Morphological and Chemical Diversity and Antioxidant Capacity of the Service Tree (Sorbus domestica L.) Fruits from Two Eco-Geographical Regions
Abstract
1. Introduction
2. Results
2.1. Climate Differences among Sampling Sites
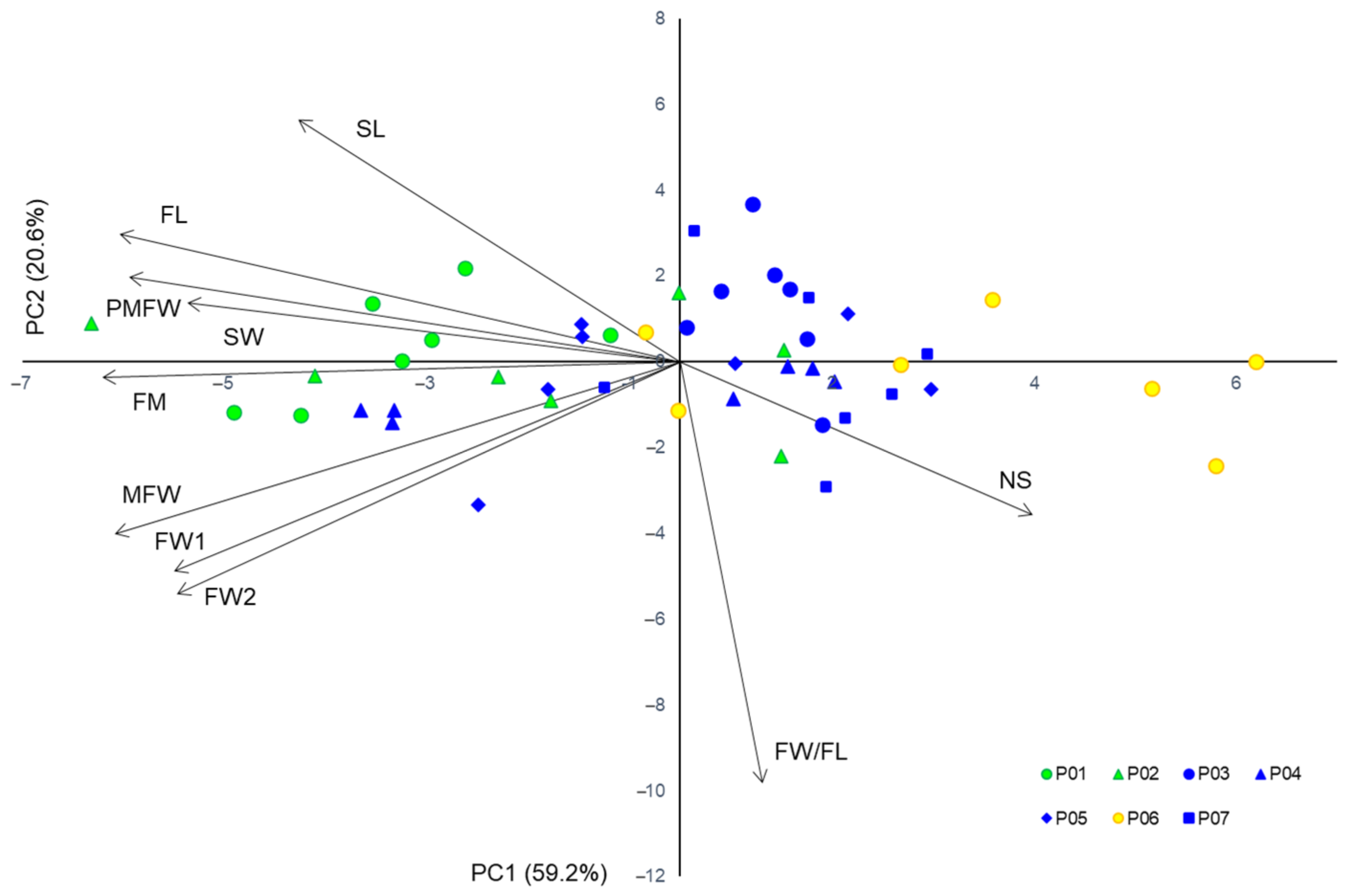
2.2. Fruit Morphometric Characteristics
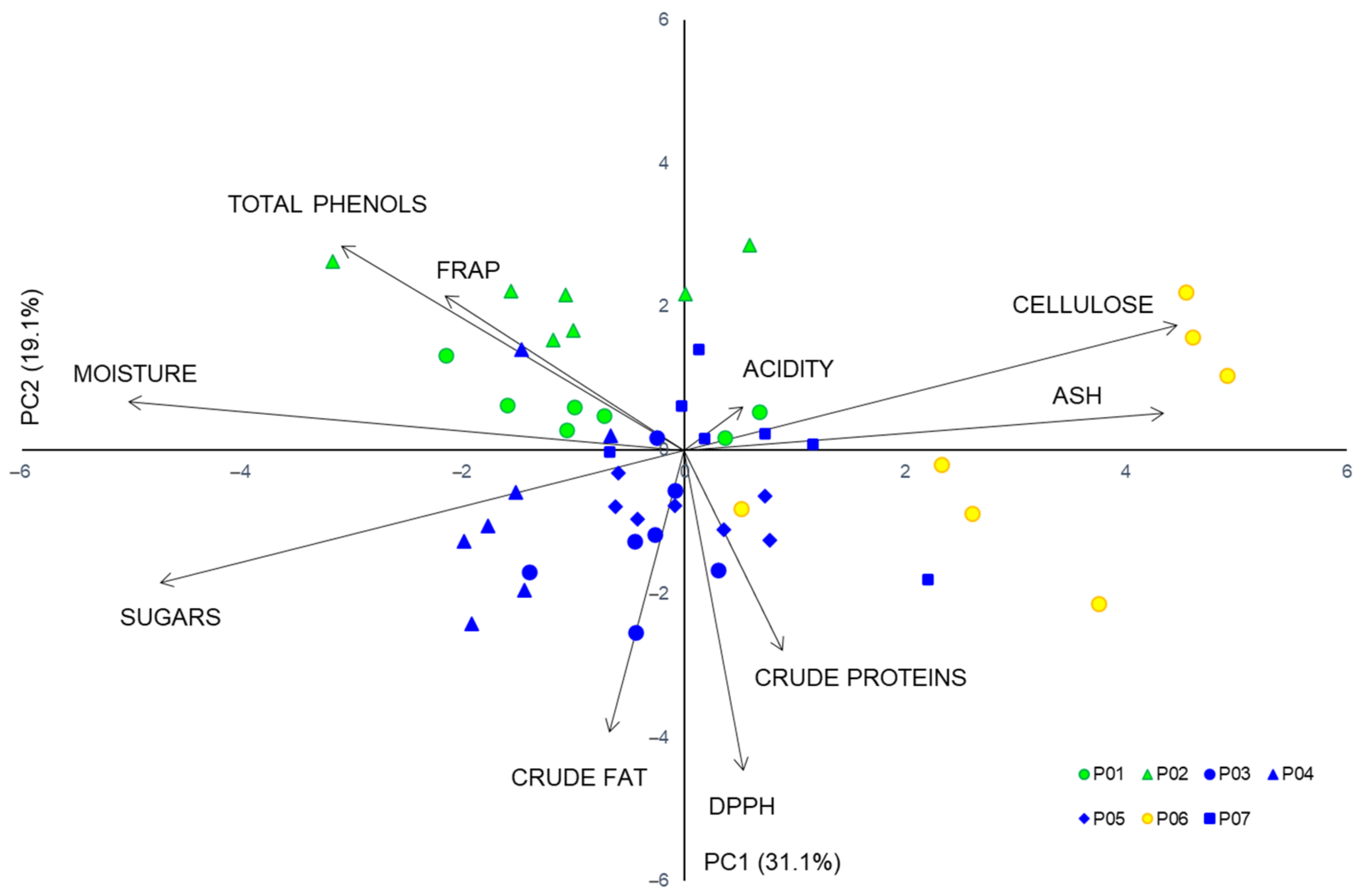
2.3. Proximate Analysis and Acidity
2.4. Total Phenolics and Antioxidant Capacity
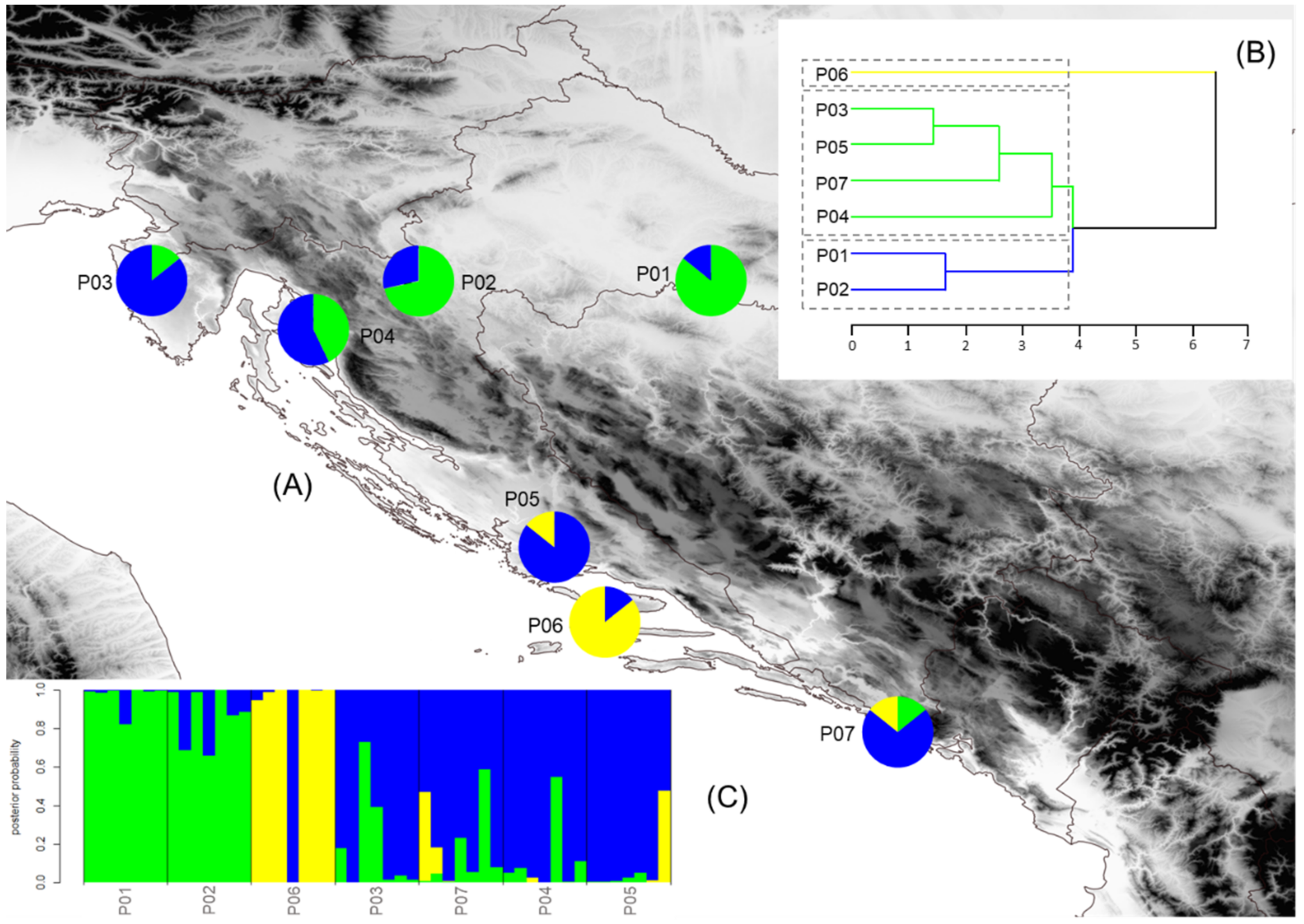
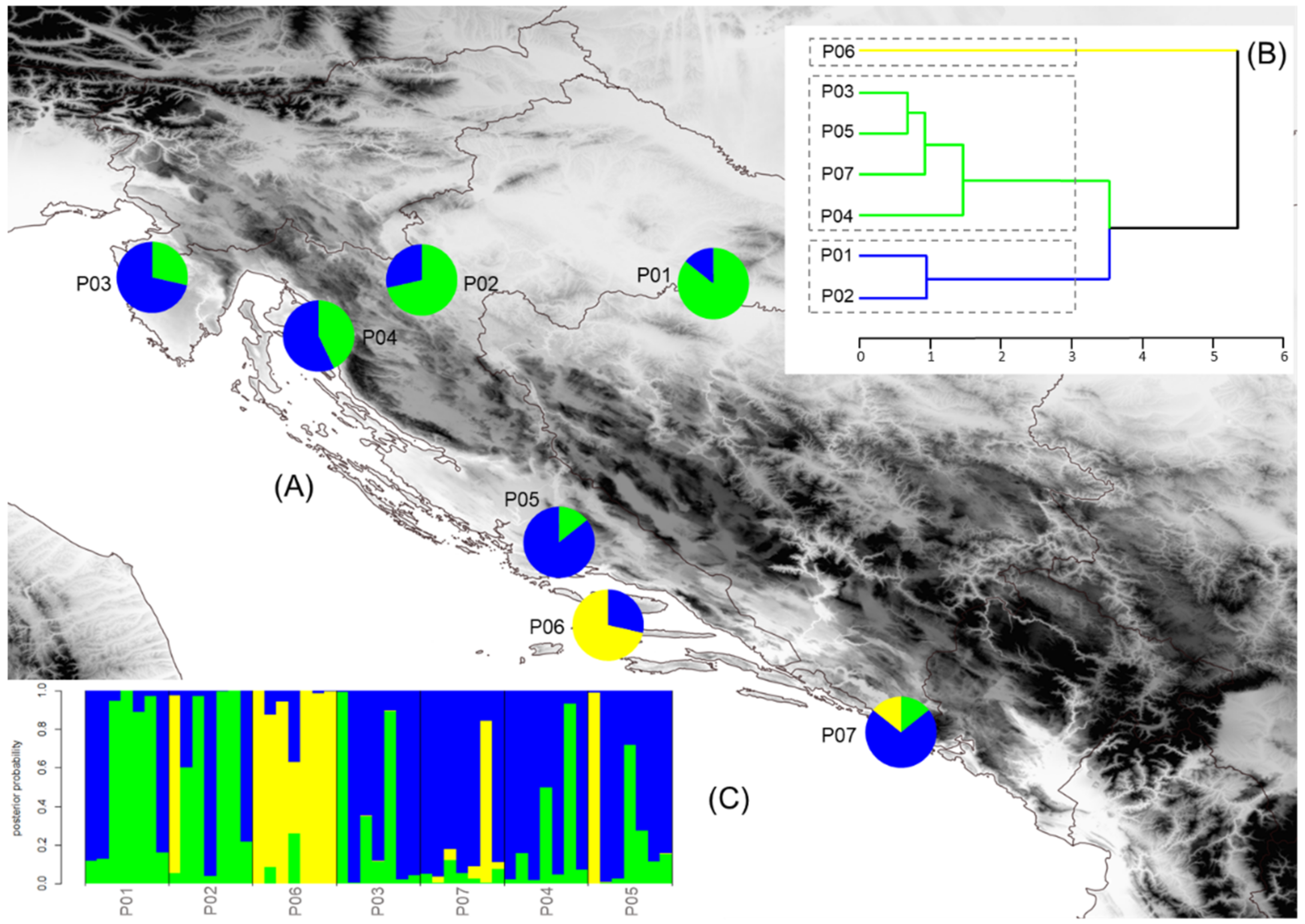
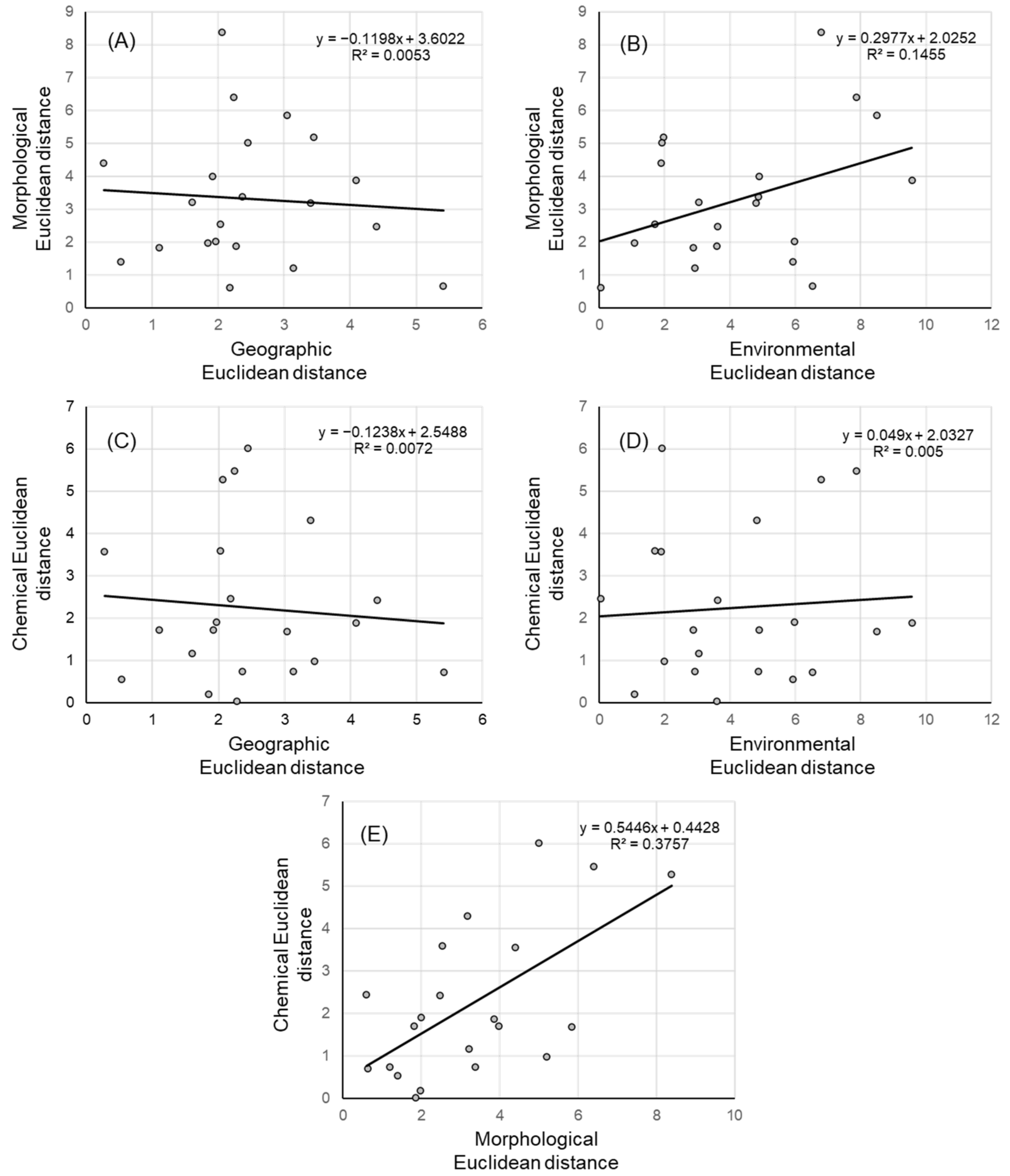
2.5. Population Structure, Isolation by Distance, and Environment
3. Discussion
3.1. Fruit Morphometric Characteristics
3.2. Proximate Composition
3.3. Total Phenolic Content and Antioxidant Activity
3.4. Population Structure, Isolation by Distance and Environment
4. Materials and Methods
4.1. Plant Material and Study Area
4.2. Morphometric Analysis
4.3. Proximate Analysis
4.4. Determination of Total Phenolics and Antioxidant Capacity
4.4.1. Fruit Extraction Procedure
4.4.2. Estimation of Total Phenolic Content Using the Folin-Ciocalteu Method
4.4.3. Evaluation of Antioxidant Activity Using the DPPH Method
4.4.4. Determination of FRAP
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drvodelić, D.; Oršanić, M.; Grahovac-Tremski, M. Rasadnička proizvodnja šumskih voćkarica u rasadnicima Hrvatskih šuma doo. Šumar. List 2020, 11–12, 597–606. [Google Scholar] [CrossRef]
- Sardeshpande, M.; Shackleton, C. Wild edible fruits: A systematic review of an under-researched multifunctional NTFP (Non-Timber Forest Products). Forests 2019, 10, 467. [Google Scholar] [CrossRef]
- Bvenura, C.; Sivakumar, D. The role of wild fruits and vegetables in delivering a balanced and healthy diet. Food Res. Int. 2017, 99, 15–30. [Google Scholar] [CrossRef]
- Stryamets, N. Non-wood forest products for livelihoods. Bosque 2012, 33, 329–332. [Google Scholar]
- Sheppard, J.P.; Chamberlain, J.; Agundez, D.; Bhattacharya, P.; Chirwa, P.W.; Gontcharov, A.; Sagona, W.C.J.; Shen, H.; Tadesse, W.; Mutke, S. Sustainable forest management beyond the timber-oriented Status Quo: Transitioning to coproduction of timber and non-wood forest products—A global perspective. Curr. For. Rep. 2020, 6, 26–40. [Google Scholar] [CrossRef]
- Rotach, P. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Service Tree (Sorbus domestica); International Plant Genetic Resources Institute: Rome, Italy, 2003; p. 6. [Google Scholar]
- Brütsch, U.; Rotach, P. Der Speierling (Sorbus domestica L.) in der Schweiz: Verbreitung, ökologie, standsortsanspruche, konkurrenzkraft und waldbauliche eignung. Schweiz. Z. Forstwes. 1993, 144, 967–991. [Google Scholar]
- George, J.-P.; Konrad, H.; Collin, E.; Thevenet, J.; Ballian, D.; Idžojtić, M.; Kamm, U.; Zhelev, P.; Geburek, T. High molecular diversity in the true service tree (Sorbus domestica) despite rareness: Data from Europe with special reference to the Austrian occurrence. Ann. Bot. 2015, 115, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- George, J.-P.; Woodman, J.; Hampton, M.; Konrad, H.; Geburek, T. True service-tree (Sorbus domestica, Rosaceae) in the British Isles: Rare but diverse. New J. Bot. 2016, 6, 21–30. [Google Scholar] [CrossRef]
- Matić, S.; Vukelić, J. Speierling und Elsbeere in den Waldern Kroatiens. Corminaria 2001, 16, 31–33. [Google Scholar]
- Paganová, V. Ecology and distribution of Service tree Sorbus domestica L. in Slovakia. Ekológia 2008, 27, 152–167. [Google Scholar]
- Poljak, I.; Idžojtić, M.; Zebec, M.; Šapić, I. Diversity and distribution of Sorbus spp. in Croatia. In Book of Abstracts of the 34th International Symposium—Eastern Alpine and Dinaric Society for Vegetation Ecology, Proceedings of International symposium—Eastern Alpine and Dinaric Society for Vegetation Ecology, Camerino, Italy, 24–28 May 2011; Carimini, L., Ed.; University of Camerino: Camerino, Italy, 2011. [Google Scholar]
