1. Introduction
Drought inhibits plant growth, thereby affecting agricultural production, and its global severity is great concern [
1]. With the continuous intensification of the greenhouse effect, the frequency of drought is increasing [
2]. Moreover, drought causes morphological and physiological changes, and even causes death [
3]. Many parts of China, a large agricultural country, are currently suffering from drought, which not only restricts the development of agriculture and forestry, but also has adverse effects on the ecological environment [
4]. Therefore, research on the drought resistance of plants has become a top priority.
Plants can respond differently to drought through complex regulatory mechanisms, which can be summarized as follows: (1) They can enhance water harvesting to escape drought, such as by developing roots, closing stomata, or lowering leaf temperature [
5]. (2) They can respond to drought by reducing transpiration, through processes such as premature leaf shedding or accelerated leaf senescence [
6]. (3) They can move prematurely from the vegetative to reproductive stage to speed up maturation and seed production before drought induces mortality [
7]. (4) They can enhance their drought resistance by improving osmotic regulation and leaf tissue elasticity [
8]. (5) The capacity for antioxidant metabolism can be increased to maintain normal growth under severe drought [
9] and (6) Genetic mutation and genetic evolution of physiological and biochemical characteristics more adapted to drought can occur in long-term drought environments [
10].
Some studies have found that morphological, physiological, molecular and other parameters can characterize the drought resistance of plants. The roots of the plant serve as sensing organs, and the leaves serve as expression organs, and together they coordinate internal or external defense mechanisms to resist drought [
11,
12,
13,
14]. To date, photosynthesis and water use efficiency have been widely used in the detection of drought tolerance in plants [
15]. From the perspective of leaf morphology, leaf water loss and curling are important indicators of plant water status and drought resistance ability, which are typical responses of plants to water deficit [
16]. Under drought conditions, stomatal conductance is the dominant factor restricting CO
2 entry into cells, and stomatal development is greatly affected by environmental factors [
17]. The accumulation of photosynthetic products in leaves is the basis of plant growth and yield. Stabilizing the photosynthetic production efficiency of crop populations is the goal of crop responses to drought [
18].
Many indicators represent the ability of plants to resist drought. The morphological indexes of plants mainly describe the root system and leaf morphology. Leaves are the most sensitive organs of plants, and a change in leaf shape, such as the degree of wilting, can indicate the adaptability of plants to drought [
19]. Physiological indexes including photosynthesis can also represent the drought resistance of plants, are widely used in the detection of drought tolerance in plants and can directly reflect the degree of water deficit [
20]. Different levels of drought inhibit photosynthesis, and plants resist drought by closing their stomata, in turn reducing transpiration and CO
2 loss to the external environment [
21]. Cells, tissues and organs will be destroyed under drought conditions, which directly affects the accumulation of dry matter and is directly reflected in the yield [
22]. Therefore, generally, old leaves of plants will fall off to resist drought. At the molecular level, drought resistance can be enhanced through the expression of specific genes, such as genes that promote the production of abscisic acid [
23,
24]. The traditional method for diagnosing drought resistance of plants is mainly based on the changes of plant morphological and physiological indexes [
25]. Although the above indicators show that plants have their own advantages in terms of drought resistance, plant morphology must change gradually and remain static for a long time to reflect drought resistance [
26]. Similar to photosynthetic indexes, morphological indexes cannot completely represent water use. Growth indicators need to be monitored for a long time, and sometimes they will be affected by light, leading to errors in the measured data [
27,
28]. Monitoring by molecular biology methods is relatively cumbersome and destructive. However, electrophysiological indexes can be used to monitor water use rapidly, online, accurately, in a timely manner, accumulatively, and nondestructively. Plants are organisms that adjust themselves to complex and changeable environments in the face of various stresses [
29]. Plant electrophysiological signals are the most rapid and effective conduction signals between plant organs and tissues [
30]. Most higher plants rely on plant electrophysiological signals to regulate their physiological functions [
31]. Studies in recent years have shown that plant electrophysiological signal transducers are ubiquitous in higher plants and are the first response of plants to various stimuli in the external environment, which is reflected in plant growth, material metabolism and other aspects [
32]. However, plant drought resistance can be analyzed by the initial response of photosynthesis, and electrophysiology has a strong correlation with this, so it can quickly reflect the photosynthetic characteristics of plants. Therefore, the study on changes of electrophysiological parameters is the basis for the realization of rapid detection of plant drought resistance.
