Debottlenecking Thermophilic Cyanobacteria Cultivation and Harvesting through the Application of Inner-Light Photobioreactor and Chitosan
Abstract
:1. Introduction
2. Results
2.1. Light Attenuation Curve and Light Exposure Characteristics of Thermosynechococcus E542
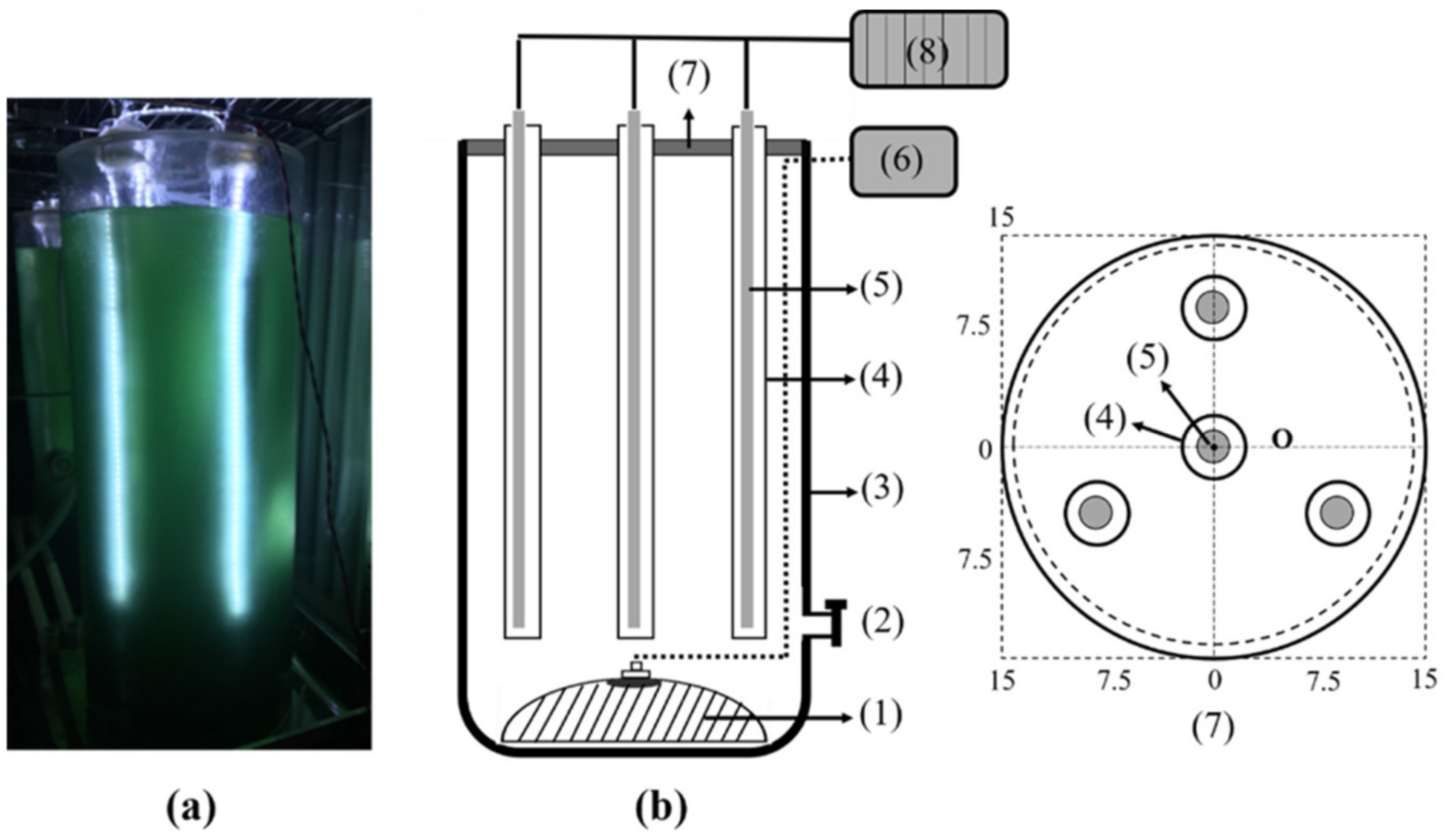
2.2. Design of the Internal Illumination Photobioreactor and Cultivation of Cyanobacteria
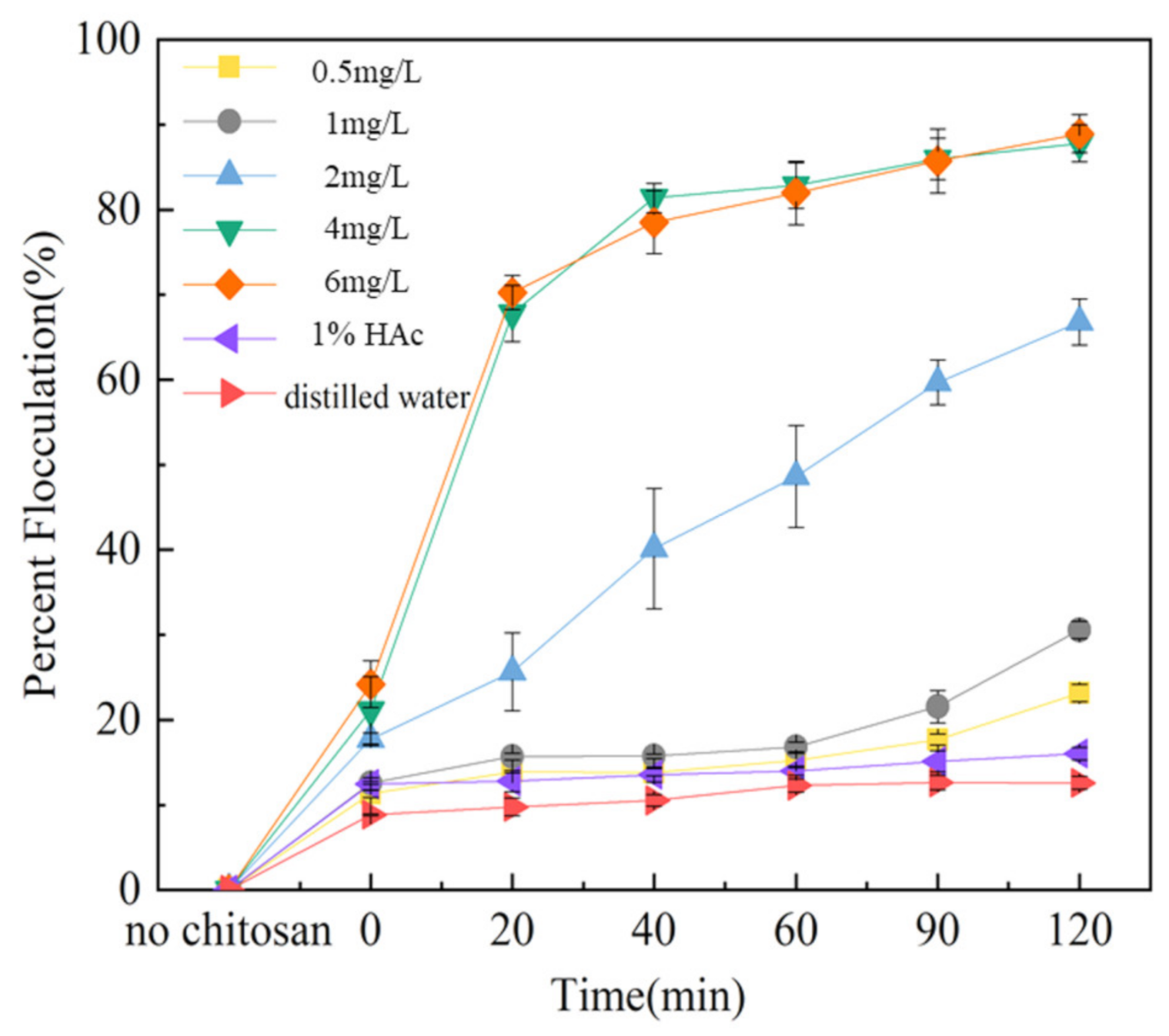
2.3. Chitosan Flocculation Results
3. Discussion
4. Materials and Methods
4.1. Strain Cultivation
4.2. Estimation of Light Attenuation
4.3. Light Attenuation Models
4.3.1. Lambert–Beer Model
4.3.2. The Hyperbolic Model
4.4. Numerical Simulation of the Model
4.5. PBR Device Construction and Operation
4.5.1. Materials
4.5.2. Cultivation of Thermosynechococcus E542 in the Internally-illuminated PBR
4.6. Preparation of the Flocculant and Measurement of Flocculation Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Kaczmarek, M.B.; Kasprzak, A.K.; Tang, J.; Shah, M.M.R.; Jin, P.; Klepacz-Smółka, A.; Cheng, J.J.; Daroch, M. Thermosynechococcaceae as a source of thermostable C-phycocyanins: Properties and molecular insights. Algal Res. 2018, 35, 223–235. [Google Scholar] [CrossRef]
- Ciebiada, M.; Kubiak, K.; Daroch, M. Modifying the cyanobacterial metabolism as a key to efficient biopolymer production in photosynthetic microorganisms. Int. J. Mol. Sci. 2020, 21, 7204. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Huang, G.; Zhang, B. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2021, 410, 124619. [Google Scholar] [CrossRef]
- Liang, Y.; Tang, J.; Luo, Y.; Kaczmarek, M.B.; Li, X.; Daroch, M. Thermosynechococcus as a thermophilic photosynthetic microbial cell factory for CO 2 utilisation. Bioresour. Technol. 2019, 278, 255–265. [Google Scholar] [CrossRef]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Gale, G.A.R.; Osorio, A.A.S.; Mills, L.A.; Wang, B.; Lea-Smith, D.J.; McCormick, A.J. Emerging species and genome editing tools: Future prospects in cyanobacterial synthetic biology. Microorganisms 2019, 7, 409. [Google Scholar] [CrossRef] [Green Version]