- Enescu, C.M.; de Rigo, D.; Houston Durrant, T.; Caudullo, G. Sorbus domestica in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; p. e019db5+. [Google Scholar]
- Jeran, N.; Židovec, V.; Dujmović Purgar, D.; Vokurka, A.; Mešić, A.; Karlović, K.; Duralija, B. Utilization value of Sorbus s.l. in Croatia and its potential enhancement. Acta Hortic. 2021, 1308, 81–88. [Google Scholar] [CrossRef]
- Pietzarka, U.; Heidecke, C.; Roloff, A. Sorbus domestica L. In Enzyklopädie der Holzgewächse; Roloff, A., Weisgeber, H., Lang, U.M., Stimm, B., Eds.; Wiley-VCH: Weinheim, Germany, 2010; pp. 1–16. [Google Scholar]
- Idžojtić, M. Dendrology: Cones, Flowers, Fruits and Seeds; Elsevier—Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; London, 2019; 800p. [Google Scholar]
- Kamm, U.; Rotach, P.; Gugerli, F.; Siroky, M.; Edwards, P.; Holderegger, R. Frequent long-distance gene flow in a rare temperate forest tree (Sorbus domestica) at the landscape scale. Heredity 2009, 103, 476–482. [Google Scholar] [CrossRef]
- Miko, M.; Gažo, J. Morphological diversity of Sorbus domestica at the level of fruits and leaves in the selected localities of Slovakia. Biologia 2003, 58, 35–39. [Google Scholar]
- Miko, M.; Gažo, J. Morphological and biological characteristics of fruits and seed of the service tree (Sorbus domestica L.). J. Fruit Ornam. Plant Res. 2004, 12, 139–146. [Google Scholar]
- Mikić, T.; Orlović, S.; Marković, M.; Kovačević, B.; Pilipović, A. Variability in service tree (Sorbus domestica L.) populations in Serbia. Lesn. Čas.–For. J. 2008, 54, 61–67. [Google Scholar]
- Miletić, R.; Paunović, S.M. Research into service tree (Sorbus domestica L.) population in Eastern Serbia. Genetika 2012, 44, 483–490. [Google Scholar] [CrossRef]
- Drvodelić, D.; Oršanić, M.; Vuković, M.; Jatoi, M.A.; Jemrić, T. Correlation of fruit size with morphophysiological properties and germination rate of the seeds of Service tree (Sorbus domestica L.). SEEFOR 2018, 9, 47–54. [Google Scholar] [CrossRef]
- Ölschläger, C.; Milde, J.; Schempp, H.; Treuter, D. Polyphenols and antioxidant capacity of Sorbus domestica L. fruits. J. Appl. Bot. Food. Qual. 2004, 78, 112–116. [Google Scholar]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. Antioxidant activities of various extracts and fractions of Sorbus domestica fruits at different maturity stages. Food Chem. 2006, 98, 599–608. [Google Scholar] [CrossRef]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. LC–DAD–MS (ESI+) analysis of the phenolic content of Sorbus domestica fruits in relation to their maturity stage. Food Chem. 2008, 106, 1234–1245. [Google Scholar] [CrossRef]
- Piagnani, M.C.; Debellini, C.; LoScalzo, R. Ohyllometry and carpometry, chemical and functional characterization of fruits of Sorbus domestica L. (service tree) selections. J. Berry Res. 2012, 2, 7–22. [Google Scholar] [CrossRef]
- Majić, B.; Šola, I.; Likić, S.; Juranović Cindrić, I.; Rusak, G. Characterisation of Sorbus domestica L. bark, fruit and seeds: Nutrient composition and antioxidant activity. Food. Technol. Biotechnol. 2015, 53, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Brus, R.; Ballian, D.; Bogunić, F.; Bobinac, M.; Idžojtić, M. Leaflet morphometric variation of service tree (Sorbus domestica L.) in the Balkan Peninsula. Plant Biosyst. 2011, 145, 278–285. [Google Scholar] [CrossRef]
- Poljak, I.; Kajba, D.; Ljubić, I.; Idžojtić, M. Morphological variability of leaves of Sorbus domestica L.in Croatia. Acta Soc. Bot. Pol. 2015, 84, 249–259. [Google Scholar] [CrossRef]
- Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica leaf extracts and their activity markers: Antioxidant potential and synergy effects in scavenging assays of multiple oxidants. Molecules 2019, 24, 2289. [Google Scholar] [CrossRef] [PubMed]
