Elevated CO2 and Reactive Oxygen Species in Stomatal Closure
Abstract
1. Introduction
2. Importance of CO2 Regulation of Stomatal Conductance
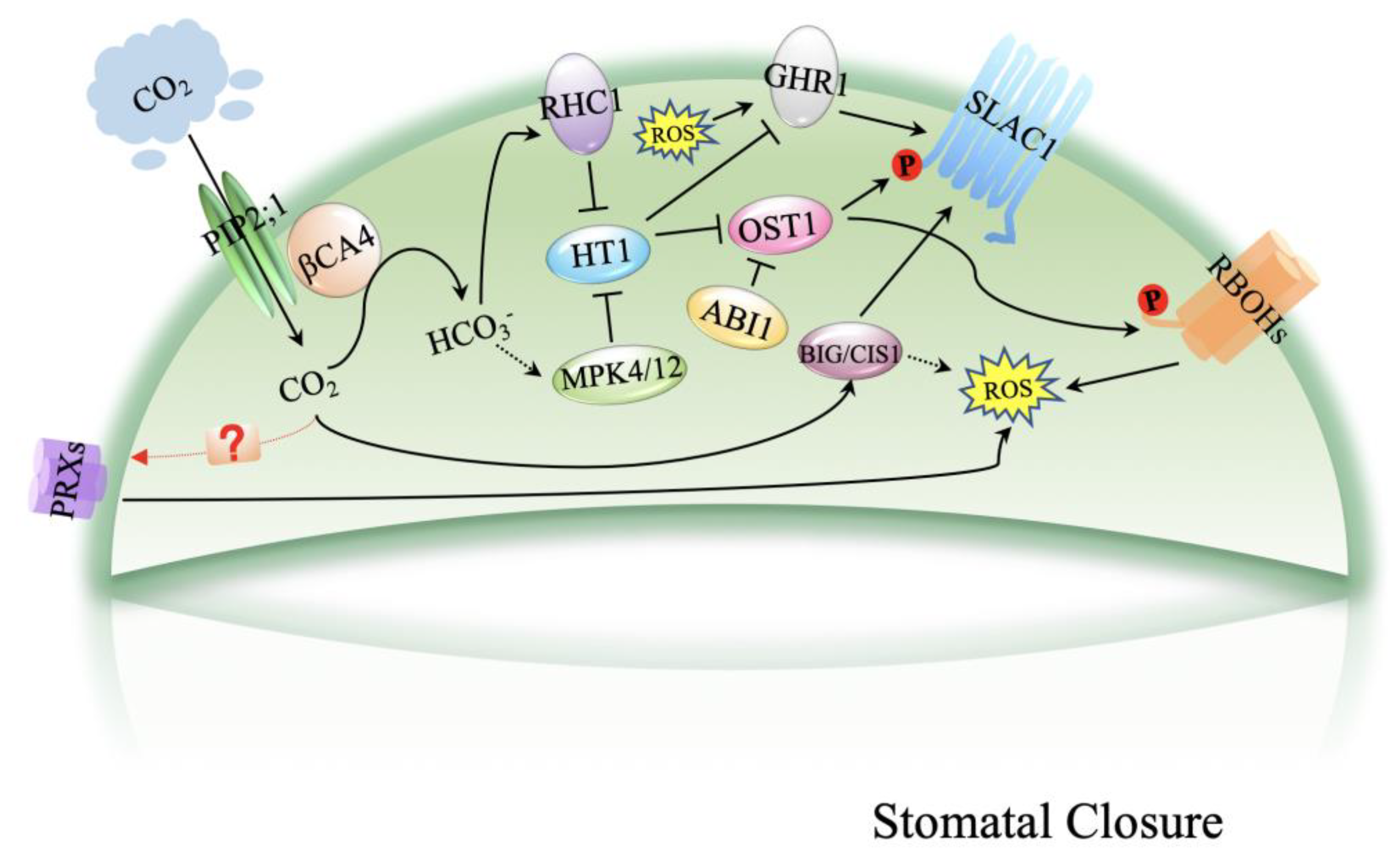
3. Mechanism of High CO2-Induced Stomatal Closure
4. ROS in Stomatal Closure
5. Function of ROS Signaling in CO2-Induced Stomatal Closure
6. ROS Are the Nodes of CO2 and ABA Signaling during Stomatal Movement
7. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 2001, 410, 327–330. [Google Scholar] [CrossRef]
- Young, J.J.; Mehta, S.; Israelsson, M.; Godoski, J.; Grill, E.; Schroeder, J.I. CO2 signaling in guard cells: Calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc. Natl. Acad. Sci. USA 2006, 103, 7506–7511. [Google Scholar] [CrossRef]
- Woodward, F.I. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 1987, 327, 617–618. [Google Scholar] [CrossRef]
- Keeling, C.D.; Bacastow, R.B.; Bainbridge, A.E.; Ekdahl, C.A.; Guenther, P.R.; Waterman, L.S.; Chin, J.F.S. Atmospheric carbon-dioxide variations at Mauna-Loa Observatory, Hawaii. Tellus 1976, 28, 538–551. [Google Scholar]
- Neftel, A.; Moor, E.; Oeschger, H.; Stauffer, B. Evidence from polar ice cores for the increase in atmospheric CO2 in the past 2 centuries. Nature 1985, 315, 45–47. [Google Scholar] [CrossRef]
- Mcelwain, J.C.; Chaloner, W.G. Stomatal density and index of fossil plants track atmospheric carbon-dioxide in the Paleozoic. Ann. Bot. 1995, 76, 389–395. [Google Scholar] [CrossRef]
- Chater, C.; Gray, J.E.; Beerling, D.J. Early evolutionary acquisition of stomatal control and development gene signalling networks. Curr. Opin. Plant Biol. 2013, 16, 638–646. [Google Scholar] [CrossRef]
- Mott, K.A. Do stomata respond to CO2 concentrations other than intercellular? Plant Physiol. 1988, 86, 200–203. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Robinson, A.P.; Clement, R.; McMurtrie, R.E. On the validation of models of forest CO2 exchange using eddy covariance data: Some perils and pitfalls. Tree Physiol. 2005, 25, 839–857. [Google Scholar] [CrossRef]
- Hu, H.H.; Boisson-Dernier, A.; Israelsson-Nordström, M.; Böhmer, M.; Xue, S.; Ries, A.; Godoski, J.; Kuhn, J.M.; Schroeder, J.I. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 2010, 12, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hou, C.; Ren, Z.; Pan, Y.; Jia, J.; Zhang, H.; Bai, F.; Zhang, P.; Zhu, H.; He, Y.; et al. A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. 2015, 6, 6057. [Google Scholar] [CrossRef]
- Hashimoto, M.; Negi, J.; Young, J.; Israelsson, M.; Schroeder, J.I.; Iba, K. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 2006, 8, 391–397. [Google Scholar] [CrossRef]
- Hashimoto-Sugimoto, M.; Negi, J.; Monda, K.; Higaki, T.; Isogai, Y.; Nakano, T.; Hasezawa, S.; Iba, K. Dominant and recessive mutations in the Raf-like kinase HT1 gene completely disrupt stomatal responses to CO2 in Arabidopsis. J. Exp. Bot. 2016, 67, 3251–3261. [Google Scholar] [CrossRef]
- Hõrak, H.; Sierla, M.; Tõldsepp, K.; Wang, C.; Wang, Y.S.; Nuhkat, M.; Valk, E.; Pechter, P.; Merilo, E.; Salojärvi, J.; et al. A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure. Plant Cell 2016, 28, 2493–2509. [Google Scholar] [CrossRef]
