Scopolia carniolica var. hladnikiana: Alkaloidal Analysis and Potential Taxonomical Implications
Abstract
:1. Introduction
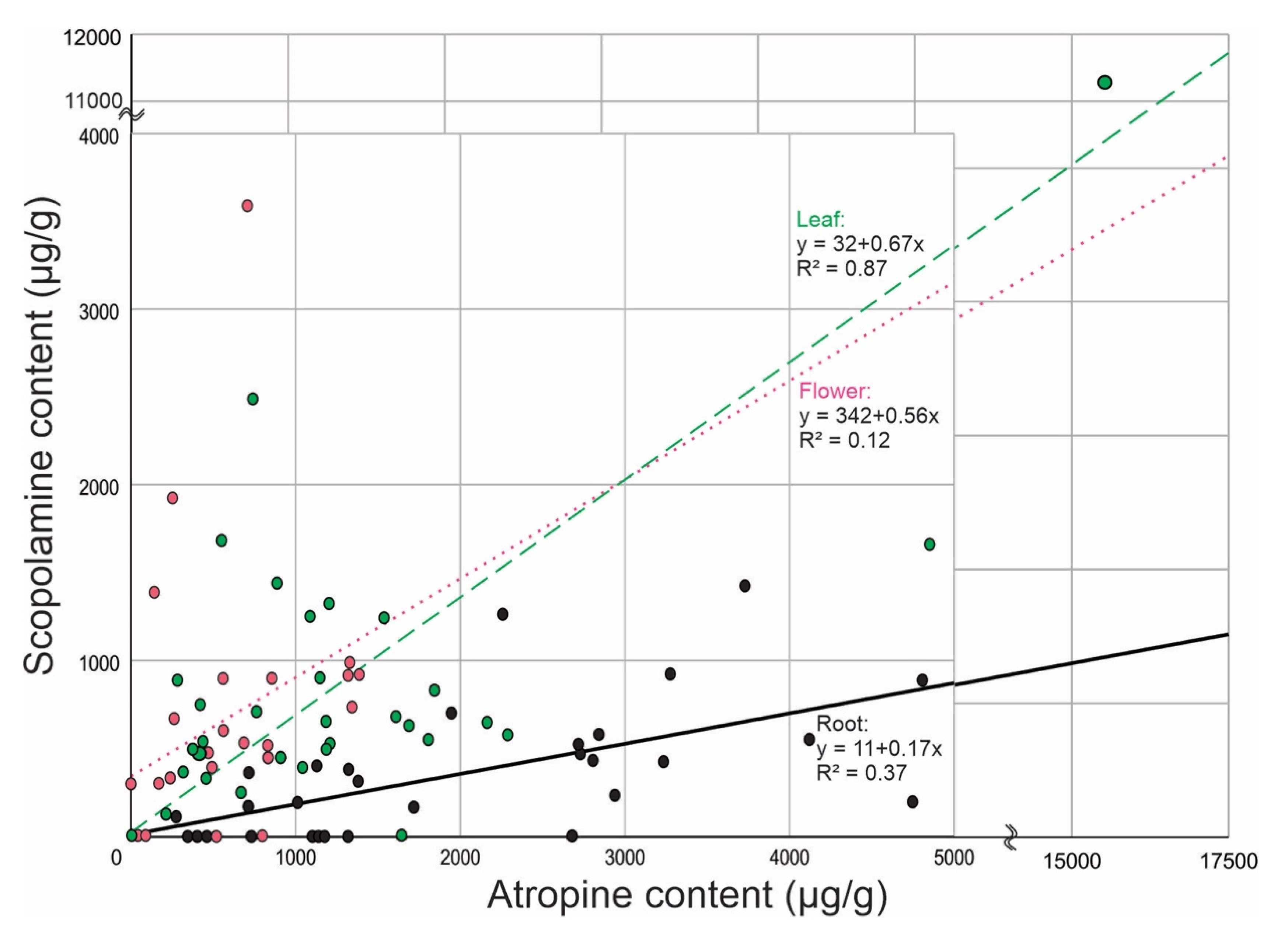
2. Results and Discussion
3. Materials and Methods
3.1. Sample Collection
3.2. Sample Preparation
3.3. Extraction
3.4. Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Praprotnik, N. Botaniki, Njihovo delo in Herbarijske Zbirke Praprotnic in Semenk v Prirodoslovnem Muzeju Slovenije. Revija Prirodosl. Muz. Slov. 2015, 83–84. [Google Scholar]
- Kocjan, J.M. Prispevek k poznavanju raširjenosti nekaterih redkih in endemičnih taksiniv v Sloveniji. Hladnikia 2001, 11, 17–24. [Google Scholar]
- Dakskobler, I. Novosti v flori zahodne, severzahodne in osrednje Slovenije. Hladnikia 2013, 31, 31–50. [Google Scholar]
- Festi, F. Scopolia carniolica Jacq. Eleusis 1996, 5, 34–45. [Google Scholar]
- Lonati, M.; Siniscalco, C. Populations status, syntaxonomy and synecology of Scopolia carniolica Jacq in the Western Alps (Piedmont, Italy). Acta Bot. Gall. 2009, 156, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Adamse, P.; van Egmond, H.P. Tropane Alkaloids in Food; Institute of Food Safety: Bedford Park, IL, USA, 2010. [Google Scholar]
- Carpa, R.; Dumitru, D.-V.; Burtescu, R.F.; Maior, M.C.; Dobrotă, C.; Olah, N.-K. Bio-chemical analysis of Datura stramonium extract. Stud. Univ. Babeş-Bolyai Biol. 2017, 62, 5–19. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Gadzikowska, M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep. 2008, 60, 439–463. [Google Scholar]
- Wink, M. Allelochemical properties or the raison d’être of alkaloids. Alkaloids Chem. Pharmacol. 1993, 43, 1–118. [Google Scholar]
- Parr, A.J.; Payne, J.; Eagles, J.; Chapman, B.T.; Robins, R.; Rhodes, M.J. Variation in tropane alkaloid accumulation within the solanaceae and strategies for its exploitation. Phytochemistry 1990, 29, 2545–2550. [Google Scholar] [CrossRef]
- Arroo, R. Transgenic Crops VI. Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Miraldi, E.; Masti, A.; Ferri, S.; Barni Comparini, I. Distribution of hyoscyamine and scopolamine in Datura stramonium. Fitoterapia 2001, 72, 644–648. [Google Scholar] [CrossRef]
- Ashtiania, F. Tropane alkaloids of Atropa belladonna L. and Atropa acuminata Royle ex Miers plants. J. Med. Plants Res. 2011, 5, 6515–6522. [Google Scholar]
- Brachet, A.; Mateus, L.; Cherkaoui, S.; Christen, P.; Gauvrit, J.-Y.; Lanteri, P.; Veuthey, J.-L. Application of central composite designs in the supercritical fluid extraction of tropane alkaloids in plant extracts. Analusis 1999, 27, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Bahmanzadegana, A.; Sefidkona, F.; Sonbolib, A. Determination of hyoscyamine and scopolamine in four Hyoscyamus species from Iran. Iran. J. Pharm. Res. 2009, 8, 65–70. [Google Scholar]
