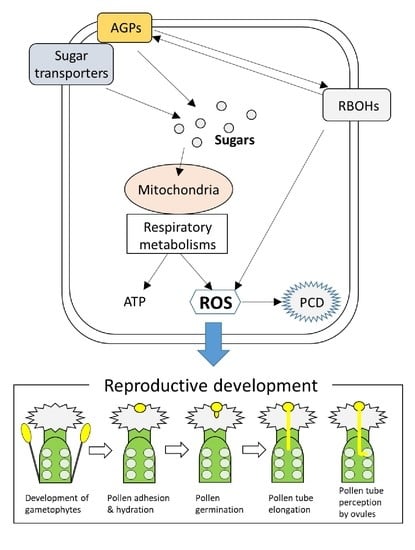
Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development
Abstract
:1. Introduction
2. Development of Male Gametophytes
3. Development of Female Gametophytes
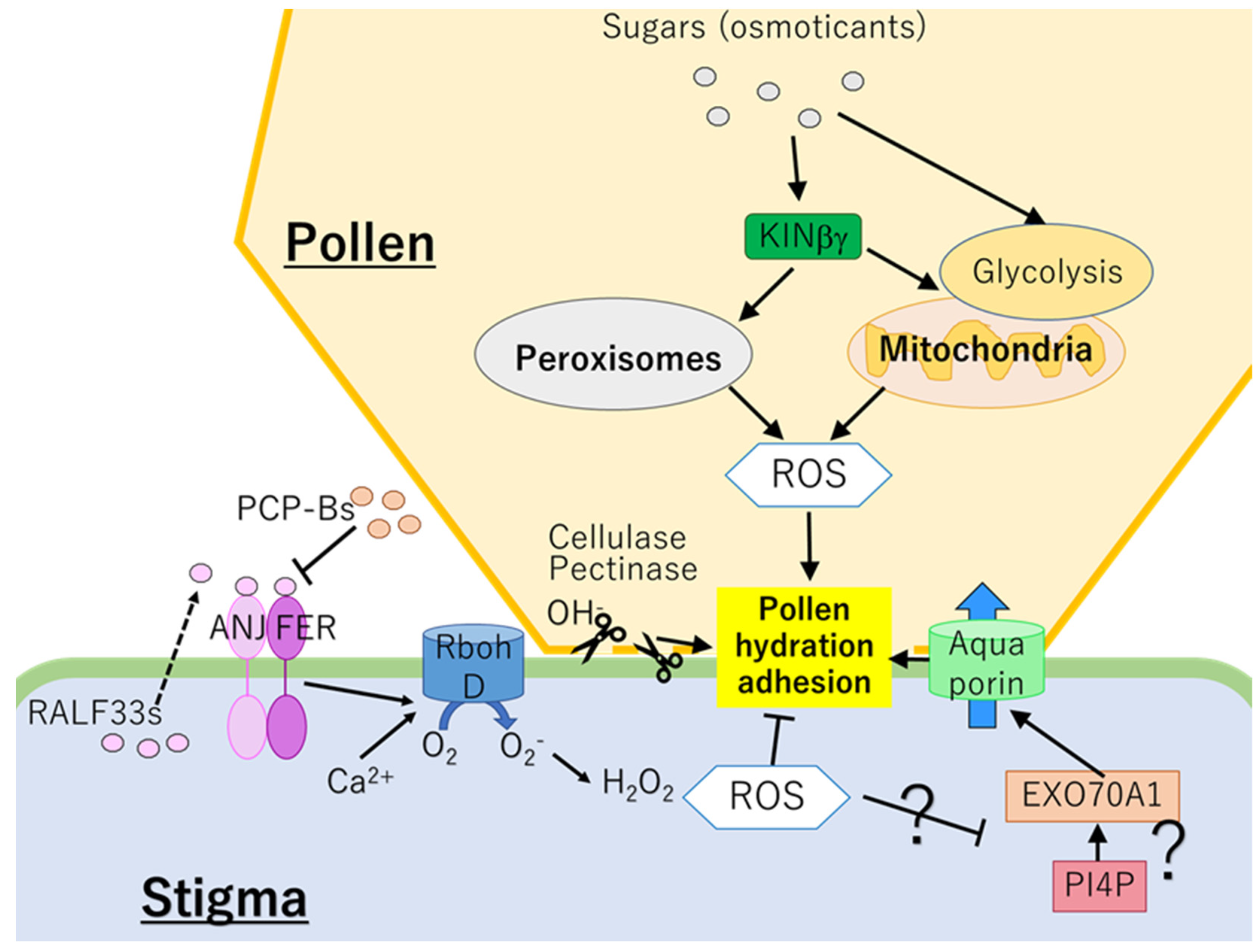
4. Pollen Adhesion and Hydration
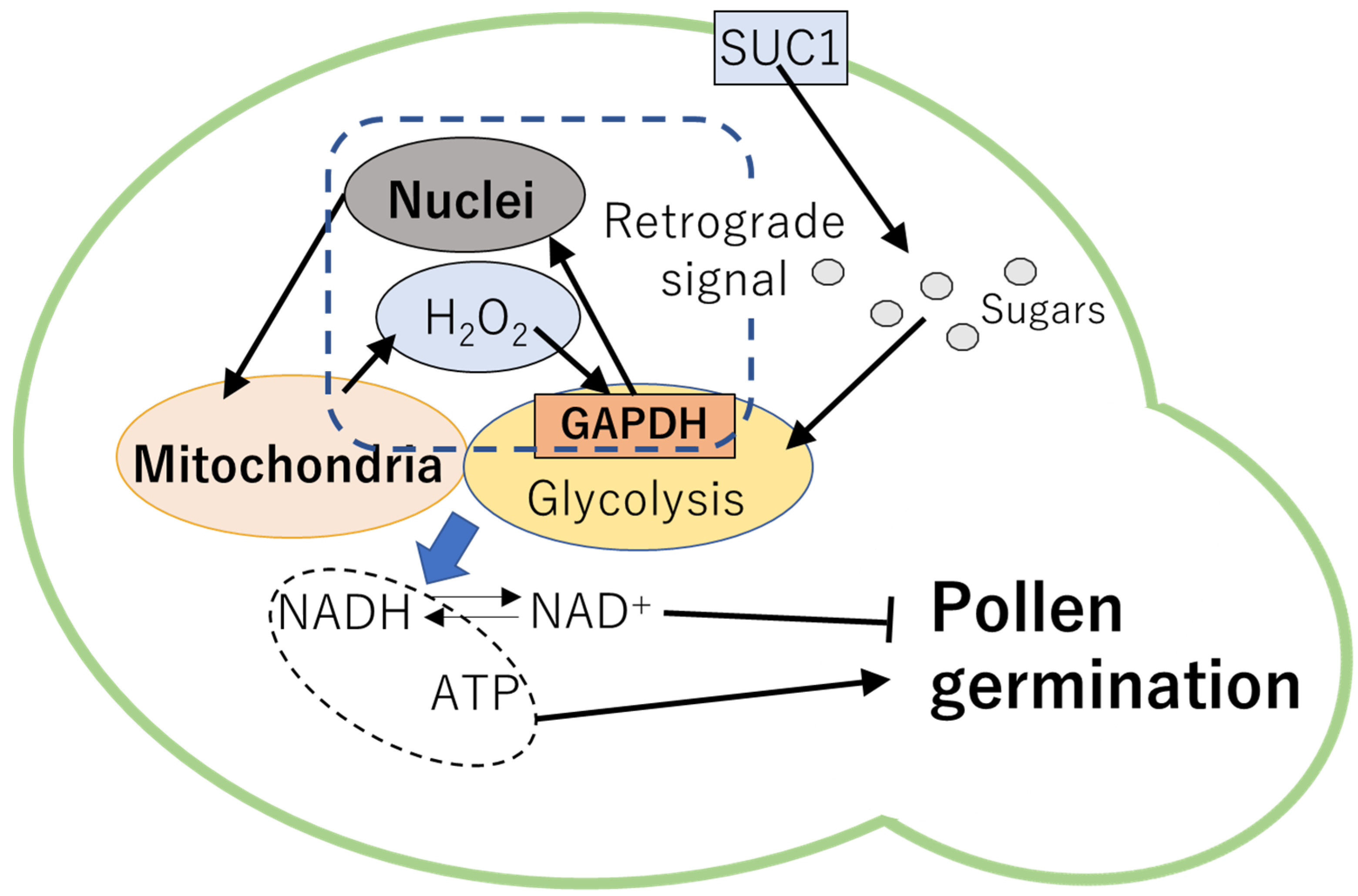
5. Pollen Germination and Pollen Tube Elongation
6. Pollen Tube Perception by the Female Gametophyte and Tube Rapture
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dwivedi, S.L.; Siddique, K.H.M.; Farooq, M.; Thornton, P.K.; Ortiz, R. Using Biotechnology-Led Approaches to Uplift Cereal and Food Legume Yields in Dryland Environments. Front. Plant Sci. 2018, 9, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Sankaranarayanan, S.; Ju, Y.; Kessler, S.A. Reactive Oxygen Species as Mediators of Gametophyte Development and Double Fertilization in Flowering Plants. Front. Plant Sci. 2020, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Zhang, X.S.; Gao, X.Q. ROS in the Male-Female Interactions During Pollination: Function and Regulation. Front. Plant Sci. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Plant Cell 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Xie, H.T.; Wan, Z.Y.; Li, S.; Zhang, Y. Spatiotemporal Production of Reactive Oxygen Species by NADPH Oxidase Is Critical for Tapetal Programmed Cell Death and Pollen Development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef] [Green Version]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Kaya, H.; Iwano, M.; Takeda, S.; Kanaoka, M.M.; Kimura, S.; Abe, M.; Kuchitsu, K. Apoplastic ROS production upon pollination by RbohH and RbohJ in Arabidopsis. Science 2015, 10, e989050. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Tang, D.; Zhu, K.; Wang, K.; Li, M.; Cheng, Z. Somatic and reproductive cell development in rice anther is regulated by a putative glutaredoxin. Plant Cell 2012, 24, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Jacobowitz, J.R.; Doyle, W.C.; Weng, J.K. PRX9 and PRX40 Are Extensin Peroxidases Essential for Maintaining Tapetum and Microspore Cell Wall Integrity during Arabidopsis Anther Development. Plant Cell 2019, 31, 848–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, S.; Zachgo, S. ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J. Cell Mol. Biol. 2008, 53, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Zafra, A.; Rejón, J.D.; Hiscock, S.J.; Alché Jde, D. Patterns of ROS Accumulation in the Stigmas of Angiosperms and Visions into Their Multi-Functionality in Plant Reproduction. Front. Plant Sci. 2016, 7, 1112. [Google Scholar] [CrossRef] [Green Version]
- Katano, K.; Oi, T.; Suzuki, N. Failure of Pollen Attachment to the Stigma Triggers Elongation of Stigmatic Papillae in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 989. [Google Scholar] [CrossRef]
- Liu, C.; Shen, L.; Xiao, Y.; Vyshedsky, D.; Peng, C.; Sun, X.; Liu, Z.; Cheng, L.; Zhang, H.; Han, Z.; et al. Pollen PCP-B peptides unlock a stigma peptide-receptor kinase gating mechanism for pollination. Science 2021, 372, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Chaiwongsar, S.; Strohm, A.K.; Roe, J.R.; Godiwalla, R.Y.; Chan, C.W.M. A cyclic nucleotide-gated channel is necessary for optimum fertility in high-calcium environments. Plant Cell 2009, 183, 76–87. [Google Scholar] [CrossRef]