- Bednorz, L. Morphological variability of leaves of Sorbus torminalis (L.) Crantz in Poland. Acta Soc. Bot. Pol. 2006, 75, 233–243. [Google Scholar] [CrossRef][Green Version]
- Bednorz, L. Morphological variability of fruits and seeds of Sorbus torminalis in Poland. Dendrobiology 2007, 57, 3–14. [Google Scholar]
- Paganová, V. Taxonomic reliability of leaf and fruit morphological characteristics of the Pyrus L. taxa in Slovakia. Hort. Sci. 2003, 30, 98–107. [Google Scholar] [CrossRef]
- Paganová, V. The occurrence and morphological characteristics of the wild pear lower taxa in Slovakia. Hort. Sci. 2009, 36, 1–13. [Google Scholar] [CrossRef]
- Reim, S.; Proft, A.; Heinz, S.; Höfer, M. Diversity of the European indigenous wild apple Malus sylvestris (L.) Mill. in the East Ore Mountains (Osterzgebirge), Germany: I. Morphological characterization. Genet. Resour. Crop Evol. 2012, 59, 1101–1114. [Google Scholar] [CrossRef]
- Rakonjac, V.; Mratinić, E.; Jovković, R.; Fotirić Akšić, M. Analysis of morphological variability in wild cherry (Prunus avium L.) genetic resources from Central Serbia. J. Agric. Sci. Tech. 2014, 16, 151–162. [Google Scholar]
- Khadivi, A.; Mohammadi, M.; Asgari, K. Morphological and pomological characterizations of sweet cherry (Prunus avium L.), sour cherry (Prunus cerasus L.) and duke cherry (Prunus × gondouinii Rehd.) to choose the promising selections. Sci. Hort. 2019, 257, 108719. [Google Scholar] [CrossRef]
- Albarouki, E.; Peterson, A. Molecular and morphological characterization of Crataegus L. species (Rosaceae) in southern Syria. Bot. J. Linn. Soc. 2007, 153, 255–263. [Google Scholar] [CrossRef]
- Yanar, M.; Ercisli, S.; Yilmaz, K.U.; Sahiner, H.; Taskin, T.; Zengin, Y.; Akgul, I.; Celik, F. Morphological and chemical diversity among hawthorn (Crataegus spp.) genotypes from Turkey. SRE 2011, 6, 35–38. [Google Scholar]
- Krüssmann, G. Handbuch der Laubgehölze; Paul Parkey in Berlin und Hamburg, Verlag für Landwirtschaft, Veterinärmedizin, Gartenbau und Forstwesen: Berlin, Germany, 1962; Volume 2. [Google Scholar]
- Hegi, G. Illustrierte Flora von Mitteleuropa; Blackwell Wissenschaft-Verlag: Berlin, Germany, 1995; pp. 353–356. [Google Scholar]
- Barengo, N.; Rudow, A.; Schwab, P. Förderung seltener Baumarten auf der Schweizer Alpennordseite; Buwal/Eidgenössische Forstdirektion and ETHZ/Professur Waldbau: Bern, Switzerland, 2001. [Google Scholar]
- Brus, R. Dendrologija za Gozdarje (Dendrology for Foresters), 2nd ed.; Biotechnical Faculty, University of Ljubljana: Ljubljana, Slovenia, 2008. [Google Scholar]
- Bignami, C. Service tree (Sorbus domestica L.). Description and use of service tree. Inf. Agrar. 2000, 56, 55–58. [Google Scholar]
- Ferrazzini, D.; Monteleone, I.; Belletti, P. Small-scale genetic diversity in oneseed hawthorn (Crataegus monogyna Jacq.). Eur. J. For. Res. 2008, 127, 407–414. [Google Scholar] [CrossRef]
- Rahmani, M.-S.; Shabanian, N.; Khadivi-Khub, A.; Woeste, K.E.; Badakhshan, H.; Alikhani, L. Population structure and genotypic variation of Crataegus pontica inferred by molecular markers. Gene 2015, 572, 123–129. [Google Scholar] [CrossRef]
- Popović, V.; Kerkez, I. Varijabilnost populacija divlje trešnje (Prunus avium L.) u Srbiji prema morfološkim svojstvima listova. Šumar. List 2016, 140, 347–355. [Google Scholar] [CrossRef]
- Popović, V.; Lučić, A.; Kerkez Janković, I.; Rakonjac, L.; Bogdan, S. Varijabilnost svojstava plodova kod provenijencija divlje trešnje (Prunus avium L.) u Srbiji. Šumar. List 2020, 144, 585–596. [Google Scholar] [CrossRef]
- Poljak, I.; Idžojtić, M.; Šapić, I.; Korijan, P.; Vukelić, J. Diversity and structure of Croatian continental and alpine-Dinaric populations of grey alder (Alnus incana/L./Moench subsp. incana): Isolation by distance and environment explains phenotypic divergence. Šumar. List 2018, 142, 19–32. [Google Scholar]
- Frankham, R. Do island populations have less genetic variation than mainland populations? Heredity 1997, 78, 311–327. [Google Scholar] [CrossRef] [PubMed]