- Boros, B.; Farkas, Á.; Jakabová, S.; Bacskay, I.; Kilar, F.; Felinger, A. LC-MS Quantitative Determination of Atropine and Scopolamine in the Floral Nectar of Datura Species. Chromatographia 2010, 71, 43–49. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Oshaghi, M.A.; Majd, A. Distribution of atropine and scopolamine in different organs and stages of development in Datura stramonium L. Acta Biol. Crac. Ser. Bot. 2006, 48, 13–18. [Google Scholar]
- Klančar, U.; Kac, J.; Mlinarič, A.; Krbavčič, A. Vsebnosti Atropina in Skopolamina v Strupenih Rastlinah Razhudnikov na Slovenskem. Zdr. Vestn. 2006, 75, 157–162. [Google Scholar]
- Berkov, S.; Philipov, S. Alkaloid Production in Diploid and Autotetraploid Plants of Datura stramonium. Pharm. Biol. 2003, 40, 617–621. [Google Scholar] [CrossRef]
- Facchini, P.J. Alkaloid Biosynthesis in Plants: Biochemistry, Cell Biology, Molecular Regulation, and Metabolic Engineering Applications. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 29–66. [Google Scholar] [CrossRef] [Green Version]
- Jouhikainen, K.; Lindgren, L.; Jokelainen, T.; Hiltunen, R.; Teeri, T.; Oksman-Caldentey, K.-M. Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 1999, 208, 545–551. [Google Scholar] [CrossRef]
- Banani, N.; Mehrafarin, A.; Hosseini Mazinani, S.M. Changes of tropane alkaloids in Black Henbane (Hyoscyamus niger L.) in Response to Different Types of Nitrogenous Fertilizers. J. Med. Plants 2017, 16, 56–67. [Google Scholar]
- Berkov, S.; Zayed, R.; Doncheva, T. Alkaloid patterns in some varieties of Datura stramonium. Fitoterapia 2006, 77, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Shonle, I.; Bergelson, J. Evolutionary ecology of the tropane alkaloids of Datura stramonium L. (solanaceae). Evolution 2000, 54, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Baricevic, D.; Umek, A.; Kreft, S.; Maticic, B.; Zupancic, A. Effect of water stress and nitrogen fertilization on the content of hyoscyamine and scopolamine in the roots of deadly nightshade (Atropa belladonna). Environ. Exp. Bot. 1999, 42, 17–24. [Google Scholar] [CrossRef]
- Jakabová, S.; Vincze, L.; Farkas, Á.; Kilar, F.; Boros, B.; Felinger, A. Determination of tropane alkaloids atropine and scopolamine by liquid chromatography–mass spectrometry in plant organs of Datura species. J. Chromatogr. A 2012, 1232, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Spurná, V.; Sovová, M.; Jirmanová, E.; Šustáčková, A. Chromosomal Characteristics and Occurrence of Main Alkaloids in Datura stramonium and Datura wrightii. Planta Med. 2007, 41, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Lipič, F.V. Topografija c.-kr. Deželnega Glavnega Mesta Ljubljane z Vidika Naravoslovja in Medicine, Zdravstvene Ureditve in Biostatike; Znanstveno Društvo za Zgodovino Zdravstvene Kulture Slovenije: Ljubljana, Slovenia, 1834. [Google Scholar]
- Koetz, M.; Santos, T.G.; Rayane, M.; Henriques, A.T. Quantification of atropine in leaves of Atropa belladonna: Development and validation of method by high-performance liquid chromatography (HPLC). Drug Anal. Res. 2017, 1, 44–49. [Google Scholar] [CrossRef]