- Katano, K.; Kataoka, R.; Fujii, M.; Suzuki, N. Differences between seedlings and flowers in anti-ROS based heat responses of Arabidopsis plants deficient in cyclic nucleotide gated channel 2. Plant Physiol. Biochem. 2018, 123, 288–296. [Google Scholar] [CrossRef]
- Zafra, A.; Rodríguez-García, M.I.; Alché Jde, D. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biol. 2010, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Traverso, J.A.; Pulido, A.; Rodríguez-García, M.I.; Alché, J.D. Thiol-based redox regulation in sexual plant reproduction: New insights and perspectives. Front. Plant Sci. 2013, 4, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, A.M.; Porterfield, D.M.; Feijó, J.A. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 2004, 131, 2707–2714. [Google Scholar] [CrossRef] [Green Version]
- Prado, A.M.; Colaço, R.; Moreno, N.; Silva, A.C.; Feijó, J.A. Targeting of pollen tubes to ovules is dependent on nitric oxide (NO) signaling. Mol. Plant 2008, 1, 703–714. [Google Scholar] [CrossRef]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric oxide: A multitasked signaling gas in plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirayesh, N.; Giridhar, M.; Ben Khedher, A.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118948. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, X.; Li, W.; Hussain, J.; Qi, G.; Liu, S. Calcium Signaling in Plant Programmed Cell Death. Cells 2021, 10, 1089. [Google Scholar] [CrossRef]
- Świeżawska-Boniecka, B.; Duszyn, M.; Kwiatkowski, M.; Szmidt-Jaworska, A.; Jaworski, K. Cross Talk Between Cyclic Nucleotides and Calcium Signaling Pathways in Plants-Achievements and Prospects. Front. Plant Sci. 2021, 12, 643560. [Google Scholar] [CrossRef] [PubMed]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Yang, J.; Eom, J.S.; Prior, M.; Sosso, D.; Hartwig, T.; Szurek, B.; Oliva, R.; Vera-Cruz, C.; White, F.F.; et al. Sugar flux and signaling in plant-microbe interactions. Plant J. Cell Mol. Biol. 2018, 93, 675–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnu, J.; Schlereth, A.; Zacharaki, V.; Działo, A.M.; Abel, C.; Feil, R.; Schmid, M.; Wahl, V. The trehalose 6-phosphate pathway impacts vegetative phase change in Arabidopsis thaliana. Plant J. 2020, 104, 768–780. [Google Scholar] [CrossRef]
- O’Hara, L.E.; Paul, M.J.; Wingler, A. How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol. Plant 2013, 6, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Iftikhar, J.; Lyu, M.; Liu, Z.; Mehmood, N.; Munir, N.; Ahmed, M.A.A.; Batool, W.; Aslam, M.M.; Yuan, Y.; Wu, B. Sugar and Hormone Dynamics and the Expression Profiles of SUT/SUC and SWEET Sweet Sugar Transporters during Flower Development in Petunia axillaris. Plant 2020, 9, 1770. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.X.; Li, Q.T.; Liu, Y.F.; Zhang, F.X.; Ma, B.; Zhang, W.K.; Man, W.Q.; Du, W.G.; Wang, G.D.; Chen, S.Y.; et al. Soybean GmbZIP123 gene enhances lipid content in the seeds of transgenic Arabidopsis plants. J. Exp. Bot. 2013, 64, 4329–4341. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.F.; Huang, X.Y.; Zhu, J.; Gao, J.F.; Zhang, H.X.; Yang, Z.N. Ruptured Pollen Grain1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plants 2008, 147, 852–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, M.L.; Holmes-Davis, R.; McCormick, S. Green sperm. Identification of male gamete promoters in Arabidopsis. Plant Physiol. 2005, 138, 2124–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, F.; Deng, W.; Pang, X.; Gao, Y.; Chan, H.; Zhang, Q.; Hu, N.; Chen, J.; Li, Z. Overexpression of the KNOX gene Tkn4 affects pollen development and confers sensitivity to gibberellin and auxin in tomato. Plant Physiol. 