Different Sensitivity Levels of the Photosynthetic Apparatus in Zea mays L. and Sorghum bicolor L. under Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Photosynthetic Pigments
2.2. Oxidative Stress Markers
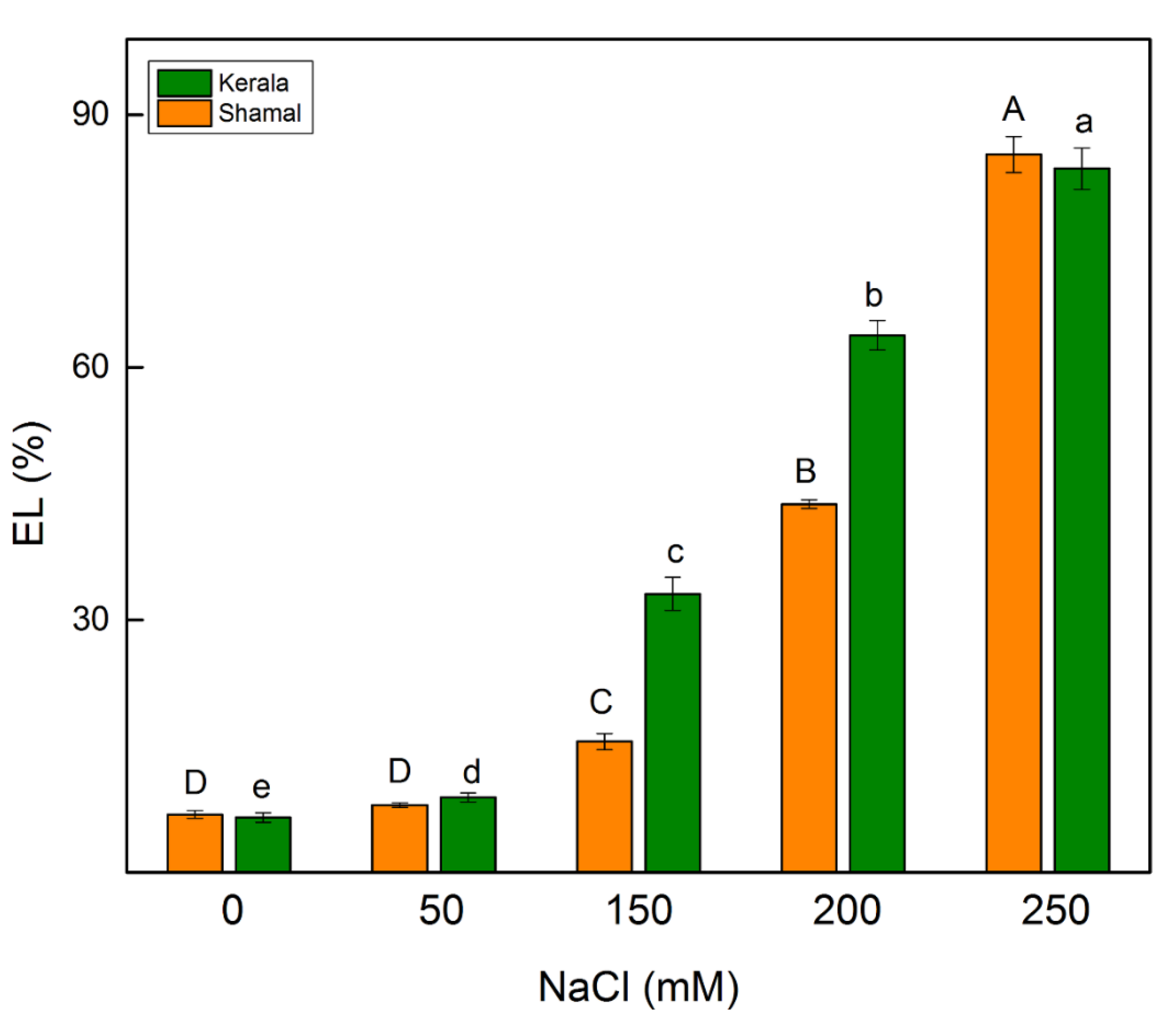
2.3. Electrolyte Leakage
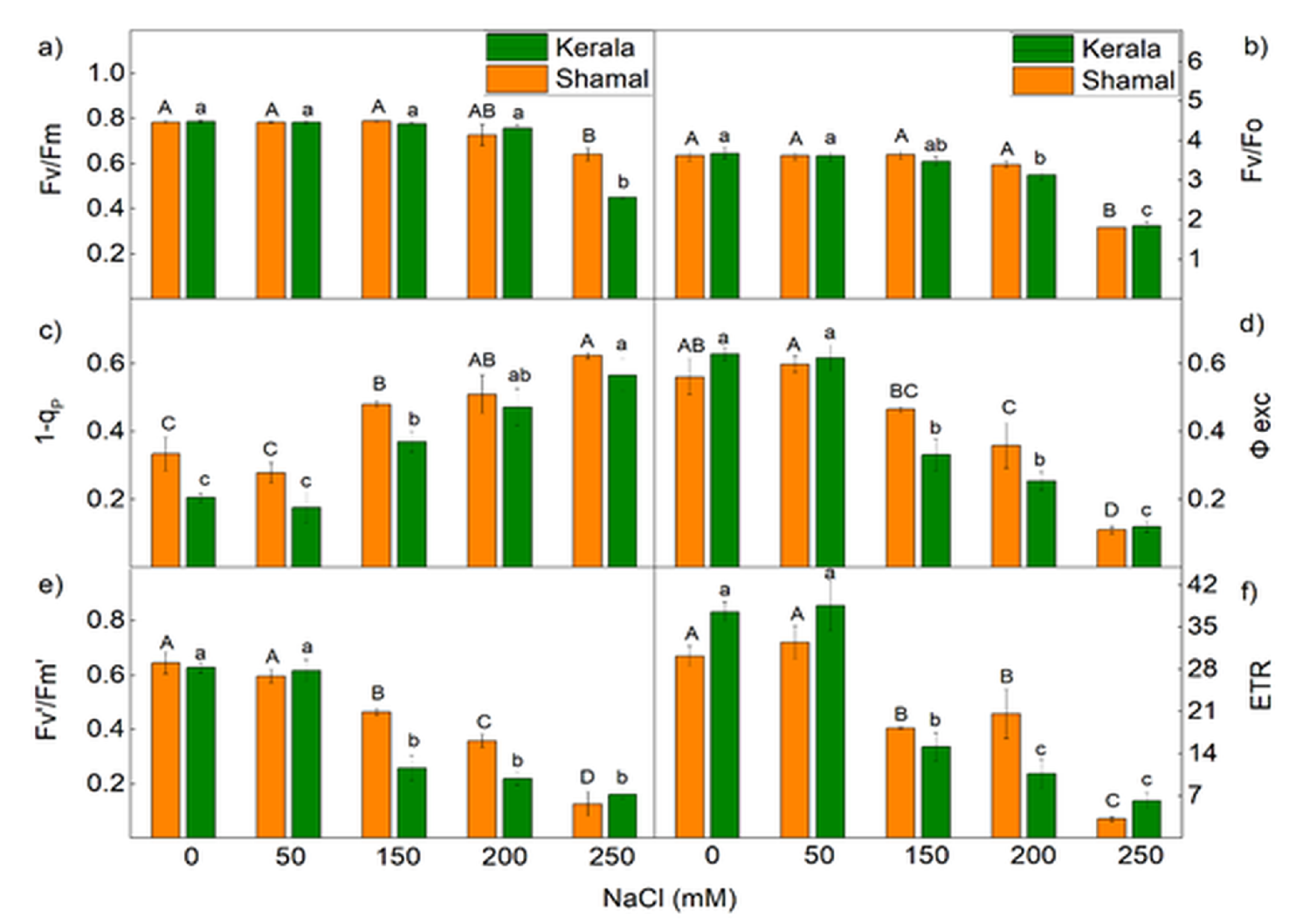
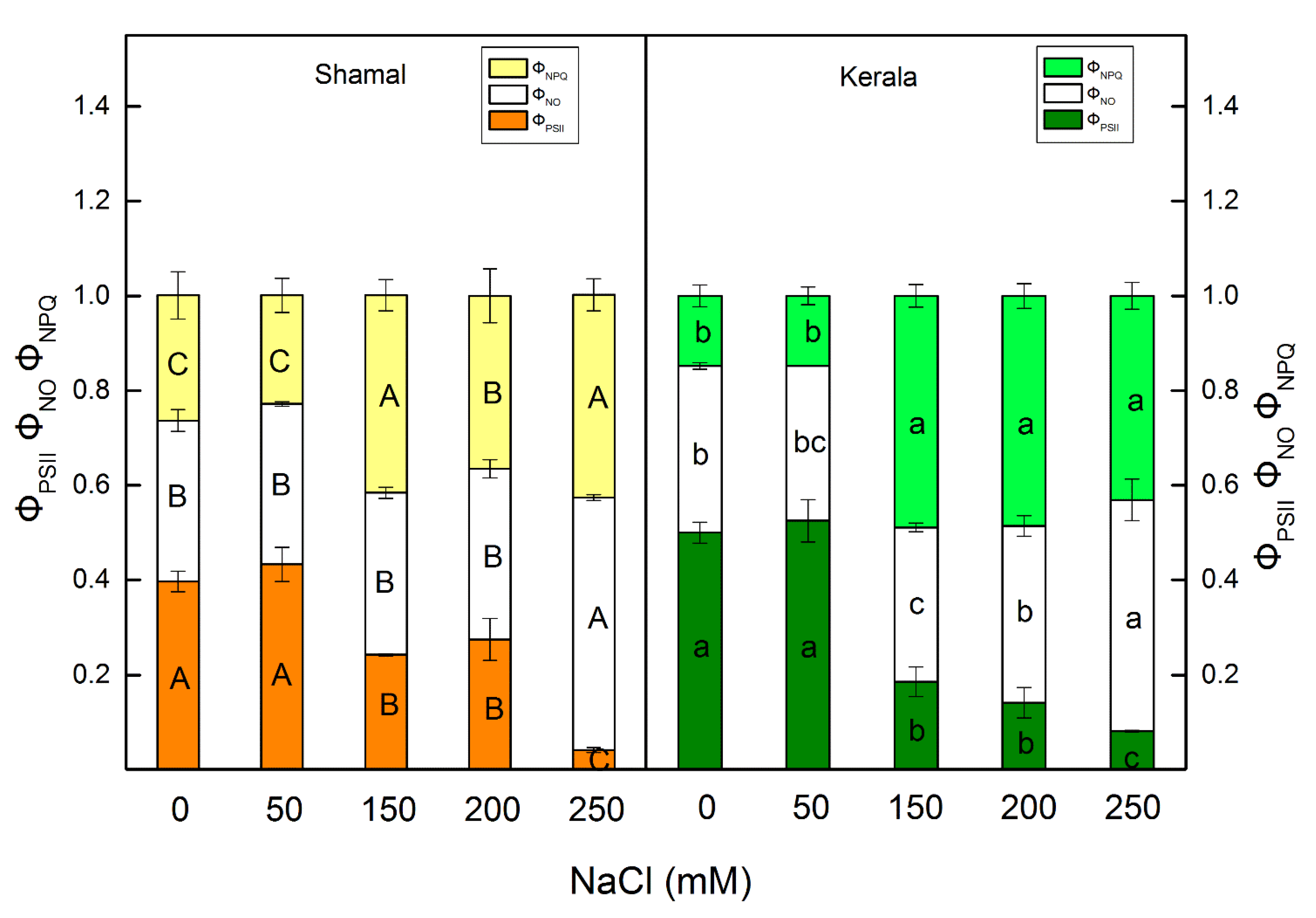
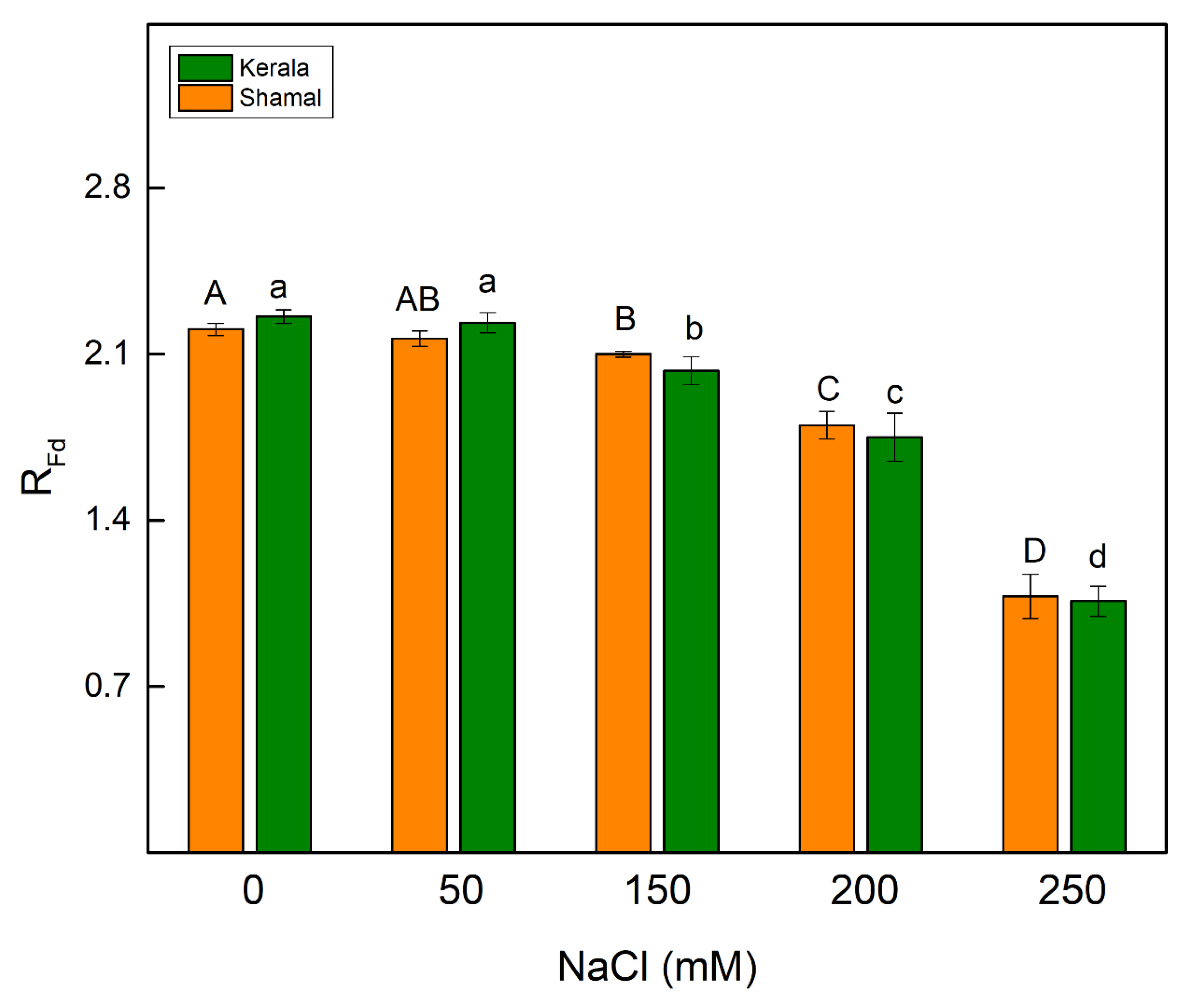
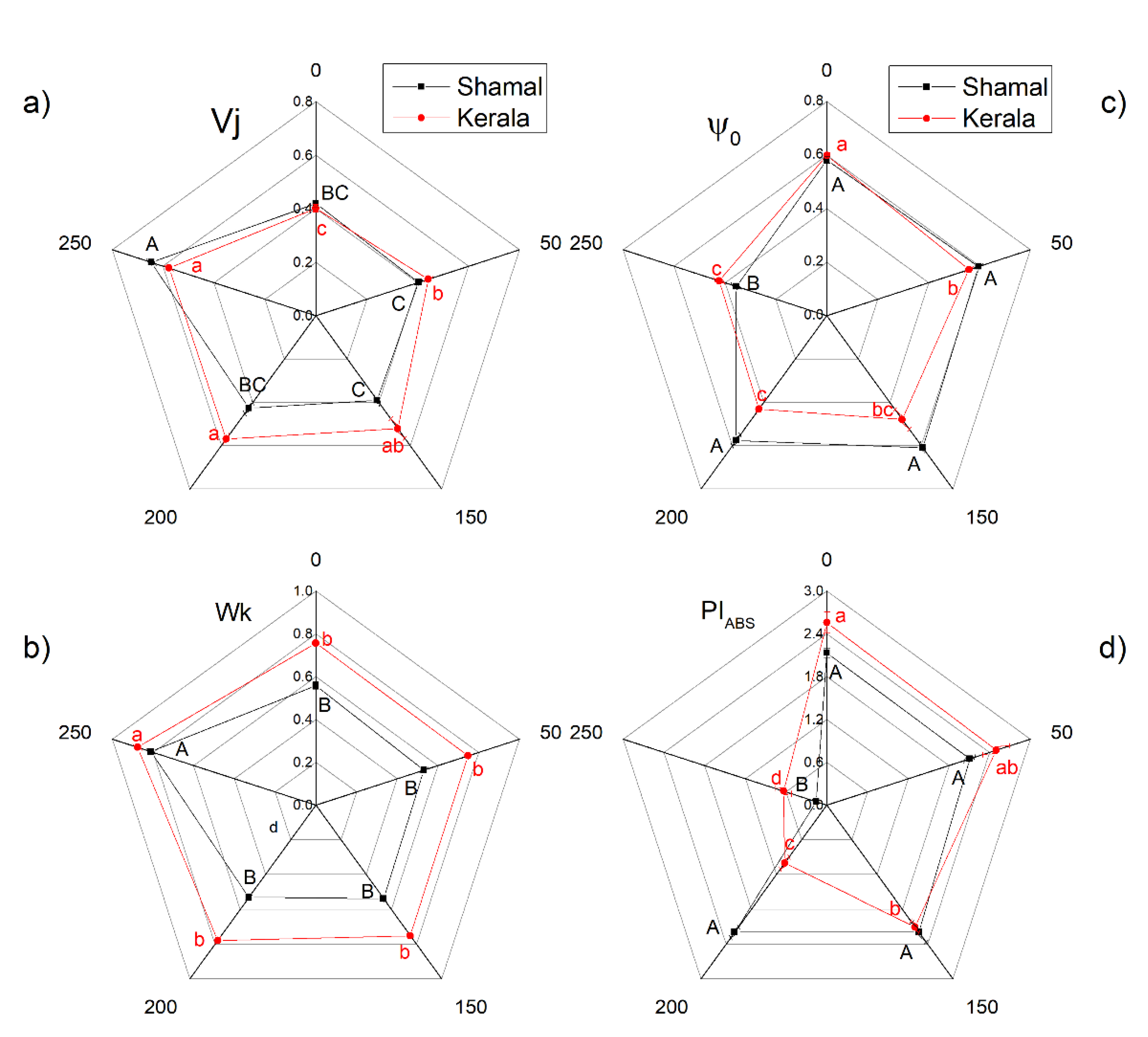
2.4. Room Temperature Chlorophyll a Fluorescence
2.5. Photooxidation of P700
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Treatments
4.2. Pigment Content
4.3. Determination of Oxidative Stress Markers
4.4. Electrolyte Leakage
4.5. Room-Temperature Chlorophyll Fluorescence
4.6. P700 Redox State Measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.; Pulatov, A.; Kempen, B.; de Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of salinity stress on growth and metabolomic rofiling of Cucumis sativus and Solanum lycopesicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Harris, P. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Omoto, E.; Taniguchi, M.; Miyake, H. Adaptation responses in C4 photosynthesis of maize under salinity. J. Plant Physiol. 2012, 169, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.H.; Siahpoosh, M.R.; Roessner, U.; Udvardi, M.; Kopka, J. Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol. Plant. 2008, 132, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.N.; Sahu, S.; Misra, M.; Singh, P.; Meera, I.; Das, N.; Kar, M.; Sahu, P. Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biol. Plant. 1997, 39, 257–262. