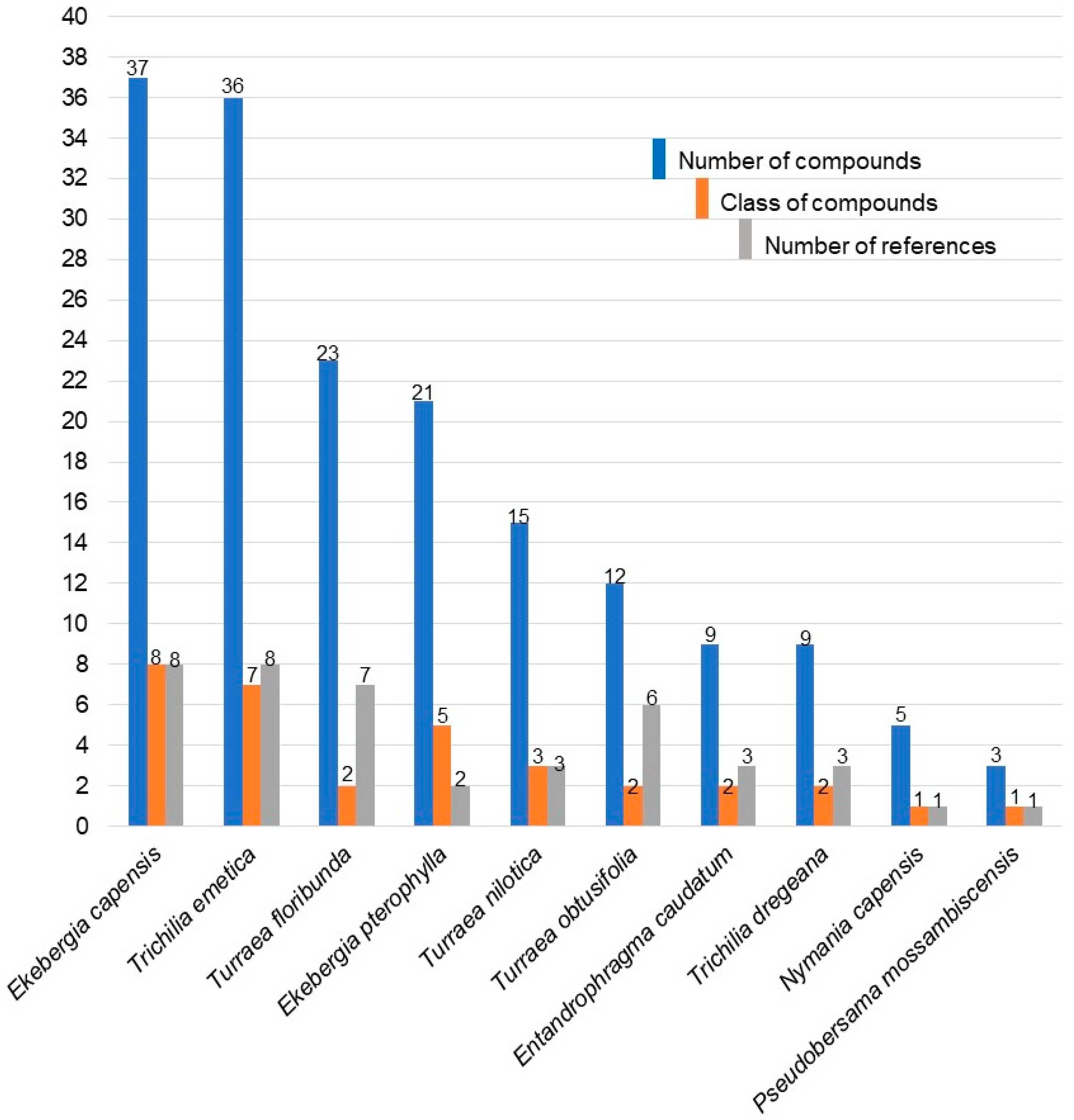
2.2. Reported Active Compounds
| Plant. | Compound | Part Extracted | References |
|---|---|---|---|
| Ekebergia capensis | Limonoids | ||
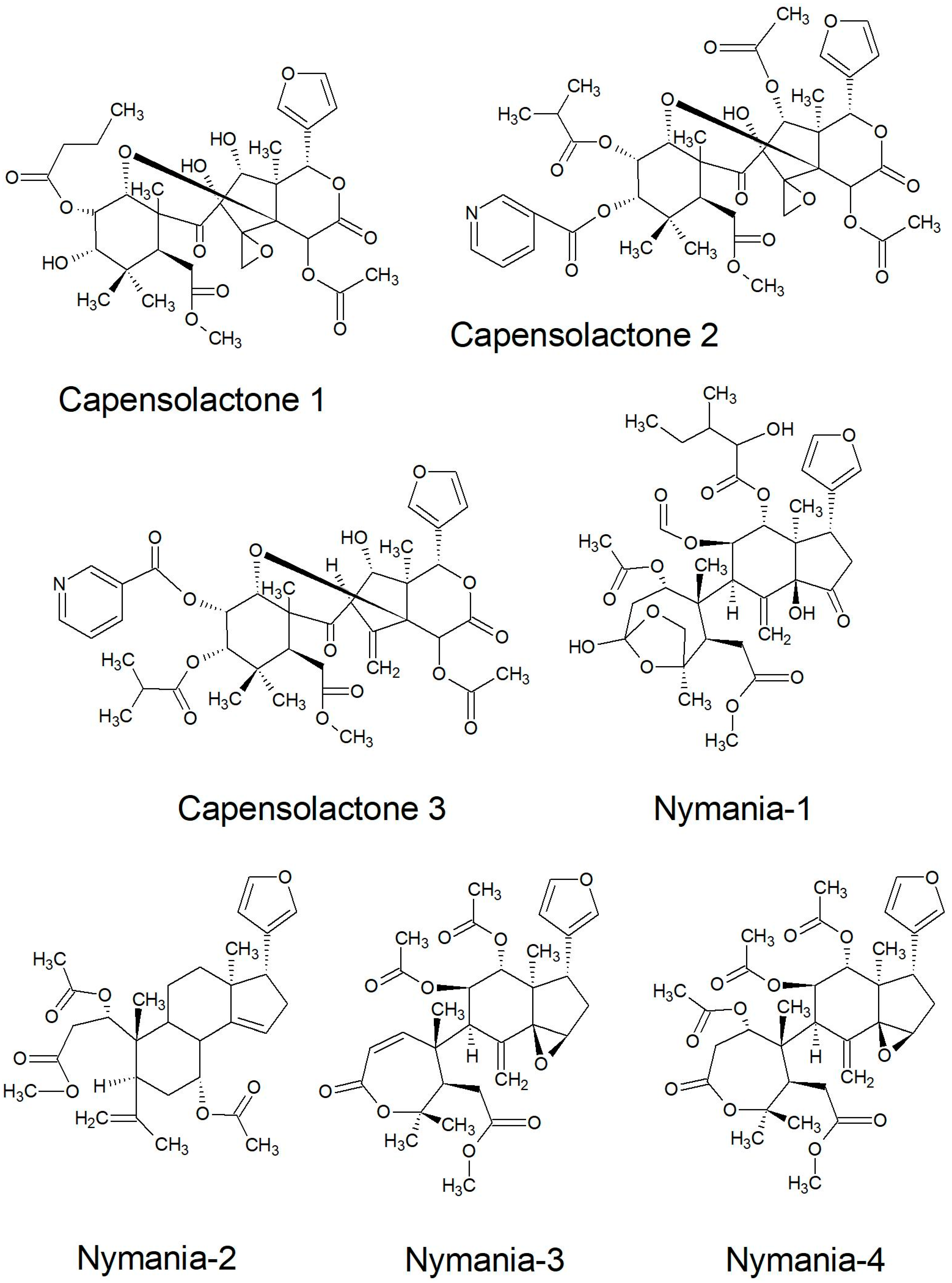
| Capensolactones 1-3 | Seed | [108] | |
| Methyl 3α-hydroxy-3-deoxy angolensate | Seed | [108] | |
| Ekebergin | Seed | [109] | |
| Ekebergins C1-C3 | Bark | [33] | |
| 7-Deacetoxy-7-oxogedunin | Bark | [33] | |
| Methylangolensate | Bark | [33] | |
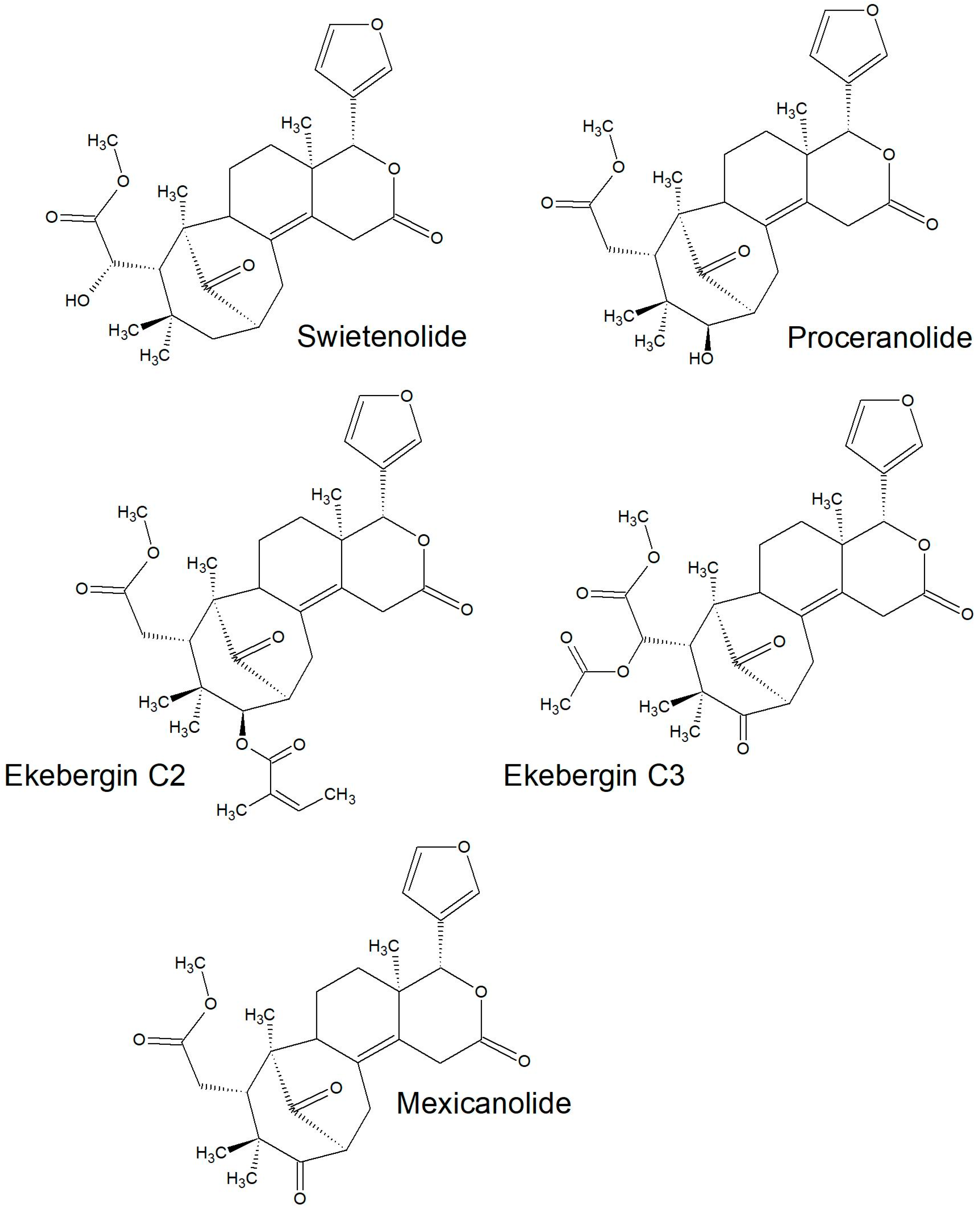
| Mexicanolide | Bark | [33] | |
| Proceranolide | Leaf and bark | [31,33] | |
| Swietenolide | Bark | [33] | |
| Triterpenes | |||
| 3,11-Dioxoolean-12-en-28-oic acid | Bark | [33] | |
| Ekebergin A | Bark and root | [31,33] | |
| Ekebergins D1-D5 | Bark | [33] | |
| Melliferone | Bark | [33] | |
| 7-Acetylneotrichilenone | Bark | [33] | |
| Lupeol | Bark | [32] | |
| 2-hydroxymethyl-2,3,22,23-tetrahydroxy-6,10,15,19,23-penta methyl-6,10,14,18-tetra cosatetraene | Bark | [31,33,34] | |
| 2,3,22,23-tetrahydroxy- 2,6,10,15,19,23-hexamethyl-6,10,14,18-tetracosatetraene | Bark and wood | [31,33,34,35] | |
| 3-Epi-oleanolic acid | Bark, root, and wood | [31,32,33,34,35] | |
| 3-Oxo-12β-hydroxy-oleanan-28,13β-olide | Bark and root | [33] | |
| Oleanolic acid | Bark, root, and wood | [31,32,33,34,35] | |
| Coumarins | |||
| Ekersenin | Bark | [33,135] | |
| 4,6-Dimethoxy-5-methylcoumarin | Bark | [33] | |
| 7-Hydroxy-6-methoxycoumarin | Wood | [35] | |
| Glycoflavonoids | |||
| kaempferol-3-O-β-D-glucopyranoside; quercetin-3-O-β-D-glucopyranoside | Leaf | [31] | |
| Phenolics | |||
| Atraric acid | Bark | [32] | |
| Sterols | |||
| β-sitosterol | Bark and wood | [32,35] | |
| β-sitosterol oleate; β-sitosterol palmate | Bark | [32] | |
| Protolimonoid | |||
| Ekebergin B | Bark | [33] | |
| Pregnane | |||
| (Z)-volkendousin | Bark | [33] | |
| Ekebergia pterophylla | Limonoids | ||
| Ekebergin | Seed | [110] | |
| Ekebergolactones and prieurianin | Seed | [110] | |
| EP1-EP6 | Seed | [111] | |
| Coumarins | |||
| Pterophyllins 1 and 2 | Bark | [108] | |
| Pterophyllins 3-5 | Wood | [108] | |
| Triterpenes | |||
| Lupeol | Leaf | [108] | |
| Oleanonic acid; β-amyrin; and β-amyrone | Bark | [108] | |
| Sterols | |||
| β-sitosterol | Bark | [108] | |
| β-sitosteryl acetate | Bark | [108] | |
| Phenolics | |||
| Atraric acid | Bark | [108] | |
| Entandrophragma caudatum | Limonoids | ||
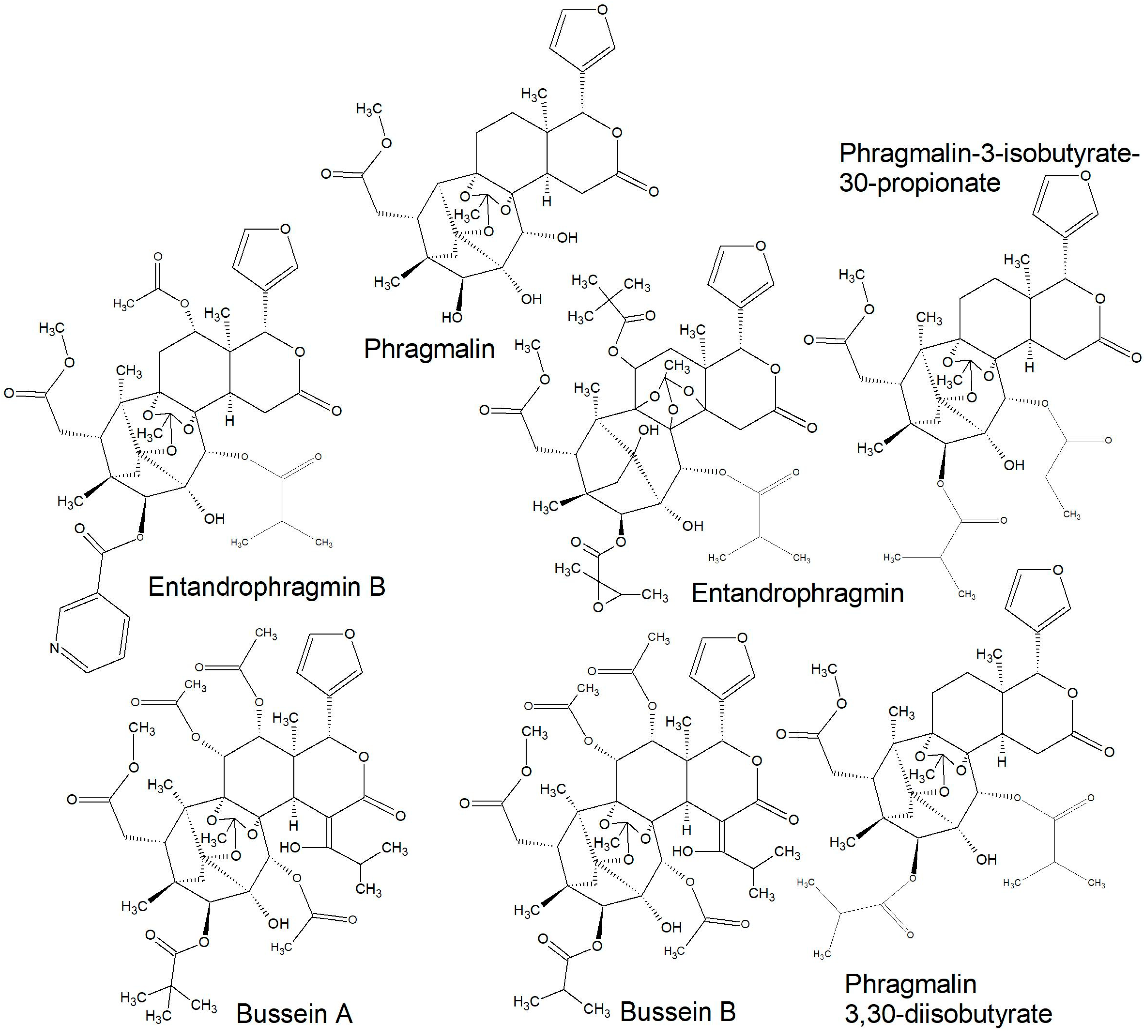
| Phragmalin; phragmalin 3,30-diisobutyrate; phragmalin 3-isobutyrate-30-propionate; Entandrophragmin B (12α-acetoxyphragmalin 3-nicotinate-30-isobutyrate) | Seed | [116] | |
| Bussein A and B; entandrophragmin | Wood | [113] | |
| Protolimonoids | |||
| 3α–turreanthin; melianone | Wood | [114] | |
| Nymania capensis | Limonoids | ||
| Nymania 1-4; Prieurianin | Bark and Wood | [112] | |
| Pseudobersama mossambicensis | Sterols | ||
| Ergosta-5,24(28)-diene-3β, 7α-diol; 24,28-epoxyergost-5-ene-3β, 7α-diol; and ergost-5-ene-3β,7 α,24,28-tetraol | Twig and leaf | [129] | |
| Trichilia dregeana | Limonoids | ||
| Dregeana-5; dregeanin; and 12-(2′-deacetyl)-dregeanin | Stem | [136] | |
| Dregeana 1-4; hispidin C | Seed | [117] | |
| Sterol | |||
| Cycloart-23-ene-3β,25-diol | Leaf | [130] | |
| Trichilia emetica | Limonoids | ||
| Trichirokin | Stem | [24] | |
| Rohituka | Stem | [24,118] | |
| Trichilin A; trichilin B; and 7-acetyltrichilin A | Stem | [137] | |
| Trichilin C; trichilin D; and trichilin G | Stem | [138] | |
| Trichilin F and trichilin G | Stem | [139] | |
| 1-acetyltrichilin; Tr-A; Tr-B; Tr-C | Stem | [140] | |
| Dregeana-4; rohituca-3; rohituca-5; rohituca-7; and Nymania-1 | Stem | [118] | |
| Sesquiterpenes | |||
| Kurubasch aldehyde | Leaf | [131] | |
| Triterpenes | |||
| Methyl-1(S),23(R)-diacetoxy-7(R),24,25-trihydroxy-20(S)-21,24-epoxy-3,4-seco-apotirucall-4(28), 14(15)-dien-3-oate | Stem | [118] | |
| Pregnane | |||
| 1-methoxy-pregnan-17(R)-1,4-dien-3,16-dione; 1-methoxy-pregnan-17(S)-1,4-dien-3,16-dione; 2,3-seco-pregnan-17(S)-2,3-dioic acid-16-oxo-dimethyl ester; 2,3,16-trihydroxy-5-pregnan-17(R)-20-yl acetate; 1-methoxy-androstan-1,4-dien-3,16-dione; 2,3-seco-androstan-2,3-dioic acid-16-oxo-dimethyl ester; 3-methoxycarbonyl-2,3-seco-androstan-3-oic acid-16-oxo-2,19-lactone; 2,3,16,20-tetrahydroxy-5-pregnane; 2,3-dihydroxypregnan-16-one | Root | [126] | |
| Phenolics | |||
| Benzoic acid; protocatechuic acid | Stem | [24] | |
| Coumarin | |||
| Scopoletin | Stem | [24] | |
| Sterols | |||
| Ergosta-5,24(28)-diene-3S,16S,20S-triol; β-sitosterol; stigmasterol; and β-sitosterol-3-O-β-D-glucopyranoside | Stem | [24] | |
| Turraea floribunda | Limonoids | ||
| Floribundin A (11β-acetoxy-3,7-diacetyl-4α-carbomethoxy- 12α-isobutyryloxy-28-nor-1-tigloyl-havanensin); | Root | [141] | |
| Floribundin B (28-nor-4α-carbomethoxy-11β-acetoxy-12α-(2-methylbutanoyloxy)-14,15-deoxyhavanensin-1,7-diacetate); Floribundin C (28-nor-4α-carbomethoxy-11β-hydroxy-12α-(2-methylbutanoyloxy)-14,15-deoxyhavanensin-1,7-diacetate); Floribundin D (2218-nor-4α-carbomethoxy-11β-acetoxy-12α-(2-methylbutanoyloxy)-14,15-deoxyhavanensin-1-acetate); Floribundin E (28-nor-4α-carbomethoxy-7-deoxy-7-oxo-11β-acetoxy-12α-(2-methylbutanoyloxy)-14,15-deoxyhavanensin-1-acetate) | Root | [142] | |
| Havanensinoids 2-4 | Root | [143] | |
