Novel Sources of Pre-Harvest Sprouting Resistance for Japonica Rice Improvement
Abstract
:1. Introduction
2. Results
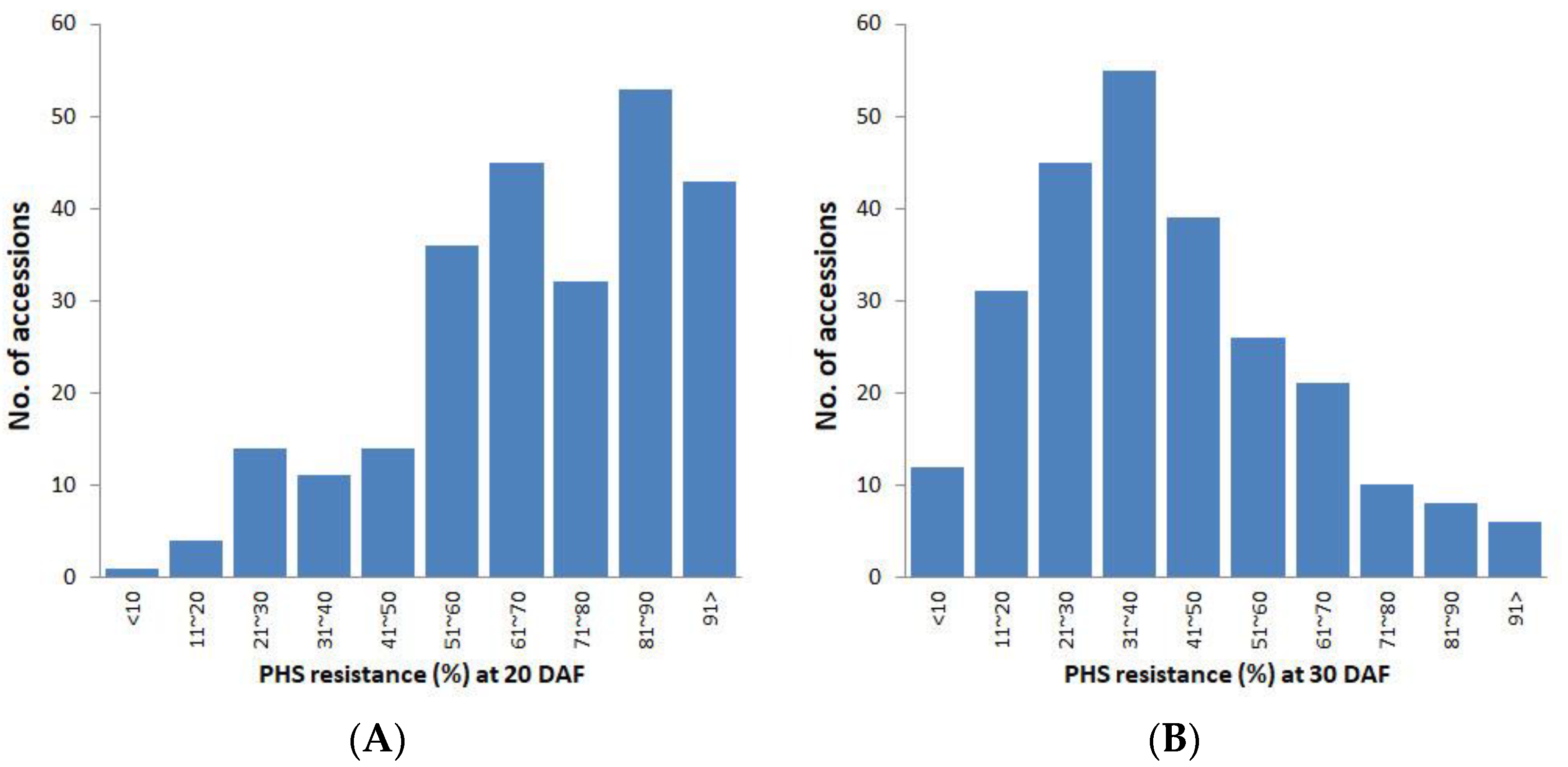
2.1. Optimal Sampling Time for PHS Screening
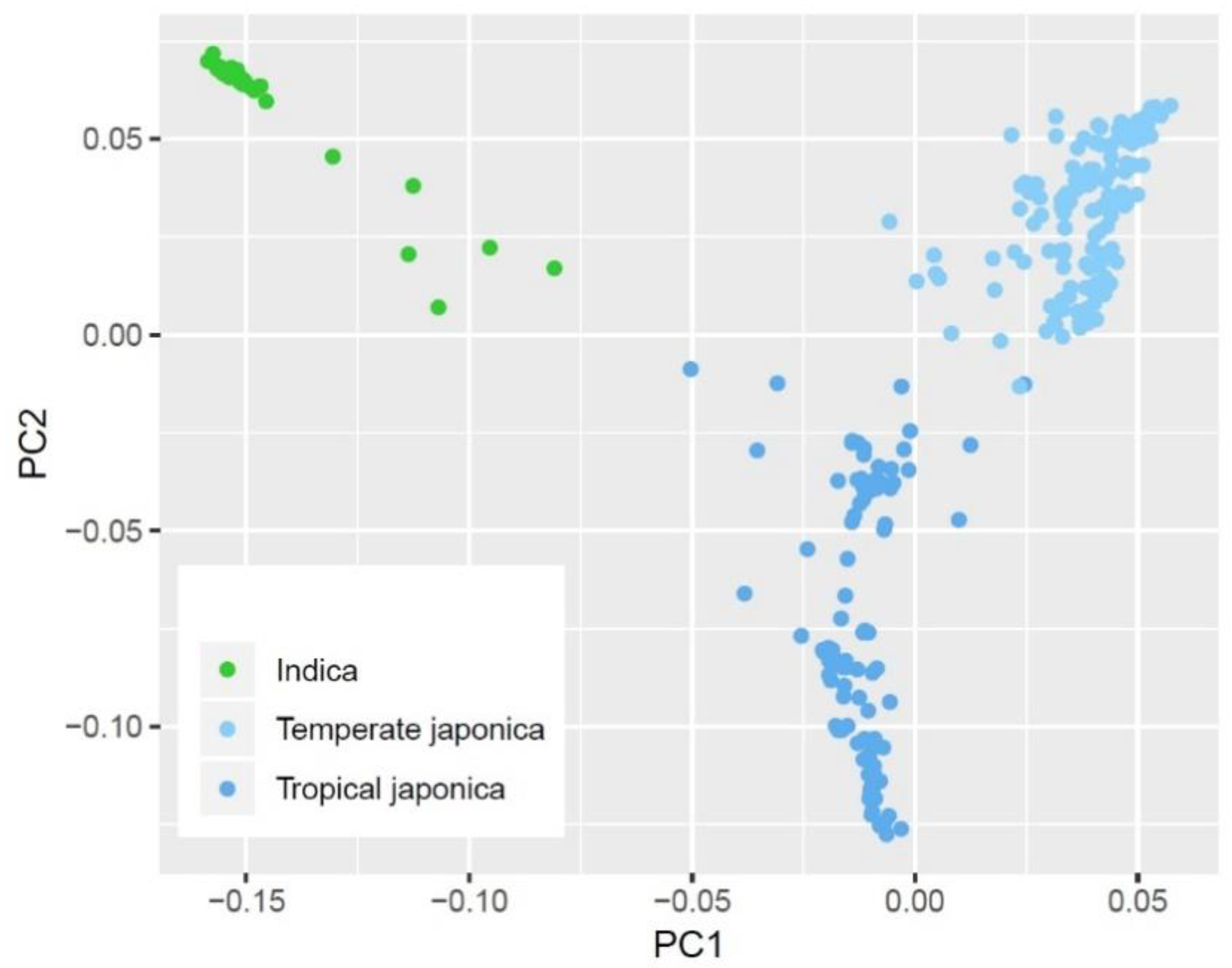
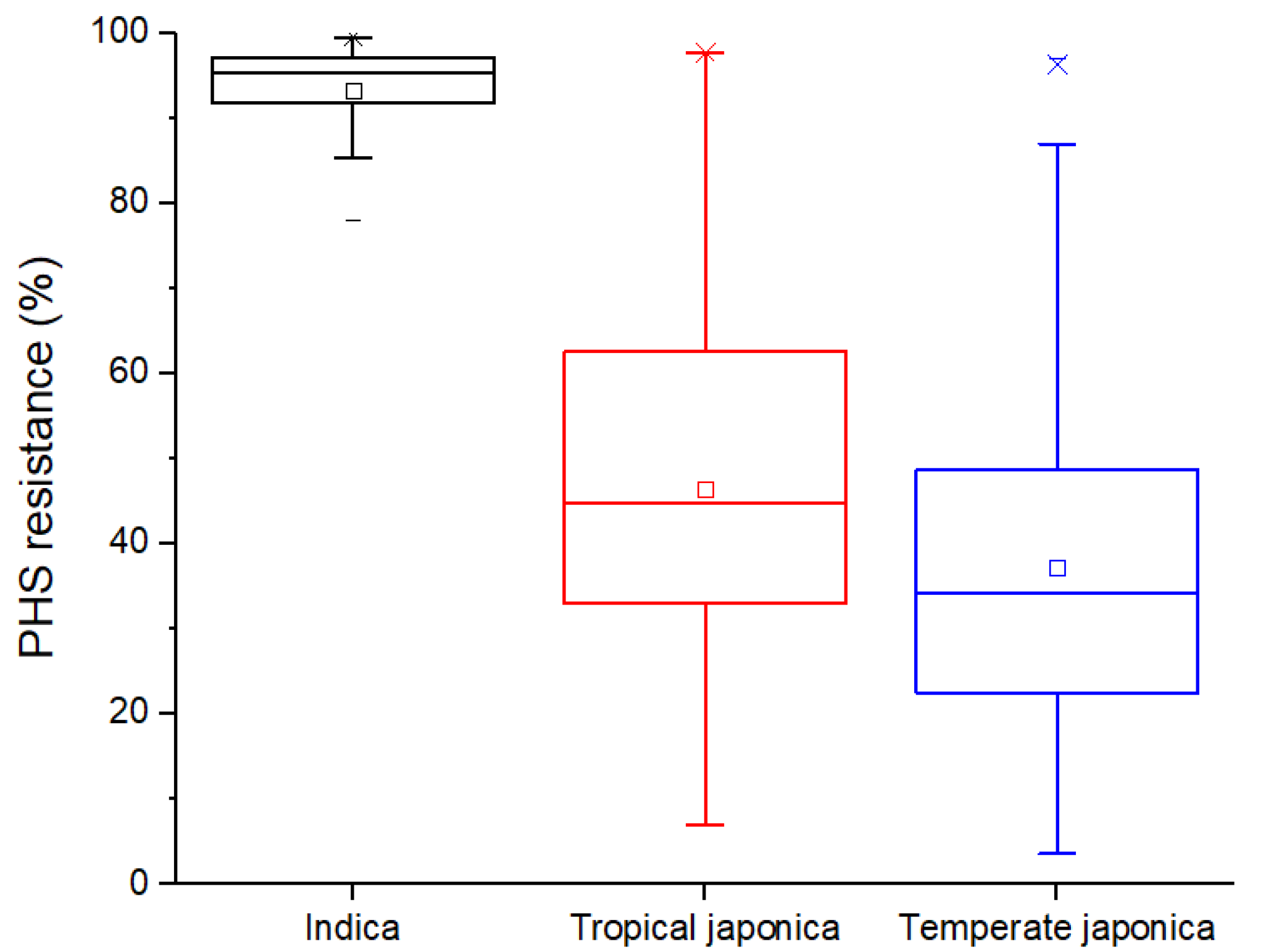
2.2. Intraspecific Variation on PHS
2.3. Loci Associated with PHS Resistance
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. PHS Screening
4.3. GWA Analysis
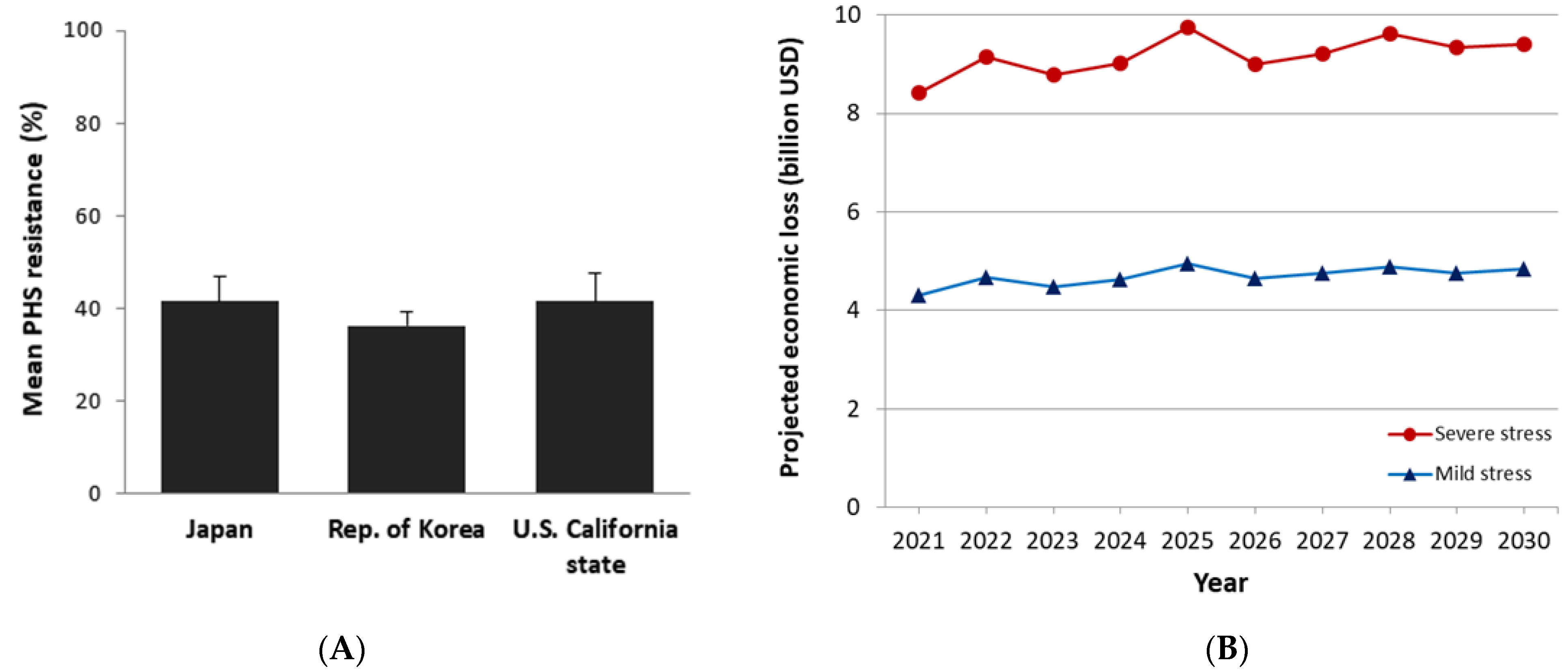
4.4. Projected Economic Loss
- 76.9% of original price when portion of damaged grains is less than 25%;
- 64.1% of original price when portion of damaged grains is less than 35%; and
- 51.3% of original price when portion of damaged grains is less than 50%.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, J.; Chu, C. Abscisic acid and the pre-harvest sprouting in cereals. Plant Signal Behav. 2008, 3, 1046–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S. Grain dormancy genes responsible for preventing pre-harvest sprouting in barley and wheat. Breed. Sci. 2018, 68, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.V.; Barrero, J.M.; Corbineau, F.; Gubler, F.; Benech-Arnold, R.L. Dormancy in cereals (not too much, not so little): About the mechanisms behind this trait. Seed Sci. Res. 2015, 25, 99–119. [Google Scholar] [CrossRef] [Green Version]
- Black, M.; Bewley, J.D.; Halmer, P. Encyclopedia of Seeds-Science, Technology and Uses, 1st ed.; CABI: Wallingford/London, UK, 2006. [Google Scholar]
- Zhu, D.; Qian, Z.; Wei, H.; Guo, B.; Xu, K.; Dai, Q.; Zhang, H.; Huo, Z. The effects of field pre-harvest sprouting on the morphological structure and physicochemical properties of rice (Oryza sativa L.) starch. Food Chem. 2019, 278, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, L.; Lu, Y.; Yang, Y.; Feng, L.; Hao, W.; Li, Q.; Fan, X.; Zhao, D.; Liu, Q. Changes in the physicochemical properties and starch structures of rice grains upon pre-harvest sprouting. Carbohydr. Polym. 2020, 234, 115893. [Google Scholar] [CrossRef]