- García-Verdugo, C.; Sajeva, M.; La Mantia, T.; Harrouni, C.; Msanda, F.; Caujapé-Castells, J. Do island plant populations really have lower genetic variation than mainland populations? Effects of selection and distribution range on genetic diversity estimates. Mol. Ecol. 2015, 24, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Morente-López, J.; García, C.; Lara-Romero, C.; García-Fernández, A.; Draper, D.; María Iriondo, J. Geography and environment shape landscape genetics of Mediterranean Alpine species Silene ciliata Poiret. (Caryophyllaceae). Front. Plant. Sci. 2018, 9, 1698. [Google Scholar] [CrossRef] [PubMed]
- Dowle, E.J.; Morgan-Richards, M.; Brescia, F.; Trewick, S.A. Correlation between shell phenotype and local environment suggests a role for natural selection in the evolution of Placostylus snails. Mol. Ecol. 2015, 24, 4205–4221. [Google Scholar] [CrossRef]
- Souci, S.W.; Fachmann, W.; Kraut, H. Die Zusammensetzung der Lebensmittel, Nährwert-Tabellen; Wissenschaftliche Verlagsgesellschaft MBH: Stuttgart, Germany, 1979. [Google Scholar]
- Raspé, O.; Findlay, C.; Jacquemart, A.-L. Sorbus aucuparia L. J. Ecol. 2000, 88, 910–930. [Google Scholar]
- Drvodelić, D.; Jemrić, T.; Oršanić, M. Jarebika (Sorbus aucuparia L.): Važnost, Uzgoj i Uporaba; University of Zagreb, Faculty of Forestry: Zagreb, Croatia, 2019. [Google Scholar]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Von Kausch-Blecken Schmeling, W. Der Speierling, 2nd ed.; Druckhaus Gottingen im Gottinger Tageblatt GmbH & Co.: Göttinger, Germany, 2000. [Google Scholar]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The Sorbus spp.—Underutilised plants for foods and nutraceuticals: Review on polyphenolic phytochemicals and antioxidant potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- Nielsen, E.; Temporiti, M.E.E.; Cella, R. Improvement of phytochemical production by plant cells and organ culture and by genetic engineering. Plant Cell. Rep. 2019, 38, 1199–1215. [Google Scholar] [CrossRef]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C.B. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected Pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food. Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Seguin, P.; Zheng, W.; Souleimanov, A. Alfalfa phytoestrogen content: Impact of plant maturity and herbage components. J. Agron. Crop. Sci. 2004, 190, 211–217. [Google Scholar] [CrossRef]
- Figueroa-Pérez, M.G.; Pérez-Ramírez, I.F.; Paredes-López, O.; Mondragón-Jacobo, C.; Reynoso-Camacho, R. Phytochemical composition and in vitro analysis of Nopal (O. Ficus-Indica) cladodes at different stages of maturity. Int. J. Food Prop. 2018, 21, 1728–1742. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Medicinal plants: Factors of influence on the content of secondary metabolites. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Borges, L.L.; Alves, S.F.; Sampaio, B.L.; Conceição, E.C.; Bara, M.T.F.; Paula, J.R. Environmental factors affecting the concentration of phenolic compounds in Myrcia tomentosa leaves. Rev. Bras. Pharmacogn. 2013, 23, 230–238. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Romero, L. Oxidative metabolism in tomato plants subjected to heat stress. J. Hortic. Sci. Biotech. 2004, 79, 560–564. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, D. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Hadi Rad, M.; Reza Asghari, M.; Hassan Asareh, M. Morphological, chemical and biochemical response to drought stress in pomegranate (Punica granatum L.) fruit. IJBPAS 2015, 4, 64–78. [Google Scholar]
- Hassani Moghaddam, E.; Esna-Ashari, M.; Shaaban, M. An investigation of the secondary metabolites and antioxidant capacity of some commercial Iranian pomegranate (Punica granatum L.) cultivars under drought stress. Herb. Med. J. 2018, 3, 14–25. [Google Scholar]