| Floribundin F (1α,7α-12α -triacetoxy-4α -carbomethoxy-11β-(2-methylpropanoyloxy)-14β,15β -epoxyhavanensin) | Bark | [121] | |
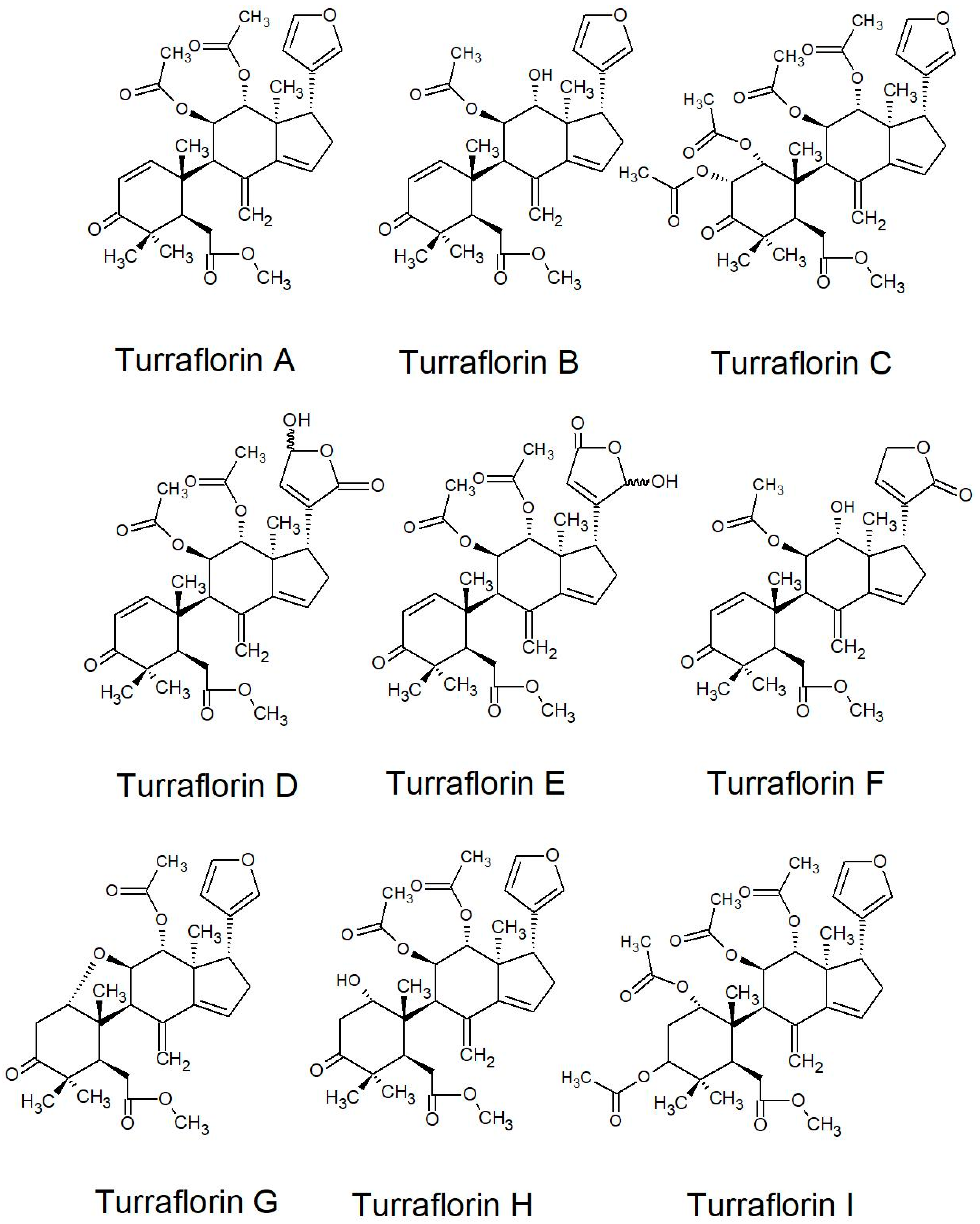
| Turraflorins A, B, and C | Seed | [144] | |
| Turraflorins A and B; turraflorins D-I | Seed | [112] | |
| 14,15-deoxytoonacilin | Seed | [145] | |
| Toonafolin A and B | Seed | [146] | |
| Sterols | |||
| Stigmasterol and sitosterol | Wood | [146] | |
| Turraea nilotica | Limonoids | ||
| Nilotin | Root | [119] | |
| Mzikonone; azadirone; 12α-acetoxy-7-deacetylazadirone; 1α,3α-diacety-7α-tigloyvilasinin | Root | [147] | |
| Protolimonoids | |||
| Niloticin; hispidol B; piscidinol A; toonapubesin F | Bark | [147] | |
| Niloticin; dihydroniloticin; and piscidinol | Bark | [120] | |
| Sterols | |||
| Sitosterol-3-O-β-D-glucopyranoside acetate; stigmasterol-3-O-βD-glucopyranoside acetate; and sitosterol-3-O-β-D-glucopyranoside | Leaf | [147] | |
| Turraea obtusifolia | Limonoids | ||
| Nymania-1 | Seed | [122,148] | |
| Prieurianin and rohitukin | Seed | [123] | |
| Prieurianin | Whole plant | [121] | |
| Protolimonoids | |||
| 7-deacetylglabretal-3-acetate | Wood | [149] | |
| Melianone; Turraeanthin | Seed | [148] | |
| Melianodiol; melianotriol; and 7,8-dihydroTurraeanthin 3-acetate | Wood | [146] | |
| Melianone; sepalin-F | Leaf | [146] |
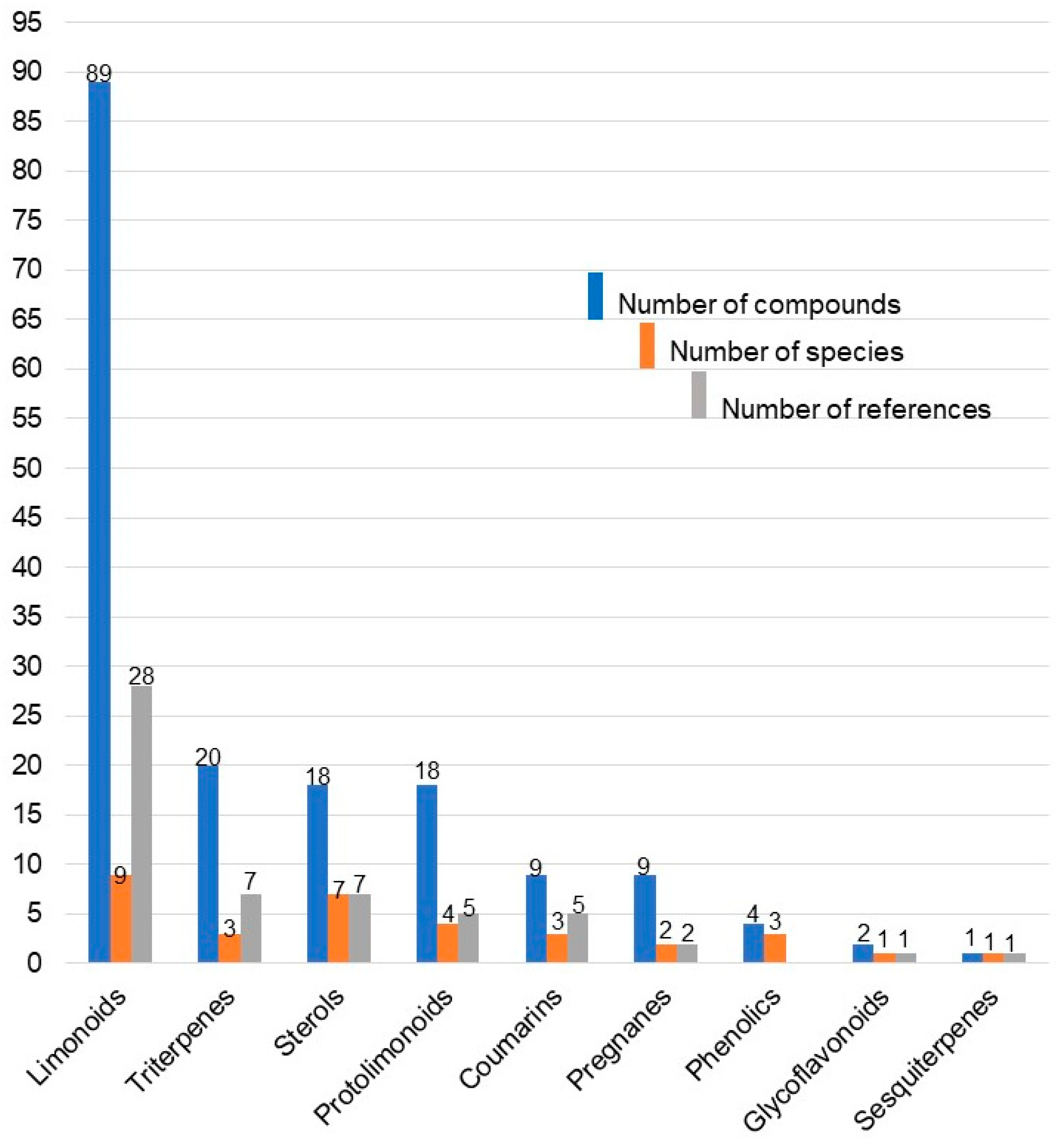
2.2.1. Class of Isolated Compounds and Parts Extracted
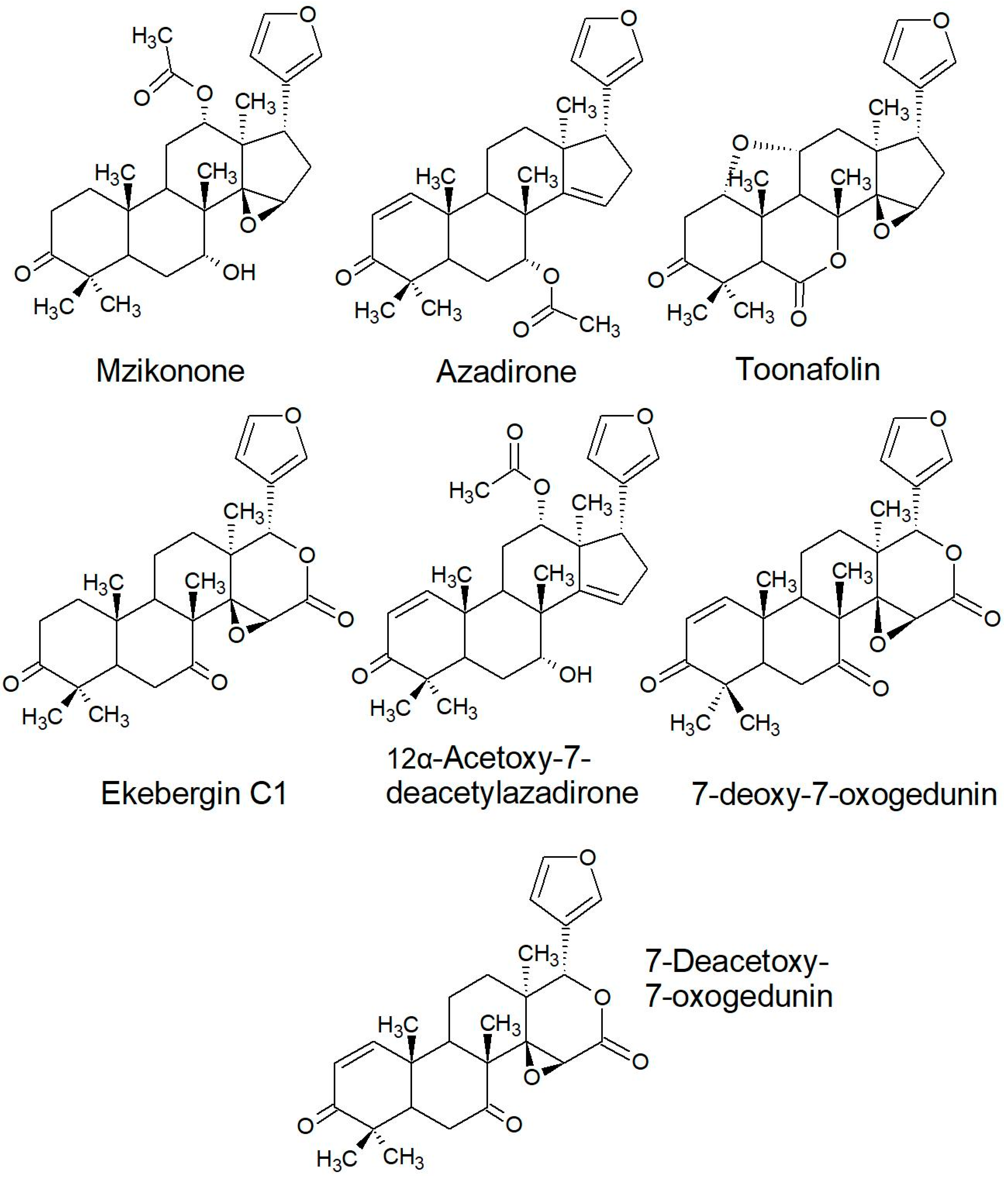
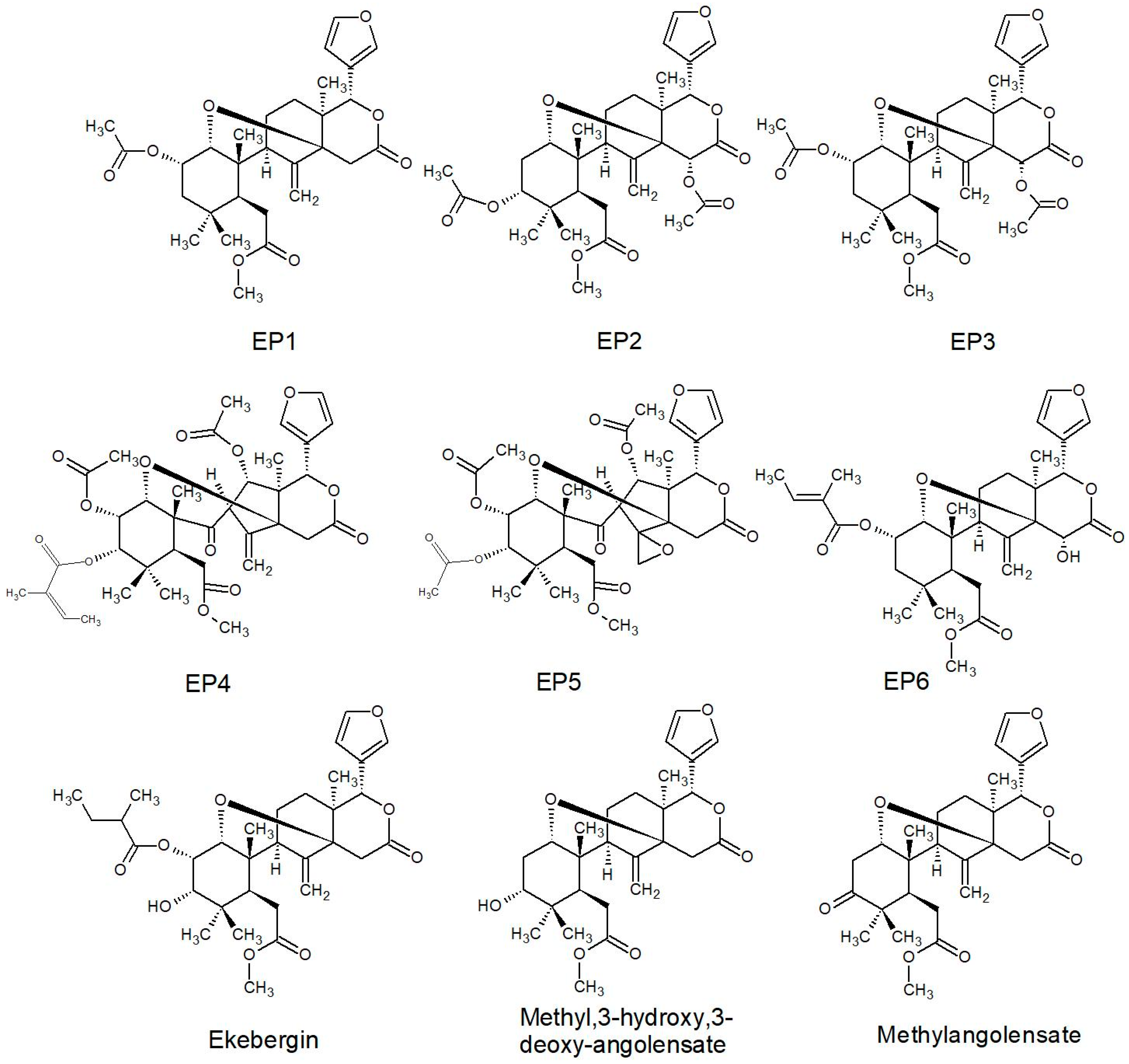
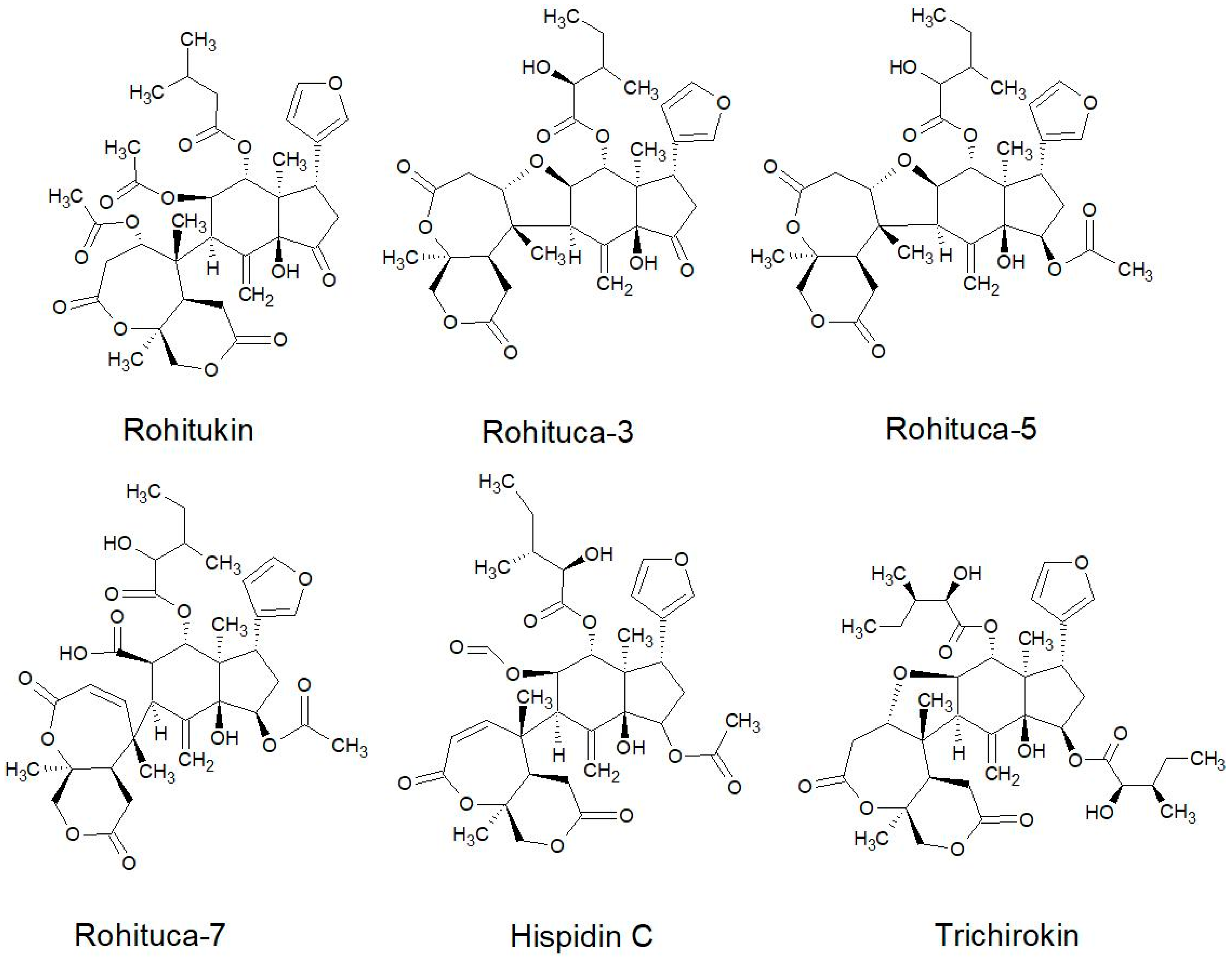
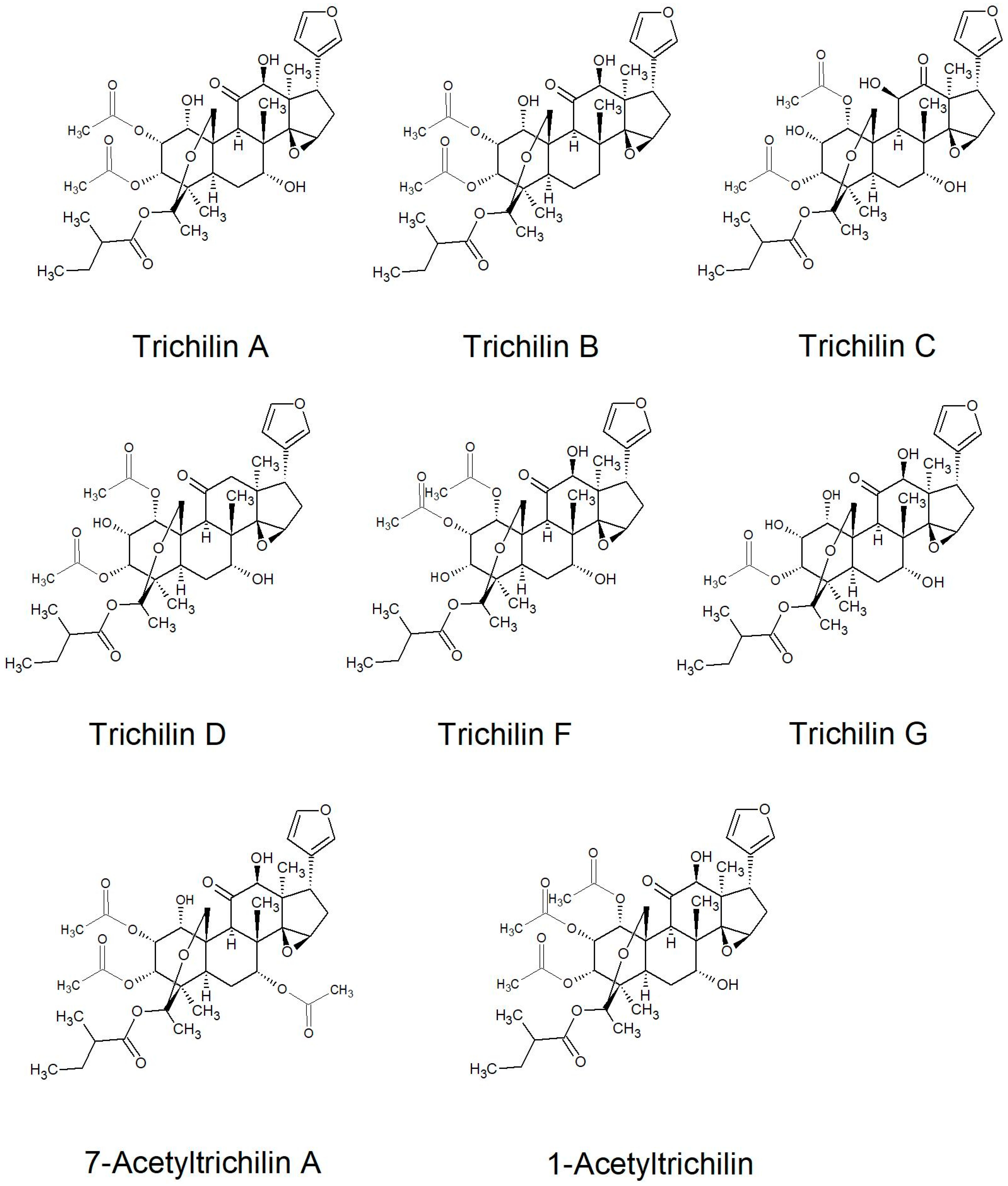
2.2.2. Structures of Some of the Isolated Compounds
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soejarto, D.D.; Fong, H.H.S.; Tan, G.T.; Zhang, H.J.; Ma, C.Y.; Franzblau, S.G.; Gyllenhaal, C.; Riley, M.C.; Kadushin, M.R.; Pezzuto, J.M. Ethnobotany/ethnopharmacology and mass bioprospecting: Issues on intellectual property and benefit-sharing. J. Ethnopharmacol. 2005, 100, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Grace, O.M.; Prendergast, H.D.V.; Jäger, A.K.; Van Staden, J.; Van Wyk, A.E. Bark medicines used in traditional healthcare in KwaZulu-Natal, South Africa: An inventory. S. Afr. J. Bot. 2003, 69, 301–363. [Google Scholar] [CrossRef]
- Williams, V.L.; Balkwill, K.; Witkowski, E.T.F. Unraveling the commercial market for medicinal plants and plant parts on the Witwatersrand. S. Afr. J. Bot. 2000, 54, 310–327. [Google Scholar] [CrossRef]
- Cunningham, A.B. Review of Ethnobotanical Literature from Eastern and Southern Africa. Available online: http://www.rbgkew.org.uk/peopleplants/regions/africa/aen1/review.htm (accessed on 23 August 2020).
- Teklehaymanot, T.; Giday, M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J. Ethnobiol. Ethnomed. 2007, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2009. [Google Scholar]
- Street, R.A.; Stirk, W.A.; Van Staden, J. South African traditional medicinal plant trade-challenges in regulating quality, safety and efficacy. J. Ethnopharmacol. 2008, 119, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2018. [Google Scholar]
- Chinyama, R.F. Biological Activities of Medicinal Plants Traditionally Used to Treat Septicaemia in the Eastern Cape, South Africa. Master’s Thesis, Nelson Mandella Metropolitan University, Port Elizabeth, South Africa, 2009. Unpublished. [Google Scholar]
- Cieśla, Ł.; Waksmundzka-Hajnos, M. Two-dimensional thin-layer chromatography in the analysis of secondary plant metabolites. J. Chromatogr. A 2009, 1216, 1035–1052. [Google Scholar] [CrossRef]
- Pappe, K.W.L. Florae Capensis Medicae Prodromus, Or, an Enumeration of South. African Plants Used as Remedies by the co.lonists of the Cape of Good Hope; W. Brittain: Cape Town, South Africa, 1868. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa Being an Account. of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal; E. & S. Livingstone: Ann Arbor, MI, USA, 1962. [Google Scholar]
- Hutchings, A.; Scott, A.H.; Lewis, G.; Cunningham, A. Zulu Medicinal Plants: An. Inventory; University Natal Press: Pietermaritzburg, South Africa, 1996. [Google Scholar]
- Mhlongo, L.S.; Van Wyk, B.-E. Zulu medicinal ethnobotany: New records from the Amandawe area of KwaZulu-Natal, South Africa. S. Afr. J. Bot. 2019, 122, 266–290. [Google Scholar] [CrossRef]
- Taylor, D.A.H. Recent developments in the biomimetic synthesis of limonoids. In Advances in Medicinal Phytochemistry; Barton, D., Ollis, W.D., Eds.; John Libbey and Co Ltd.: Blissfield, MI, USA, 1986; pp. 179–186. [Google Scholar]
- Paritala, V.; Chiruvella, K.K.; Thammineni, C.; Ghanta, R.G.; Mohammed, A. Phytochemicals and antimicrobial potentials of mahogany family. Rev. Bras. Farmacogn. 2015, 25, 61–83. [Google Scholar] [CrossRef]
- Champagne, D.E.; Koul, O.; Isman, M.B.; Scudder, G.G.E.; Towers, G.H.N. Biological activity of limonoids from the Rutales. Phytochemistry 1992, 31, 377–394. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Nakatani, M. Antifeeding activity of limonoids from Khaya senegalensis (Meliaceae). J. Appl. Entomol. 2003, 127, 236–239. [Google Scholar] [CrossRef]
- Greger, H.; Pacher, T.; Brem, B.; Bacher, M.; Hofer, O. Insecticidal flavaglines and other compounds from Fijian Aglaia species. Phytochemistry 2001, 57, 57–64. [Google Scholar] [CrossRef]