- Barnard, A.; Smith, M.F. The effect of rainfall and temperature on the preharvest sprouting tolerance of winter wheat in the dryland production areas of the Free State Province. Field Crop. Res. 2009, 112, 158–164. [Google Scholar] [CrossRef]
- Kang, S.; Shon, J.; Kim, H.-S.; Kim, S.-J.; Choi, J.-S.; Park, J.-H.; Yoon, Y.; Sim, J.; Yang, W. Analysis of genetic variation in pre-harvest sprouting at different cumulative temperatures after heading of rice. Korean J. Crop Sci. 2018, 63, 8–17. [Google Scholar] [CrossRef]
- Soper, J.F.; Cantrell, R.G.; Dick, J.W. Sprouting damage and kernel color relationships in durum wheat. Crop Sci. 1989, 29, 895–898. [Google Scholar] [CrossRef]
- Himi, E.; Noda, K. Red grain colour gene (R) of wheat is a Myb-type transcription factor. Euphytica 2005, 143, 239–242. [Google Scholar] [CrossRef]
- Himi, E.; Maekawa, M.; Miura, H.; Noda, K. Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theor. Appl. Genet. 2011, 122, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Y.; Foley, M.E.; Horvath, D.P.; Anderson, J.V.; Feng, J.; Zhang, L.; Mowry, C.R.; Ye, H.; Suttle, J.C.; Kadowaki, K.; et al. Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics 2011, 189, 1515–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Chai, C.; Qian, Q.; Li, C.; Tang, J.; Sun, L.; Huang, Z.; Guo, X.; Sun, C.; Liu, M.; et al. Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J. 2008, 54, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, J.; Yu, Y.; Kong, L.; Liu, Y.; Liu, Z.; Li, H.; Wei, P.; Liu, M.; Zhou, H.; et al. Identification and characterization of the rice pre-harvest sprouting mutants involved in molybdenum cofactor biosynthesis. New Phytol. 