2019, 281, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, G.; Islam, M.R.; Fu, W.; Feng, B.; Tao, L.; Fu, G. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biol. 2019, 19, 525. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.G.; Elleman, C.J.; Doughty, J. Pollen coatings—Chimaeric genetics and new functions. Sex. Plant Reprod. 2000, 12, 302–309. [Google Scholar] [CrossRef]
- Chapman, L.A.; Goring, D.R. Misregulation of phosphoinositides in Arabidopsis thaliana decreases pollen hydration and maternal fertility. Sex. Plant Reprod. 2011, 24, 319–326. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Pollen tube growth: Where does the energy come from? BMC Plant Biol. 2014, 9, e977200. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, A.G.; Inatsugi, R.; Jiao, J.; Kotake, T.; Kuwata, K.; Ootani, K.; Okuda, S.; Sankaranarayanan, S.; Sato, Y.; Maruyama, D.; et al. The AMOR Arabinogalactan Sugar Chain Induces Pollen-Tube Competency to Respond to Ovular Guidance. PLoS ONE 2016, 26, 1091–1097. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Li, J.; Ma, L.; Wang, H.; Zhou, H.; Ni, E.; Jiang, D.; Liu, Z.; Zhuang, C. OsAGO2 controls ROS production and the initiation of tapetal PCD by epigenetically regulating OsHXK1 expression in rice anthers. Proc. Natl. Acad. Sci. USA 2019, 116, 7549–7558. [Google Scholar] [CrossRef] [Green Version]
- Carrizo García, C.; Nepi, M.; Pacini, E. It is a matter of timing: Asynchrony during pollen development and its consequences on pollen performance in angiosperms-a review. Protoplasma 2017, 254, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Higashiyama, T. Arabinogalactan proteins and their sugar chains: Functions in plant reproduction, research methods, and biosynthesis. Plant Reprod. 2018, 31, 67–75. [Google Scholar] [CrossRef]
- Ge, Z.; Cheung, A.Y.; Qu, L.J. Pollen tube integrity regulation in flowering plants: Insights from molecular assemblies on the pollen tube surface. Plant Reprod. 2019, 222, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breygina, M.; Klimenko, E. ROS and Ions in Cell Signaling during Sexual Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 9476. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kang, J.; Wu, J.; Zhu, Y.; Wang, X. Identification of tapetum-specific genes by comparing global gene expression of four different male sterile lines in Brassica oleracea. Plant Mol. Biol. 2015, 87, 541–554. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Singh, M.B.; Bhalla, P.L. Anther ontogeny in Brachypodium distachyon. Protoplasma 2015, 252, 439–450. [Google Scholar] [CrossRef]
- Xing, S.; Lauri, A.; Zachgo, S. Redox regulation and flower development: A novel function for glutaredoxins. Plant Biol. 2006, 8, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Scholz-Starke, J.; Büttner, M.; Sauer, N. AtSTP6, a new pollen-specific H+-monosaccharide symporter from Arabidopsis. Plant Physiol. 2003, 131, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Lauterbach, C.; Niedermeier, M.; Besenbeck, R.; Stadler, R.; Sauer, N. Immunolocalization of the PmSUC1 sucrose transporter in Plantago major flowers and reporter-gene analyses of the PmSUC1 promoter suggest a role in sucrose release from the inner integument. Plant Biol. 2007, 9, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Firon, N.; Nepi, M.; Pacini, E. Water status and associated processes mark critical stages in pollen development and functioning. Ann. Bot. 2012, 109, 1201–1214. [Google Scholar] [CrossRef] [Green Version]