- Jakobson, L.; Vaahtera, L.; Tõldsepp, K.; Nuhkat, M.; Wang, C.; Wang, Y.S.; Hõrak, H.; Valk, E.; Pechter, P.; Sindarovska, Y.; et al. Natural variation in Arabidopsis Cvi-0 accession reveals an important role of MPK12 in guard cell CO2 signaling. PLoS Biol. 2016, 14, e2000322. [Google Scholar] [CrossRef]
- Tõldsepp, K.; Zhang, J.; Takahashi, Y.; Sindarovska, Y.; Hõrak, H.; Ceciliato, P.H.O.; Koolmeister, K.; Wang, Y.S.; Vaahtera, L.; Jakobson, L.; et al. Mitogen-activated protein kinases MPK4 and MPK12 are key components mediating CO2-induced stomatal movements. Plant J. 2018, 96, 1018–1035. [Google Scholar] [CrossRef]
- Zhang, J.; De-oliveira-Ceciliato, P.; Takahashi, Y.; Schulze, S.; Dubeaux, G.; Hauser, F.; Azoulay-Shemer, T.; Tõldsepp, K.; Kollist, H.; Rappel, W.J.; et al. Insights into the molecular mechanisms of CO2-mediated regulation of stomatal movements. Curr. Biol. 2018, 28, 1356–1363. [Google Scholar] [CrossRef]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Song, Y.; Miao, Y.; Song, C.P. Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytol. 2014, 201, 1121–1140. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Chater, C.; Peng, K.; Movahedi, M.; Dunn, J.A.; Walker, H.J.; Liang, Y.K.; McLachlan, D.H.; Casson, S.; Isner, J.C.; Wilson, I.; et al. Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr. Biol. 2015, 25, 2709–2716. [Google Scholar] [CrossRef]
- Hanstein, S.; Beer, D.; de Felle, H.H. Miniaturised carbon dioxide sensor designed for measurements within plant leaves. Sens. Actuators B Chem. 2001, 81, 107–114. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ort, D.R. More than taking the heat: Crops and global change. Curr. Opin. Plant. Biol. 2010, 13, 241–248. [Google Scholar] [CrossRef]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.J.; Iba, K.; Schroeder, J.I. CO2 Sensing and CO2 Regulation of Stomatal Conductance: Advances and Open Questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef]
- Wroblewitz, S.; Hüther, L.; Manderscheid, R.; Weigel, H.J.; Wätzig, H.; Dänicke, S. Effect of rising atmospheric carbon dioxide concentration on the protein composition of cereal grain. J. Agric. Food Chem. 2014, 62, 6616–6625. [Google Scholar] [CrossRef]
- Weigel, H.J. Plant quality declines as CO2 levels rise. Elife 2014, 3, e03233. [Google Scholar] [CrossRef]
- Lu, Z.M.; Percy, R.G.; Qualset, C.O.; Zeiger, E. Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. J. Exp. Bot. 1998, 49, 453–460. [Google Scholar] [CrossRef]
- Bahar, B.; Yildirim, M.; Barutcular, C. Relationships between stomatal conductance and yield components in spring durum wheat under Mediterranean conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 45–48. [Google Scholar]
- Prashar, A.; Yildiz, J.; McNicol, J.W.; Bryan, G.J.; Jones, H.G. Infra-red thermography for high through-put field phenotyping in Solanum tuberosum. PLoS ONE 2013, 8, e65816. [Google Scholar] [CrossRef] [PubMed]
- Frommer, W.B. CO2mmon sense. Science 2010, 327, 275–276. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Yarmolinsky, D.; Buchholtz, L.; von Oka, Y.; Sly, W.; Ryba, N.J.P.; Zuker, C.S. The taste of carbonation. Science 2009, 326, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Moroney, J.V.; Bartlett, S.G.; Samuelsson, G. Carbonic anhydrases in plants and algae. Plant. Cell Environ. 2001, 24, 141–153. [Google Scholar] [CrossRef]