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. Plant Physiol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.; Das, A. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Baiguz, A.; Havat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Labudda, M.; Tokarz, K.; Tokarz, B.; Muszyńska, E.; Gietler, M.; Górecka, M.; Różańska, E.; Rybarczyk-Płońska, A.; Fidler, J.; Prabucka, B.; et al. Reactive oxygen species metabolism and photosynthetic performance in leaves of Hordeum vulgare plants co-infested with Heterodera flipjevi and Aceria tosichella. Plant Cell Rep. 2020, 39, 1719–1741. [Google Scholar] [CrossRef]
- Stefanov, M.; Biswal, A.K.; Misra, M.; Misra, A.N.; Apostolova, E.L. Responses of photosynthetic apparatus to salt stress: Structure, Function and Protection. In Handbook of Plant & Crop Stress, 4th ed.; Pessarakli, M., Ed.; Taylor & Francis: New York, NY, USA; CRC Press instead Taylor & Francis: Abingdon-on-Thames, UK, 2021; pp. 233–250. [Google Scholar]
- Sun, Z.W.; Ren, L.K.; Fan, J.W.; Li, Q.; Wang, K.J.; Guo, M.M.; Wang, L.; Li, J.; Zhang, G.X.; Yang, Z.Y.; et al. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant Soil Environ. 2016, 62, 515–521. [Google Scholar]
- Unnep, R.; Zsiros, O.; Salymosi, K.; Kovacs, L.; Lambrev, P.H.; Toth, T. The ultrastructure and flexibility of thylakoid membranes in leaves and isolated chloroplasts as revealed by small angle neutron scattering. Biochim. Biophys. Acta 2014, 1837, 1572–1580. [Google Scholar] [CrossRef] [Green Version]