- Ariizumi, T.; Toriyama, K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant Biol. 2011, 62, 437–460. [Google Scholar] [CrossRef]
- Hanamata, S.; Kurusu, T.; Kuchitsu, K. Roles of autophagy in male reproductive development in plants. Front. Plant Sci. 2014, 5, 457. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Ferraz, R.; Dupree, P.; Showalter, A.M.; Coimbra, S. Three Decades of Advances in Arabinogalactan-Protein Biosynthesis. Front. Plant Sci. 2020, 11, 610377. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.X.; Huang, X.Y.; Yang, J.; Guan, Y.F.; Yang, Z.N. Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod. 2013, 26, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Ikezaki, M.; Fujibe, T.; Aida, M.; Tian, C.E.; Ueno, Y.; Yamamoto, K.T.; Machida, Y.; Nakamura, K.; Ishiguro, S. Arabidopsis auxin response factor6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol. 2010, 51, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Xu, Z.; Song, J.; Conner, K.; Vizcay Barrena, G.; Wilson, Z.A. Arabidopsis MYB26/MALE STERILE35 regulates secondary thickening in the endothecium and is essential for anther dehiscence. Plant J. Cell Mol. Biol. 2007, 19, 534–548. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, S.; Kawai-Oda, A.; Ueda, J.; Nishida, I.; Okada, K. The defective in anther dehiscience gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 2001, 13, 2191–2209. [Google Scholar] [CrossRef] [Green Version]
- Cecchetti, V.; Altamura, M.M.; Brunetti, P.; Petrocelli, V.; Falasca, G.; Ljung, K.; Costantino, P.; Cardarelli, M. Auxin controls Arabidopsis anther dehiscence by regulating endothecium lignification and jasmonic acid biosynthesis. Plant J. Cell Mol. Biol. 2013, 74, 411–422. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xiang, Y.; Li, C.; Yu, G. Modulatory Role of Reactive Oxygen Species in Root Development in Model Plant of Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 485932. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.D.; Ogé, L.; Hamama, L.; Atanassova, R. The Sugar-Signaling Hub: Overview of Regulators and Interaction with the Hormonal and Metabolic Network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.V.; Distéfano, A.M.; Zabaleta, E.J.; Pagnussat, G.C. New insights into the functional roles of reactive oxygen species during embryo sac development and fertilization in Arabidopsis thaliana. Plant Signal Behav. 2013, 8, e25714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.V.; Fiol, D.F.; Sundaresan, V.; Zabaleta, E.J.; Pagnussat, G.C. Oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 2013, 25, 1573–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratibha, P.; Singh, S.K. Gametophyte Development Needs Mitochondrial Coproporphyrinogen III Oxidase Function. Plant Physiol. 2017, 174, 258–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-García, G.; Vielle-Calzada, J.P. A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell 2004, 16, 2614–2628. [Google Scholar] [CrossRef] [Green Version]