- Simmonds, M.S.J.; Stevenson, P.C.; Porter, E.A.; Veitch, N.C. Insect Antifeedant Activity of Three New Tetranortriterpenoids from Trichilia Pallida. J. Nat. Prod. 2001, 64, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Kim, M.-R.; Kim, J.-H.; Takeoka, G.R.; Kim, T.-W.; Park, B.-S. Antimalarial activity of anthothecol derived from Khaya anthotheca (Meliaceae). Phytomedicine 2008, 15, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, W.; Laphookhieo, S.; Koysomboon, S.; Chantrapromma, K. Antimalarial, antimycobacterial and cytotoxic limonoids from Chisocheton siamensis. Phytomedicine 2008, 15, 1130–1134. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Parel, B.; Coombes, P.H. The chemistry of the Meliaceae and Ptaeroxylaceae of southern and eastern Africa and Madagascar. Currr. Org. Chem. 2000, 4, 1011–1054. [Google Scholar] [CrossRef]
- Tsopgni, W.D.T.; Happi, G.M.; Stammler, H.-G.; Neumann, B.; Mbobda, A.S.W.; Kouam, S.F.; Frese, M.; Azébazé, A.G.B.; Lenta, B.N.; Sewald, N. Chemical constituents from the bark of the Cameroonian mahogany Trichilia emetica Vahl (Meliaceae). Phytochem. Lett. 2019, 33, 49–54. [Google Scholar] [CrossRef]
- Wang, X.N.; Yin, S.; Fan, C.Q.; Lin, L.P.; Ding, J.; Yue, J.M. Eight new limonoids from Turraea Pubescens. Tetrahedron 2007, 63, 8234–8241. [Google Scholar] [CrossRef]
- Tan, Q.-G.; Luo, X.-D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef] [PubMed]
- De Wet, H.; Ngubane, S.C. Traditional herbal remedies used by women in a rural community in northern Maputaland (South Africa) for the treatment of gynaecology and obstetric complaints. S. Afr. J. Bot. 2014, 94, 129–139. [Google Scholar] [CrossRef]
- Diarra, N.; van’t Klooster, C.; Togola, A.; Diallo, D.; Willcox, M.; de Jong, J. Ethnobotanical study of plants used against malaria in Sélingué subdistrict, Mali. J. Ethnopharmacol. 2015, 166, 352–360. [Google Scholar] [CrossRef]
- Mabona, U.; Van Vuuren, S.F. Southern African medicinal plants used to treat skin diseases. S. Afr. J. Bot. 2013, 87, 175–193. [Google Scholar] [CrossRef]
- Neuwinger, H.D. African Traditional Medicine: A Dictionary of Plant Use and Applications. With Supplement: SEARCH system for Diseases; Medpharm: Stuttgart, Germany, 2000. [Google Scholar]
- Irungu, B.N.; Orwa, J.A.; Gruhonjic, A.; Fitzpatrick, P.A.; Landberg, G.; Kimani, F.; Midiwo, J.; Erdélyi, M.; Yenesew, A. Constituents of the roots and leaves of Ekebergia capensis and their potential antiplasmodial and cytotoxic activities. Molecules 2014, 19, 14235–14246. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.A.; Mahomed, H.A.; Lourine, S. A comparison of extractives from the bark of Ekebergia capensis and Ekebergia senegalensis. S. Afr. J. Bot. 1997, 5, 259–260. [Google Scholar] [CrossRef]
- Murata, T.; Miyase, T.; Muregi, F.W.; Naoshima-Ishibashi, Y.; Umehara, K.; Warashina, T.; Kanou, S.; Mkoji, G.M.; Terada, M.; Ishih, A. Antiplasmodial triterpenoids from Ekebergia capensis. J. Nat. Prod. 2008, 71, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Moriyasu, M.; Ichimaru, M.; Tachibana, Y.; Kato, A.; Mathenge, S.G.; Nganga, J.N.; Juma, F.D. Acyclic triterpenoids from Ekebergia capensis. Phytochemistry 1996, 42, 803–807. [Google Scholar] [CrossRef]
- Sewram, V.; Raynor, M.W.; Mulholland, D.A.; Raidoo, D.M. The uterotonic activity of compounds isolated from the supercritical fluid extract of Ekebergia capensis. J. Pharm. Biomed. Anal. 2000, 24, 133–145. [Google Scholar] [CrossRef]
- Oyedeji-Amusa, M.O.; Van Vuuren, S.; Van Wyk, B.E. Antimicrobial activity and toxicity of extracts from the bark and leaves of South African indigenous Meliaceae against selected pathogens. S. Afr. J. Bot. 2020, 133, 83–90. [Google Scholar] [CrossRef]
- Kokwaro, J.O. Medicinal Plants of East Africa; University of Nairobi Press: Nairobi, Kenya, 2009. [Google Scholar]
- Komane, B.M.; Olivier, E.I.; Viljoen, A.M. Trichilia emetica (Meliaceae)—A review of traditional uses, biological activities and phytochemistry. Phytochem. Lett. 2011, 4, 1–9. [Google Scholar] [CrossRef]
- Rabe, T.; Van Staden, J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997, 56, 81–87. [Google Scholar] [CrossRef]
- Lovett, J.C.; Ruffo, C.K.; Gereau, R.E.; Taplin, J.R.D. Field Guide to the Moist Forest Trees of Tanzania; Society for Environmental Exploration: London, UK, 2006. [Google Scholar]
- Batten, A.; Bokelmann, H. Wild Flowers of the Eastern Cape Province; Books Of Africa: London, UK, 1966. [Google Scholar]
- Kerharo, J.; Bouquet, A. Plantes Médicinales Et Toxiques De La Côte d’Ivoire-Haute-Volta: Mission D’étude De La Pharmacopée Indigène En A.O.F.; Vigot: Paris, France, 1950. [Google Scholar]
- Mabogo, D.E.N. The Ethnobotany of the Vhavenda. Ph.D. Thesis, Pretoria University, Pretoria, South Africa, 1990. [Google Scholar]
- Opio, D.R.; Andama, E.; Kureh, G.T. Ethnobotanical survey of antimalarial plants in areas of: Abukamola, Angeta, Oculokori and Omarari of Alebtong district in Northern Uganda. Eur. J. Med. Plants 2017, 21, 1–14. [Google Scholar] [CrossRef]
- Koch, A.; Tamez, P.; Pezzuto, J.; Soejarto, D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J. Ethnopharmacol. 2005, 101, 95–99. [Google Scholar] [CrossRef]
- Kremnitz, W.A.; Knies, M.; Kremnitz, M. Kalahari: Aus Dem Pflanzenreich: Floristische Und Ethnobotanische Betrachtungen; Ambro Lacus: München, Germany, 1988. [Google Scholar]