- Faraji, S.; Hadadinejad, M.; Abdosii, V.; Basaki, T.; Karami, S. Screening pomegranate (Punica granatum L.) genotypes for drought tolerance using physiological and phytochemical characteristics. Fruits 2020, 75, 130–140. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and Olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Herrera, R.; Pacheco-López, B.; Peragón, J. Water stress, irrigation and concentrations of pentacyclic triterpenes and phenols in Olea europaea L. cv. Picual Olive trees. Antioxidants 2019, 8, 294. [Google Scholar] [CrossRef]
- Lipan, L.; Cano-Lamadrid, M.-C.; Hernández, F.; Sendra, E.; Corell, M.; Vázquez-Araújo, L.; Moriana, A.; Carbonell-Barrachina, A.A. Long-term correlation between water deficit and quality markers in hydroSOStainable almonds. Agronomy 2020, 20, 1470. [Google Scholar] [CrossRef]
- Weidner, S.; Karolak, M.; Kramarać, M.; Kosinska, A.; Amarowicz, R. Phenolic compounds and properties of antioxidants in grapevine roots (Vitis vinifera L.) under drought stress followed by recovery. Acta Soc. Bot. Pol. 2009, 78, 97–103. [Google Scholar] [CrossRef]
- Dong-Ping, X.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.; Li, H. Natural antioxidants in foods and medicinal extractions, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar]
- Drvodelić, D.; Jemrić, T.; Oršanić, M. Oskoruša: Važnost, Uporaba i Uzgoj; University of Zagreb, Faculty of Forestry: Zagreb, Croatia, 2015. [Google Scholar]
- Maki, M.; Yamashiro, T.; Matsumura, S. High levels of genetic diversity in island populations of the island endemic Suzukia luchuensis (Labiatae). Heredity 2003, 91, 300–305. [Google Scholar] [CrossRef]
- Hufford, K.M.; Mazer, S.J.; Hodges, S.A. Genetic variation among mainland and island populations of a native perennial grass used in restoration. AoB PLANTS 2014, 6, plt055. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. Isolation by distance under diverse systems of mating. Genetics 1946, 31, 39–59. [Google Scholar] [CrossRef]
- Savolainen, O.; Kuittinen, H. Small Population Processes. In Forest Conservation Genetics—Principles and Practice; Young, A., Boshier, D., Boyle, T., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 91–100. [Google Scholar]
- Arnaud-Haond, S.; Teixeira, S.; Massa, S.I.; Billot, C.; Saenger, P.; Coupland, G.; Duarte, C.M.; Serrão, A. Genetic structure at range edge: Low diversity and high inbreeding in Southeast Asian mangorve (Avicennia marina) populations. Mol. Ecol. 2006, 15, 3515–3525. [Google Scholar] [CrossRef]
- Kamm, U.; Gugerli, F.; Rotach, P.; Edwards, P.; Holderegger, R. Open areas in a landscape enhance pollen-mediated gene flow of a tree species: Evidence from northern Switzerland. Landsc. Ecol. 2010, 25, 903–911. [Google Scholar] [CrossRef]
- Kamm, U.; Gugerli, F.; Rotach, P.; Edwards, P.; Holderegger, R. Genetic consequences of local tree densities—Implications for the management of naturally rare, insect pollinated species in temperate forests. Forest Ecol. Manag. 2011, 262, 1047–1053. [Google Scholar] [CrossRef]
- Oddou-Muratorio, S.; Petit, R.J.; Le Guerroue, B.; Guesnet, D.; Demesure, B. Pollen- versus seed-mediated gene flow in a scattered forest tree species. Evolution 2001, 55, 1123–1135. [Google Scholar] [CrossRef]
- Oddou-Muratorio, S.; Klein, E.K.; Austerlitz, F. Pollen-flow in the wild service tree, Sorbus torminalis (L.) Crantz. II. Pollen dispersal and heterogeneity in mating success inferred from parent-offspring analysis. Mol. Ecol. 2005, 14, 4441–4452. [Google Scholar] [CrossRef] [PubMed]
- Oddou-Muratorio, S.; Klein, E.K.; Demesure-Musch, B.; Austerlitz, F. Real-time patterns of pollen flow in the wild service tree, Sorbus torminalis (Rosaceae). III. Mating patterns and the ecological maternal neighbourhood. Am. J. Bot. 2006, 93, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Guo, W.; Xu, W.; Wei, V.; Wang, R. Leaf morphology correlates with water and light availability: What consequences for simple and compound leaves? Prog. Nat. Sci. 2009, 19, 1789–1798. [Google Scholar] [CrossRef]
