Will Plant Genome Editing Play a Decisive Role in “Quantum-Leap” Improvements in Crop Yield to Feed an Increasing Global Human Population?
Abstract
1. Introduction
2. The Green Revolution and Its Genes
3. Theoretical Limits on Crop Productivity. A Complex System Cannot Be Predictably Modified but Can Be Replaced by a Functionally Similar Complex System
4. Complexity of Agronomic Traits
5. Nitrogen Input
6. Is It Possible to Transfer Nitrogen-Fixing Genes from Legumes to Non-Legumes?
7. Taking Advantage of Plant–Microbe Interactions
8. Plant-Mediated Strategies for Shaping the Rhizosphere Microbiome
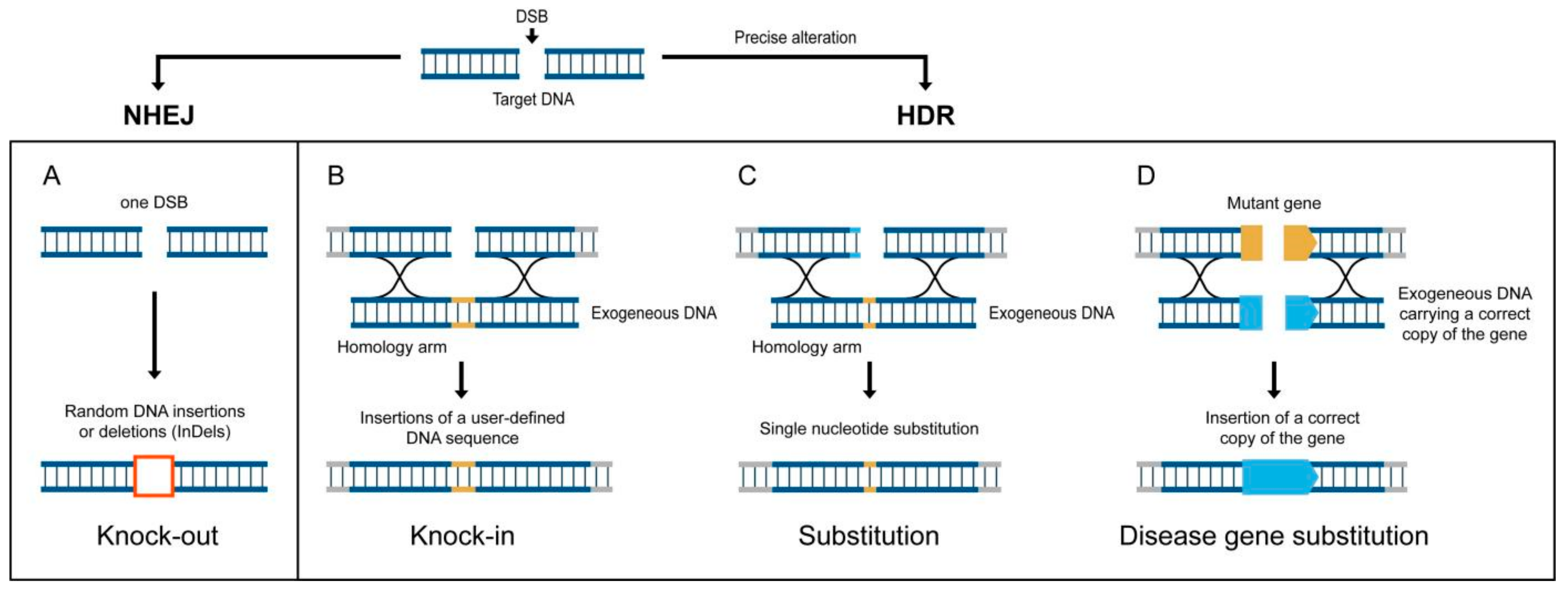
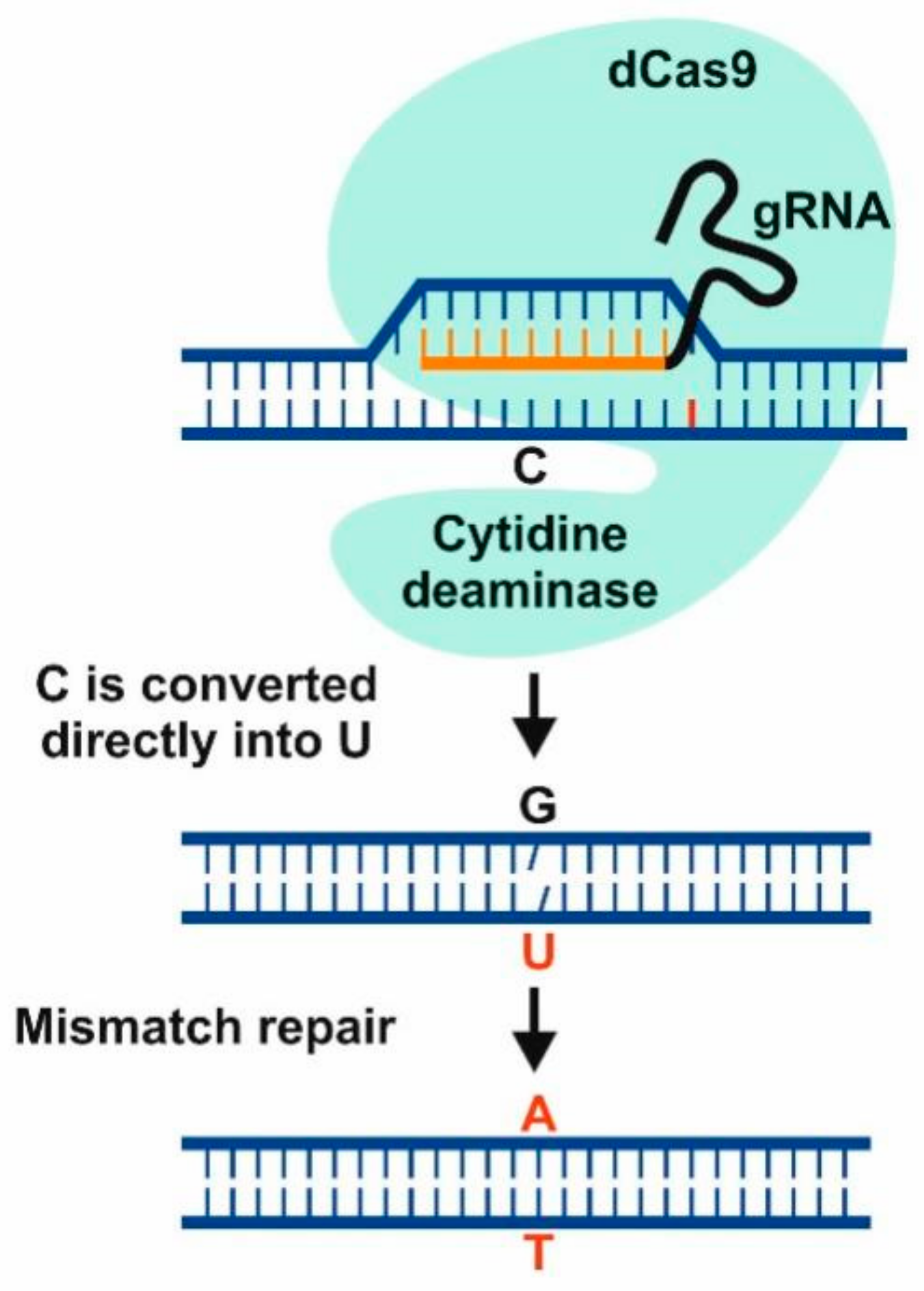
9. Genome Editing Technologies for “Quantum-Leap” Improvements in Yield-Limited Crops Are Ready, but Where Are the Targets?