- Duncan, A.C.; Jäger, A.K.; van Staden, J. Screening of Zulu medicinal plants for angiotensin converting enzyme (ACE) inhibitors. J. Ethnopharmacol. 1999, 68, 63–70. [Google Scholar] [CrossRef]
- Tefera, B.N.; Kim, Y.-D. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J. Ethnobiol. Ethnomed. 2019, 15, 25. [Google Scholar] [CrossRef]
- Jeruto, P.; Too, E.; Mwamburi, L.A.; Amuka, O. An inventory of medicinal plants used to treat gynaecological obstetric-urino-genital disorders in South Nandi Sub County in Kenya. J. Nat. Sci. Res. 2015, 5, 136–152. [Google Scholar]
- Tuasha, N.; Petros, B.; Asfaw, Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J. Ethnobiol. Ethnomed. 2018, 14, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J. NaturAfrica: The Herbalist Handbook: African Flora Medicinal Plants. Natural Healers Foundation; Thorolds Contemporary Africana, Law & General Booksellers (Frank R. Thorold Pty Ltd.): Sandton, South Africa, 1990. [Google Scholar]
- Van Wyk, A.E.; Van den Berg, E.; Coates Palgrave, M.; Jordaan, M. Dictionary of Names for Southern African Trees. Scientific Names of Indigenous Trees, Shrubs and Climbers with Common Names from 30 Languages; Briza Publications: Pretoria, South Africa, 2011. [Google Scholar]
- Arnold, H.J.; Gulumian, M. Pharmacopoeia of traditional medicine in Venda. J. Ethnopharmacol. 1984, 12, 35–74. [Google Scholar] [CrossRef]
- Bryant, A.T. Zulu Medicine and Medicine-Men; C. Struik Publishers: Cape Town, South Africa, 1966. [Google Scholar]
- Pooley, E. Complete Field Guide to Trees of Natal, Zululand & Transkei; Natal Flora Publications Trust: Durban, South Africa, 1993. [Google Scholar]
- Adjanohoun, É.J. Contribution Aux Études Ethnobotaniques et Floristiques en République Populaire du Bénin; Agence de Coopération Culturelle et Technique: Paris, France, 1989. [Google Scholar]
- Irvine, F.R. Woody Plants of Ghana; Oxford University Press: Ann Arbor, MI, USA, 1961. [Google Scholar]
- Kewessa, G.; Abebe, T.; Demessie, A. Indigenous knowledge on the use and management of medicinal trees and shrubs in Dale District, Sidama Zone, Southern Ethiopia. Ethnobot. Res. Appl. 2015, 14, 171–182. [Google Scholar] [CrossRef]
- Njoroge, G.N.; Bussmann, R.W. Herbal usage and informant consensus in ethnoveterinary management of cattle diseases among the Kikuyus (Central Kenya). J. Ethnopharmacol. 2006, 108, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, J. A preliminary check list of Zulu names of plants: With short notes. Bantu Stud. 1941, 15, 277–301. [Google Scholar] [CrossRef]
- Prozesky, E.A.; Meyer, J.J.M.; Louw, A.I. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected South African plants. J. Ethnopharmacol. 2001, 76, 239–245. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Ethnobotanical study of medicinal flora utilised by traditional healers in the management of sexually transmitted infections in Sesheke District, Western Province, Zambia. Rev. Bras. Farmacogn. 2016, 26, 268–274. [Google Scholar] [CrossRef]
- Archer, F.M. Ethnobotany of Namaqualand: The Richtersveld. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1994. [Google Scholar]
- von Koenen, E. Medicinal, Poisonous, and Edible Plants in Namibia; Klaus Hess Publishers (Klaus Heß Verlag): Göttingen, Germany, 2001. [Google Scholar]
- Maroyi, A. Trichilia dregeana Sond. In PROTA 14: Vegetable Oils/Oléagineux (CD-Rom); Van der Vossen, H.A.M., Mkamilo, G.S., Eds.; PROTA: Wageningen, The Netherlands, 2007. [Google Scholar]
- Desta, B. Ethiopian traditional herbal drugs. Part II: Antimicrobial activity of 63 medicinal plants. J. Ethnopharmacol. 1993, 39, 129–139. [Google Scholar] [CrossRef]
- Gelfland, M.; Mavi, S.; Drummond, R.B.; Ndemera, B. The Traditional Medical Practitioner in Zimbabwe: His Principles of Practice and Pharmacopoeia; Mambo Press: Gweru, Zimbabwe, 1985. [Google Scholar]
- Amusan, O.O.G.; Dlamini, P.S.; Msonthi, J.D.; Makhubu, L.P. Some herbal remedies from Manzini region of Swaziland. J. Ethnopharmacol. 2002, 79, 109–112. [Google Scholar] [CrossRef]
- Burkill, H.M. The Useful Plants of West. Tropical Africa (New Edition of Dalziel); Royal Botanic Gardens, Kew: Richmond, GB, USA, 1997. [Google Scholar]
- Assi, L.A.; Lazare, A.A. Plants Used in Traditional Medicine in West Africa; Editions Frison Roche: Paris, France, 1991. [Google Scholar]
- Mashungwa, G.N.; Mmolotsi, R.M. Trichilia emetica Vahl. In Plant Resources of Tropical Africa/Ressources Végétales De l’Afrique Tropicale; van der Vossen, H.A.M., Mkamilo, G.S., Eds.; PROTA: Wageningen, The Netherlands, 2007; Available online: http://www.prota4u.org/search.asp (accessed on 15 August 2020).
- Iwu, M.M. Handbook of African Medicinal Plants; CRC Press: New York, NY, USA, 2014. [Google Scholar]
- Adeniji, K.O.; Amusan, O.O.G.; Dlamini, P.S.; Enow-Orock, E.G.; Gamedze, S.T.; Gbile, Z.O.; Langa, A.D.; Makhubu, L.P.; Mahunnah, R.L.A.; Mshana, R.N. Traditional Medicine and Pharmacopoeia Contribution to Ethnobotanical and Floristic Studies in Swaziland; Science and Technology Research and Communications Organisation: Eswatini, Swaziland, 2000. [Google Scholar]
- Germano, M.P.; D’angelo, V.; Sanogo, R.; Catania, S.; Alma, R.; De Pasquale, R.; Bisignano, G. Hepatoprotective and antibacterial effects of extracts from Trichilia emetica Vahl.(Meliaceae). J. Ethnopharmacol. 2005, 96, 227–232. [Google Scholar] [CrossRef]