- Demesa-Arévalo, E.; Vielle-Calzada, J.P. The classical arabinogalactan protein AGP18 mediates megaspore selection in Arabidopsis. Plant Cell 2013, 25, 1274–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Lizarraga, L.; Bottomley, L.A.; Carson Meredith, J. Effect of water absorption on pollen adhesion. J. Colloid Interface Sci. 2015, 442, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, A.V.; Matveyeva, N.P.; Yermakov, I.P. Reactive oxygen species are involved in regulation of pollen wall cytomechanics. Plant Biol. 2014, 16, 252–257. [Google Scholar] [CrossRef]
- Bunzel, M. Chemistry and occurrence of hydroxycinnamate oligomers. Phytochem. Rev. 2010, 9, 47–64. [Google Scholar] [CrossRef]
- Gao, X.Q.; Liu, C.Z.; Li, D.D.; Zhao, T.T.; Li, F.; Jia, X.N.; Zhao, X.Y.; Zhang, X.S. The Arabidopsis KINβγ Subunit of the SnRK1 Complex Regulates Pollen Hydration on the Stigma by Mediating the Level of Reactive Oxygen Species in Pollen. PLoS Genet. 2016, 12, e1006228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiscock, S.J.; Allen, A.M. Diverse cell signalling pathways regulate pollen-stigma interactions: The search for consensus. New Phytol. 2008, 179, 286–317. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Romero-Puertas, M.C.; Sandalio, L.M.; Olmedilla, A. The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J. Exp. Bot. 2015, 66, 2869–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katano, K.; Honda, K.; Suzuki, N. Integration between ROS Regulatory Systems and Other Signals in the Regulation of Various Types of Heat Responses in Plants. Int. J. Mol. Sci. 2018, 19, 3370. [Google Scholar] [CrossRef] [Green Version]
- McInnis, S.M.; Desikan, R.; Hancock, J.T.; Hiscock, S.J. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: Potential signalling crosstalk? New Phytol. 2006, 172, 221–228. [Google Scholar] [CrossRef]
- Lan, X.; Yang, J.; Abhinandan, K.; Nie, Y.; Li, X.; Li, Y.; Samuel, M.A. Flavonoids and ROS Play Opposing Roles in Mediating Pollination in Ornamental Kale (Brassica oleracea var. acephala). Mol. Plant 2017, 10, 1361–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safavian, D.; Zayed, Y. RNA Silencing of Exocyst Genes in the Stigma Impairs the Acceptance of Compatible Pollen in Arabidopsis. Plant J. Cell Mol. Biol. 2015, 169, 2526–2538. [Google Scholar] [CrossRef] [Green Version]
- Del Campillo, E.; Lewis, L.N. Occurrence of 9.5 cellulase and other hydrolases in flower reproductive organs undergoing major cell wall disruption. Plant Physiol. 1992, 99, 1015–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, M.A.; Chong, Y.T.; Haasen, K.E.; Aldea-Brydges, M.G.; Stone, S.L.; Goring, D.R. Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell 2009, 21, 2655–2671. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Xi, F.; Zhang, X.; Zhang, J.; Guo, W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007, 26, 4053–4065. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, X.; Yue, P.; Guo, W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol. Biol. Cell 2007, 18, 4483–4492. [Google Scholar] [CrossRef] [Green Version]
- Crepin, N.; Rolland, F. SnRK1 activation, signaling, and networking for energy homeostasis. Curr. Opin. Plant Biol. 2019, 51, 29–36. [Google Scholar] [CrossRef]
- Broeckx, T.; Hulsmans, S.; Rolland, F. The plant energy sensor: Evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 2016, 67, 6215–6252. [Google Scholar] [CrossRef]
- Barros, J.A.S.; Siqueira, J.A.B.; Cavalcanti, J.H.F.; Araújo, W.L.; Avin-Wittenberg, T. Multifaceted Roles of Plant Autophagy in Lipid and Energy Metabolism. Trends Plant Sci. 2020, 25, 1141–1153. [Google Scholar] [CrossRef]