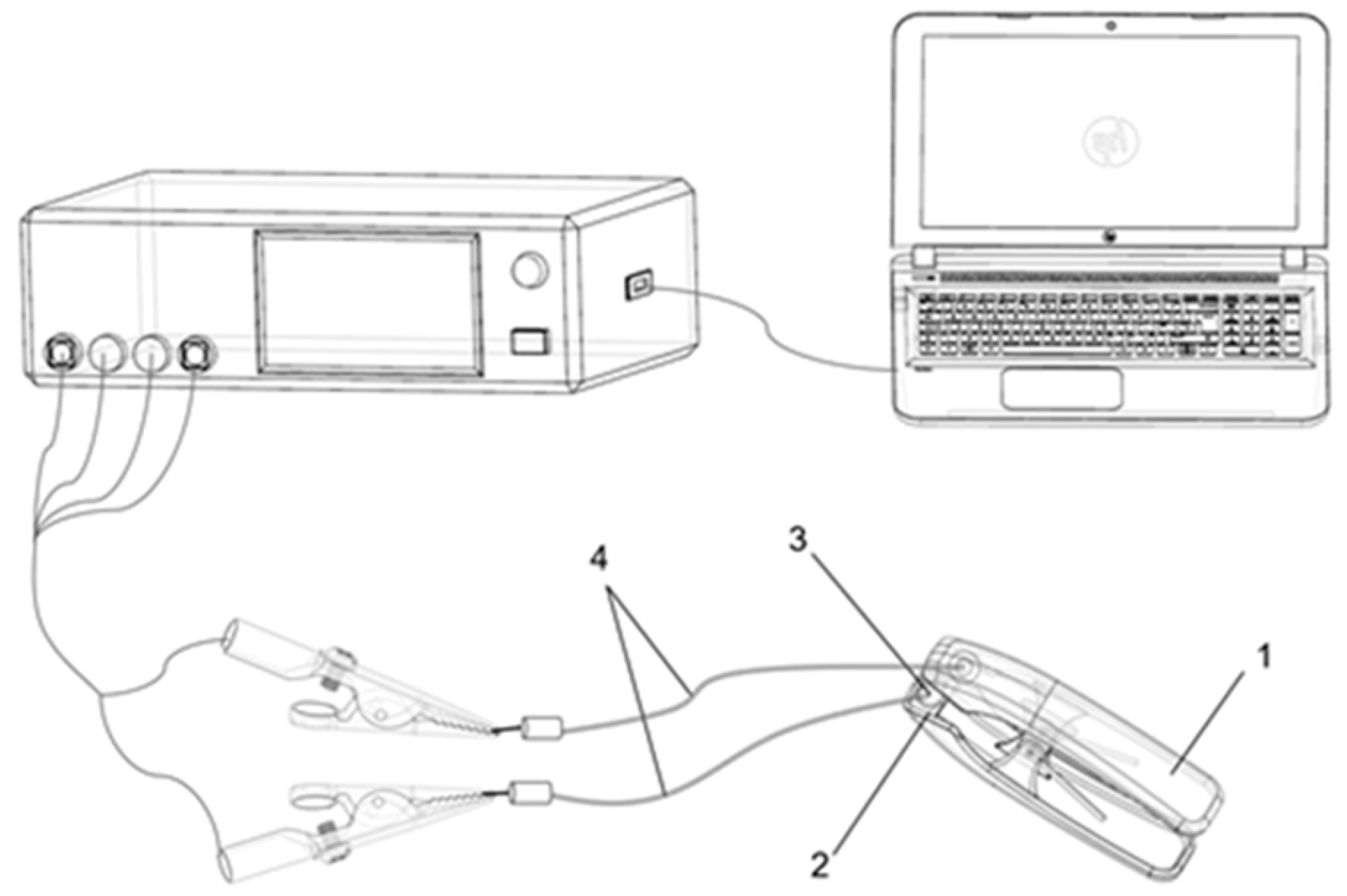
Plant electrophysiological signals can quickly represent the internal growth conditions of plants and changes in the external environment, and thus have important physiological significance [
31]. The capacitance (
Cp), leaf resistance (R), leaf impedance (Z) are the most common electrical parameters to evaluate the varying physiological status of plants [
33]. When plant leaves are stimulated by the external environment, the cell membrane permeability changes immediately. Therefore, the electrolyte concentration in leaf cells inside and outside changes, leading to changes in
Cp, R, and Z in the leaves, and the water inside the cells also changes. The passive electrical properties of plant leaves (
Cp, R, and Z) can reflect plant water metabolism [
34]. Many scholars have explored the response of plant electrophysiology through potential signals, resulting in vague biological significance. Studies of plant electrophysiology were most common until the late 1950s [
35,
36,
37]. Electrical, hydrodynamic, and chemical signal transduction parameters also appear to be regulated when mimosa leaves are subjected to injury stress [
38]. The transmission of electrical signals has an effect on photosynthesis and elicits a specific response in leaf photosynthesis [
39,
40,
41]. Studies have shown a significant correlation between the impedance value of leaves and the relative water content, which can be used to accurately evaluate the water content of plants [
42].
In recent years, plant electrical signals have been widely used in the field of plant stress resistance physiology in China. Li and Mao used impedance and capacitance to monitor the moisture content of tomato leaves in real time [
43]. This research group has also successfully used electrical indicators to detect water status. Zhang et al. defined leaf tension and developed a model of the relationships between leaf tension, tissue water potential and physiological capacitance, which could be successfully applied to rapidly acquire water requirement information in
Brassica napus during drought [
44]. Xing et al. used the relationship between leaf tension and dry weight biomass to rapidly predict the rehydration time points of leaves under different drought treatments through online monitoring of electrophysiological parameters [
45]. We also established a coupling model of leaf clamping force and physiological impedance and found that these parameters play an important role in photosynthesis and water use efficiency. In addition, the use of physiological impedance to monitor water status in plants is nondestructive and can determine the water demand of plants in a timely manner [
46]. Since plant cell volume is closely related to physiological capacitance, impedance, resistance and capacitive resistance, electrophysiological characteristics are increasingly used in the diagnosis of plant water status [
29,
44]. Recently, many cutting-edge technologies have been applied to plant electrophysiology, and an increasing number of studies have been conducted on gene expression related to electrical signals [
47].
Mulberry (
Morus alba L.,
M. alba) and paper mulberry (
Broussonetia papyrifera (L.) Vent.,
B. papyrifera), perennial tree species belonging to Moraceae, are characterized by a higher growth rate and greater adaptability to adverse environments than other species in this family [
48]. They are widely valuable natural resources. The species
M. alba., which is native to China and is now cultivated throughout the world [
49], is also an economically important perennial tree, as it serves as the sole food source of the domesticated silkworm. In addition, it adds value through the production of edible fruit, timber and several pharmaceutically important chemicals [
50].