- Davies, F.K.; Jinkerson, R.E.; Posewitz, M.C. Toward a photosynthetic microbial platform for terpenoid engineering. Photosynth. Res. 2015, 123, 265–284. [Google Scholar] [CrossRef]
- Jung, E.E.; Jain, A.; Voulis, N.; Doud, D.F.R.; Angenent, L.T.; Erickson, D. Stacked optical waveguide photobioreactor for high density algal cultures. Bioresour. Technol. 2014, 171, 495–499. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Wu, W.T. A novel photobioreactor with transparent rectangular chambers for cultivation of microalgae. Biochem. Eng. J. 2009, 46, 300–305. [Google Scholar] [CrossRef]
- Ahsan, S.S.; Gumus, A.; Jain, A.; Angenent, L.T.; Erickson, D. Integrated hollow fiber membranes for gas delivery into optical waveguide based photobioreactors. Bioresour. Technol. 2015, 192, 845–849. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Xue, S.; Zhang, Q.; Wu, X.; Yan, C.; Cong, W. A novel photobioreactor structure using optical fibers as inner light source to fulfill flashing light effects of microalgae. Bioresour. Technol. 2013, 138, 141–147. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Wan, M.; Yan, Y.; Feng, F.; Qu, X.; Wang, J.; Shen, G.; Li, W.; Fan, J.; et al. Novel flat-plate photobioreactors for microalgae cultivation with special mixers to promote mixing along the light gradient. Bioresour. Technol. 2014, 159, 8–16. [Google Scholar] [CrossRef]
- Degen, J.; Uebele, A.; Retze, A.; Schmid-Staiger, U.; Trösch, W. A novel airlift photobioreactor with baffles for improved light utilization through the flashing light effect. J. Biotechnol. 2001, 92, 89–94. [Google Scholar] [CrossRef]
- Wang, L.L.; Tao, Y.; Mao, Z.X. A novel flat plate algal bioreactor with horizontal baffles: Structural optimization and cultivation performance. Bioresour. Technol. 2014, 164, 20–27. [Google Scholar] [CrossRef]