- Hiroi, K.; Sone, M.; Sakazono, S.; Osaka, M.; Masuko-Suzuki, H.; Matsuda, T.; Suzuki, G.; Suwabe, K.; Watanabe, M. Time-lapse imaging of self- and cross-pollinations in Brassica rapa. Ann. Bot. 2013, 112, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Sivitz, A.B.; Reinders, A.; Ward, J.M. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008, 147, 92–100. [Google Scholar] [CrossRef]
- Hashida, S.N.; Takahashi, H.; Takahara, K.; Kawai-Yamada, M.; Kitazaki, K.; Shoji, K.; Goto, F.; Yoshihara, T.; Uchimiya, H. NAD+ accumulation during pollen maturation in Arabidopsis regulating onset of germination. Mol. Plant 2013, 6, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Holtgrefe, S.; Gohlke, J.; Starmann, J.; Druce, S.; Klocke, S.; Altmann, B.; Wojtera, J.; Lindermayr, C.; Scheibe, R. Regulation of plant cytosolic glyceraldehyde 3-phosphate dehydrogenase isoforms by thiol modifications. Physiol. Plant. 2008, 133, 211–228. [Google Scholar] [CrossRef]
- Wojtera-Kwiczor, J.; Groß, F.; Leffers, H.M.; Kang, M.; Schneider, M.; Scheibe, R. Transfer of a Redox-Signal through the Cytosol by Redox-Dependent Microcompartmentation of Glycolytic Enzymes at Mitochondria and Actin Cytoskeleton. Front. Plant Sci. 2012, 3, 284. [Google Scholar] [CrossRef] [Green Version]
- Zachgo, S.; Hanke, G.T.; Scheibe, R. Plant cell microcompartments: A redox-signaling perspective. Biol. Chem. 2013, 394, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.; Desikan, R.; Harrison, J.; Bright, J.; Hooley, R.; Neill, S. Doing the unexpected: Proteins involved in hydrogen peroxide perception. J. Exp. Bot. 2006, 57, 1711–1718. [Google Scholar] [CrossRef]
- Lennon, K.A.; Roy, S.; Hepler, P.K.; Lord, E.M. The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. Sex. Plant Reprod. 1998, 11, 49–59. [Google Scholar] [CrossRef]
- Crawford, B.C.; Ditta, G.; Yanofsky, M.F. The NTT gene is required for transmitting-tract development in carpels of Arabidopsis thaliana. Curr. Biol. 2007, 17, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, T.; Feron, R.; Wolters-Arts, M.; Edqvist, J.; Gerats, T.; Derksen, J.; Mariani, C. STIG1 controls exudate secretion in the pistil of petunia and tobacco. Plant Physiol. 2005, 138, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.J.; Liu, H.K.; McCormick, S.; Tang, W.H. Tomato Pistil Factor STIG1 Promotes in vivo Pollen Tube Growth by Binding to Phosphatidylinositol 3-Phosphate and the Extracellular Domain of the Pollen Receptor Kinase LePRK2. Plant Cell 2014, 26, 2505–2523. [Google Scholar] [CrossRef] [Green Version]
- Wrzaczek, M.; Vainonen, J.P.; Stael, S.; Tsiatsiani, L.; Help-Rinta-Rahko, H.; Gauthier, A.; Kaufholdt, D.; Bollhöner, B.; Lamminmäki, A.; Staes, A.; et al. GRIM REAPER peptide binds to receptor kinase PRK5 to trigger cell death in Arabidopsis. Plant Cell 2015, 34, 55–66. [Google Scholar]
- Pereira, A.M.; Pereira, L.G.; Coimbra, S. Arabinogalactan proteins: Rising attention from plant biologists. Plant Reprod. 2015, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Wang, H.; Wu, H.M. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 1995, 82, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Meng, D.; Piñeros, M.A.; Mao, Y.; Dandekar, M.A.; Cheng, L. A Sugar Transporter Takes Up both Hexose and Sucrose for Sorbitol-Modulated In Vitro Pollen Tube Growth in Apple. Plant Cell 2020, 32, 449–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadege, M.; Dupuis, I.I.; Kuhlemeier, C. Ethanolic fermentation: New functions for an old pathway. Trends Plant Sci. 1999, 4, 320–325. [Google Scholar] [CrossRef]