B. papyrifera, which is a fast-growing tree mainly distributed in Asian and Pacific countries, can be utilized in many applications. It is well known for its bark fibers, which are used to manufacture high-quality paper, cloth, ropes, animal feed, bioenergy feedstock and traditional Chinese medicines [
51,
52]. Based on previous studies [
53], the two plants have great differences in drought resistance and different mechanisms of water use efficiency. This paper can be used as a comparison material to further study the different characteristics of their electrophysiological responses to drought.
The main contents of this paper are as follows: First, we study the responses of the electrophysiological, photosynthetic and growth parameters of the two plant species to different water treatments. Second, we study the relationships between the electrophysiological, photosynthetic and growth parameters. Finally, we study the different mechanisms underlying the response to drought between the two plant species. Based on these studies, this paper mainly aims to explore the feasibility of using electrophysiological parameters to characterize plant water metabolism, and to clarify the response mechanism of two plant species to drought by combining electrophysiological, photosynthetic and growth parameters.
3. Discussion
Almost all life activities in plants involve water. Plant physiological information can be represented by the leaf physical parameters which can be directly measured and ensure the accuracy and improve the convenience of drought resistance research of plants [
54]. The determination of electrophysiological indexes provides a more convenient method for the study of water status in cells. Electrical signals of plants are considered to be the fastest response of plants to environmental changes and are closely related to the life activities of plants such as material and energy metabolism, development, stress resistance and signal transduction [
27,
30]. When plant cells lose water and contract, resulting in smaller cell size, the cell expansion pressure must change. Capacitance is related to the degree of expansion and contraction of plant cells [
55]. At the same time, the change of water content will inevitably lead to the change of membrane permeability and ion concentration inside and outside the cell, which is closely related to the resistance and impedance [
56]. When plants are subjected to different degrees of environmental stress, the water status, ion concentration and membrane permeability of cells will change immediately, and the electrical signals of plants will also change. Therefore, the electrophysiological indexes have the theoretical basis to reflect the water status of plant leaves [
57,
58]. In this paper, the response of
M. alba to drought can be characterized by photosynthetic parameters rather than growth parameters. Drought can cause a decrease in plant cell turgor pressure, which leads to a decrease in components of the plant growth rate, such as plant height, stem thickness and leaf area [
59]. Drought has a strong impact on plant growth and development and causes death in severe cases. In addition, the response of
B. papyrifera to drought can be characterized by growth parameters rather than photosynthetic parameters. Drought will affect plant photosynthesis, leaf photosynthesis is an important physiological activity of plants, and the accumulated products of photosynthesis play an important role in plant growth and development [
60]. However, electrophysiological parameters can characterize the response of
M. alba and
B. papyrifera to drought. The degree of drought in plants is related to the electrophysiological characteristics of leaves. Therefore, electrophysiological parameters can be used to quickly and nondestructively determine the water status of leaves.
To characterize the effect of drought, the growth curves were analyzed initially using the logistic model, the adequacy of which is discussed. Under different water conditions, the growth models of different plant species are also different [
61]. The sigmoidal model measures the growth of plants over time and thus can be used to analyze the temporal growth trend of plants [
62]. In this study, a 4-parameter logistic model was used to fit the growth parameters of
M. alba and
B. papyrifera, such as X
0, a, GR
50, DT
log, DT
s, and R
2, under different drought levels, and the physiological parameters plant height, leaf length, leaf width and leaf area were analyzed. The results obtained by the 4-parameter logistic model in this study revealed obvious patterns in the growth parameters of
B. papyrifera under different drought levels. Changes in the plant height, leaf length, leaf width and leaf area parameters of
B. papyrifera showed obvious regularity with the decrease in soil moisture. These results reveal specific life phenomena in plants; that is,
B. papyrifera grows fast and has a long-life cycle, and its growth is affected by drought at any treatment time. In contrast, the life cycle of
M. alba is shorter, its photosynthesis is lower, and its overall growth is slower. Moreover, the logarithmic growth period of