- Boon, R.; Pooley, E. Pooley’s Trees of Eastern South Africa: A Complete Guide; Flora and Fauna Publications Trust: Cambridge, MA, USA, 2010. [Google Scholar]
- Schmidt, E.; Lotter, M.; McCleland, W. Trees and Shrubs of Mpumalanga and Kruger National Park; Jacana Media: Johannesburg, South Africa, 2002. [Google Scholar]
- Weiss, E.A. Some indigenous plants used domestically by East African coastal fishermen. Econ. Bot. 1979, 33, 35–51. [Google Scholar] [CrossRef]
- Corrigan, B.M.; Van Wyk, B.-E.; Geldenhuys, C.J.; Jardine, J.M. Ethnobotanical plant uses in the KwaNibela Peninsula, St Lucia, South Africa. S. Afr. J. Bot. 2011, 77, 346–359. [Google Scholar] [CrossRef]
- Sanogo, R. Medicinal plants traditionally used in Mali for dysmenorrhea. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 90–96. [Google Scholar] [CrossRef]
- Oliver, B. Nigeria’s useful plants. Part 11. Medicinal plants. Niger. Field 1960, 25, 46–48. [Google Scholar]
- Chhabra, S.C.; Mahunnah, R.L.A.; Mshiu, E.N. Plants used in traditional medicine in Eastern Tanzania. VI. Angiosperms (Sapotaceae to Zingiberaceae). J. Ethnopharmacol. 1993, 39, 83–103. [Google Scholar] [CrossRef]
- Palmer, E.; Pitman, N. Trees of Southern Africa: Covering All Known Indigenous Species in the Republic of South Africa, South-West. Africa, Botswana, Lesotho & Swaziland; A. A. Balkema: Ann Arbor, MI, USA, 1972; Volumes 1–2. [Google Scholar]
- Bhat, R.B.; Jacobs, T.V. Traditional herbal medicine in Transkei. J. Ethnopharmacol. 1995, 48, 7–12. [Google Scholar] [CrossRef]
- Mathias, M.E. Some medicinal plants of the Hehe (Southern highlands province, Tanzania). Taxon 1982, 31, 488–494. [Google Scholar] [CrossRef]
- Chinemana, F.; Drummond, R.B.; Mavi, S.; De Zoysa, I. Indigenous plant remedies in Zimbabwe. J. Ethnopharmacol. 1985, 14, 159–172. [Google Scholar] [CrossRef]
- Madikizela, B.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Ethnopharmacological study of plants from Pondoland used against diarrhoea. J. Ethnopharmacol. 2012, 141, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Luseba, D.; Tshisikhawe, M.P. Medicinal plants used in the treatment of livestock diseases in Vhembe region, Limpopo province, South Africa. J. Med. Plants Res. 2013, 7, 593–601. [Google Scholar]
- Bussmann, R.W.; Sharon, D. Traditional medicinal plant use in Northern Peru: Tracking two thousand years of healing culture. J. Ethnobiol. Ethnomed. 2006, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.H.; Prentice, C.A.; Hawker, L.C.; Snyman, E.E.; Tomalin, M.; Crouch, N.R.; Pottas-Bircher, C. Medicinal and Magical Plants of Southern Africa: An Annotated Checklist; South African National Biodiversity Institute (SANBI Publishing): Pretoria, South Africa, 2002; Volume 13. [Google Scholar]
- Williams, V.L.; Balkwill, K.; Witkowski, E.T.F. A lexicon of plants traded in the Witwatersrand umuthi shops, South Africa. Bothalia 2001, 31, 71–98. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Nepal, B.K.; Kshhetri, H.B.; Rai, S.K.; Bussmann, R.W. Ethnomedicine in Himalaya: A case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J. Ethnobiol. Ethnomed. 2006, 2, 27. [Google Scholar] [CrossRef]
- Semenya, S.S.; Potgieter, M.J. Ethnobotanical survey of medicinal plants used by Bapedi traditional healers to treat erectile dysfunction in the Limpopo Province, South Africa. J. Med. Plants Res. 2013, 7, 349–357. [Google Scholar]
- Yineger, H.; Kelbessa, E.; Bekele, T.; Lulekal, E. Ethnoveterinary medicinal plants at Bale Mountains National Park, Ethiopia. J. Ethnopharmacol. 2007, 112, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.D. Trials in bad taste. Nature 1994, 372, 410–411. [Google Scholar] [CrossRef]
- Sheldon, J.W.; Balick, M.J.; Laird, S.A.; Milne, G.M. Medicinal plants: Can utilization and conservation coexist? Adv. Econ. Bot. 1997, 12, 1–104. [Google Scholar]
- Ahmad, M.; Sultana, S.; Fazl-i-Hadi, S.; Ben Hadda, T.; Rashid, S.; Zafar, M.; Khan, M.A.; Khan, M.P.Z.; Yaseen, G. An Ethnobotanical study of Medicinal Plants in high mountainous region of Chail valley (District Swat-Pakistan). J. Ethnobiol. Ethnomed. 2014, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Amjad, M.S.; Ahmad, K.; Altaf, M.; Umair, M.; Abbasi, A.M. Ethnomedicinal knowledge of the rural communities of Dhirkot, Azad Jammu and Kashmir, Pakistan. J. Ethnobiol. Ethnomed. 2019, 15, 45. [Google Scholar] [CrossRef]
- Ojha, S.N.; Tiwari, D.; Anand, A.; Sundriyal, R.C. Ethnomedicinal knowledge of a marginal hill community of Central Himalaya: Diversity, usage pattern, and conservation concerns. J. Ethnobiol. Ethnomed. 2020, 16, 29. [Google Scholar] [CrossRef]
- Tugume, P.; Kakudidi, E.K.; Buyinza, M.; Namaalwa, J.; Kamatenesi, M.; Mucunguzi, P.; Kalema, J. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J. Ethnobiol. Ethnomed. 2016, 12, 5. [Google Scholar] [CrossRef]
- Umair, M.; Altaf, M.; Abbasi, A.M. An ethnobotanical survey of indigenous medicinal plants in Hafizabad district, Punjab-Pakistan. PLoS ONE 2017, 12, e0177912. [Google Scholar] [CrossRef]
- Magwede, K.; Van Wyk, B.-E.; Van Wyk, A.E. An inventory of Vhavenda useful plants. S. Afr. J. Bot. 2019, 122, 57–89. [Google Scholar] [CrossRef]