10. Success Stories of CRISPR/Cas9
11. Reference Genomes and Assessment of Genomic Variation
12. The Research Bottleneck in Plant Sciences Is Shifting from Genotyping to Phenotyping
13. The Use of Locally Available Resources to Adapt to Climate Variability and Change
14. De Novo Domestication of Wild Plants
15. Reference, Pan-, and Super-Pan Genome Sequences Provide a Strong Basis for the Location of Domestication Syndrome Genes
16. Conclusions. Must I Be Cruel Only to Be Kind?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tortell, P.D. Earth 2020: Science, society, and sustainability in the Anthropocene. Proc. Natl. Acad. Sci. USA 2020, 117, 8683–8691. [Google Scholar] [CrossRef]
- Harte, J. Patient Earth; Socolow, R.H., Ed.; Holt, Rinehart, and Winston: New York, NY, USA, 1971. [Google Scholar]
- Meadows, D.; Meadows, D.; Randers, J.; Behrens, W.W., III. The Limits to Growth. A Report for the Club of Rome’s Project on the Predicament of Mankind; Universe Books New York: New York, NY, USA, 1972. [Google Scholar]
- Tollefson, J. The hard truths of climate change—By the numbers. Nature 2019, 573, 324–327. [Google Scholar] [CrossRef] [PubMed]
- NOAA’s National Centers for Environmental Information (NCEI). Temperature Change and Carbon Dioxide Change. Available online: https://www.ncdc.noaa.gov/global-warming/temperature-change (accessed on 12 February 2018).
- Hartfield, G.; Blunden, J.; Arndt, D. State of the climate in 2017. Bull. Am. Meteorol. Soc. 2018, 99, Si–S310. [Google Scholar] [CrossRef]
- Pöschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem. Int. Ed. Engl. 2005, 44, 7520–7540. [Google Scholar] [CrossRef]
- Ramankutty, N.; Mehrabi, Z.; Waha, K.; Jarvis, L.; Kremen, C.; Herrero, M.; Rieseberg, L.H. Trends in Global Agricultural Land Use: Implications for Environmental Health and Food Security. Annu. Rev. Plant Biol. 2018, 69, 789–815. [Google Scholar] [CrossRef] [PubMed]
- Fanzo, J.; Covic, N.; Dobermann, A.; Henson, S.; Herrero, M.; Pingali, P.; Staal, S. A research vision for food systems in the 2020s: Defying the status quo. Glob. Food Secur. 2020, 26, 100397. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, L.; Bravo, G.; Clough, Y.; Nicholas, K. From population to production: 50 years of scientific literature on how to feed the world. Glob. Food Secur. 2020, 24, 100346. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Science Breakthroughs to Advance Food and Agricultural Research by 2030; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Searchinger, T.; Waite, R.; Hanson, C.; Ranganathan, J.; Matthews, E. Creating a Sustainable Food Future: A Menu of Solutions to Feed Nearly 10 Billion People by 2050. Final Report. 2019. Available online: https://www.wri.org/research/creating-sustainable-food-future (accessed on 19 July 2019).
- Gerten, D.; Heck, V.; Jägermeyr, J.; Bodirsky, B.; Fetzer, I.; Jalava, M.; Kummu, M.; Lucht, W.; Rockström, J.; Schaphoff, S.; et al. Feeding ten billion people is possible within four terrestrial planetary boundaries. Nat. Sustain. 2020, 3, 200–208. [Google Scholar] [CrossRef]
- Borelli, T.; Hunter, D.; Padulosi, S.; Amaya, N.; Meldrum, G.; de Oliveira Beltrame, D.; Samarasinghe, G.; Wasike, V.W.; Güner, B.; Tan, A.; et al. Local Solutions for Sustainable Food Systems: The Contribution of Orphan Crops and Wild Edible Species. Agronomy 2020, 10, 231. [Google Scholar] [CrossRef]
- Zafar, K.; Sedeek, K.E.M.; Rao, G.S.; Khan, M.Z.; Amin, I.; Kamel, R.; Mukhtar, Z.; Zafar, M.; Mansoor, S.; Mahfouz, M.M. Genome Editing Technologies for Rice Improvement: Progress, Prospects, and Safety Concerns. Front. Genome Ed. 2020, 2, 5. [Google Scholar] [CrossRef]
- Zaman, Q.; Lia, C.; Chenga, H.; Hua, Q. Genome editing opens a new era of genetic improvement in polyploid crop. Crop. J. 2019, 7, 141–150. [Google Scholar] [CrossRef]
- Piesse, M. Global Food and Water Security in 2050: Demographic Change and Increased Demand. Strategic Analysis Paper. 2020. Available online: https://www.futuredirections.org.au/wp-content/uploads/2020/02/Global-Food-and-Water-Security-in-2050-Demographic-Change-and-Increased-Demand.pdf (accessed on 4 February 2020).
- Weber, A.P.M.; Bar-Even, A. Update: Improving the Efficiency of Photosynthetic Carbon Reactions. Plant Physiol. 2019, 179, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Fraser, E.D.G. The challenge of feeding a diverse and growing population. Physiol. Behav. 2020, 221, 112908. [Google Scholar] [CrossRef] [PubMed]
- Rockstrom, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S., 3rd; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockstrom, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Lade, S.; Steffen, W.; de Vries, W.; Carpenter, S.; Donges, J.; Gerten, D.; Hoff, H.; Newbold, T.; Richardson, K.; Rockström, J. Human impacts on planetary boundaries amplified by Earth system interactions. Nat. Sustain. 2020, 3, 119–128. [Google Scholar] [CrossRef]
- Biermann, F.; Kim, R. Framework: A CriticalAppraisal of Approaches toDefine a “Safe OperatingSpace” for Humanity. Annu. Rev. Environ. Resour. 2020, 45, 4.1–4.25. [Google Scholar] [CrossRef]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef]
- Global Sustainable Development Report 2019: The Future Is Now—Science for Achieving Sustainable Development. 2019. Available online: https://sustainabledevelopment.un.org/globalsdreport/2019 (accessed on 11 September 2019).