- Zeeshan, M.; Lu, M.; Shar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes diferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.R. Possible role of photosystem II in environmental perturbations of photosynthesis. Physiol. Plant. 1991, 81, 563–570. [Google Scholar] [CrossRef]
- Hakala, M.; Tuominen, I.; Keranen, M.; Tyystjärvi, T.; Tyystjärvi, E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim. Biophys. Acta 2005, 1706, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Jiang, B.; Wang, B.; Kuang, T. Photosystem II photochemistry and photosynthetic pigment composition in salt adapted chalophyte Artemisia anethifolia grown under outdoor conditions. J. Plant Physiol. 2003, 160, 403–408. [Google Scholar] [CrossRef]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Jajoo, A. Changes in photosystem II in response to salt stress. In Ecophysiol. Responses Plants under Salt Stress; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer: New York, NY, USA, 2013; pp. 149–168. [Google Scholar]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Hessini, K.; Jeddi, K.; Siddique, K.; Cruz, C. Drought and salinity: A comparison of their effects on the ammonium-preferring species Spartina Alterniflora. Physiol. Plant. 2021, 172, 431–440. [Google Scholar] [CrossRef]
- Jusovic, M.; Velitchkova, M.Y.; Misheva, S.P.; Börner, A.; Apostolova, E.L.; Dobrikova, A.G. Photosynthetic responses of a wheat mutant (Rht-B1c) with altered DELLA proteins to salt stress. J. Plant Growth Regul. 2018, 37, 645–656. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Rashkov, G.; Ivanova, K.; Markovska, Y.; Apostolova, E.L. Effects of salinity on the photosynthetic apparatus of two Paulownia lines. Plant Physiol. Biochem. 2016, 101, 54–59. [Google Scholar] [CrossRef]