M. alba passed quickly, so drought had no effect on its leaf growth.
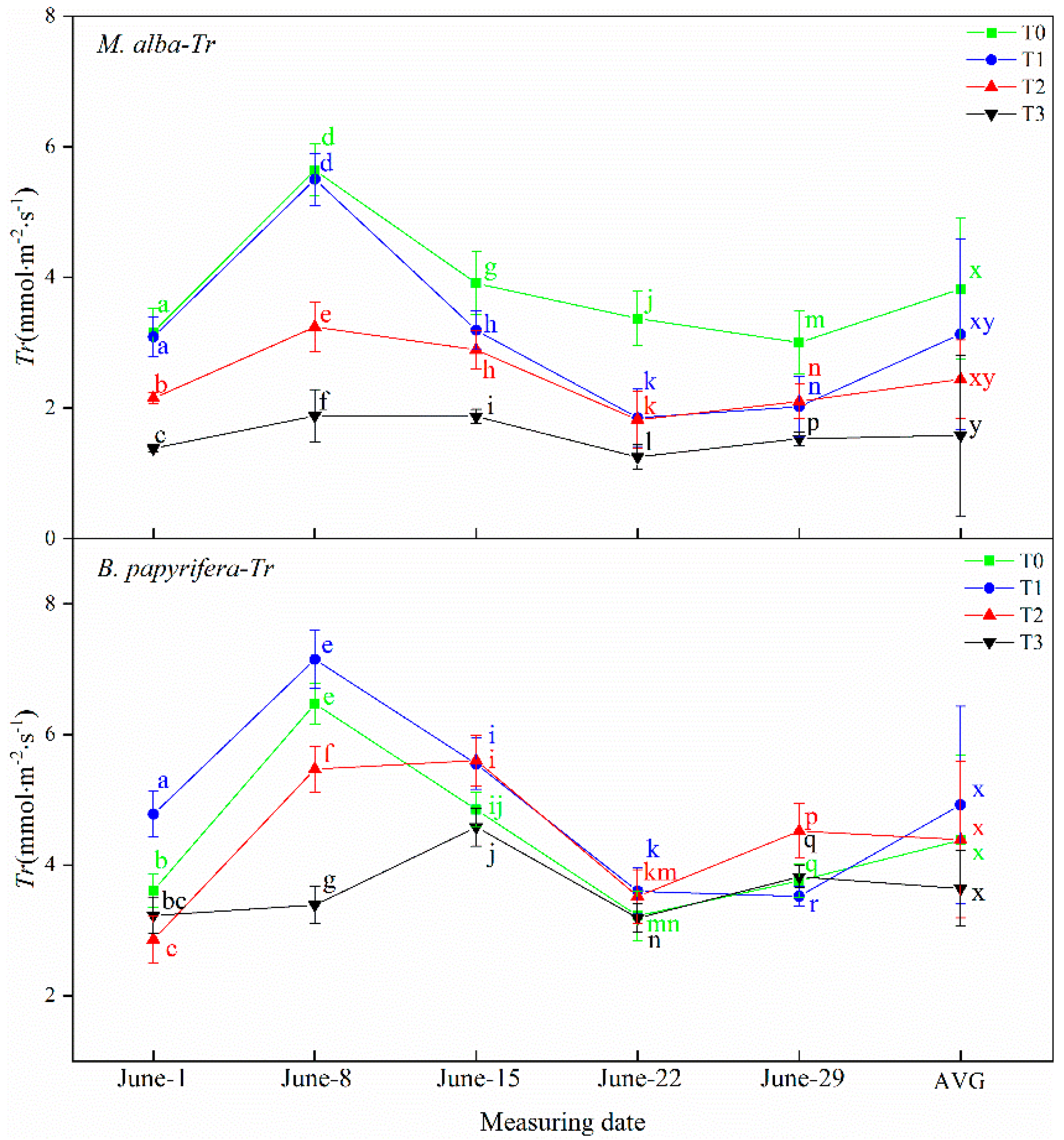
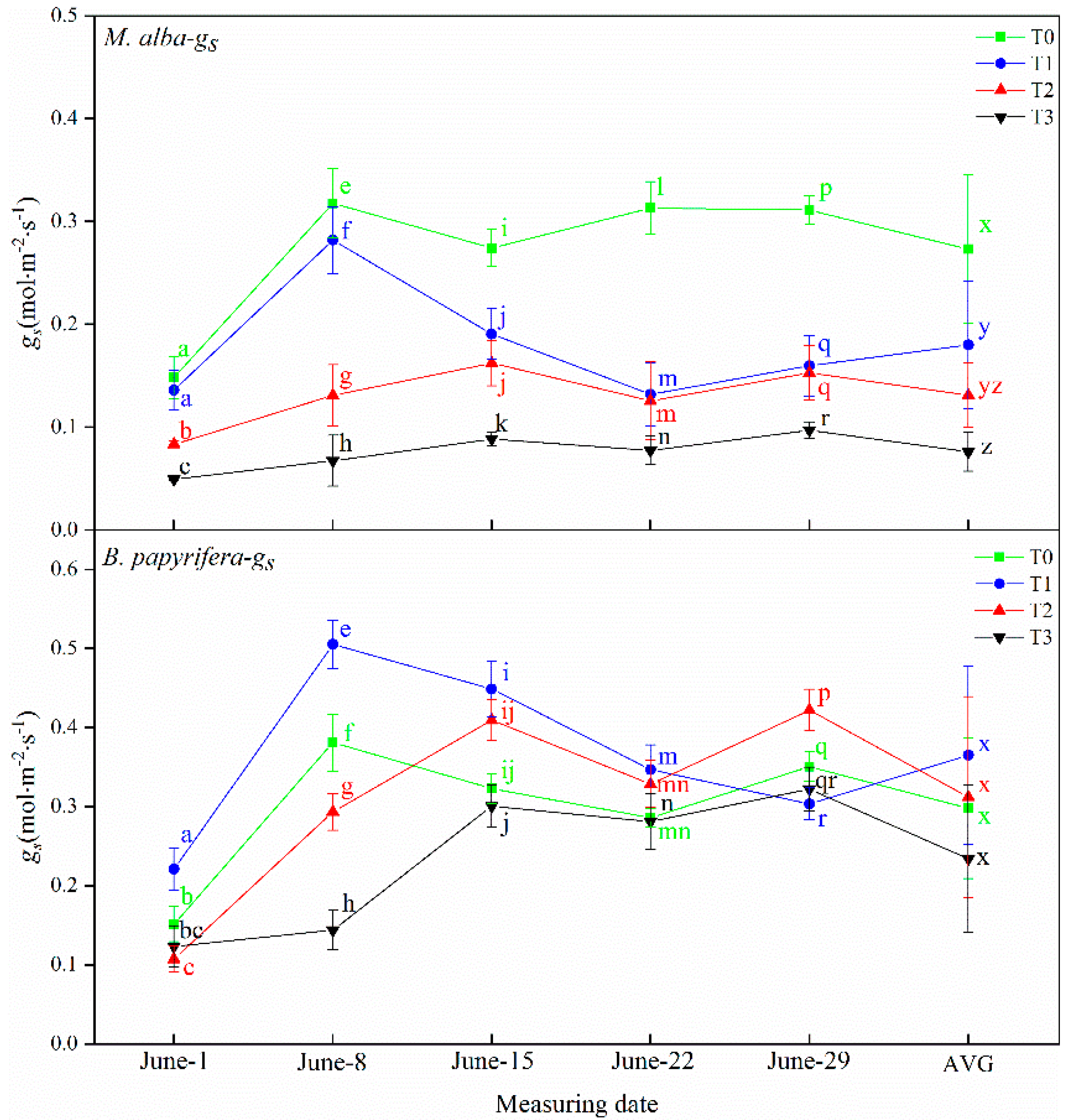
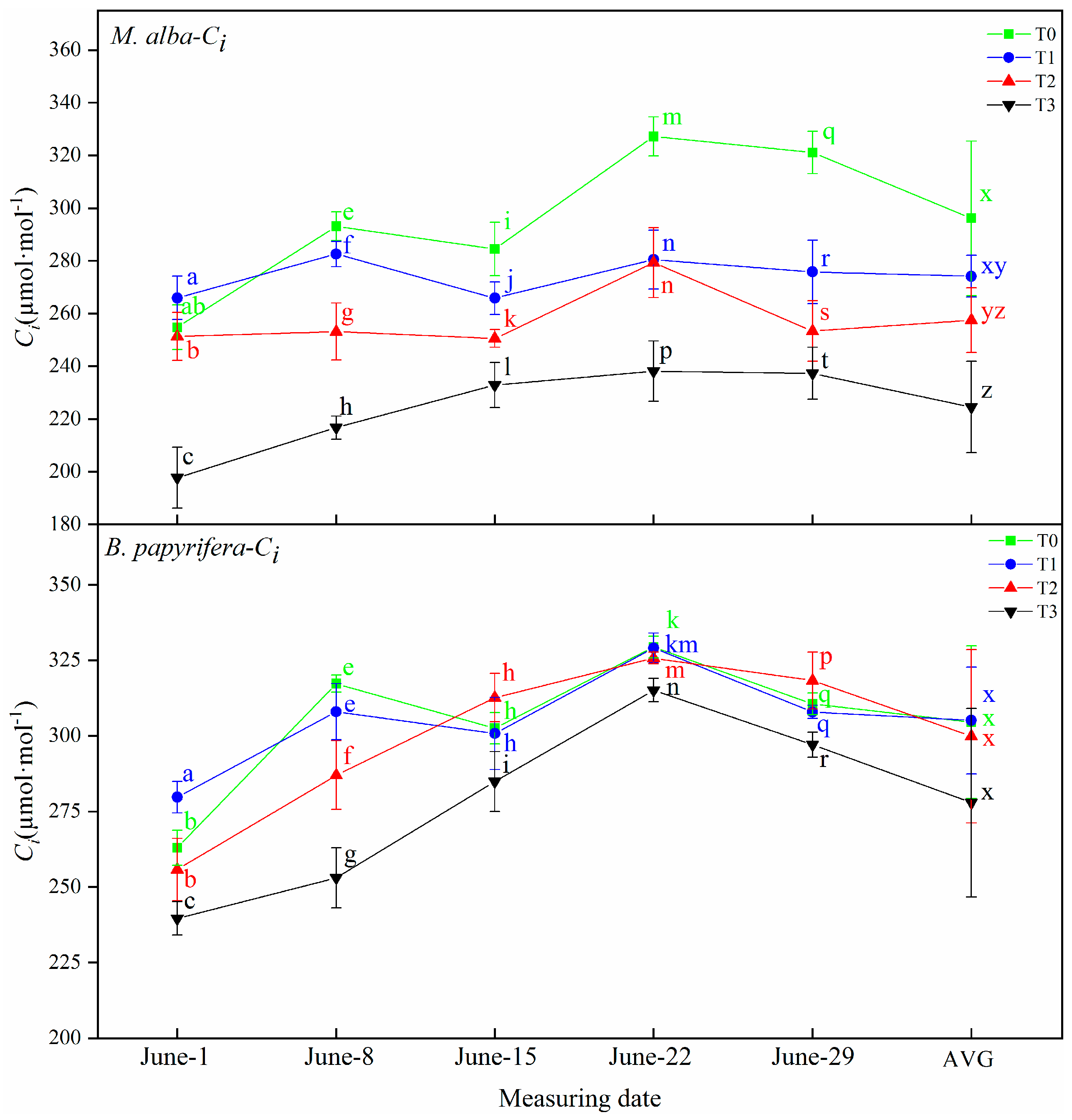
Drought resistance in plants can be determined through their photosynthetic and physiological characteristics. The main driving force for the growth of plants is photosynthesis, which supplies the energy and carbon required for the biosynthesis of organic compounds necessary for development [
63]. Increasing photosynthetic energy use efficiency and enhanced photosynthetic capacity may be the most successful mechanisms for alien species invasion and for adaptability to adverse environments [
64]. In response to water deficiency, stomatal closure occurs, which reduces the net photosynthetic rate and transpiration rate and decreases the intercellular CO
2 concentration [
65]. In this study, the leaf area of
B. papyrifera is larger than
M. alba, and the photosynthesis is stronger than
M. alba, the photosynthetic parameters of
M. alba responded to soil moisture at different time under different drought treatments, different from
M. alba, the photosynthetic parameters of
B. papyrifera only responded to soil water in the early stage, but did not respond to soil water in the later stage, indicating that
B. papyrifera has strong adaptability. Although the area of
M. alba leaves was small, the photosynthetic parameters exhibited obvious differences under different drought levels, indicating that
M. alba has a more obvious response than
B. papyrifera to water.
B. papyrifera continues to grow fast and can be regarded as a “pool”. It needs to consume energy for growth. The carbohydrates produced via photosynthesis cannot be consumed through growth, thus inhibiting photosynthesis. Therefore, photosynthetic parameters cannot characterize the response of
B. papyrifera to drought.
M. alba leaves are small and grow slowly, so the pool is small, which affects photosynthesis. Photosynthesis does not affect the growth of individual leaves. The substance produced via photosynthesis are used to promote the growth of new leaves.