- Capstaff, N.M.; Miller, A.J. Improving the Yield and Nutritional Quality of Forage Crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef]
- Richards, N.; Nielsen, B.D.; Finno, C.J. Nutritional and Non-nutritional Aspects of Forage. Vet. Clin. Equine Pract. 2021, 37, 43–61. [Google Scholar] [CrossRef]
- Kulkarni, K.P.; Tayade, R.; Asekova, S.; Song, J.T.; Shannon, J.G.; Lee, J.D. Harnessing the Potential of Forage Legumes, Alfalfa, Soybean, and Cowpea for Sustainable Agriculture and Global Food Security. Front. Plant Sci. 2018, 9, 1314. [Google Scholar] [CrossRef]
- Hu, H.; Scheben, A.; Edwards, D. Advances in Integrating Genomics and Bioinformatics in the Plant Breeding Pipeline. Agriculture 2018, 8, 75. [Google Scholar] [CrossRef]
- Zhu, X.G.; Ort, D.R.; Parry, M.A.J.; von Caemmerer, S. A wish list for synthetic biology in photosynthesis research. J. Exp. Bot. 2020, 71, 2219–2225. [Google Scholar] [CrossRef]
- Rönspies, M.; Dorn, A.; Schindele, P.; Puchta, H. CRISPR–Cas-mediated chromosome engineering for crop improvement and synthetic biology. Nat. Plants 2021, 7, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, P.; Dessimoz, C. Gene Ontology: Pitfalls, Biases, and Remedies. Methods Mol. Biol. 2017, 1446, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Danchin, A.; Ouzounis, C.; Tokuyasu, T.; Zucker, J.D. No wisdom in the crowd: Genome annotation in the era of big data—Current status and future prospects. Microb. Biotechnol. 2018, 11, 588–605. [Google Scholar] [CrossRef]
- Armanda, D.T.; Guinéea, J.B.; Tukkerac, A. The second green revolution: Innovative urban agriculture’s contribution to food security and sustainability—A review. Glob. Food Secur. 2019, 22, 13–24. [Google Scholar] [CrossRef]
- Kwon, C.T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef]
- Van-Erp, H. Can We Create a New Green Revolution to Avert the Coming Global Food Crisis? Ann. Agric. Crop. Sci. 2016, 1, 1001. Available online: https://austinpublishinggroup.com/agriculture-crop-sciences/fulltext/aacs-v1-id1001.php (accessed on 7 September 2016).
- Lynch, J. Roots of the second green revolution. Aust. Bot. 2007, 55, 493–512. [Google Scholar] [CrossRef]
- Wurschum, T.; Langer, S.M.; Longin, C.F.H.; Tucker, M.R.; Leiser, W.L. A modern Green Revolution gene for reduced height in wheat. Plant J. 2017, 92, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Monna, L.; Kitazawa, N.; Yoshino, R.; Suzuki, J.; Masuda, H.; Maehara, Y.; Tanji, M.; Sato, M.; Nasu, S.; Minobe, Y. Positional cloning of rice semidwarfing gene, sd-1: Rice “green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res. 2002, 9, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Sasaki, A.; Ashikari, M.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Swapan, D.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Green revolution: A mutant gibberellin-synthesis gene in rice. Nature 2002, 416, 701–702. [Google Scholar] [CrossRef]
- Wang, B.; Li, J. Understanding the Molecular Bases of Agronomic Trait Improvement in Rice. Plant Cell 2019, 31, 1416–1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, S.; Chao, S.; Sun, Q.; Liu, S.; Xia, G. A Genome-Wide Association Study of Highly Heritable Agronomic Traits in Durum Wheat. Front. Plant Sci. 2019, 10, 919. [Google Scholar] [CrossRef]
- Thomas, S.G. Novel Rht-1 dwarfing genes: Tools for wheat breeding and dissecting the function of DELLA proteins. J. Exp. Bot. 2017, 68, 354–358. [Google Scholar] [CrossRef]
- Grant, N.P.; Mohan, A.; Sandhu, D.; Gill, K.S. Inheritance and Genetic Mapping of the Reduced Height (Rht18) Gene in Wheat. Plants 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ma, J.; Hou, L.; Shi, S.; Sun, J.; Li, G.; Zhao, C.; Xia, H.; Zhao, S.; Wang, X.; et al. Transcriptome profiling provides insights into molecular mechanism in Peanut semi-dwarf mutant. BMC Genom. 2020, 21, 211. [Google Scholar] [CrossRef]
- Chen, X.; Xu, P.; Zhou, J.; Tao, D.; Yu, D. Mapping and breeding value evaluation of a semi-dominant semi-dwarf gene in upland rice. Plant Divers. 2018, 40, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Serrano, A.; Cantos, C.; Assmann, S.M. The Role of Dwarfing Traits in Historical and Modern Agriculture with a Focus on Rice. Cold Spring Harb. Perspect. Biol. 2019, 11, a034645. [Google Scholar] [CrossRef]
- Tomlinson, L.; Yang, Y.; Emenecker, R.; Smoker, M.; Taylor, J.; Perkins, S.; Smith, J.; MacLean, D.; Olszewski, N.E.; Jones, J.D.G. Using CRISPR/Cas9 genome editing in tomato to create a gibberellin-responsive dominant dwarf DELLA allele. Plant Biotechnol. J. 2019, 17, 132–140. [Google Scholar] [CrossRef]
- Jia, X.; Yu, L.; Tang, M.; Tian, D.; Yang, S.; Zhang, X.; Traw, M.B. Pleiotropic changes revealed by in situ recovery of the semi-dwarf gene sd1 in rice. J. Plant Physiol. 2020, 248, 153141. [Google Scholar] [CrossRef]
- Kumar, J.; Gupta, D.S.; Gupta, S.; Dubey, S.; Gupta, P.; Kumar, S. Quantitative trait loci from identification to exploitation for crop improvement. Plant Cell Rep. 2017, 36, 1187–1213. [Google Scholar] [CrossRef]
- Liu, F.; Wang, P.; Zhang, X.; Li, X.; Yan, X.; Fu, D.; Wu, G. The genetic and molecular basis of crop height based on a rice model. Planta 2018, 247, 1–26. [Google Scholar] [CrossRef]
- Lieberman-Lazarovich, M.; Yahav, C.; Israeli, A.; Efroni, I. Deep Conservation of cis-Element Variants Regulating Plant Hormonal Responses. Plant Cell 2019, 31, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Nadolska-Orczyk, A.; Rajchel, I.K.; Orczyk, W.; Gasparis, S. Major genes determining yield-related traits in wheat and barley. Theor. Appl. Genet. 2017, 130, 1081–1098. [Google Scholar] [CrossRef]
- Norman, B. The Green Revolution, Peace, and Humanity—Nobel Lecture. Available online: http://www.nobelprize.org/prizes/peace/1970/borlaug/lecture/ (accessed on 11 August 2021).