| Flower | Leaf | Root | Total | ||
|---|---|---|---|---|---|
| Scopolamine | Mean | 619 | 1103 | 346 | 692 |
| N | 28 | 31 | 31 | 90 | |
| SD | 748 | 1964 | 379 | 1273 | |
| Atropine | Mean | 492 | 1615 | 1945 | 1379 |
| N | 28 | 31 | 31 | 90 | |
| SD | 460 | 2737 | 1328 | 1887 | |
| Total alkaloids (Scopolamine + atropine) | Mean | 1110 | 2717 | 2291 | 2070 |
| N | 28 | 31 | 31 | 90 | |
| SD | 1004 | 4621 | 1585 | 2967 | |
| Bohor Mountain | Ljubljana Botanical Garden | Pekel Pri Borovnici | ||
|---|---|---|---|---|
| Scopolamine | Mean | 816 | 585 | 570 |
| N | 28 | 31 | 31 | |
| SD | 1695 | 794 | 381 | |
| Atropine | Mean | 1942 | 758 | 1445 |
| N | 28 | 31 | 31 | |
| SD | 2428 | 965 | 1204 | |
| Hladnikiana | Hybrid? | Normal | Striped Flowers | ||
|---|---|---|---|---|---|
| Scopolamine | Mean | 617 | 638 | 906 | 386 |
| N | 28 | 31 | 31 | 3 | |
| SD | 1748 | 388 | 863 | 50 | |
| Atropine | Mean | 1313 | 1934 | 987 | 1363 |
| N | 28 | 31 | 31 | 3 | |
| SD | 2446 | 1269 | 1137 | 1313 | |
| 2019 | 2020 | ||
|---|---|---|---|
| Scopolamine | Mean | 659 | 728 |
| N | 28 | 31 | |
| SD | 1613 | 730 | |
| Atropine | Mean | 1446 | 1304 |
| N | 28 | 31 | |
| SD | 2357 | 1163 | |
| Scopolamine | Atropine | ||||
|---|---|---|---|---|---|
| Source of variability | df | Sig. | Sig. | Sig. | Sig. |
| Corrected model | 23 | 0.391 | 0.201 | 0.167 | 0.014 |
| Intercept | 1 | 0.000 | 0.007 | 0.000 | 0.000 |
| organ | 2 | 0.744 | 0.054 | 0.095 | 0.006 |
| Location | 2 | 0.134 | 0.124 | 0.083 | 0.051 |
| Type | 3 | 0.235 | 0.211 | 0.981 | 0.985 |
| year | 1 | 0.629 | 0.949 | 0.541 | 0.375 |
| Location × type | 0 | na | na | ||
| organ × location | 4 | 0.452 | 0.380 | ||
| Location × year | 1 | 0.130 | 0.459 | ||
| organ × type | 6 | 0.683 | 0.657 | ||
| Type × year | 2 | 0.373 | 0.367 | ||
| organ × year | 2 | 0.328 | 0.503 | ||
| Error | 66 | ||||
| Total | 90 | ||||
| Corrected total | 89 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatur, K.; Ravnikar, M.; Kreft, S. Scopolia carniolica var. hladnikiana: Alkaloidal Analysis and Potential Taxonomical Implications. Plants 2021, 10, 1643. https://doi.org/10.3390/plants10081643
Fatur K, Ravnikar M, Kreft S. Scopolia carniolica var. hladnikiana: Alkaloidal Analysis and Potential Taxonomical Implications. Plants. 2021; 10(8):1643. https://doi.org/10.3390/plants10081643
Chicago/Turabian StyleFatur, Karsten, Matjaž Ravnikar, and Samo Kreft. 2021. "Scopolia carniolica var. hladnikiana: Alkaloidal Analysis and Potential Taxonomical Implications" Plants 10, no. 8: 1643. https://doi.org/10.3390/plants10081643
APA StyleFatur, K., Ravnikar, M., & Kreft, S. (2021). Scopolia carniolica var. hladnikiana: Alkaloidal Analysis and Potential Taxonomical Implications. Plants, 10(8), 1643. https://doi.org/10.3390/plants10081643