2019, 222, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Tang, J.; Gao, S.; Cheng, X.; Du, L.; Chu, C. Control of rice pre-harvest sprouting by glutaredoxinmediated abscisic acid signaling. Plant J. 2019, 100, 1036–1051. [Google Scholar] [CrossRef]
- Sugimoto, K.; Takeuchi, Y.; Ebana, K.; Miyao, A.; Hirochika, H.; Hara, N.; Ishiyama, K.; Kobayashi, M.; Ban, Y.; Hattori, T.; et al. Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. USA 2010, 107, 5792–5797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Famoso, A.N.; Zhao, K.; Clark, R.T.; Tung, C.-W.; Wright, M.H.; Bustamante, C.; Kochian, L.V.; McCouch, S.R. Genetic architecture of aluminum tolerance in rice (Oryza sativa) determined through genome-wide association analysis and QTL mapping. PLoS Genet. 2011, 7, e1002221. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Tung, C.; Eizenga, G.; Wright, M.H.; Ali, M.L.; Price, A.H.; Norton, G.J.; Islam, M.R.; Reynolds, A.; Mezey, J.; et al. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat. Commun. 2011, 2, 467. [Google Scholar] [CrossRef]
- Nakamura, S.; Abe, F.; Kawahigashi, H.; Nakazono, K.; Tagiri, A.; Matsumoto, T.; Utsugi, S.; Ogawa, T.; Handa, H.; Ishida, H.; et al. A wheat homolog of Mother of FT and TFL1 acts in the regulation of germination. Plant Cell 2011, 23, 3215–3229. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-R.; Torollo, G.; Yoon, M.-R.; Kwak, J.; Lee, C.-K.; Prahalada, G.D.; Choi, I.-R.; Yeo, U.-S.; Jeong, O.-Y.; Jena, K.K. Loss-of-function alleles of heading date 1 (Hd1) are associated with adaptation of temperate japonica rice plants to the tropical region. Front. Plant Sci. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Kwak, J.; Yoon, M.R.; Lee, J.S.; Hay, F.R. Contrasting tocol ratios associated with seed longevity in rice sub-populations. Seed Sci. Res. 2017, 27, 273–280. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kwak, J.; Cho, J.-H.; Chebotarov, D.; Yoon, M.-R.; Lee, J.-S.; Hamilton, R.S.; Hay, F.R. A high proportion of beta-tocopherol in vitamin E is associated with poor seed longevity in rice produced under temperate conditions. Plant Genet. Resour. 2019, 17, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.-H.; Wu, X.; Chen, B.-C.; Ma, J.-Q.; Gao, J. Effects of low light on agronomic and physiological characteristics of rice including grain yield and quality. Rice Sci. 2014, 21, 243–251. [Google Scholar] [CrossRef]
- Lee, G.-A.; Jeon, Y.-A.; Lee, H.-S.; Hyun, D.Y.; Lee, J.-R.; Lee, M.-C.; Lee, S.-Y.; Ma, K.-H.; Koh, H.-J. New genetic loci associated with preharvest sprouting and its evaluation based on the model equation in rice. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Zheng, X.; Luo, J.; Gaut, B.S.; Ge, S. Multilocus analysis of nucleotide variation of Oryza sativa and its wild relatives: Severe bottleneck during domestication of rice. Mol. Biol. Evol. 2007, 24, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.-X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Londo, J.; Chiang, Y.; Hung, K.; Chiang, T.; Schaal, B. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc. Natl. Acad. Sci. USA 2006, 103, 9578–9583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Liu, K.D.; Yang, G.P.; Saghai Maroof, M.A.; Xu, C.G.; Zhou, Z.Q. Molecular marker diversity and hybrid sterility in indica-japonica rice crosses. Theor. Appl. Genet. 1997, 95, 112–118. [Google Scholar] [CrossRef]
- Liu, H.Y.; Xu, C.G.; Zhang, Q. Male and female gamete abortions, and reduced affinity between the uniting gametes as the causes for sterility in an indica/japonica hybrid in rice. Sex Plant Reprod. 2004, 17, 55–62. [Google Scholar] [CrossRef]