- Denance, N.; Sanchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2018, 19, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Pilet-Nayel, M.L.; Moury, B.; Caffier, V.; Montarry, J.; Kerlan, M.C.; Fournet, S.; Durel, C.E.; Delourme, R. Quantitative Resistance to Plant Pathogens in Pyramiding Strategies for Durable Crop Protection. Front. Plant Sci. 2017, 8, 1838. [Google Scholar] [CrossRef]
- Wiesner-Hanks, T.; Nelson, R. Multiple Disease Resistance in Plants. Annu. Rev. Phytopathol. 2016, 54, 229–252. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Rant, J.C. Fitness costs and trade-offs of disease resistance and their consequences for breeding arable crops. Plant Pathol. 2013, 62 (Suppl. 1), 83–95. [Google Scholar] [CrossRef]
- Comont, D.; Knight, C.; Crook, L.; Hull, R.; Beffa, R.; Neve, P. Alterations in Life-History Associated with Non-target-site Herbicide Resistance in Alopecurus myosuroides. Front. Plant Sci. 2019, 10, 837. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Bass, C. Does resistance really carry a fitness cost? Curr. Opin. Insect Sci. 2017, 21, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Cao, J.; Zhang, J.; Xia, F.; Ke, Y.; Zhang, H.; Xie, W.; Liu, H.; Cui, Y.; Cao, Y.; et al. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Koller, T.; Brunner, S.; Herren, G.; Sanchez-Martin, J.; Hurni, S.; Keller, B. Field grown transgenic Pm3e wheat lines show powdery mildew resistance and no fitness costs associated with high transgene expression. Transgenic Res. 2019, 28, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.S.; Chen, L.Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Daviere, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Van De Velde, K.; Ruelens, P.; Geuten, K.; Rohde, A.; Van Der Straeten, D. Exploiting DELLA Signaling in Cereals. Trends Plant Sci. 2017, 22, 880–893. [Google Scholar] [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lison, P.; Nemri, A.; Harberd, N.P.; Jones, J.D. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.J.; Alborn, H.T.; Block, A.K.; Christensen, S.A.; Hunter, C.T.; Rering, C.C.; Seidl-Adams, I.; Stuhl, C.J.; Torto, B.; Tumlinson, J.H. Interactions Among Plants, Insects, and Microbes: Elucidation of Inter-Organismal Chemical Communications in Agricultural Ecology. J. Agric. Food Chem. 2018, 66, 6663–6674. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Karasov, T.L.; Chae, E.; Herman, J.J.; Bergelson, J. Mechanisms to Mitigate the Trade-Off between Growth and Defense. Plant Cell 2017, 29, 666–680. [Google Scholar] [CrossRef]
- Zhang, L.; Watson, L.T. Analysis of the fitness effect of compensatory mutations. HFSP J. 2009, 3, 47–54. [Google Scholar] [CrossRef]
- Wang, R.; Angenent, G.C.; Seymour, G.; de Maagd, R.A. Revisiting the Role of Master Regulators in Tomato Ripening. Trends Plant Sci. 2020, 25, 291–301. [Google Scholar] [CrossRef]
- Wang, Y.; Richards, M.; Dorus, S.; Priest, B.K.; Bryson, J.J. Compensatory mutation can drive gene regulatory network evolution. BioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2019.12.18.881276v2.full (accessed on 18 May 2018). [CrossRef]
- Bugbee, B.; Monje, O. The limits of crop productivity: Validating theoretical estimates and determining the factors that limit crop yields in optimal environments. Bioscience 1992, 42, 494–502. [Google Scholar] [CrossRef]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Lawson, T. Natural genetic variation in photosynthesis: An untapped resource to increase crop yield potential? Plant J. 2020, 101, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Moses, T. Shedding Light on the Power of Light. Plant Physiol. 2019, 179, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Shmakov, N.A.; Vasiliev, G.V.; Shatskaya, N.V.; Doroshkov, A.V.; Gordeeva, E.I.; Afonnikov, D.A.; Khlestkina, E.K. Identification of nuclear genes controlling chlorophyll synthesis in barley by RNA-seq. BMC Plant Biol. 2016, 16, 245. [Google Scholar] [CrossRef]
- Barajas-Lopez Jde, D.; Blanco, N.E.; Strand, A. Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim. Biophys. Acta 2013, 1833, 425–437. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Dann, M.; Leister, D. Enhancing (crop) plant photosynthesis by introducing novel genetic diversity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160380. [Google Scholar] [CrossRef]
- Leister, D. Genetic Engineering, Synthetic Biology and the Light Reactions of Photosynthesis. Plant Physiol. 2019, 179, 778–793. [Google Scholar] [CrossRef]
- Batista-Silva, W.; da Fonseca-Pereira, P.; Martins, A.; Zsogon, A.; Nunes-Nesi, A.; Araujo, W. Engineering Improved Photosynthesis in the Era of Synthetic Biology. Plant Comm. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Simkin, A.J. Genetic Engineering for Global Food Security: Photosynthesis and Biofortification. Plants 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Han, Y.; Liu, G.; An, B.; Yang, J.; Yang, G.; Li, Y.; Zhu, Y. Overexpression of sedoheptulose-1,7-bisphosphatase enhances photosynthesis and growth under salt stress in transgenic rice plants. Funct. Plant Biol. 2007, 34, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 2016, 6, 32741. [Google Scholar] [CrossRef]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawson, T.; Parry, M.A.J.; Raines, C.A. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160384. [Google Scholar] [CrossRef]
- South, P.F.; Cavanagh, A.P.; Liu, H.W.; Ort, D.R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 2019, 363, eaat9077. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Doucha, J.; Livansky, K.; Kotrbacek, V.; Zachleder, V. Production of Chlorella biomass enriched by selenium and its use in animal nutrition: A review. Appl. Microbiol. Biotechnol. 2009, 83, 1001–1008. [Google Scholar] [CrossRef]
- Biswas, T.; Bhushan, S.; Prajapati, S.K.; Ray Chaudhuri, S. An eco-friendly strategy for dairy wastewater remediation with high lipid microalgae-bacterial biomass production. J. Environ. Manag. 2021, 286, 112196. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pages, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Barros de Medeiros, V.P.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, A.; Namsaraev, Z.; Maltsev, Y.; Gusev, E.; Kulikovskiy, M.; Petrushkina, M.; Kuzmin, D. Simultaneous increase in cellular content and volumetric concentration of lipids in Bracteacoccus bullatus cultivated at reduced nitrogen and phosphorus concentrations. J. Appl. Phycol. 2018, 30, 2237–2246. [Google Scholar] [CrossRef]