- Fabre, N.; Reiter, I.M.; Becuwe-Linka, N.; Genty, B.; Rumeau, D. Characterization and expression analysis of genes encoding alpha and beta carbonic anhydrases in Arabidopsis. Plant. Cell Environ. 2007, 30, 617–629. [Google Scholar] [CrossRef]
- Hu, H.; Rappel, W.J.; Occhipinti, R.; Ries, A.; Böhmer, M.; You, L.; Xiao, C.; Engineer, C.B.; Boron, W.F.; Schroeder, J.I. Distinct cellular locations of carbonic anhydrases mediate CO2 control of stomatal movements. Plant Physiol. 2015, 169, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, H.H.; Qin, X.; Zeise, B.; Xu, D.Y.; Rappel, W.J.; Boron, W.F.; Schroeder, J.I. Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as CARBONIC ANHYDRASE 4 interactor. Plant Cell 2016, 28, 568–582. [Google Scholar] [CrossRef]
- Xue, S.W.; Hu, H.H.; Ries, A.; Merilo, E.; Kollist, H.; Schroeder, J.I. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. Embo J. 2011, 30, 1645–1658. [Google Scholar] [CrossRef]
- Matrosova, A.; Bogireddi, H.; Mateo-Peñas, A.; Hashimoto-Sugimoto, M.; Iba, K.; Schroeder, J.I.; Israelsson-Nordström, M. The HT1 protein kinase is essential for red light-induced stomatal opening and genetically interacts with OST1 in red light and CO2-induced stomatal movement responses. New Phytol. 2015, 208, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Hagiwara, S. Cytosolic calcium regulates ion channels in the plasma-membrane of Vicia faba guard-cells. Nature 1989, 338, 427–430. [Google Scholar] [CrossRef]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Negi, J.; Wang, C.; Isogai, Y.; Schroeder, J.I.; Iba, K. The transmembrane region of guard cell SLAC1 channels perceives CO2 signals via an ABA-independent pathway in Arabidopsis. Plant. Cell 2016, 28, 557–567. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Miao, Y.; Hauser, F.; McCammon, J.A.; Rappel, W.J.; Schroeder, J.I. Identification of SLAC1 anion channel residues required for CO2/bicarbonate sensing and regulation ofstomatal movements. Proc. Natl. Acad. Sci. USA 2018, 115, 11129–11137. [Google Scholar] [CrossRef] [PubMed]
- Geiger, D.; Scherzer, S.; Mumm, P.; Stange, A.; Marten, I.; Bauer, H.; Ache, P.; Matschi, S.; Liese, A.; Al-Rasheid, K.A.S.; et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 2009, 106, 21425–21430. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.P.; Wang, C.; He, J.N.; Liao, H.; Duan, Y.; Zhu, Z.Q.; Guo, Y.; Chen, Z.Z.; Gong, Z.Z. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 2012, 24, 2546–2561. [Google Scholar] [CrossRef] [PubMed]
- Jammes, F.; Song, C.; Shin, D.J.; Munemasa, S.; Takeda, K.; Gu, D.; Cho, D.; Lee, S.; Giordo, R.; Sritubtim, S.; et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl Acad. Sci. USA 2009, 106, 20520–20525. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.J.; Chu, S.P.; Harrington, C.L.; Schumacher, K.; Hoffmann, T.; Tang, Y.Y.; Grill, E.; Schroeder, J.I. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 2001, 411, 1053–1057. [Google Scholar] [CrossRef]
- Tang, M.; Zhao, X.; Hu, Y.; Zeng, M.; Wang, K.; Dong, N.; Ma, X.; Bai, L.; Song, C.P. Arabidopsis guard cell CO2/HCO3− response mutant screening by an aequorin-based calcium imaging system. Plant. Methods 2020, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.A.R.; McAinsh, M.R.; Mansfield, T.A.; Hetherington, A.M. Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant. J. 1996, 9, 297–304. [Google Scholar] [CrossRef]