- Rice Genome Annotation Project. Available online: http://rice.uga.edu/ (accessed on 20 April 2021).
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, K.; Lin, F.; Romero-Gamboa, S.P.; Saha, P.; Goh, H.-J.; An, G.; Jung, K.-H.; Hazen, S.P.; Bartley, L.E. Rice Genome-Scale Network Integration Reveals Transcriptional Regulators of Grass Cell Wall Synthesis. Front. Plant Sci. 2019, 10, 1275. [Google Scholar] [CrossRef]
- Lee, T.; Oh, T.; Yan, S.; Shin, J. RiceNet v2: An improved network prioritization server for rice genes. Nucleic Acids Res. 2015, 43, W122–W127. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Feng, J.; Zhang, L.; Zhang, J.; Mispan, M.S.; Cao, Z.; Beighley, D.H.; Yang, J.; Gu, X.-Y. Map-Based Cloning of Seed Dormancy1-2 Identified a Gibberellin Synthesis Gene Regulating the Development of Endosperm-Imposed Dormancy in Rice. Plant Physiol. 2015, 169, 2152–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Cai, S.; Graybosch, R.; Chen, C.; Bai, G. Quantitative trait loci for resistance to pre-harvest sprouting in U.S. hard white winter wheat Rio Blanco. Theor. Appl. Genet. 2008, 117, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sehgal, V.S.; Li, J.; Lin, M.; Gill, B.S.; Harold, T.; Bai, G. Cloning and characterization of a critical regulator for pre-harvest sprouting (PHS) resistance in wheat. Genetics 2013, 195, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hou, X. Antagonistic regulation of aba and ga in metabolism and signaling pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.; Liu, C.; Hou, X.; Yu, H. Mother of FT and TFL1 regulates seed germination through a negative feedback loop modulating aba signaling in arabidopsis. Plant Cell 2010, 22, 1733–1748. [Google Scholar] [CrossRef] [Green Version]