- Petrushkina, M.; Gusev, E.; Sorokin, B.; Zotko, N.; Mamaeva, A.; Filimonova, A.; Kuzmin, D. Fucoxanthin production by heterokont microalgae. Algal Res. 2017, 24, 387–393. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The impact of the phytochromes on photosynthetic processes. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.R.; Rufty, T.W.; Lewis, R.S. Increasing Photosynthesis: Unlikely Solution for World Food Problem. Trends Plant Sci. 2019, 24, 1032–1039. [Google Scholar] [CrossRef]
- Tshikunde, N.M.; Mashilo, J.; Shimelis, H.; Odindo, A. Agronomic and Physiological Traits, and Associated Quantitative Trait Loci (QTL) Affecting Yield Response in Wheat (Triticum aestivum L.): A Review. Front. Plant Sci. 2019, 10, 1428. [Google Scholar] [CrossRef]
- Kaiser, J. Growth spurt for height genetics. Science 2020, 370, 645. [Google Scholar] [CrossRef]
- Manolio, T.A.; Collins, F.S.; Cox, N.J.; Goldstein, D.B.; Hindorff, L.A.; Hunter, D.J.; McCarthy, M.I.; Ramos, E.M.; Cardon, L.R.; Chakravarti, A.; et al. Finding the missing heritability of complex diseases. Nature 2009, 461, 747–753. [Google Scholar] [CrossRef]
- Abiola, O.; Angel, J.M.; Avner, P.; Bachmanov, A.A.; Belknap, J.K.; Bennett, B.; Blankenhorn, E.P.; Blizard, D.A.; Bolivar, V.; Brockmann, G.A.; et al. The nature and identification of quantitative trait loci: A community’s view. Nat. Rev. Genet. 2003, 4, 911–916. [Google Scholar] [CrossRef]
- Ahmar, S.; Saeed, S.; Khan, M.H.U.; Ullah Khan, S.; Mora-Poblete, F.; Kamran, M.; Faheem, A.; Maqsood, A.; Rauf, M.; Saleem, S.; et al. A Revolution toward Gene-Editing Technology and Its Application to Crop Improvement. Int. J. Mol. Sci. 2020, 21, 5665. [Google Scholar] [CrossRef]
- Cao, S.; Xu, D.; Hanif, M.; Xia, X.; He, Z. Genetic architecture underpinning yield component traits in wheat. Theor. Appl. Genet. 2020, 133, 1811–1823. [Google Scholar] [CrossRef]
- Nordborg, M.; Weigel, D. Next-generation genetics in plants. Nature 2008, 456, 720–723. [Google Scholar] [CrossRef]
- Birchler, J.A. Editing the Phenotype: A Revolution for Quantitative Genetics. Cell 2017, 171, 269–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falcon, C.M.; Kaeppler, S.M.; Spalding, E.P.; Miller, N.D.; Haase, N.; AlKhalifah, N.; Bohn, M.; Buckler, E.S.; Campbell, D.A.; Ciampitti, I.; et al. Relative utility of agronomic, phenological, and morphological traits for assessing genotype-by-environment interaction in maize inbreds. Crop. Sci. 2020, 60, 62–81. [Google Scholar] [CrossRef]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Curatti, L.; Rubio, L.M. Challenges to develop nitrogen-fixing cereals by direct nif-gene transfer. Plant Sci. 2014, 225, 130–137. [Google Scholar] [CrossRef]
- Bloch, S.; Ryu, M.-H.; Ozaydin, B.; Broglie, R. Harnessing atmospheric nitrogen for cereal crop production. Curr. Opin. Biotechnol. 2020, 62, 181–188. [Google Scholar] [CrossRef]
- Xiang, N.; Guo, C.; Liu, J.; Xu, H.; Dixon, R.; Yang, J.; Wang, Y.P. Using synthetic biology to overcome barriers to stable expression of nitrogenase in eukaryotic organelles. Proc. Natl. Acad. Sci. USA 2020, 117, 16537–16545. [Google Scholar] [CrossRef]
- Leach, J.E.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the Phytobiome. Cell 2017, 169, 587–596. [Google Scholar] [CrossRef]
- Arif, I.; Batool, M.; Schenk, P.M. Plant Microbiome Engineering: Expected Benefits for Improved Crop Growth and Resilience. Trends Biotechnol. 2020, 38, 1385–1396. [Google Scholar] [CrossRef]
- Kumar, A.; Dubey, A. Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Delaux, P.M.; Schornack, S. Plant evolution driven by interactions with symbiotic and pathogenic microbes. Science 2021, 371, eaba6605. [Google Scholar] [CrossRef] [PubMed]
- Pankievicz, V.C.S.; Irving, T.B.; Maia, L.G.S.; Ane, J.M. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 2019, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Shelake, R.M.; Pramanik, D.; Kim, J.Y. Exploration of Plant-Microbe Interactions for Sustainable Agriculture in CRISPR Era. Microorganisms 2019, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Kawamura, A.; Kihara, T.; Hara, T.; Takita, E.; Shibata, D. Overexpression of mitochondrial citrate synthase in Arabidopsis thaliana improved growth on a phosphorus-limited soil. Plant Cell Physiol. 2000, 41, 1030–1037. [Google Scholar] [CrossRef]
- Yang, H.; Knapp, J.; Koirala, P.; Rajagopal, D.; Peer, W.A.; Silbart, L.K.; Murphy, A.; Gaxiola, R.A. Enhanced phosphorus nutrition in monocots and dicots over-expressing a phosphorus-responsive type I H+-pyrophosphatase. Plant Biotechnol. J. 2007, 5, 735–745. [Google Scholar] [CrossRef]
- Gevaudant, F.; Duby, G.; von Stedingk, E.; Zhao, R.; Morsomme, P.; Boutry, M. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol. 2007, 144, 1763–1776. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef]
- Jansing, J.; Schiermeyer, A.; Schillberg, S.; Fischer, R.; Bortesi, L. Genome Editing in Agriculture: Technical and Practical Considerations. Int. J. Mol. Sci. 2019, 20, 2888. [Google Scholar] [CrossRef]
- Van Eck, J. Applying gene editing to tailor precise genetic modifications in plants. J. Biol. Chem. 2020, 295, 13267–13276. [Google Scholar] [CrossRef]
- Ansari, W.A.; Chandanshive, S.U.; Bhatt, V.; Nadaf, A.B.; Vats, S.; Katara, J.L.; Sonah, H.; Deshmukh, R. Genome Editing in Cereals: Approaches, Applications and Challenges. Int. J. Mol. Sci. 2020, 21, 4040. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Rahman, M.U.; Mukhtar, Z.; Zafar, Y.; Zhang, B. A critical look on CRISPR-based genome editing in plants. J. Cell. Physiol. 2020, 235, 666–682. [Google Scholar] [CrossRef]
- Friedland, A.E.; Baral, R.; Singhal, P.; Loveluck, K.; Shen, S.; Sanchez, M.; Marco, E.; Gotta, G.M.; Maeder, M.L.; Kennedy, E.M.; et al. Characterization of Staphylococcus aureus Cas9: A smaller Cas9 for all-in-one adeno-associated virus delivery and paired nickase applications. Genome Biol. 2015, 16, 257. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Lee, C.M.; Gasiunas, G.; Davis, T.H.; Cradick, T.J.; Siksnys, V.; Bao, G.; Cathomen, T.; Mussolino, C. Streptococcus thermophilus CRISPR-Cas9 Systems Enable Specific Editing of the Human Genome. Mol. Ther. 2016, 24, 636–644. [Google Scholar] [CrossRef]
- Razzaq, A.; Saleem, F.; Kanwal, M.; Mustafa, G.; Yousaf, S.; Imran Arshad, H.M.; Hameed, M.K.; Khan, M.S.; Joyia, F.A. Modern Trends in Plant Genome Editing: An Inclusive Review of the CRISPR/Cas9 Toolbox. Int. J. Mol. Sci. 2019, 20, 4045. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas System: Recent Advances and Future Prospects for Genome Editing. Trends Plant Sci. 2019, 24, 1102–1125. [Google Scholar] [CrossRef]