- Lemmens, R.H.M.J. Dalbergia trichocarpa Baker. In Plant Resources of Tropical Africa/Ressources Végétales De l’Afrique Tropicale; van der Vossen, H.A.M., Mkamilo, G.S., Eds.; PROTA: Wageningen, The Netherlands, 2008; Available online: http://www.prota4u.org/search.asp:PROTA (accessed on 15 August 2020).
- Von Breitenbach, F.W. The Indigenous Trees of Southern Africa; Government Printer: Pretoria, South Africa, 1965; Volume 4. [Google Scholar]
- Jamieson, J.S. Examination of the bark and seed oil of Trichilia emetica. S. Afr. J. Sci. 1916, 53, 496–498. [Google Scholar]
- Coates Palgrave, K. Trees of Southern Africa; Struik Publishers: Cape Town, South Africa, 2002. [Google Scholar]
- Saka, J.D.K.; Msonthi, J.D. Nutritional value of edible fruits of indigenous wild trees in Malawi. For. Ecol. Manag. 1994, 64, 245–248. [Google Scholar] [CrossRef]
- Pakia, M. African Traditional Plant Knowledge Today: An. Ethnobotanical Study of the Digo at the Kenya Coast; Lit Verlag Berlin: Münster, Germany, 2006. [Google Scholar]
- Mulholland, D.A.; Lourine, S.E.; Taylor, D.A.H.; Dean, F.M. Coumarins from Ekebergia pterophylla. Phytochemistry 1998, 47, 1641–1644. [Google Scholar] [CrossRef]
- Taylor, D.A.H. Ekebergin, a limonoid extractive from Ekebergia capensis. Phytochemistry 1981, 20, 2263–2265. [Google Scholar] [CrossRef]
- Kehrli, A.R.H.; Taylor, D.A.H.; Niven, M. Limonoids from Ekebergia pterophylla seed. Phytochemistry 1990, 29, 153–159. [Google Scholar] [CrossRef]
- Taylor, A.R.H.; Taylor, D.A.H. Limonoids from Ekebergia pterophylla. Phytochemistry 1984, 23, 2676–2677. [Google Scholar] [CrossRef]
- MacLachlan, L.K.; Taylor, D.A.H. Limonoids from Nymania capensis. Phytochemistry 1982, 21, 1701–1703. [Google Scholar] [CrossRef]
- Adesida, G.A.; Taylor, D.A.H. The chemistry of the genus Entandrophragma. Phytochemistry 1967, 6, 1429–1433. [Google Scholar] [CrossRef]
- Bevan, C.W.L.; Ekong, D.E.U.; Halsall, T.G.; Toft, P. West African timbers. Part XX. The structure of turraeanthin, an oxygenated tetracyclic triterpene monoacetate. J. Chem. Soc. C Org. 1967, 820–828. [Google Scholar] [CrossRef]
- Mouthé Happi, G.; Tchaleu Ngadjui, B.; Green, I.R.; Fogué Kouam, S. Phytochemistry and pharmacology of the genus Entandrophragma over the 50 years from 1967 to 2018: A ‘golden’ overview. J. Pharm. Pharmacol. 2018, 70, 1431–1460. [Google Scholar] [CrossRef]
- Ansell, S.M.; Taylor, D.A.H. Limonoids from the seed of Entandrophragma caudatum. Phytochemistry 1988, 27, 1218–1220. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Taylor, D.A.H. Limonoids from the seed of the natal mahogany, Trichilia dregeana. Phytochemistry 1980, 19, 2421–2425. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L.; da S. Bolzani, V.; Dagne, E.; Hofmann, G.A.; Johnson, R.K.; McCabe, F.L.; Mattern, M.R.; Kingston, D.G.I. Limonoids showing selective toxicity to DNA repair-deficient yeast and other constituents of Trichilia Emetica. J. Nat. Prod. 1998, 61, 179–184. [Google Scholar] [CrossRef]
- Bentley, M.D.; Adul, G.O.; Alford, A.R.; Huang, F.-Y.; Gelbaum, L.; Hassanali, A. An insect antifeedant limonoid from Turraea Nilotica. J. Nat. Prod. 1995, 58, 748–750. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Taylor, D.A.H. Protolimonoids from Turraea nilotica. Phytochemistry 1988, 27, 1220–1221. [Google Scholar] [CrossRef]
- Akinniyi, J.A.; Connolly, J.D.; Mulholland, D.A.; Rycroft, D.S.; Taylor, D.A.H. Limonoid extractives from Turraea floribunda and T. obtusifolia. Phytochemistry 1986, 25, 2187–2189. [Google Scholar] [CrossRef]
- Fraser, L.-A.; Mulholland, D.A.; Taylor, D.A.H. The chemotaxonomic significance of the limonoid, nymania-1, in Turraea obtusifolia. S. Afr. J. Bot. 1995, 61, 281–282. [Google Scholar] [CrossRef][Green Version]
- Sarker, S.D.; Savchenko, T.; Whiting, P.; Šik, V.; Dinan, L. Two limonoids from Turraea obtusifolia (Meliaceae), prieurianin and rohitukin, antagonise 20-hydroxyecdysone action in a Drosophila cell line. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 1997, 35, 211–217. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The chemistry and pharmacology of citrus limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef]
- Obbo, C.J.D.; Makanga, B.; Mulholland, D.A.; Coombes, P.H.; Brun, R. Antiprotozoal activity of Khaya anthotheca, (Welv.) C.D.C. a plant used by chimpanzees for self medication. J. Ethnopharmacol. 2013, 147, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Malafronte, N.; Sanogo, R.; Vassallo, A.; De Tommasi, N.; Bifulco, G.; Dal Piaz, F. Androstanes and pregnanes from Trichilia emetica ssp. suberosa JJ de Wilde. Phytochemistry 2013, 96, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Goodwin, B.; Wilson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Jones, G.L. From Petri Dish to Patient: Bioavailability Estimation and Mechanism of Action for Antimicrobial and Immunomodulatory Natural Products. Front. Microbiol. 2019, 10, 2470. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L.; Samaranayake, G.; Kingston, D.G.I.; Hoffmann, G.; Johnson, R.K. Bioactive Ergost-5-ene-3β/, 7α-diol Derivatives from Pseudobersama mossambicensis. J. Nat. Prod. 1992, 55, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Eldeen, I.M.S.; Van Heerden, F.R.; Van Staden, J. Biological activities of cycloart-23-ene-3, 25-diol isolated from the leaves of Trichilia dregeana. S. Afr. J. Bot. 2007, 73, 366–371. [Google Scholar] [CrossRef]