M. alba leaves are abundant. Therefore, photosynthetic characters can characterize the response of
M. alba to drought.
Plants differ in their physiological capacitance response to water stress. Burdon-Sanderson noted the electrical phenomena that accompany irritation of the leaf of
Dionaea muscipula [
66,
67], prompting studies of plant electrical signals. Previous studies showed that direct changes in plant electrophysiological information such as Z, R and
Cp could directly reflect changes in plant water [
68,
69]. Jamaludin et al. study showed that there was a significant correlation between the impedance value of leaves and relative water content, and the water content of plants could be accurately evaluated by measuring the impedance [
42]. Yang et al. studied the relationship between plants and air heat in the greenhouse and found that the growth status of plants could be determined by measuring the changes of plant leaf temperature, transpiration and surface electrophysiology of plants [
70]. By studying wheat, Briggs found that there was a logarithmic relationship between the water content of wheat and its physiological resistance, and the water content of wheat could be calculated indirectly by measuring the physiological resistance [
71]. Ksenzhek et al. studied the physiological resistance of the main vein of maize in different directions, and the results showed that the physiological resistance of the leaf gradually decreased with the wilting of the leaf [
72]. In addition, the water status data of plant leaves obtained by photosynthesis-transpiration and water potential did not directly reflect the intracellular water status [
73,
74]. Through the study of scholars, it is found that the monitoring and analysis of the changes of electrical signals and physiological and biochemical aspects of plants under drought stress, and the test of the response of electrophysiological characteristics of plants to drought stress, so as to achieve a comprehensive, timely and accurate characterization of the drought resistance of crops under drought stress. In this study, the leaf electrophysiological parameters (
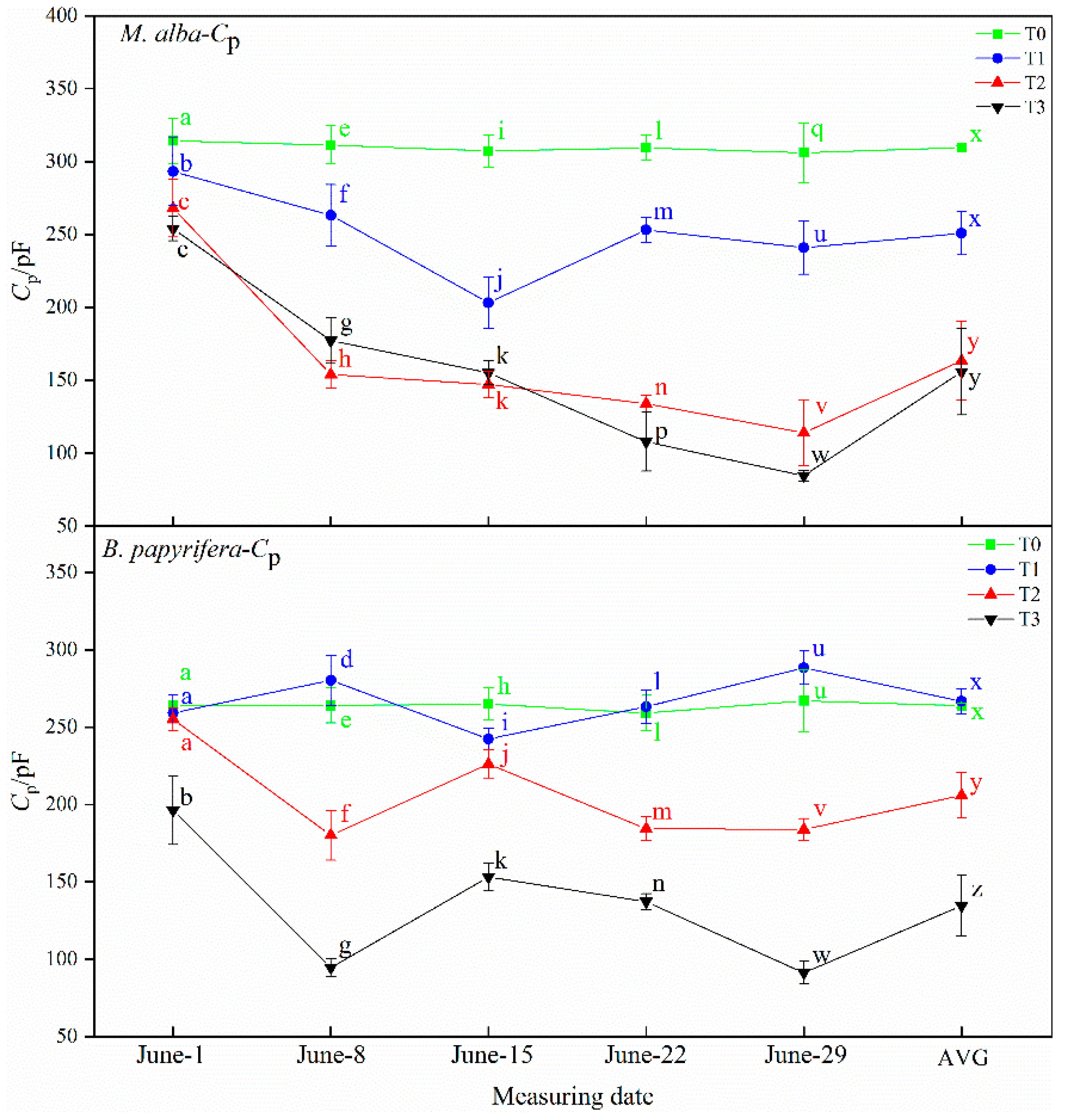
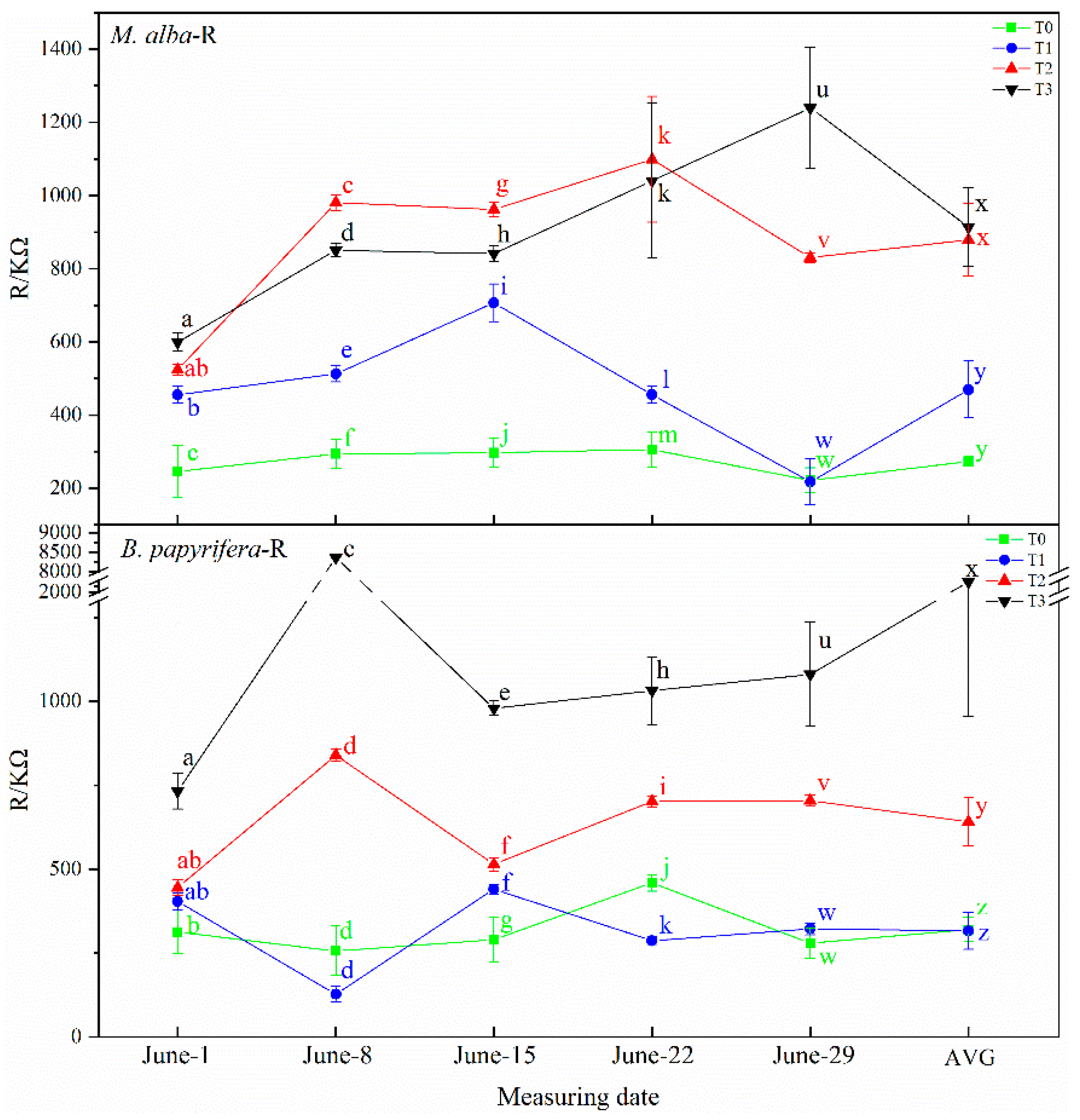
Cp, R and Z) of
M. alba and
B. papyrifera accurately revealed the diversity of intracellular water metabolism in plant leaves and could potentially be applied in the acquisition of plant water information. The results of this study revealed obvious characteristics of the electrophysiological parameters of
M. alba and
B. papyrifera With a gradual increase in plant water shortage severity, the capacitance value of
M. alba and
B. papyrifera decreased gradually and the water content of leaves decreased gradually, and with the increase in resistance and impedance, stomata closed, transpiration decreased, leaf water use efficiency increased, and soil water decreased. Therefore, the electrophysiological index can respond to soil water shortages.