- Es, I.; Gavahian, M.; Marti-Quijal, F.J.; Lorenzo, J.M.; Mousavi Khaneghah, A.; Tsatsanis, C.; Kampranis, S.C.; Barba, F.J. The application of the CRISPR-Cas9 genome editing machinery in food and agricultural science: Current status, future perspectives, and associated challenges. Biotechnol. Adv. 2019, 37, 410–421. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Liang, Z.; Shi, W.; Gao, C.; Xia, G. From Genetic Stock to Genome Editing: Gene Exploitation in Wheat. Trends Biotechnol. 2018, 36, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Q.; He, F.; Akhunova, A.; Chao, S.; Trick, H.; Akhunov, E. Transgenerational CRISPR-Cas9 Activity Facilitates Multiplex Gene Editing in Allopolyploid Wheat. CRISPR J. 2018, 1, 65–74. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Armario Najera, V.; Twyman, R.M.; Christou, P.; Zhu, C. Applications of multiplex genome editing in higher plants. Curr. Opin. Biotechnol. 2019, 59, 93–102. [Google Scholar] [CrossRef]
- Hashimoto, R.; Ueta, R.; Abe, C.; Osakabe, Y.; Osakabe, K. Efficient Multiplex Genome Editing Induces Precise, and Self-Ligated Type Mutations in Tomato Plants. Front. Plant Sci. 2018, 9, 916. [Google Scholar] [CrossRef]
- Zhou, J.; Xin, X.; He, Y.; Chen, H.; Li, Q.; Tang, X.; Zhong, Z.; Deng, K.; Zheng, X.; Akher, S.A.; et al. Multiplex QTL editing of grain-related genes improves yield in elite rice varieties. Plant Cell Rep. 2019, 38, 475–485. [Google Scholar] [CrossRef]
- Wilson, F.M.; Harrison, K.; Armitage, A.D.; Simkin, A.J.; Harrison, R.J. CRISPR/Cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid strawberry. Plant Methods 2019, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sapkota, M.; van der Knaap, E. Perspectives of CRISPR/Cas-mediated cis-engineering in horticulture: Unlocking the neglected potential for crop improvement. Hortic. Res. 2020, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480.e8. [Google Scholar] [CrossRef]
- Xu, W.; Fu, W.; Zhu, P.; Li, Z.; Wang, C.; Wang, C.; Zhang, Y.; Zhu, S. Comprehensive Analysis of CRISPR/Cas9-Mediated Mutagenesis in Arabidopsis thaliana by Genome-wide Sequencing. Int. J. Mol. Sci. 2019, 20, 4125. [Google Scholar] [CrossRef]
- Young, J.; Zastrow-Hayes, G.; Deschamps, S.; Svitashev, S.; Zaremba, M.; Acharya, A.; Paulraj, S.; Peterson-Burch, B.; Schwartz, C.; Djukanovic, V.; et al. CRISPR-Cas9 Editing in Maize: Systematic Evaluation of Off-target Activity and Its Relevance in Crop Improvement. Sci. Rep. 2019, 9, 6729. [Google Scholar] [CrossRef]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Banakar, R.; Eggenberger, A.L.; Lee, K.; Wright, D.A.; Murugan, K.; Zarecor, S.; Lawrence-Dill, C.J.; Sashital, D.G.; Wang, K. High-frequency random DNA insertions upon co-delivery of CRISPR-Cas9 ribonucleoprotein and selectable marker plasmid in rice. Sci. Rep. 2019, 9, 19902. [Google Scholar] [CrossRef]
- Jacobsen, T.; Ttofali, F.; Liao, C.; Manchalu, S.; Gray, B.N.; Beisel, C.L. Characterization of Cas12a nucleases reveals diverse PAM profiles between closely-related orthologs. Nucleic Acids Res. 2020, 48, 5624–5638. [Google Scholar] [CrossRef]
- Murugan, K.; Seetharam, A.S.; Severin, A.J.; Sashital, D.G. CRISPR-Cas12a has widespread off-target and dsDNA-nicking effects. J. Biol. Chem. 2020, 295, 5538–5553. [Google Scholar] [CrossRef]
- Graham, N.; Patil, G.B.; Bubeck, D.M.; Dobert, R.C.; Glenn, K.C.; Gutsche, A.T.; Kumar, S.; Lindbo, J.A.; Maas, L.; May, G.D.; et al. Plant Genome Editing and the Relevance of Off-Target Changes. Plant Physiol. 2020, 183, 1453–1471. [Google Scholar] [CrossRef]
- Bharat, S.; Li, S.; Li, J.; Yan, L.; Xia, L. Base editing in plants: Current status and challenges. Crop. J. 2020, 8, 384–395. [Google Scholar] [CrossRef]
- Kempton, H.R.; Qi, L.S. When genome editing goes off-target. Science 2019, 364, 234–236. [Google Scholar] [CrossRef]
- Wienert, B.; Wyman, S.K.; Richardson, C.D.; Yeh, C.D.; Akcakaya, P.; Porritt, M.J.; Morlock, M.; Vu, J.T.; Kazane, K.R.; Watry, H.L.; et al. Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq. Science 2019, 364, 286–289. [Google Scholar] [CrossRef]
- Zuo, E.; Sun, Y.; Wei, W.; Yuan, T.; Ying, W.; Sun, H.; Yuan, L.; Steinmetz, L.M.; Li, Y.; Yang, H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 2019, 364, 289–292. [Google Scholar] [CrossRef]
- Jin, S.; Zong, Y.; Gao, Q.; Zhu, Z.; Wang, Y.; Qin, P.; Liang, C.; Wang, D.; Qiu, J.L.; Zhang, F.; et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 2019, 364, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Konermann, S.; Brigham, M.D.; Trevino, A.E.; Joung, J.; Abudayyeh, O.O.; Barcena, C.; Hsu, P.D.; Habib, N.; Gootenberg, J.S.; Nishimasu, H.; et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 2015, 517, 583–588. [Google Scholar] [CrossRef]
- Kwik, M.; Hainzl, S.; Oppelt, J.; Tichy, B.; Koller, U.; Bernardinelli, E.; Steiner, M.; Zara, G.; Nofziger, C.; Weis, S.; et al. Selective Activation of CNS and Reference PPARGC1A Promoters Is Associated with Distinct Gene Programs Relevant for Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 3296. [Google Scholar] [CrossRef]
- Weng, M.L.; Becker, C.; Hildebrandt, J.; Neumann, M.; Rutter, M.T.; Shaw, R.G.; Weigel, D.; Fenster, C.B. Fine-Grained Analysis of Spontaneous Mutation Spectrum and Frequency in Arabidopsis thaliana. Genetics 2019, 211, 703–714. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Huang, J.; Zhang, X.; Yuan, Y.; Chen, J.Q.; Hurst, L.D.; Tian, D. Parent-progeny sequencing indicates higher mutation rates in heterozygotes. Nature 2015, 523, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Xu, X.W.; Wang, R.R.; Peng, W.L.; Cai, L.; Song, J.M.; Li, W.; Luo, X.; Niu, L.; Wang, Y.; et al. Contributions of Zea mays subspecies mexicana haplotypes to modern maize. Nat. Commun. 2017, 8, 1874. [Google Scholar] [CrossRef]
- Sedeek, K.E.M.; Mahas, A.; Mahfouz, M. Plant Genome Engineering for Targeted Improvement of Crop Traits. Front. Plant Sci. 2019, 10, 114. [Google Scholar] [CrossRef]
- Kalinina, N.O.; Khromov, A.; Love, A.J.; Taliansky, M.E. CRISPR Applications in Plant Virology: Virus Resistance and Beyond. Phytopathology 2020, 110, 18–28. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Liu, H.; Li, Z. Highly efficient DNA-free plant genome editing using virally delivered CRISPR-Cas9. Nat. Plants 2020, 6, 773–779. [Google Scholar] [CrossRef]