- Mellema, S.; Eichenberger, W.; Rawyler, A.; Suter, M.; Tadege, M.; Kuhlemeier, C. The ethanolic fermentation pathway supports respiration and lipid biosynthesis in tobacco pollen. Plant J. Cell Mol. Biol. 2002, 30, 329–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gass, N.; Glagotskaia, T.; Mellema, S.; Stuurman, J.; Barone, M.; Mandel, T.; Roessner-Tunali, U.; Kuhlemeier, C. Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia. Plant Cell 2005, 17, 2355–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.; Bergonci, T.; Zhao, Y.; Zou, Y.; Du, S.; Liu, M.C.; Luo, X.; Ruan, H.; García-Valencia, E.L.; Zhong, S.; et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 2017, 358, 1596–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.; Zhao, Y.; Liu, M.C.; Zhou, L.Z.; Wang, L.; Zhong, S.; Hou, S.; Jiang, J.; Liu, T.; Huang, Q.; et al. LLG2/3 Are Co-receptors in BUPS/ANX-RALF Signaling to Regulate Arabidopsis Pollen Tube Integrity. Science 2019, 29, 3256–3265.e5. [Google Scholar] [CrossRef] [PubMed]
- Mecchia, M.A. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 2017, 358, 1600–1603. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Liu, C.; Fu, R.; Zhang, M.; Li, H.; Shen, L.; Wei, Q.; Sun, X.; Xu, L.; Ni, B.; et al. Lorelei-Like Gpi-Anchored Proteins 2/3 Regulate Pollen Tube Growth as Chaperones and Coreceptors for ANXUR/BUPS Receptor Kinases in Arabidopsis. Mol. Plant 2019, 12, 1612–1623. [Google Scholar] [CrossRef]
- Boisson-Dernier, A.; Franck, C.M.; Lituiev, D.S.; Grossniklaus, U. Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc. Natl. Acad. Sci. USA 2015, 112, 12211–12216. [Google Scholar] [CrossRef] [Green Version]
- Lassig, R.; Gutermuth, T.; Bey, T.D.; Konrad, K.R.; Romeis, T. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J. Cell Mol. Biol. 2014, 78, 94–106. [Google Scholar] [CrossRef]
- Zhu, L.; Chu, L.C.; Liang, Y.; Zhang, X.Q.; Chen, L.Q.; Ye, D. The Arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required for normal growth of pollen tubes in the pistil. Plant J. Cell Mol. Biol. 2018, 95, 474–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messenger, D.J.; McLeod, A.R.; Fry, S.C. The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ. 2009, 32, 1–9. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.; Fu, G.; Tao, L. Novel roles of hydrogen peroxide (H₂O₂) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Sogo, A.; Tobe, H. Intermittent pollen-tube growth in pistils of alders (Alnus). Plant Physiol. 2005, 102, 8770–8775. [Google Scholar] [CrossRef] [Green Version]
- Cucinotta, M.; Colombo, L.; Roig-Villanova, I. Ovule development, a new model for lateral organ formation. Front. Plant Sci. 2014, 5, 117. [Google Scholar] [CrossRef] [Green Version]
- Pagnussat, G.C.; Yu, H.J.; Sundaresan, V. Cell-fate switch of synergid to egg cell in Arabidopsis eostre mutant embryo sacs arises from misexpression of the BEL1-like homeodomain gene BLH1. Plant Cell 2007, 19, 3578–3592. [Google Scholar] [CrossRef] [Green Version]
- Huck, N.; Moore, J.M.; Federer, M.; Grossniklaus, U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 2003, 130, 2149–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.M.; Lopes, A.L.; Coimbra, S. Arabinogalactan Proteins as Interactors along the Crosstalk between the Pollen Tube and the Female Tissues. Front. Plant Sci. 2016, 7, 1895. [Google Scholar] [CrossRef]