- Akram, A.; Ashraf, M. Improvement in growth, chlorophyll pigments and photosynthetic performance in salt-stressed plants of sunflower (Helianthus annuus L.) by foliar application of 5- aminolevulinic acid. Agrochimica 2011, 55, 94–104. [Google Scholar]
- Santos, C. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hort. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Dubis, B.; Sokólski, M.M.; Załuski, D.; Bórawski, P.; Szempliński, W. Productivity and energy balance of maize and sorghum grown for biogas in a large-area farm in Poland: An 11-year field experiment. Ind. Crop. Prod. 2020, 148, 112326. [Google Scholar] [CrossRef]
- Labuschange, M.T. A review of cereal grain proteomics and its potential for sorghum improvement. J. Cereal Sci. 2018, 84, 151–158. [Google Scholar] [CrossRef]
- Gong, F.; Wu, X.; Zhang, H.; Chen, Y.; Wang, W. Making better maize plants for sustainable grain production in a changing climate. Front. Plant Sci. 2015, 6, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef] [Green Version]
- Mundia, C.W.; Secchi, S.; Akamani, K.; Wang, G. A regional comparison of factors affecting global sorghum production: The case of North America, Asia and Africa’s sahel genes and salt tolerance: Bringing them together. Sustainability 2019, 11, 2135. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Xu, W.; Rodriguez, D.; Sun, Y. Growth and physiological responses of maize and sorghum genotypes to salt stress. ISRN Agron. 2012, 41, 12. [Google Scholar] [CrossRef] [Green Version]