- Maminata, T.; Lin, Z.; Ming, C.; Olsen, C.E.; Nacoulma, O.; Guissou, P.I.; Quedrago, Q.J.; Guigemde, R.T.; Brogger, S.C. Cytotoxic kurubasch aldehyde from Trichilia emetica. Nat. Prod. Res. 2007, 21, 13–17. [Google Scholar]
- Mulholland, D.A.; Lourine, S.E. Limonoids from Ekebergia capensis. Phytochemistry 1998, 47, 1357–1361. [Google Scholar] [CrossRef]
- Pergomet, J.L.; Di Liberto, M.G.; Derita, M.G.; Bracca, A.B.J.; Kaufman, T.S. Activity of the pterophyllins 2 and 4 against postharvest fruit pathogenic fungi. Comparison with a synthetic analog and related intermediates. Fitoterapia 2018, 125, 98–105. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Brodie, P.J.; Callmander, M.W.; Randrianaivo, R.; Rakotonandrasana, S.; Rasamison, V.E.; Rakotobe, E.; Kingston, D.G.I. Astrotricoumarin, an antiproliferative 4′-hydroxy-2′,3′-dihydroprenylated methylcoumarin from an Astrotrichilia sp. from the Madagascar Dry Forest. Nat. Prod. Commun. 2011, 6, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Okogun, J.I.; Enyenihi, V.U.; Ekong, D.E.U. Spectral studies on coumarins and the determination of the constitution of ekersenin by total synthesis. Tetrahedron 1978, 34, 1221–1224. [Google Scholar] [CrossRef]
- Connolly, J.D.; Okorie, D.A.; de Wit, L.D.; Taylor, D.A.H. Structure of dregeanin and rohitukin, limonoids from the subfamily Melioideae of the family Meliaceae. An unusually high absorption frequency for a six-membered lactone ring. J. Chem. Soc. Chem. Commun. 1976, 22, 909–910. [Google Scholar] [CrossRef]
- Nakatani, M.; James, J.C.; Nakanishi, K. Isolation and structures of trichilins, antifeedants against the Southern army worm. J. Am. Chem. Soc. 1981, 103, 1228–1230. [Google Scholar] [CrossRef]
- Nakatani, M.; Iwashita, T.; Naoki, H.; Hase, T. Structure of a limonoid antifeedant from Trichilia roka. Phytochemistry 1985, 24, 195–196. [Google Scholar] [CrossRef]
- Nakatani, M.; Nakanishi, K. Structures of insect antifeeding limonoids, trichilins F and G, from Trichilia roka. Heterocycles 1993, 36, 725–731. [Google Scholar] [CrossRef]
- Nakatani, M.; Iwashita, T.; Mizukawa, K.; Hase, T. Trichilinin, a new hexacyclic limonoid from Trichilia roka. Heterocycles 1987, 26, 43–46. [Google Scholar] [CrossRef]
- Torto, B.; Hassanali, A.; Nyandat, E.; Bentley, M.D. A limonoid from Turraea floribunda. Phytochemistry 1996, 42, 1235–1237. [Google Scholar] [CrossRef]
- Torto, B.; Bentley, M.D.; Cole, B.J.W.; Hassanali, A.; Huang, F.-Y.; Gelbaum, L.; Vanderveer, D.G. Limonoids from Turraea floribunda. Phytochemistry 1995, 40, 239–243. [Google Scholar] [CrossRef]
- Ndung’u, M.W.; Kaoneka, B.; Hassanali, A.; Lwande, W.; Hooper, A.M.; Tayman, F.; Zerbe, O.; Torto, B. New mosquito larvicidal tetranortriterpenoids from Turraea wakefieldii and Turraea floribunda. J. Agric. Food Chem. 2004, 52, 5027–5031. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.A.; Mulholland, D.A.; Nair, J.J. Limonoids from the seed of Turraea floribunda. Phytochemistry 1994, 35, 455–458. [Google Scholar] [CrossRef]
- Mullholland, D.A.; Fraser, L.; Nair, J.J. A new limonoid from the seed of Turraea floribunda. Planta Med. 1992, 58, 712. [Google Scholar] [CrossRef]
- Akerman, L.A. Extractives from the Meliaceae and Icacinaceae. Ph.D. Thesis, University of Natal, Durban, South Africa, 1990. [Google Scholar]
- Irungu, B.N.; Adipo, N.; Orwa, J.A.; Kimani, F.; Heydenreich, M.; Midiwo, J.O.; Björemark, P.M.; Håkansson, M.; Yenesew, A.; Erdélyi, M. Antiplasmodial and cytotoxic activities of the constituents of Turraea robusta and Turraea nilotica. J. Ethnopharmacol. 2015, 174, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, N. Extractives from the Meliaceae and Annonaceae. Ph.D. Thesis, University of Natal, Durban, South Africa, 1997. [Google Scholar]
- Mulholland, D.A.; Monkhe, T.V. Two glabretal-type triterpenoids from the heartwood of Aglaia ferruginaea. Phytochemistry 1993, 34, 579–580. [Google Scholar] [CrossRef]
- Moffett, R. Sesotho Plant and Animal Names and Plants Used by the Basotho; Sun Media: Stellenbosch, South Africa, 2010. [Google Scholar]


| Taxa | Traditional Use | References | ||
|---|---|---|---|---|
| Functional Use | Part Use | Method of Use | ||
| Ekebergia capensis | Tanning, furniture, brush, broom heads and handle, beams, planks, wagon, ship and boat building, light construction, poles and tool handles, light flooring, joinery, interior trim, vehicle bodies, sporting goods, toys, novelties | Wood | Wood is used in furniture industry | [12,40] |
| Firewood and charcoal production | Wood | Wood is used in cooking | [12,40] | |
| Animal feed | Fruit and leaf | Birds feed on fleshy parts of the fruit, while the leaf is used as a fodder | [12,40] | |
| Shades and wind break | Whole plant | It serves as an ornamental tree planted in gardens and roadsides for shades, as well as for wind break and soil conservation | [40,43] | |
| Edible caterpillar | Whole plant | Caterpillars are gathered from the plant and prepared as food | [43,101] | |
| Ekebergia pterophylla | Garden tree | Whole plant | Whole plant is used as an ornamental garden tree as well as a bonsai | [76] |