- Mollet, J.C.; Park, S.Y.; Nothnagel, E.A.; Lord, E.M. A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. Plant Cell 2000, 12, 1737–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Jauh, G.Y.; Mollet, J.C.; Eckard, K.J.; Nothnagel, E.A.; Walling, L.L.; Lord, E.M. A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix. Plant Cell 2000, 12, 151–164. [Google Scholar]
- Tunc-Ozdemir, M.; Rato, C.; Brown, E.; Rogers, S.; Mooneyham, A.; Frietsch, S.; Myers, C.T.; Poulsen, L.R.; Malhó, R.; Harper, J.F. Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS ONE 2013, 8, e55277. [Google Scholar]
- Gao, Q.F.; Gu, L.L.; Wang, H.Q.; Fei, C.F.; Fang, X.; Hussain, J.; Sun, S.J.; Dong, J.Y.; Liu, H.; Wang, Y.F. Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 3096–3101. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.L.; Gao, Q.F.; Wang, Y.F. Cyclic nucleotide-gated channel 18 functions as an essential Ca(2+) channel for pollen germination and pollen tube growth in Arabidopsis. Plant Signal. Behav. 2017, 12, e1197999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, H.; Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 2016, 531, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Kao, Y.; Michard, E.; Simon, A.A.; Li, D.; Wudick, M.M.; Lizzio, M.A.; Feijó, J.A.; Chang, C. Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat. Commun. 2020, 11, 4082. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Kita, D.; Johnson, E.A.; Aggarwal, M.; Gates, L.; Wu, H.M.; Cheung, A.Y. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 2014, 5, 3129. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Kita, D.; Li, C.; Cheung, A.Y.; Wu, H.M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 2010, 107, 17821–17826. [Google Scholar] [CrossRef] [Green Version]
- Nissen, K.S.; Willats, W.G.T.; Malinovsky, F.G. Understanding CrRLK1L Function: Cell Walls and Growth Control. Trends Plant Sci. 2016, 21, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yeh, F.L.; Cheung, A.Y.; Duan, Q.; Kita, D.; Liu, M.C.; Maman, J.; Luu, E.J.; Wu, B.W.; Gates, L.; et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 2015, 4, e06587. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.G.; Liang, L.; Jia, P.F.; Wang, Y.C.; Li, H.J. Integration of ovular signals and exocytosis of a Ca(2+) channel by MLOs in pollen tube guidance. Nat. Plants 2020, 6, 143–153. [Google Scholar] [CrossRef]
- Jones, D.S.; Yuan, J.; Smith, B.E.; Willoughby, A.C.; Kumimoto, E.L.; Kessler, S.A. Mildew Resistance Locus O Function in Pollen Tube Reception Is Linked to Its Oligomerization and Subcellular Distribution. Nat. Plants 2017, 175, 172–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiyono, H.; Katano, K.; Suzuki, N. Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development. Plants 2021, 10, 1652. https://doi.org/10.3390/plants10081652
Kiyono H, Katano K, Suzuki N. Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development. Plants. 2021; 10(8):1652. https://doi.org/10.3390/plants10081652
Chicago/Turabian StyleKiyono, Hanako, Kazuma Katano, and Nobuhiro Suzuki. 2021. "Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development" Plants 10, no. 8: 1652. https://doi.org/10.3390/plants10081652
APA StyleKiyono, H., Katano, K., & Suzuki, N. (2021). Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development. Plants, 10(8), 1652. https://doi.org/10.3390/plants10081652