- Igartua, E.; Gracia, M.P.; Lasa, J.M. Field responses of grain sorghum to a salinity gradient. F. Crop. Res. 1995, 42, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.M.F.; Salama, K.H.A. Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 2004, 52, 113–122. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dabrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, A.; Zivcak, M.; Tripathi, D.K.; Yadav, S.; Kalaji, H.M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Cha-um, S.; Kirdmanee, C. Effect of salt stress on proline accumulation, photosynthetic ability and growth characters in two maize cultivars. Pak. J. Bot. 2009, 41, 87–98. [Google Scholar]
- Qu, C.; Liu, C.; Gong, X.; Li, C.; Hong, M.; Wang, L.; Hong, F. Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environ. Exp. Bot. 2012, 75, 134–141. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. South. Afr. J. Bot. 2017, 108, 261–265. [Google Scholar] [CrossRef]
- Eckardt, N.A. A new chlorophyll degradation pathway. Plant Cell 2009, 21, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.; Aro, E. Grana stacking and protection of Photosystem II in thylakoid membranes of higher plant leaves under sustained high irradiance: An hypothesis. Photosynth. Res. 1994, 41, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Stoichkova, K.; Busheva, M.; Apostolova, E.; Andreeva, A. Changes in the energy distribution in mutant thylakoid membranes of pea with modified pigment content II. Changes due to magnesium ions concentration. J. Photochem. Photobiol. B 2006, 83, 11–20. [Google Scholar] [CrossRef]