- Schwartz, A.; Ilan, N.; Grantz, D.A. Calcium Effects on Stomatal movement in Commelina communis L. use of EGTA to modulate stomatal response to light, KCl and CO2. Plant. Physiol. 1988, 87, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.E.; Siegel, R.S.; Valerio, G.; Brandt, B.; Schroeder, J.I. Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus–response analyses. Ann. Bot. 2012, 109, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Ehonen, S.; Yarmolinsky, D.; Kollist, H.; Kangasjärvi, J. Reactive Oxygen Species, Photosynthesis, and Environment in the Regulation of Stomata. Antioxid Redox Signal. 2019, 30, 1220–1237. [Google Scholar] [CrossRef] [PubMed]
- Sierla, M.; Waszczak, C.; Vahisalu, T.; Kangasjärvi, J. Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 2016, 171, 1569–1580. [Google Scholar] [CrossRef]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Parihar, P.; Singh, S.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Reactive oxygen species signaling and stomatal movement: Current updates and future perspectives. Redox. Biol. 2017, 11, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C.; Fluhr, R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 1997, 9, 1559–1572. [Google Scholar] [CrossRef]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Pinontoan, R.; Kawano, T.; Muto, S. Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 2001, 42, 1383–1388. [Google Scholar] [CrossRef]
- Khokon, M.A.R.; Okuma, E.I.J.I.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Okamoto, H.; Okuma, E.; Shiba, H.; Kamada, H.; Hasegawa, P.M.; Murata, Y. SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant. J. 2013, 73, 91–104. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhard, N.; Angel Torres, M.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. Embo J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.P.; Boutrot, F.; Rathjen, J.P.; Zipfel, C. ASPARTATE OXIDASE plays an important role in Arabidopsis stomatal immunity. Plant. Physiol 2012, 159, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: Implications for plant adaptive responses. J. Exp. Bot. 2014, 65, 1271–1283. [Google Scholar] [CrossRef]
- An, Z.; Jing, W.; Liu, Y.; Zhang, W. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 2008, 59, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Liu, G.; Hou, L.; Wang, L.; Liu, X. Regulatory function of polyamine oxidase- generated hydrogen peroxide in ethylene-induced stomatal closure in Arabidopsis thaliana. J. Integr. Agric. 2013, 12, 251–262. [Google Scholar] [CrossRef]
- Kimura, S.; Kaya, H.; Kawarazaki, T.; Hiraoka, G.; Senzaki, E.; Michikawa, M.; Kuchitsu, K. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta 2012, 1823, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Steinhorst, L.; Kudla, J. Calcium and reactive oxygen species rule the waves of signaling. Plant. Physiol. 2013, 163, 471–485. [Google Scholar] [CrossRef]