- Emenecker, R.J.; Strader, L.C. Auxin-abscisic acid interactions in plant growth and development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Lee, K.E.; Lee, E.S.; Matin, M.N.; Lee, D.S.; Yun, J.S.; Kim, J.B.; Kang, S.G. The genetic constitutions of complementary genes Pp and Pb determine the purple color variation in pericarps with cyanidin-3-O-glucoside depositions in black rice. J. Plant Biol. 2013, 56, 24–31. [Google Scholar] [CrossRef]
- Kobayashi, A.; Sugimoto, K.; Hayashi, T.; Kondo, M.; Tomita, K. Development of a near isogenic line of ‘Koshihikari’ with a seed dormancy gene and an evaluation of its resistance to heat-induced quality decline. Breed. Res. 2016, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- The 3000 Rice Genomes Project. The 3000 rice genomes project. Gigascience 2014, 3, 7. [Google Scholar]
- Lee, J.-S.; Valdez, R.; Punzalan, M.; Pacleb, M.; Kretzschmar, T.; McNally, K.; Ismail, A.M.; Cruz, P.S.; Hamilton, R.S.; Hay, F.R. Variation in seed longevity among diverse Indica rice varieties. Ann. Bot. 2019, 124, 447–460. [Google Scholar] [CrossRef]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansueto, L.; Fuentes, R.R.; Borja, F.N.; Detras, J.; Abriol-Santos, J.M.; Chebotarov, D.; Sanciangco, M.; Palis, K.; Copetti, D.; Poliakov, A.; et al. Rice SNP-seek database update: New SNPs, indels, and queries. Nucleic Acids Res. 2017, 45, D1075–D1081. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, 7. [Google Scholar] [CrossRef]
- Li, J.; Ji, L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 2005, 95, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Starmer, J.; Martin, E.R. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet. Epidemiol. 2008, 32, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Rice SNP-Seek Database. Available online: https://snp-seek.irri.org (accessed on 3 May 2021).