- Apostolova, E.; Dobrikova, A.; Ivanova, P.; Petkanchin, I.; Taneva, S. Relationship between the organization of the PSII supercomplex and the functions of the photosynthetic apparatus. J. Photochem. Photobiol. B 2006, 83, 114–122. [Google Scholar] [CrossRef]
- Shu, S.; Guo, R.; Sun, J.; Yuan, Y. Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol. Plant. 2012, 146, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Yang, Z.; Liu, M.; Wang, B. Identification and transcriptomic profiling of genus involved in increasing sugar content during salt stress in sweet sorghum leave. BMC Genom. 2015, 16, 534. [Google Scholar] [CrossRef] [Green Version]
- Wani, A.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.; Inaba, M.; Yokota, A.; Murata, N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001, 20, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Jogeswar, G.; Pallela, R.; Jakka, N.M.; Reddy, P.S.; Rao, J.V.; Sreenivasulu, N. Antioxidative response in different sorghum species under short-term salinity stress. Acta Physiol. Plant. 2006, 28, 465–475. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Ngara, R.; Ndimba, R.; Borch-Jensen, J.; Jensen, O.N.; Ndimba, B. Identification and profiling of salinity stress-responsive proteins in Sorghum bicolor seedlings. J. Proteom. 2012, 75, 4139–4150. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.B.D.; Alencar, N.L.M.; Prisco, J.T.; Gomes-Filho, E. Accumulation of organic and inorganic solutes in NaCl-stressed sorghum seedlings from aged and primed seeds. Sci. Agric. 