- Lenaghan, S.; Stewart, C. An Automated Protoplast Transformation System. In Plant Genome Editing with CRISPR Systems. Part of the Methods in Molecular Biology Book Series (MIMB); Humana Press: New York, NY, USA, 2019; Volume 1917, pp. 355–363. [Google Scholar]
- Hussain, Q.; Shi, J.; Scheben, A.; Zhan, J.; Wang, X.; Liu, G.; Yan, G.; King, G.J.; Edwards, D.; Wang, H. Genetic and signalling pathways of dry fruit size: Targets for genome editing-based crop improvement. Plant Biotechnol. J. 2020, 18, 1124–1140. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, R.; Huang, G.; Li, Y.; Melaku, G.; Zhang, S. Developing superior alleles of yield genes in rice by artificial mutagenesis using the CRISPR/Cas9 system. Crop. J. 2018, 6, 475–481. [Google Scholar] [CrossRef]
- Miao, C.; Xiao, L.; Hua, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. USA 2018, 115, 6058–6063. [Google Scholar] [CrossRef]
- Adamski, N.M.; Borrill, P.; Brinton, J.; Harrington, S.A.; Marchal, C.; Bentley, A.R.; Bovill, W.D.; Cattivelli, L.; Cockram, J.; Contreras-Moreira, B.; et al. A roadmap for gene functional characterisation in crops with large genomes: Lessons from polyploid wheat. Elife 2020, 9, e55646. [Google Scholar] [CrossRef] [PubMed]
- Igartua, E.; Contreras-Moreira, B.; Casas, A.M. TB1: From domestication gene to tool for many trades. J. Exp. Bot. 2020, 71, 4621–4624. [Google Scholar] [CrossRef]
- Alonge, M.; Soyk, S.; Ramakrishnan, S.; Wang, X.; Goodwin, S.; Sedlazeck, F.J.; Lippman, Z.B.; Schatz, M.C. RaGOO: Fast and accurate reference-guided scaffolding of draft genomes. Genome Biol. 2019, 20, 224. [Google Scholar] [CrossRef]
- Bayer, P.E.; Golicz, A.A.; Scheben, A.; Batley, J.; Edwards, D. Plant pan-genomes are the new reference. Nat. Plants 2020, 6, 914–920. [Google Scholar] [CrossRef]
- Danilevicz, M.F.; Tay Fernandez, C.G.; Marsh, J.I.; Bayer, P.E.; Edwards, D. Plant pangenomics: Approaches, applications and advancements. Curr. Opin. Plant Biol. 2020, 54, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Garg, V.; Roorkiwal, M.; Golicz, A.A.; Edwards, D.; Varshney, R.K. Super-Pangenome by Integrating the Wild Side of a Species for Accelerated Crop Improvement. Trends Plant Sci. 2020, 25, 148–158. [Google Scholar] [CrossRef]
- Michael, T.P.; VanBuren, R. Building near-complete plant genomes. Curr. Opin. Plant Biol. 2020, 54, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Peluso, P.; Shi, J.; Liang, T.; Stitzer, M.C.; Wang, B.; Campbell, M.S.; Stein, J.C.; Wei, X.; Chin, C.S.; et al. Improved maize reference genome with single-molecule technologies. Nature 2017, 546, 524–527. [Google Scholar] [CrossRef]
- Mir, R.R.; Reynolds, M.; Pinto, F.; Khan, M.A.; Bhat, M.A. High-throughput phenotyping for crop improvement in the genomics era. Plant Sci. 2019, 282, 60–72. [Google Scholar] [CrossRef]
- Alqudah, A.M.; Haile, J.K.; Alomari, D.Z.; Pozniak, C.J.; Kobiljski, B.; Borner, A. Genome-wide and SNP network analyses reveal genetic control of spikelet sterility and yield-related traits in wheat. Sci. Rep. 2020, 10, 2098. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Kulwal, P.L.; Jaiswal, V. Association mapping in plants in the post-GWAS genomics era. Adv. Genet. 2019, 104, 75–154. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Chris, C.S.M. Gene Ontology Data Archive. Available online: https://zenodo.org/record/3980761#.YN4uqyBR2bg (accessed on 2 July 2018).
- Tello-Ruiz, M.K.; Naithani, S.; Gupta, P.; Olson, A.; Wei, S.; Preece, J.; Jiao, Y.; Wang, B.; Chougule, K.; Garg, P.; et al. Gramene 2021: Harnessing the power of comparative genomics and pathways for plant research. Nucleic Acids Res. 2020, 49, D1452–D1463. [Google Scholar] [CrossRef]
- Howe, K.L.; Contreras-Moreira, B.; De Silva, N.; Maslen, G.; Akanni, W.; Allen, J.; Alvarez-Jarreta, J.; Barba, M.; Bolser, D.M.; Cambell, L.; et al. Ensembl Genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Res. 2020, 48, D689–D695. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, L.; Colantuono, C.; Diretto, G.; Fiore, A.; Chiusano, M.L. Bioinformatics Resources for Plant Abiotic Stress Responses: State of the Art and Opportunities in the Fast Evolving-Omics Era. Plants 2020, 9, 591. [Google Scholar] [CrossRef]
- Pan, Q.; Wei, J.; Guo, F.; Huang, S.; Gong, Y.; Liu, H.; Liu, J.; Li, L. Trait ontology analysis based on association mapping studies bridges the gap between crop genomics and Phenomics. BMC Genom. 2019, 20, 443. [Google Scholar] [CrossRef] [PubMed]
- Wimalanathan, K.; Lawrence-Dill, C.J. Gene Ontology Meta Annotator for Plants. BioRxiv 2019. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Chen, J.; Zhang, X.; Guo, M.; Yu, G. A Literature Review of Gene Function Prediction by Modeling Gene Ontology. Front. Genet. 2020, 11, 400. [Google Scholar] [CrossRef]
- Wimalanathan, K.; Friedberg, I.; Andorf, C.M.; Lawrence-Dill, C.J. Maize GO Annotation-Methods, Evaluation, and Review (maize-GAMER). Plant Direct 2018, 2, e00052. [Google Scholar] [CrossRef]
- Borrill, P. Blurring the boundaries between cereal crops and model plants. New Phytol. 2019, 228, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Xaviera, A.; Beckett, T.; Beyer, S.; Chen, L.; Chikssa, H.; Cross, V.; Moreira, F.F.; French, E.; Gaire, R.; et al. Identification, deployment, and transferability of quantitative trait loci from genome-wide association studies in plants. Curr. Plant Biol. 2020, 24, 100145. [Google Scholar] [CrossRef]
- Liu, H.J.; Yan, J. Crop genome-wide association study: A harvest of biological relevance. Plant J. 2019, 97, 8–18. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Tang, Y.; Wang, D.; Chen, X.; Yao, Y.; Guo, Y. Genome-Wide Identification, Comprehensive Gene Feature, Evolution, and Expression Analysis of Plant Metal Tolerance Proteins in Tobacco Under Heavy Metal Toxicity. Front. Genet. 2019, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Mabhaudhi, T.; Chimonyo, V.G.P.; Hlahla, S.; Massawe, F.; Mayes, S.; Nhamo, L.; Modi, A.T. Prospects of orphan crops in climate change. Planta 2019, 250, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Gruber, K. Agrobiodiversity: The living library. Nature 2017, 544, S8–S10. [Google Scholar] [CrossRef]
- Castaneda-Alvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef]
- Shelef, O.; Weisberg, P.J.; Provenza, F.D. The Value of Native Plants and Local Production in an Era of Global Agriculture. Front. Plant Sci. 2017, 8, 2069. [Google Scholar] [CrossRef]