- Li, F.F.; Yang, Z.H.; Zeng, R.; Yang, G.; Chang, X.; Yan, J.B.; Hou, Y.L. Microalgae capture of CO2 from actual flue gas discharged from a combustion chamber. Ind. Eng. Chem. Res. 2011, 50, 6496–6502. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Bekasova, O.D. Two Distinct Photoprocesses in Cyanobacterial Bilin Pigments: Energy Migration in Light-Harvesting Phycobiliproteins versus Photoisomerization in Phytochromes. Photochem. Photobiol. 2020, 96, 750–767. [Google Scholar] [CrossRef] [Green Version]
- Ungerer, J.; Lin, P.; Chen, H.-Y.; Pakrasi, H.B. Adjustments to Photosystem Stoichiometry and Electron Transfer Proteins Are Key to the Remarkably Fast Growth of the Cyanobacterium Synechococcus elongatus UTEX 2973. ASM mBio 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.J. Radiocarbon uptake: Its relation to net particulate carbon production. Limnol. Oceanogr. 1978, 23, 179–184. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Wan, M.; Wang, R.; Huang, J.; Zhang, K.; Guo, J.; Bai, W.; Li, Y. Comparative study on light attenuation models of Galdieria sulphuraria for efficient production of phycocyanin. J. Appl. Phycol. 2020, 32, 165–174. [Google Scholar] [CrossRef]
- Divakaran, R.; Pillai, V.N.S. Flocculation of algae using chitosan. J. Appl. Phycol. 2002, 14, 419–422. [Google Scholar] [CrossRef]
- Beach, E.S.; Eckelman, M.J.; Cui, Z.; Brentner, L.; Zimmerman, J.B. Preferential technological and life cycle environmental performance of chitosan flocculation for harvesting of the green algae Neochloris oleoabundans. Bioresour. Technol. 2012, 121, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Lesage, G.; Nghiem, L.D. Synergistic effect of dual flocculation between inorganic salts and chitosan on harvesting microalgae Chlorella vulgaris. Environ. Technol. Innov. 2020, 17, 100622. [Google Scholar] [CrossRef]
- Komárek, J.; Johansen, J.R.; Šmarda, J.; Strunecký, O. Phylogeny and taxonomy of synechococcus–like cyanobacteria. Fottea 2020, 20, 171–191. [Google Scholar] [CrossRef]
- Hou, J.; Li, X.; Kaczmarek, M.B.M.B.; Chen, P.; Li, K.; Jin, P.; Liang, Y.; Daroch, M. Accelerated CO2 Hydration with Thermostable Sulfurihydrogenibium azorense Carbonic Anhydrase-Chitin Binding Domain Fusion Protein Immobilised on Chitin Support. Int. J. Mol. Sci. 2019, 20, 1494. [Google Scholar] [CrossRef] [Green Version]