- Bouman, B.A.M.; Kropff, M.J.; Wopereis, M.C.S.; ten Berge, H.F.M.; van Laar, H.H. ORYZA2000: Modeling Lowland Rice, 1st ed.; IRRI Books: Los Baños, Philippines, 2001. [Google Scholar]
- Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea. Available online: www.mafra.go.kr (accessed on 31 March 2021).


| No | Locus ID | Position | Expression during Seed Development 1 | Annotation | Network Gene | Gene Ontology |
|---|---|---|---|---|---|---|
| RiceNet v2 | RiceNet v2 | |||||
| RCRN | RCRN | |||||
| 1 | LOC_Os01g03740 | 1566554–1562612 | Seed-5 DAP 2 | Nuclease PA3 | LOC_Os05g31750/ LOC_Os02g36390/ LOC_Os06g13040 | Response to abscisic acid stimulus and abiotic stresses/pollen exine formation/photoperiodism |
| LOC_Os02g36390/ LOC_Os10g39640/ LOC_Os05g31750/ | Sugar transporter/cell wall growth/response to abiotic stimulus | |||||
| 2 | LOC_Os01g03730 | 1566138–1569472 | Seed-5 DAP | Nuclease PA3 | LOC_Os01g65440/ LOC_Os02g09220/ LOC_Os06g17830 | Response to stress/oxidation reduction/DNA demethylation and repair |
| LOC_Os01g65440/ LOC_Os08g15020/ LOC_Os01g46370 | Response to stress/MYB transcription factor/lipid metabolism | |||||
| 3 | LOC_Os01g03820 | 1597486–1604610 | Embryo-25 DAP | C2 domain containing protein | LOC_Os08g02140/ LOC_Os06g46670/ LOC_Os01g07760 | Protein ubiquitination/G-protein coupled receptor protein signaling pathway/positive regulation of seed germination and abscisic acid mediated signaling pathway |
| LOC_Os08g08190/ LOC_Os01g53160/ LOC_Os08g02140 | Unknown molecular function/plasma membrane/response to abiotic stimulus/protein modification | |||||
| 4 | LOC_Os01g03840 | 1625159–1626771 | Embryo-25 DAP | ZOS1-02–C2H2 zinc finger protein | LOC_Os08g41950/ LOC_Os05g38120/ LOC_Os01g18360 | Flower development; specification of floral organ number/regulation of transcription, DNA-dependent/auxin mediated signaling pathway |
| LOC_Os05g38120/ LOC_Os02g57790/ LOC_Os01g72020/ | Cell differentiatio/post-embryonic development/DNA binding transcription factor/homeodomain protein/nucleic acid binding/flower development | |||||
| 5 | LOC_Os01g03890 | 1657315–1658388 | Seed-5 DAP | DUF260 domain containing protein | N/A | N/A |
| N.S. (0.01) | ||||||
| 6 | LOC_Os01g03914 | 1677129–1673010 | Embryo-25 DAP | Cation efflux family protein | LOC_Os05g25310/ LOC_Os01g62760/ LOC_Os03g16170 | Long-chain fatty acid metabolic process/negative regulation of abscisic acid mediated signaling pathway/positive regulation of seed germination and gibberellic acid mediated signaling pathway; release of seed from dormancy |
| LOC_Os10g02210/ LOC_Os06g21820/ LOC_Os07g23944 | Peptide transporter/protein binding/multicellular development/carbohydrate hydrolase | |||||
| 7 | LOC_Os01g03950 | 1698203–1703090 | Seed-5 DAP | Glycosyl hydrolase, family 31 | LOC_Os07g43390/ LOC_Os06g09630/ LOC_Os04g56070 | Maltose, carbohydrate, starch and glucose metabolic processes/fatty acid biosynthetic process; embryonic development/response to auxin stimulus |