- Vitasse, Y.; Lenz, A.; Kollas, C.; Randin, C.F.; Hoch, G.; Korner, C. Genetic vs. non-genetic responses of leaf morphology and growth to elevation in temperate tree species. Funct. Ecol. 2013, 28, 243–252. [Google Scholar] [CrossRef]
- Sander, N.L.; da Silva, C.J.; Duarte, A.V.M.; Zago, B.W.; Galbiati, C.; Viana, I.G.; de Arruda, J.C.; Dardengo, J.E.; Poletine, J.P.; Siqueira Leite, M.H.; et al. The influence of environmental features on the morphometric variation in Mauritia flexuosa L.f. fruits and seeds. Plants 2020, 9, 1304. [Google Scholar] [CrossRef]
- Dubuc-Messier, G.; Réale, D.; Perret, P.; Charmantier, A. Environmental heterogeneity and population differences in blue tits personality traits. Behav. Ecol. 2017, 28, 448–459. [Google Scholar] [CrossRef]
- DeWoody, J.; Trewin, H.; Taylor, G. Genetic and morphological differentiation in Populus nigra L.: Isolation by colonization or isolation by adaptation? Mol. Ecol. 2015, 24, 2641–2655. [Google Scholar] [CrossRef] [PubMed]
- WinFolia, T.M. WinFOLIA Software Program; Version PRO 2005b; Regent Instruments Inc.: Quebec, QC, Canada, 2001. [Google Scholar]
- WinSeedle, T.M. For Seed and Conifer Needles Analysis; Version 2011a; Regent Instruments Inc.: Quebec, QC, Canada, 2010. [Google Scholar]
- AOAC International. Fruits and Fruit Products—Moisture in Fruits and Fruit Products; AOAC Official method 925.03; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC International. Fruits and Fruit Products—Ash Content in Fruits and Fruit Products; AOAC Official method 923.03; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC International. Fruits and Fruit Products—Crude Proteins in Fruits and Fruit Products; AOAC Official method 992.15; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC International. Fruits and Fruit Products—Total Fat in Fruits and Fruit Products; AOAC Official method 989.05; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC International. Fruits and Fruit Products—Cellulose in Fruits and Fruit Products; AOAC Official method 973.18; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC International. Fruits and Fruit Products—Sucrose in Fruits and Fruit Products; AOAC Official method 925.35; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid and radical scavenging activity of Rubus, Ribes and Aronia. JFS Food Chem. Toxycol. 2004, 69, 164–169. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Methods in Ecology; Blackwell Scientific Publications: Hoboken, NJ, USA; Oxford, UK, 1994. [Google Scholar]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables: Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Kazazić, S.P. Antioksidacijska i antiradiklaska aktivnost flavonoida. Arh. Hig. Rada Toksikol. 2004, 55, 279–290. [Google Scholar] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tolić, M.T.; Jurčević, I.L.; Panjkota Krbavčić, I.; Marković, K.; Vahčić, N. Antioxidant properties of Chokeberry products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [PubMed]
- Mrkonjić, Z. Fitohemijska Karakterizacija i Biohemijska Ispitivanja Plodova Vrsta Roda Sorbus L. 1753 (Rosaceae, Maloideae) Kao Izvora Prirodnih Nutraceutika. Ph.D. Thesis, Faculty of Science, University of Novi Sad, Novi Sad, Serbia, 2017. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; Freeman and Co.: San Francisco, CA, USA, 1989. [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System); Version 8.0; StatSoft, Inc.: Tulsa, OK, USA, 2001. [Google Scholar]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Smouse, P.E.; Long, J.C.; Sokal, R. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst. Zool. 1986, 35, 627–632. [Google Scholar] [CrossRef]