- Price, G.D.; Maeda, S.; Omata, T.; Badger, M.R. Modes of active inorganic carbon uptake in the cyanobacterium, Synechococcus sp. PCC7942. Funct. Plant Biol. 2002, 29, 131–149. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J.; Sánchez-Baracaldo, P. The possible evolution and future of CO2-concentrating mechanisms. J. Exp. Bot. 2017, 68, 3701–3716. [Google Scholar] [CrossRef] [Green Version]
- Riaz, S.; Xiao, M.; Chen, P.; Li, M.; Cui, Y.; Darocha, M. The Genome Copy Number of the Thermophilic Cyanobacterium Thermosynechococcus elongatus E542 Is Controlled by Growth Phase and Nutrient Availability. Appl. Environ. Microbiol. 2021, 87, 1–13. [Google Scholar] [CrossRef]
- Eberly, J.O.; Ely, R.L. Photosynthetic accumulation of carbon storage compounds under CO2 enrichment by the thermophilic cyanobacterium Thermosynechococcus elongatus. J. Ind. Microbiol. Biotechnol. 2012, 39, 843–850. [Google Scholar] [CrossRef]
- Bergmann, P.; Trösch, W. Repeated fed-batch cultivation of Thermosynechococcus elongatus BP-1 in flat-panel airlift photobioreactors with static mixers for improved light utilization: Influence of nitrate, carbon supply and photobioreactor design. Algal Res. 2016, 17, 79–86. [Google Scholar] [CrossRef]
- Chu, H.H.M.; Narindri, B.; Hsueh, H.T.; Chu, H.H.M. Improvement of Thermosynechococcus sp. CL-1 performance on biomass productivity and CO2 fixation via growth factors arrangement. J. Photochem. Photobiol. B Biol. 2020, 205, 111822. [Google Scholar] [CrossRef]
- Klepacz-Smółka, A.; Pietrzyk, D.; Szeląg, R.; Głuszcz, P.; Daroch, M.; Tang, J.; Ledakowicz, S. Effect of light colour and photoperiod on biomass growth and phycocyanin production by Synechococcus PCC 6715. Bioresour. Technol. 2020, 313, 123700. [Google Scholar] [CrossRef]
- Labeeuw, L.; Commault, A.S.; Kuzhiumparambil, U.; Emmerton, B.; Nguyen, L.N.; Nghiem, L.D.; Ralph, P.J. A comprehensive analysis of an effective flocculation method for high quality microalgal biomass harvesting. Sci. Total Environ. 2021, 752, 141708. [Google Scholar] [CrossRef]