| LOC_Os01g67220/ LOC_Os01g07120/ LOC_Os06g34690 | Carbohydrate hydrolase/response to abiotic stress stimulus/DNA binding transcription factor/ | |||||
| 8 | LOC_Os04g08460 | 4557188–4559281 | Seed-5 DAP | OsFBX115–F-box domain containing protein | N/A | N/A |
| N.S. (0.05) | ||||||
| 9 | LOC_Os04g08470 | 4571423–4564403 | Seed-5 DAP | OsFBX116–F-box domain containing protein | N/A | N/A |
| N.S. (0.01) | ||||||
| 10 | LOC_Os04g08570 | 4652730–4651469 | Seed-10 DAP | Uncharacterized PE-PGRS family protein | LOC_Os01g01350/ LOC_Os03g04360/ LOC_Os10g01560 | Protein transport/response to abscisic acid stimulus/abscisic acid mediated signaling pathway; seed germination |
| LOC_Os07g13830/ LOC_Os12g30500/ LOC_Os03g31430 | Putative protein ubiquination/protein binding/lipid metabolic process |
| Continent | Region | No. of Accession | Ecotype | ||
|---|---|---|---|---|---|
| Indica | Temperate Japonica | Tropical Japonica | |||
| Africa | Eastern Africa | 7 | 3 | 0 | 4 |
| Western Africa | 7 | 2 | 0 | 5 | |
| Northern Africa | 16 | 2 | 2 | 12 | |
| Asia | Eastern Asia | 71 | 0 | 66 | 5 |
| South-Eastern Asia | 54 | 6 | 6 | 42 | |
| Southern Asia | 27 | 8 | 8 | 11 | |
| Europe | Eastern Europe | 9 | 0 | 9 | 0 |
| Central Europe | 7 | 0 | 7 | 0 | |
| Southern Europe | 36 | 0 | 35 | 1 | |
| Western Europe | 7 | 1 | 4 | 2 | |
| Northern Europe | 1 | 0 | 1 | 0 | |
| North America | North America | 13 | 1 | 8 | 4 |
| Oceania | Oceania | 4 | 0 | 4 | 0 |
| South America | South America | 18 | 1 | 6 | 11 |
| Total | 277 | 24 | 156 | 97 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Chebotarov, D.; McNally, K.L.; Pede, V.; Setiyono, T.D.; Raquid, R.; Hyun, W.-J.; Jeung, J.-U.; Kohli, A.; Mo, Y. Novel Sources of Pre-Harvest Sprouting Resistance for Japonica Rice Improvement. Plants 2021, 10, 1709. https://doi.org/10.3390/plants10081709
Lee J-S, Chebotarov D, McNally KL, Pede V, Setiyono TD, Raquid R, Hyun W-J, Jeung J-U, Kohli A, Mo Y. Novel Sources of Pre-Harvest Sprouting Resistance for Japonica Rice Improvement. Plants. 2021; 10(8):1709. https://doi.org/10.3390/plants10081709
Chicago/Turabian StyleLee, Jae-Sung, Dmytro Chebotarov, Kenneth L. McNally, Valerien Pede, Tri Deri Setiyono, Rency Raquid, Woong-Jo Hyun, Ji-Ung Jeung, Ajay Kohli, and Youngjun Mo. 2021. "Novel Sources of Pre-Harvest Sprouting Resistance for Japonica Rice Improvement" Plants 10, no. 8: 1709. https://doi.org/10.3390/plants10081709
APA StyleLee, J.-S., Chebotarov, D., McNally, K. L., Pede, V., Setiyono, T. D., Raquid, R., Hyun, W.-J., Jeung, J.-U., Kohli, A., & Mo, Y. (2021). Novel Sources of Pre-Harvest Sprouting Resistance for Japonica Rice Improvement. Plants, 10(8), 1709. https://doi.org/10.3390/plants10081709