- Manly, B.F.J. Randomization, Bootstrap and Monte Carlo Methods in Biology, 3rd ed.; Chapman & Hall/CRC, Taylor & Francis Group: Boca Raton, FL, USA, 2007; p. 480. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’: Community Ecology Package. Version 2.4-2. 2017. Available online: https://github.com/vegandevs/vegan (accessed on 24 June 2021).
- McGarigal, K.; Cushman, S.; Stafford, S. Multivariate Statistics for Wildlife and Ecology Research; Springer Verlag: New York, NY, USA, 2000; 283p. [Google Scholar]
- Douaihy, B.; Sobierajska, K.; Jasińska, A.K.; Boratyńska, K.; Ok, T.; Romo, A.; Machon, N.; Didukh, Y.; Dagher-Kharrat, M.B.; Boratyński, A. Morphological versus molecular markers to describe variability in Juniperus excelsa subsp. excelsa (Cupressaceae). AoB Plants 2012, 2012, pls003. [Google Scholar] [CrossRef] [PubMed]
- Boratyński, A.; Jasińska, A.K.; Marcysiak, K.; Mazur, M.; Romo, A.M.; Boratyńska, K.; Sobierajska, K.; Iszkuło, G. Morphological differentiation supports the genetic pattern of the geographic structure of Juniperus thurifera (Cupressaceae). Plant Syst. Evol. 2013, 299, 773–784. [Google Scholar] [CrossRef]
- Sobierajska, K.; Boratyńska, K.; Jasińska, A.; Dering, M.; Ok, T.; Douaihy, B.; Dagher-Kharrat, M.B.; Romo, A.; Boratyński, A. Effect of the Aegean Sea barrier between Europe and Asia on differentiation in Juniperus drupacea (Cupressaceae). Bot. J. Linn. Soc. 2016, 180, 365–385. [Google Scholar] [CrossRef]
- Manni, F.; Guerard, E.; Heyer, E. Geographic patterns of (genetic, morphologic, linguistic) variation: How barriers can be detected by ‘Monmonier’s algorithm’. Hum. Biol. 2004, 76, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Koutecky, P. MorphoTools: A set of R functions for morphometric analysis. Plant Syst. Evol. 2015, 301, 1115–1121. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistics in Biological Research, 4th ed.; W.H. Freeman and Co.: New York, NY, USA, 2012; p. 973. [Google Scholar]

| Population | m | FL | MFW | PMFW | FW1 | FW2 | FW/FL | SL | SW | NS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | |
| P01 | 9.53f | 27.34 | 2.37f | 10.24 | 2.40f | 11.15 | 0.99f | 17.72 | 1.91c | 12.67 | 1.56c | 16.12 | 1.02a | 9.84 | 7.26e | 9.06 | 5.51e | 10.83 | 1.35ab | 51.85 |
| P02 | 8,91e | 32.22 | 2.26e | 16.03 | 2.31a | 12.89 | 0.92e | 19.30 | 1.87d | 15.67 | 1.53c | 14.22 | 1.03a | 10.75 | 6.68c | 13.66 | 5.23d | 14.33 | 1.48c | 54.40 |
| P03 | 7.01a | 26.03 | 2.09a | 7.36 | 2.04c | 8.92 | 0.85a | 15.32 | 1.60a | 13.15 | 1.28a | 14.71 | 0.98e | 10.41 | 6.72cd | 11.05 | 4.85a | 13.72 | 1.46bc | 37.14 |
| P04 | 7.49d | 31.45 | 2.15d | 11.56 | 2.31a | 12.02 | 0.85a | 21.12 | 1.92c | 13.81 | 1.46b | 17.33 | 1.08d | 6.97 | 6.39a | 9.42 | 5.07b | 10.65 | 1.25a | 47.59 |
| P05 | 6.86a | 30.95 | 2.06a | 11.44 | 2.21e | 12.56 | 0.88d | 18.42 | 1.76b | 15.55 | 1.46b | 15.20 | 1.07cd | 8.53 | 6.80d | 9.54 | 5.12b | 9.59 | 1.97d | 46.18 |
| P06 | 5.09b | 36.12 | 1.88b | 14.96 | 1.98b | 12.43 | 0.75b | 20.90 | 1.61a | 13.46 | 1.25a | 16.14 | 1.06bc | 9.18 | 6.48ab | 10.19 | 4.42c | 14.56 | 3.66e | 25.49 |
| P07 | 6.34c | 28.54 | 1.99c | 11.84 | 2.09d | 10.92 | 0.78c | 19.47 | 1.75b | 12.67 | 1.32d | 18.41 | 1.06b | 11.83 | 6.53b | 13.55 | 4.87a | 14.09 | 2.00d | 56.25 |
| Total | 7.32 | 36.04 | 2.37 | 10.24 | 2.19 | 13.48 | 0.86 | 20.78 | 1.77 | 15.62 | 1.41 | 18.07 | 1.04 | 10.17 | 6.70 | 11.71 | 5.01 | 14.08 | 1.88 | 60.06 |
| Variable | Components of the Variance | df | F | Percent of Variability | p-Value |
|---|---|---|---|---|---|
| m | Population | 6 | 5.27 | 25.44 | 0.000401 |
| Tree (Population) | 42 | 62.22 | 41.04 | 0.000000 | |
| Error | 33.52 | ||||
| FL | Population | 6 | 4.75 | 22.68 | 0.000898 |
| Tree (Population) | 42 | 59.47 | 41.68 | 0.000000 | |
| Error | 35.64 | ||||
| MFW | Population | 6 | 5.24 | 22.49 | 0.000422 |
| Tree (Population) | 42 | 45.13 | 36.34 | 0.000000 | |
| Error | 41.17 | ||||