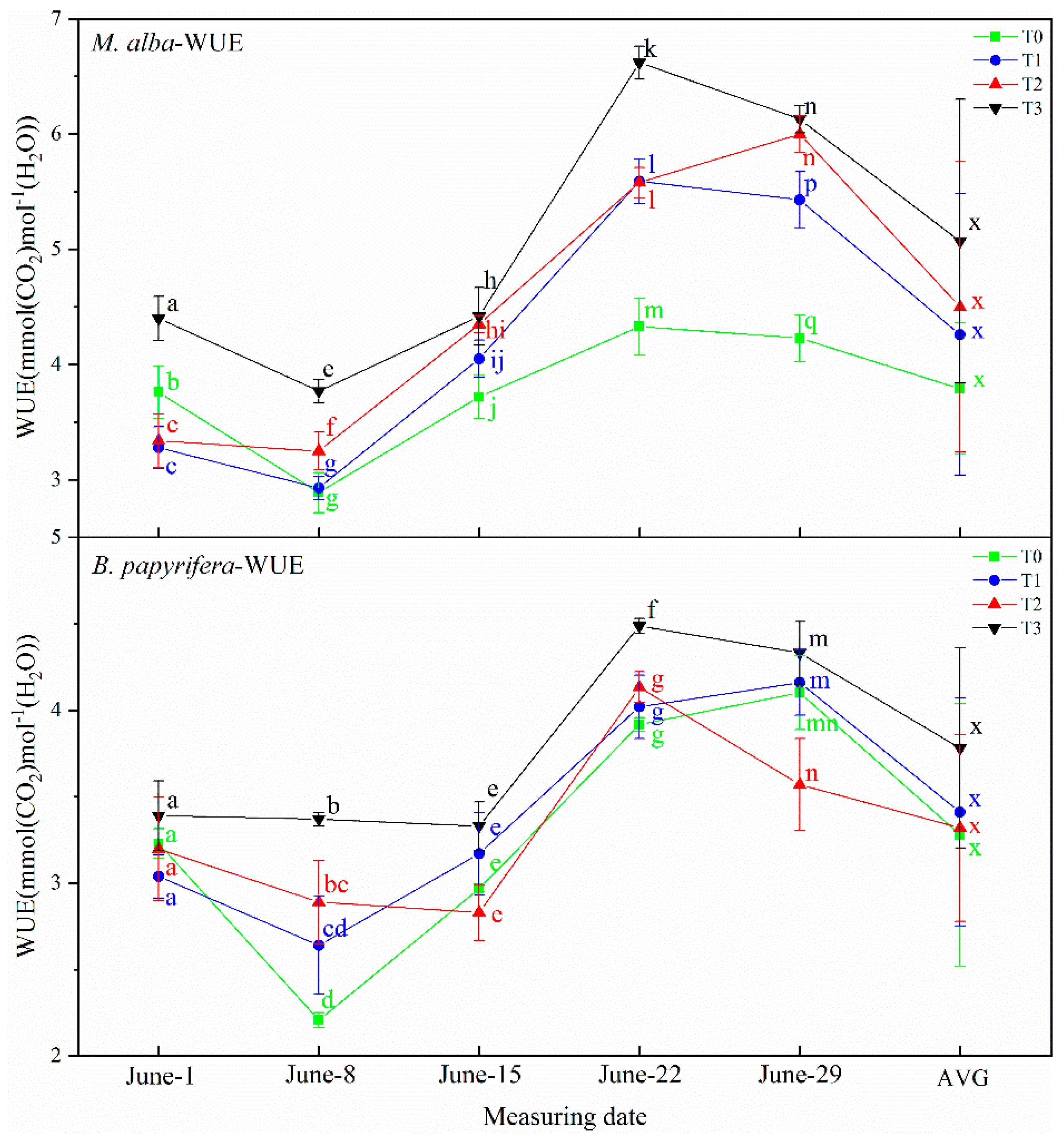
In this study, correlation analysis between photosynthetic and electrophysiological parameters of
M. alba and
B. papyrifera was performed for the first time (
Table 6 and
Table 7). Plant physiological resistance and intercellular CO
2 concentration are strongly negatively correlated in the two species. The physiological resistance of
M. alba and
B. papyrifera is also strongly negatively correlated with stomatal conductance; the greater the physiological resistance is, the smaller the intercellular CO
2 concentration. This explains why physiological resistance determines stomatal resistance and physiological resistance leads to greater stomatal resistance and smaller intercellular CO
2 concentrations. The osmotic potential of leaf cells will change immediately upon exposure to drought, so the electrolyte concentration will also change, resulting in changes in leaf capacitance, leaf resistance and leaf impedance. The unique relationships between WUE and electrophysiological parameters indicate the water deficit of plants. The physiological capacitance of
M. alba and
B. papyrifera is negatively correlated with WUE; the higher the physiological capacitance is, the lower the WUE, indicating that as the physiological capacitance increases, the stomata open, stomatal conductance and the transpiration rate increase, the leaf WUE decreases, and the demand for soil moisture is weakened. At the same time, WUE reflects the unique relationship between the physiological impedance and photosynthetic index of the two plants, because the physiological impedance of plants functions to maintain the internal stability of plant cells [
42]. The higher the physiological impedance is, the more stomatal closure will reduce transpiration, while WUE will increase. Therefore, the greater the physiological impedance is, the higher the WUE is and the lower the soil water is, and the electrophysiological index can reflect the soil water demand. This confirms the results of the experiment, which showed that the electrophysiological parameters of
M. alba reflected the water requirement. Furthermore, because the electrophysiological parameters were strongly correlated with the photosynthetic parameters, the photosynthetic parameters could also reflect the water demand of
M. alba. The water requirements of
B. papyrifera can also be inferred by the electrophysiological indicators. However, the electrophysiological indexes were not related to the photosynthetic indexes, so the photosynthetic indexes cannot reflect the water demand of plants. The water demand of
B. papyrifera can be reflected only by the electrophysiological indexes and growth indexes.
Concluded from the above, plant electrophysiological parameters have more advantages in characterizing plant drought resistance. Therefore, electrophysiological parameters can substitute for growth, and photosynthetic parameters to characterize the response of mulberry and paper mulberry to drought.