- Rojsitthisak, P.; Burut-Archanai, S.; Pothipongsa, A.; Powtongsook, S. Repeated phosphate removal from recirculating aquaculture system using cyanobacterium remediation and chitosan flocculation. Water Environ. J. 2017, 31, 598–602. [Google Scholar] [CrossRef]
- Pathania, R.; Srivastava, S. Synechococcus elongatus BDU 130192, an Attractive Cyanobacterium for Feedstock Applications: Response to Culture Conditions. Bioenergy Res. 2020, in press. [Google Scholar] [CrossRef]
- Lama, S.; Muylaert, K.; Karki, T.B.; Foubert, I.; Henderson, R.K.; Vandamme, D. Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour. Technol. 2016, 220, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Liang, Y.; Jiang, D.; Li, L.; Luo, Y.; Shah, M.R.; Daroch, M. Temperature-controlled thermophilic bacterial communities in hot springs of western Sichuan, China. BMC Microbiol. 2018, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Jiang, D.; Luo, Y.; Liang, Y.; Li, L.; Shah, M.M.R.; Daroch, M. Potential new genera of cyanobacterial strains isolated from thermal springs of western Sichuan, China. Algal Res. 2018, 31, 14–20. [Google Scholar] [CrossRef]
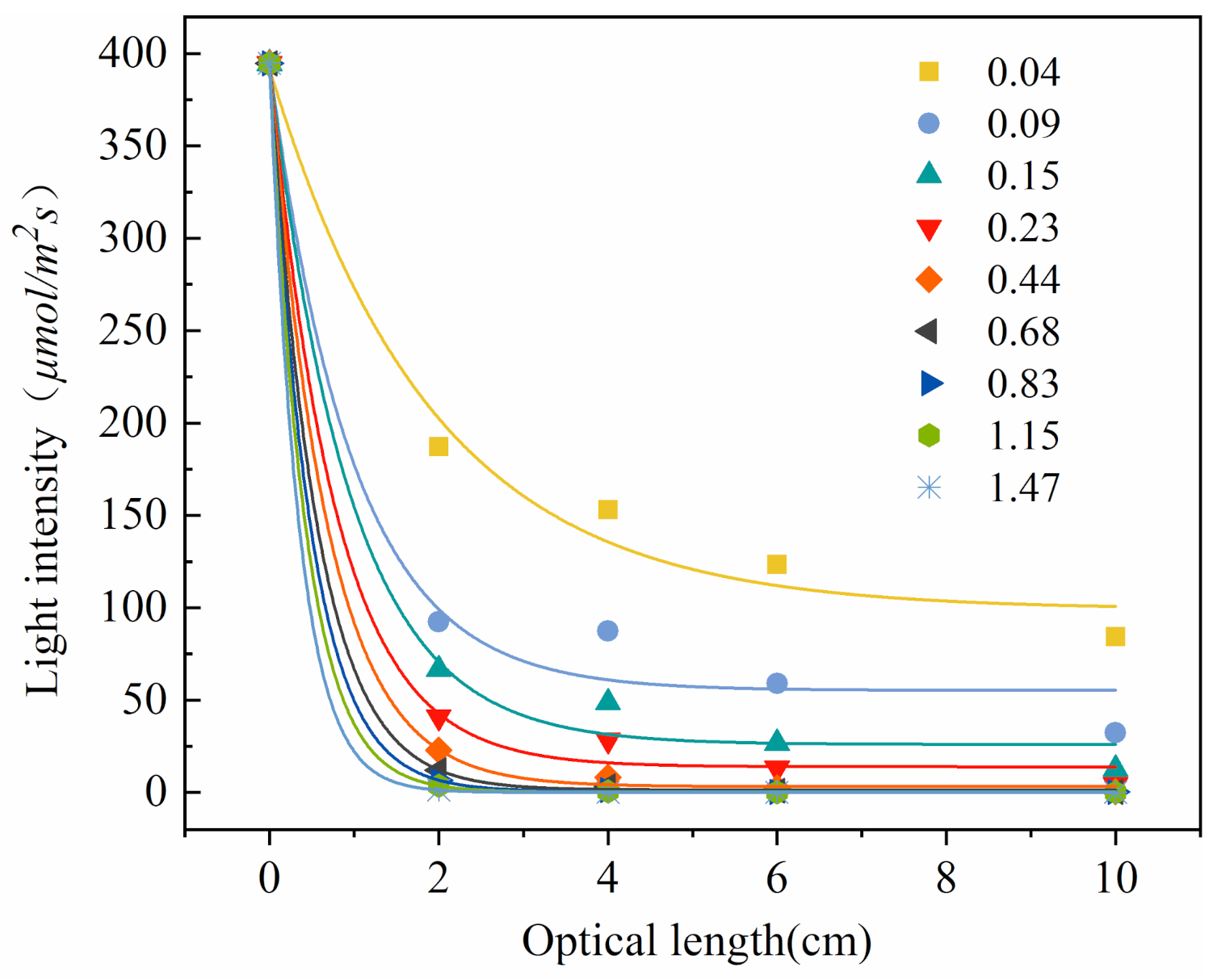





| Model: ExpDec1 | I = A1 exp(−r/t1) + y0 | |
|---|---|---|
| OD685nm | Fitting Formula | R2 |
| 0.04 | I = 292.78exp(−r/1.92) + 99.07 | 0.96788 |
| 0.09 | I = 338.93exp(−r/0.98) + 55.29 | 0.97105 |
| 0.15 | I = 368.38exp(−r/0.95) + 26.03 | 0.99044 |
| 0.23 | I = 380.67exp(−r/0.78) + 13.93 | 0.9955 |
| 0.44 | I = 391.42exp(−r/0.67) + 3.24 | 0.99963 |
| 0.68 | I = 393.31exp(−r/0.56) + 1.36 | 0.99993 |
| 0.83 | I = 394.07exp(−r/0.48) + 0.60 | 0.99999 |
| 1.15 | I = 394.41exp(−r/0.42) + 0.26 | 1 |
| 1.47 | I = 394.58exp(−r/0.35) + 0.09 | 1 |
| No. | Component | Parameters | |
|---|---|---|---|
| (1) | Diffuser stone | dmax = 25 cm | h = 15 cm |
| (2) | Harvesting valve | d1 = 1.5 cm | - |
| (3) | Reactor | V = 40 L | h = 100 cm |
| (4) | Glass tube | d2 = 5 cm | h = 80 cm |
| (5) | LED | 24 V | 14 w/m |
| (6) | Air pump | 0.045 Mpa | 50 L/min |
| (7) | Reactor cover | d = 30 cm | h = 2 cm |