| PMFW | Population | 6 | 6.34 | 16.57 | 0.000084 |
| Tree (Population) | 42 | 17.27 | 20.49 | 0.000000 | |
| Error | 62.94 | ||||
| FW1 | Population | 6 | 4.48 | 17.66 | 0.001365 |
| Tree (Population) | 42 | 37.19 | 34.57 | 0.000000 | |
| Error | 47.77 | ||||
| FW2 | Population | 6 | 4.50 | 18.36 | 0.001325 |
| Tree (Population) | 42 | 40.08 | 35.82 | 0.000000 | |
| Error | 45.82 | ||||
| FW/FL | Population | 6 | 1.32 | 2.48 | 0.270911 |
| Tree (Population) | 42 | 62.51 | 53.80 | 0.000000 | |
| Error | 43.73 | ||||
| SL | Population | 6 | 1.52 | 4.48 | 0.196602 |
| Tree (Population) | 42 | 85.93 | 60.12 | 0.000000 | |
| Error | 35.40 | ||||
| SW | Population | 6 | 5.20 | 18.65 | 0.000445 |
| Tree (Population) | 42 | 30.35 | 30.09 | 0.000000 | |
| Error | 51.26 | ||||
| NS | Population | 6 | 13.27 | 47.02 | 0.000000 |
| Tree (Population) | 42 | 50.24 | 26.29 | 0.000000 | |
| Error | 26.69 |
| Population | Water | Crude Protein | Sugar | Ash | Crude Fat | Cellulose | Acidity | Total Phenols | DPPH | FRAP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | M | CV | |
| P01 | 69.50d | 2.78 | 15.32ac | 14.47 | 49.94a | 4.81 | 1.90ab | 16.39 | 0.59d | 16.01 | 5.93ab | 10.68 | 0.74a | 13.14 | 11.11c | 63.19 | 11.99a | 10.50 | 2.41ac | 12.58 |
| P02 | 67.81ad | 2.18 | 12.49b | 20.29 | 49.44a | 8.49 | 2.01ab | 44.11 | 0.60bd | 9.22 | 6.01ab | 20.12 | 0.64a | 19.79 | 12.10c | 16.80 | 4.40b | 54.74 | 2,91bc | 1.71 |
| P03 | 66.52ac | 1.59 | 16.85a | 18.61 | 50.70a | 6.95 | 2.19a | 14.23 | 0.80ac | 12.98 | 5.54ab | 6.42 | 0.70a | 8.25 | 9.44bc | 38.23 | 17.76c | 19.42 | 2.49ab | 4.20 |
| P04 | 67.05a | 2.09 | 12.50b | 8.41 | 55.60c | 4.99 | 1.44b | 20.05 | 0.85c | 19.53 | 5.33ab | 8.58 | 0.68a | 10.04 | 6.57ab | 50.33 | 13.61a | 47.36 | 2.69abc | 5.94 |
| P05 | 64.24bc | 3.01 | 16.67a | 14.73 | 49.47a | 3.90 | 2.29ac | 8.09 | 0.78ac | 7.47 | 5.13a | 11.20 | 0.67a | 5.24 | 5.62a | 42.17 | 13.76a | 25.88 | 2.76bc | 12.32 |
| P06 | 56.80e | 6.28 | 15.63ac | 19.56 | 42.88b | 7.66 | 2.91c | 33.90 | 0.72ab | 15.62 | 9.06c | 32.34 | 0.64a | 28.20 | 3.50a | 57.44 | 11.70a | 12.13 | 2.50ab | 12.77 |
| P07 | 64.05b | 3.99 | 13.41bc | 21.96 | 47.50a | 6.83 | 2.13ab | 41.78 | 0.71ab | 22.04 | 6.65b | 10.83 | 0.67a | 11.26 | 6.82ab | 53.65 | 12.57a | 39.97 | 2.50ab | 23.37 |
| Total | 65.14 | 6.70 | 14.69 | 20.27 | 49.36 | 9.33 | 2.12 | 34.23 | 0.72 | 19.52 | 6.24 | 27.95 | 0.68 | 14.90 | 7.88 | 57.48 | 11.81 | 44.00 | 2.61 | 13.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poljak, I.; Vahčić, N.; Liber, Z.; Tumpa, K.; Pintar, V.; Zegnal, I.; Vidaković, A.; Valković, B.; Kajba, D.; Idžojtić, M. Morphological and Chemical Diversity and Antioxidant Capacity of the Service Tree (Sorbus domestica L.) Fruits from Two Eco-Geographical Regions. Plants 2021, 10, 1691. https://doi.org/10.3390/plants10081691
Poljak I, Vahčić N, Liber Z, Tumpa K, Pintar V, Zegnal I, Vidaković A, Valković B, Kajba D, Idžojtić M. Morphological and Chemical Diversity and Antioxidant Capacity of the Service Tree (Sorbus domestica L.) Fruits from Two Eco-Geographical Regions. Plants. 2021; 10(8):1691. https://doi.org/10.3390/plants10081691
Chicago/Turabian StylePoljak, Igor, Nada Vahčić, Zlatko Liber, Katarina Tumpa, Valentino Pintar, Ivana Zegnal, Antonio Vidaković, Bernarda Valković, Davorin Kajba, and Marilena Idžojtić. 2021. "Morphological and Chemical Diversity and Antioxidant Capacity of the Service Tree (Sorbus domestica L.) Fruits from Two Eco-Geographical Regions" Plants 10, no. 8: 1691. https://doi.org/10.3390/plants10081691
APA StylePoljak, I., Vahčić, N., Liber, Z., Tumpa, K., Pintar, V., Zegnal, I., Vidaković, A., Valković, B., Kajba, D., & Idžojtić, M. (2021). Morphological and Chemical Diversity and Antioxidant Capacity of the Service Tree (Sorbus domestica L.) Fruits from Two Eco-Geographical Regions. Plants, 10(8), 1691. https://doi.org/10.3390/plants10081691