- Drerup, M.M.; Schlücking, K.; Hashimoto, K.; Manishankar, P.; Steinhorst, L.; Kuchitsu, K.; Kudla, J. The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant 2013, 6, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Komori, T.; Kobori, M.; Nakahira, Y.; Shiina, T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 2008, 53, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Sakuragi, Y.; Stael, S.; Tikkanen, M.; Allahverdiyeva, Y.; Paakkarinen, V.; Aro, E.; Suorsa, M.; Scheller, H.V.; Vener, A.V.; et al. Light regulation of CaS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. Febs J. 2008, 275, 1767–1777. [Google Scholar] [CrossRef]
- Weinl, S.; Held, K.; Schlcking, K.; Steinhorst, L.; Kuhlgert, S.; Hippler, M.; Kudla, J. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol. 2008, 179, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Chen, J.; Liu, T.W.; Han, A.D.; Simon, M.; Dong, X.J.; He, J.X.; Zheng, H.L. Regulation of the calcium-sensing receptor in both stomatal movement and photosynthetic electron transport is crucial for water use efficiency and drought tolerance in Arabidopsis. J. Exp. Bot. 2014, 65, 223–234. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Hou, Y.J.; Zhao, Y.; Hsu, C.C.; Yuan, F.; Zhu, X.; Tao, W.A.; Song, C.P.; Zhu, J.K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S- nitrosylation of OST1. Proc. Natl. Acad. Sci. USA 2015, 112, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ueoka-Nakanishi, H.; Sazuka, T.; Nakanishi, Y.; Maeshima, M.; Mori, H.; Hisabori, T. Thioredoxin h regulates calcium dependent protein kinases in plasma membranes. Febs J. 2013, 280, 3220–3231. [Google Scholar] [CrossRef]
- Sierla, M.; Hõrak, H.; Overmyer, K.; Waszczak, C.; Yarmolinsky, D.; Maierhofer, T.; Vainonen, J.P.; Salojärvi, J.; Denessiouk, K.; Laanemets, K.; et al. The Receptor-like pseudokinase GHR1 is required for stomatal closure. Plant. Cell 2018, 30, 2813–2837. [Google Scholar] [CrossRef]
- Kolla, V.A.; Vavasseur, A.; Raghavendra, A.S. Hydrogen peroxide production is an early event during bicarbonate induced stomatal closure in abaxial epidermis of Arabidopsis. Planta 2007, 225, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, R.X.; Kim, D.S.; Sun, P.; Liu, H.; Liu, Z.; Hetherington, A.M.; Liang, Y.K. ROS of distinct sources and salicylic acid separate elevated CO2-mediated stomatal movements in Arabidopsis. Front. Plant Sci. 2020, 11, 542. [Google Scholar] [CrossRef]
- Fahnenstich, H.; Scarpeci, T.E.; Valle, E.M.; Flügge, U.I.; Maurino, V.G. Generation of hydrogen peroxide in chloroplasts of Arabidopsis overexpressing glycolate oxidase as an inducible system to study oxidative stress. Plant Physiol. 2008, 148, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Altschmied, L.; Chory, J. Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes Dev. 1994, 8, 339–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruegger, M.; Dewey, E.; Hobbie, L.; Brown, D.; Bernasconi, P.; Turner, J.; Muday, G.; Estelle, M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 1997, 9, 745–757. [Google Scholar]
- Gil, P.; Dewey, E.; Friml, J.; Zhao, Y.; Snowden, K.C.; Putterill, J.; Palme, K.; Estelle, M.; Chory, J. BIG: A calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. 2001, 15, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Kanyuka, K.; Praekelt, U.; Franklin, K.A.; Billingham, O.E.; Hooley, R.; Whitelam, G.C.; Halliday, K.J. Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J. 2003, 35, 57–70. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Hernández-Abreu, E.; Sánchez-Calderón, L.; Pérez-Torres, A.; Rampey, R.A.; Bartel, B.; Herrera-Estrella, L. An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol. 2005, 137, 681–691. [Google Scholar] [CrossRef]