| (8) | Power supply | Voltage range 12–24 V | Output current 1.5–6.5 A |
| OD685nm | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.04 | 0.09 | 0.15 | 0.23 | 0.44 | 0.68 | 0.83 | 1.15 | 1.47 | |
| rs | 5.06 | 3.18 | 2.62 | 2.45 | 2.32 | 2.30 | 2.29 | 2.29 | 2.29 |
| R-rs | 9.94 | 11.82 | 12.38 | 12.55 | 12.68 | 12.70 | 12.71 | 12.71 | 12.71 |
| VL:VD | 1:2 | 1:4 | 1:5 | 1:5 | 1:5 | 1:6 | 1:6 | 1:6 | 1:6 |
| VD/V | 0.66 | 0.79 | 0.83 | 0.84 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| Component | Material | Supplier | |
|---|---|---|---|
| (1) | Diffuser stone | Emery | Danies Aquarium, China |
| (2) | Harvesting valve | Acrylic | Chuang Yi, China |
| (3) | Reactor | Acrylic | Chuang Yi, China |
| (4) | Glass tube | Glass | Hua Xing, China |
| (5) | LED | LED | Kaloulight, China |
| (6) | Air pump | - | Belos, China |
| (7) | Reactor cover | Acrylic | Chuang Yi, China |
| (8) | Power supply | - | Bao an Qua Hao, China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, P.; Russel, M.; Tang, J.; Jin, P.; Daroch, M. Debottlenecking Thermophilic Cyanobacteria Cultivation and Harvesting through the Application of Inner-Light Photobioreactor and Chitosan. Plants 2021, 10, 1540. https://doi.org/10.3390/plants10081540
Zhang H, Chen P, Russel M, Tang J, Jin P, Daroch M. Debottlenecking Thermophilic Cyanobacteria Cultivation and Harvesting through the Application of Inner-Light Photobioreactor and Chitosan. Plants. 2021; 10(8):1540. https://doi.org/10.3390/plants10081540
Chicago/Turabian StyleZhang, Hairuo, Pengyu Chen, Mohammad Russel, Jie Tang, Peng Jin, and Maurycy Daroch. 2021. "Debottlenecking Thermophilic Cyanobacteria Cultivation and Harvesting through the Application of Inner-Light Photobioreactor and Chitosan" Plants 10, no. 8: 1540. https://doi.org/10.3390/plants10081540
APA StyleZhang, H., Chen, P., Russel, M., Tang, J., Jin, P., & Daroch, M. (2021). Debottlenecking Thermophilic Cyanobacteria Cultivation and Harvesting through the Application of Inner-Light Photobioreactor and Chitosan. Plants, 10(8), 1540. https://doi.org/10.3390/plants10081540