| Entandrophragma caudatum | Furniture, cabinet making, carving canoes | Wood | Wood is light and durable, hence high demand by the furniture industry | [8] |
| Tanning | Wood | Wood sap is used for tanning | [43] | |
| Toy | Fruit | Fruit pericarp is used to make ‘zwihwilili’ with which children like to play | [43,101] | |
| Shade | Whole plant | Whole plant is favoured for shade | [43] | |
| Animal feed | Seed | Seed is eaten by antelope | [75] | |
| Nymania capensis | Forage | Leaf | Source of forage for goats | [89] |
| Pseudobersa mamossam bicensis | Buildings and charcoal | Wood | Wood is used in making poles in local house buildings, as well as firewood and making charcoal | [102] |
| Trichilia dregeana | Furniture and carving | Wood | Wood is used for carving, repair of ships, and for making household furniture | [8,55,75] |
| Craftwork | Wood | NR | [101] | |
| Food condiments | Fruit | Fruit content is cooked with vegetables, fruit pulp is eaten as sour milk, while the oil made from the fruit pulp is used in cooking vegetables and other relishes | [43,65] | |
| Polish | Fruit and seed | Oil made from seed and fruit pulp is used to polish women’s clothes made from leather, furniture, and other household implements made from wood | [43] | |
| Soap and cosmetics | Seed | Oil from seeds is used to make soap, cosmetics, and candles | [43,75,103] | |
| Forage or fertilizer | Seed | Residue from seeds after oil extraction is used as a fertilizer or animal feed | [65] | |
| Fruit | Fruits are eaten by birds and bats | [75] | ||
| Drink | Seed aril | Seed aril is pounded and made into a sauce or sweet drink | [65] | |
| Shade | Whole plant | Whole plant is used to create shady avenue and as an ornamental tree | [65,75] | |
| Fishing | Seed | Bright-coloured seed is used as bait for fishing | [65] | |
| Trichilia emetica | Shade | Whole plant | Whole plant is used for shade | [43,75] |
| Soap and cosmetics | Seed | Oil from seed is used to make soap, body ointment, and candle | [8,71,76,103,104] | |
| Fertilizer | Seed | Residue from seed after oil extraction is used as a fertilizer | [71] | |
| Kola | Seed aril | Seed aril is eaten as a substitute for kola | [71] | |
| Forage | Leaf | Leaf is eaten by cattle and goats | [71] | |
| Fruit | Fruit is eaten by baboons, antelopes, and monkeys | [75] | ||
| Seed | Seed is eaten by birds | [75,76] | ||
| Carvings | Wood | Wood is used in carving furniture, household implements, musical instruments, canoes, and as chew-stick | [8,71,75,76,105] | |
| Dyeing | Bark | Pinkish or light red-brown dye is obtained from the beaten boiled bark | [4,71,75] | |
| Cooking | Seed aril | Seed aril is soaked and cooked together with squash or sweet potatoes | [8,75,101] | |
| Multivitamin | Seed | Juice is made from the seeds and other edible plants to control malnutrition | [106] | |
| Beverage | Fruit | NR | [101] | |
| Turraea floribunda | Traps | Wood | Wood is used for making traps | [107] |
| Ornamental | Whole plant | Tree is used as an ornamental plant in humid, frost-free subtropical and tropical gardens, and as a greenhouse plant in temperate countries | [76,82] | |
| Turraea nilotica | Handicrafts | Stem/branches | Stems/branches are used for handicrafts and domestics purposes | [107] |
| Firewood | Branches | Branches are sorted for firewood | [107] | |
| Turraea obtusifolia | Ornamental | Whole plant | Plant is used as an ornamental container plant in landscape designs, as well as an attractive garden plant | |
| Insect repellent | Leaf | Insect repellent | [87,101] | |
| Series | Limonoids |
|---|---|
| Ketone and Azadirone | Mzikonone; Azadirone; Toonafolin; Ekebergin C1; 12α-Acetoxy-7-deacetylazadirone; 7-deoxy-7-oxogedunin; 7-deacetoxy-7-oxogedunin |
| Capensolactones and Nymania | Capensolactone 1-3; Nymania 1-4 |
| Dregeanin | Dregeanin; Dregeana 1-5; 12-(2′-deacetyl)-dregeanin |
| EP * | EP1-6; Ekebergin; Methyl,3-hydroxy,3-deoxy-angolensate; Methlyangolensate |
| Floribundin ** and Havanen | Floribundin A-F; Havanensinoid 2-4 |
| Orphan | 1α,3α-diacetyl-7α-tigloylvilasinin; 14,15-deoxytoonacilin; Nilotin; Prieurianin |
| Phragmalin and Phragmin | Entandrophragmin B; Phragmalin 3-isobutyrate-30-propionate; Entandrophragmin; Phragmalin 3,30-diisobutyrate; Phgramalin; Bussein A and B |
| Rohituka | Rohituca 3, 5 and 7; Hispidin C; Trichirokin |
| Swietenolide/Ekebergin | Swietenolide; Proceranolide; Ekebergin C2-C3; Mexicanolide |
| Trichilin | Trichilin A-G; 7-Acetyltrichilin A; 1-Acetyltrichilin |
| Turraflorin | Turraflorin A-I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyedeji-Amusa, M.O.; Sadgrove, N.J.; Van Wyk, B.-E. The Ethnobotany and Chemistry of South African Meliaceae: A Review. Plants 2021, 10, 1796. https://doi.org/10.3390/plants10091796
Oyedeji-Amusa MO, Sadgrove NJ, Van Wyk B-E. The Ethnobotany and Chemistry of South African Meliaceae: A Review. Plants. 2021; 10(9):1796. https://doi.org/10.3390/plants10091796
Chicago/Turabian StyleOyedeji-Amusa, Mariam Oyefunke, Nicholas J. Sadgrove, and Ben-Erik Van Wyk. 2021. "The Ethnobotany and Chemistry of South African Meliaceae: A Review" Plants 10, no. 9: 1796. https://doi.org/10.3390/plants10091796
APA StyleOyedeji-Amusa, M. O., Sadgrove, N. J., & Van Wyk, B.-E. (2021). The Ethnobotany and Chemistry of South African Meliaceae: A Review. Plants, 10(9), 1796. https://doi.org/10.3390/plants10091796