- Paciorek, T.; Zažímalová, E.; Ruthardt, N.; Petrášek, J.; Stierhof, Y.D.; Kleine-Vehn, J.; Morris, D.A.; Emans, N.; Jürgens, G.; Geldner, N.; et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 2005, 435, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Hearn, T.J.; Ruiz, M.C.M.; Abdul-Awal, S.M.; Wimalasekera, R.; Stanton, C.R.; Haydon, M.J.; Theodoulou, F.L.; Hannah, M.A.; Alex, A.R.W. BIG regulates dynamic adjustment of circadian period in Arabidopsis thaliana. Plant Physiol. 2018, 178, 358–371. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Suzuki, M.; Fukaki, H.; Morita-Terao, M.; Tasaka, M.; Komeda, Y. CRM1/BIG-mediated auxin action regulates Arabidopsis inflorescence development. Plant. Cell Physiol. 2007, 48, 1275–1290. [Google Scholar] [CrossRef][Green Version]
- He, J.; Zhang, R.X.; Peng, K.; Tagliavia, C.; Li, S.; Xue, S.; Liu, A.; Hu, H.; Zhang, J.; Hubbard, K.E.; et al. The BIG protein distinguishes the process of CO2-induced stomatal closure from the inhibition of stomatal opening by CO2. New Phytol. 2018, 218, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Merilo, E.; Laanemets, K.; Hu, H.; Xue, S.; Jakobson, L.; Tulva, I.; Gonzalez-Guzman, M.; Rodriguez, P.L.; Schroeder, J.I.; Broschè, M.; et al. PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol. 2013, 162, 1652–1668. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.; Hetherington, A.M. Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiol. 1997, 114, 1557–1560. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Li, X.; Zhang, H.; Zhang, G.; Liu, Y.; Zhou, Y.; Xia, X.; Chen, Z.; Yu, J. Guard cell hydrogen peroxide and nitric oxide mediate elevated CO2-induced stomatal movement in tomato. New Phytol. 2015, 208, 342–353. [Google Scholar] [CrossRef]
- Jiang, C.; Mithani, A.; Belfield, E.J.; Mott, R.; Hurst, L.D.; Harberd, N.P. Environmentally responsive genome-wide accumulation of de novo Arabidopsis thaliana mutations and epimutations. Genome Res. 2014, 24, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Forgione, I.; Wołoszyńska, M.; Pacenza, M.; Chiappetta, A.; Greco, M.; Araniti, F.; Abenavoli, M.R.; Lijsebettens, M.V.; Bitonti, M.B.; Bruno, L. Hypomethylated drm1 drm2 cmt3 mutant phenotype of Arabidopsis thaliana is related to auxin pathway impairment. Plant Sci. 2019, 280, 383–396. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Bai, L. Elevated CO2 and Reactive Oxygen Species in Stomatal Closure. Plants 2021, 10, 410. https://doi.org/10.3390/plants10020410
Ma X, Bai L. Elevated CO2 and Reactive Oxygen Species in Stomatal Closure. Plants. 2021; 10(2):410. https://doi.org/10.3390/plants10020410
Chicago/Turabian StyleMa, Xiaonan, and Ling Bai. 2021. "Elevated CO2 and Reactive Oxygen Species in Stomatal Closure" Plants 10, no. 2: 410. https://doi.org/10.3390/plants10020410
APA StyleMa, X., & Bai, L. (2021). Elevated CO2 and Reactive Oxygen Species in Stomatal Closure. Plants, 10(2), 410. https://doi.org/10.3390/plants10020410