- Lemmon, Z.H.; Reem, N.T.; Dalrymple, J.; Soyk, S.; Swartwood, K.E.; Rodriguez-Leal, D.; Van Eck, J.; Lippman, Z.B. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 2018, 4, 766–770. [Google Scholar] [CrossRef]
- Ravindran, S. QnAs with Zachary B. Lippman. Proc. Natl. Acad. Sci. USA 2020, 117, 14624–14625. [Google Scholar] [CrossRef]
- Van Tassel, D.L.; Tesdell, O.; Schlautman, B.; Rubin, M.J.; DeHaan, L.R.; Crews, T.E.; Streit Krug, A. New Food Crop Domestication in the Age of Gene Editing: Genetic, Agronomic and Cultural Change Remain Co-evolutionarily Entangled. Front. Plant Sci. 2020, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Yan, J. De Novo Domestication: An Alternative Route toward New Crops for the Future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Piperno, D.R.; Allaby, R.G.; Purugganan, M.D.; Andersson, L.; Arroyo-Kalin, M.; Barton, L.; Climer Vigueira, C.; Denham, T.; Dobney, K.; et al. Current perspectives and the future of domestication studies. Proc. Natl. Acad. Sci. USA 2014, 111, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Allaby, R. Domestication Syndrome in Plants. Encycl. Glob. Archaeol. 2014, 2182, 2184. [Google Scholar] [CrossRef]
- Kantar, M.; Nashoba, A.; Anderson, J.; Blackman, B.; Rieseberg, L. The Genetics and Genomics of Plant Domestication. BioScience 2017, 67, 971–982. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Erskine, W.; Berger, J.D.; Nelson, M.N. Phenotypic characterisation and linkage mapping of domestication syndrome traits in yellow lupin (Lupinus luteus L.). Theor. Appl. Genet. 2020, 133, 2975–2987. [Google Scholar] [CrossRef]
- Lowry, D.B.; Hoban, S.; Kelley, J.L.; Lotterhos, K.E.; Reed, L.K.; Antolin, M.F.; Storfer, A. Breaking RAD: An evaluation of the utility of restriction site-associated DNA sequencing for genome scans of adaptation. Mol. Ecol. Resour. 2017, 17, 142–152. [Google Scholar] [CrossRef]
- Barrera-Redondo, J.; Pinero, D.; Eguiarte, L.E. Genomic, Transcriptomic and Epigenomic Tools to Study the Domestication of Plants and Animals: A Field Guide for Beginners. Front. Genet. 2020, 11, 742. [Google Scholar] [CrossRef]
- Schreiber, M.; Stein, N.; Mascher, M. Genomic approaches for studying crop evolution. Genome Biol. 2018, 19, 140. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, Q.; Lu, H.; Li, Y.; Wang, A.; Tian, Q.; Zhan, Q.; Lu, Y.; Zhang, L.; Huang, T.; et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018, 50, 278–284. [Google Scholar] [CrossRef]
- Boden, S.A.; Østergaard, L. How can developmental biology help feed a growing population? Development 2019, 146, dev172965. [Google Scholar] [CrossRef]
- Trevaskis, B. Developmental Pathways Are Blueprints for Designing Successful Crops. Front. Plant Sci. 2018, 9, 745. [Google Scholar] [CrossRef]
- Metje-Sprink, J.; Sprink, T.; Hartung, F. Genome-edited plants in the field. Curr. Opin. Biotechnol. 2020, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ganivet, E. Growth in human population and consumption both need to be addressed to reach an ecologically sustainable future. Environ. Dev. Sustain. 2020, 22, 4979–4998. [Google Scholar] [CrossRef]
- Klein Goldewijk, K.; Beusen, A.; Van Drecht, G.; De Vos, M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob. Ecol. Biogeogr. 2011, 20, 73–86. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Venter, O.; Lee, J.; Jones, K.R.; Robinson, J.G.; Possingham, H.P.; Allan, J.R. Protect the last of the wild. Nature 2018, 563, 27–30. [Google Scholar] [CrossRef]
- The 2020 Living Planet Report. Available online: www.livingplanetindex.org/home/index (accessed on 10 September 2020).
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef]
- Fiore, M.C.; Mercati, F.; Spina, A.; Blangiforti, S.; Venora, G.; Dell’Acqua, M.; Lupini, A.; Preiti, G.; Monti, M.; Pe, M.E.; et al. High-Throughput Genotype, Morphology, and Quality Traits Evaluation for the Assessment of Genetic Diversity of Wheat Landraces from Sicily. Plants 2019, 8, 116. [Google Scholar] [CrossRef]
- Taranto, F.; D’Agostino, N.; Rodriguez, M.; Pavan, S.; Minervini, A.P.; Pecchioni, N.; Papa, R.; De Vita, P. Whole Genome Scan Reveals Molecular Signatures of Divergence and Selection Related to Important Traits in Durum Wheat Germplasm. Front. Genet. 2020, 11, 217. [Google Scholar] [CrossRef]
- Crossley, M.S.; Burke, K.D.; Schoville, S.D.; Radeloff, V.C. Recent collapse of crop belts and declining diversity of US agriculture since 1840. Glob. Chang. Biol. 2021, 27, 151–164. [Google Scholar] [CrossRef]
- Smith, P. Malthus is still wrong: We can feed a world of 9–10 billion, but only by reducing food demand. Proc. Nutr. Soc. 2015, 74, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Leridon, H. World population outlook: Explosion or implosion? Popul. Soc. 2020, 573, 1–4. [Google Scholar] [CrossRef]
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Gilbert, N. Environment: The disappearing nutrient. Nature 2009, 461, 716–718. [Google Scholar] [CrossRef]
- Lidicker, W.Z., Jr. A Scientist’s Warning to humanity on human population growth. Glob. Ecol. Conserv. 2020, 24, e01232. [Google Scholar] [CrossRef]
- Taylor, K.B. The passing of western civilization. Futures 2020, 122, 102582. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Prospects 2019; United Nations: New York, NA, USA, 2019; Volume I, Available online: https://population.un.org/wpp/ (accessed on 17 June 2019).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzdin, A.V.; Patrushev, M.V.; Sverdlov, E.D. Will Plant Genome Editing Play a Decisive Role in “Quantum-Leap” Improvements in Crop Yield to Feed an Increasing Global Human Population? Plants 2021, 10, 1667. https://doi.org/10.3390/plants10081667
Buzdin AV, Patrushev MV, Sverdlov ED. Will Plant Genome Editing Play a Decisive Role in “Quantum-Leap” Improvements in Crop Yield to Feed an Increasing Global Human Population? Plants. 2021; 10(8):1667. https://doi.org/10.3390/plants10081667
Chicago/Turabian StyleBuzdin, Anton V., Maxim V. Patrushev, and Eugene D. Sverdlov. 2021. "Will Plant Genome Editing Play a Decisive Role in “Quantum-Leap” Improvements in Crop Yield to Feed an Increasing Global Human Population?" Plants 10, no. 8: 1667. https://doi.org/10.3390/plants10081667
APA StyleBuzdin, A. V., Patrushev, M. V., & Sverdlov, E. D. (2021). Will Plant Genome Editing Play a Decisive Role in “Quantum-Leap” Improvements in Crop Yield to Feed an Increasing Global Human Population? Plants, 10(8), 1667. https://doi.org/10.3390/plants10081667