2011, 68, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Račková, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can chlorophylla fluorescence parameters be used as bio-indicators to distinguish between drought andsalinity stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Kalaji, M.H.; Baczewska, A.H.; Pawluśkiewicz, B.; Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Paunov, M.; Goltsev, V. Delayed chlorophyll a cluorescence, MR 820, and gas exchange changes in perennial ryegrass under salt stress. J. Lumin. 2017, 183, 322–333. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Bussotti, F.; Pollastrini, M.; Piekut, K.; Kowalik, W.; Wróbel, J.; Kalaji, H.M. Photosynthetic efficiency of microcystis ssp. under salt stress. Environ. Exp. Bot. 2021, 186, 104459. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B.; Paunov, M.; Alexantrov, V.; Goltsev, V.; Kalaji, M.H. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in perennial ryegrass. J. Photochem. Photobiol. B. 2016, 157, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Baycu, G.; Sperdouli, I.; Eroglu, H.; Eleftheriou, E.P. Arbuscular mycorrhizal symbiosis enhances photosynthesis in the medicinal herb Salvia fruticosa by improving photosystem II photochemistry. Plants 2020, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.E.; de Siqueira, D.L.; Martinez, C.A.; Puiatti, M. Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminum stress. Plant Physiol. 2000, 157, 513–520. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Markovska, Y.; Apostolova, E.L. Effect of high light intensity on the photosynthetic apparatus of two hybrid lines of Paulownia grown on soils with different salinity. Photosynthetica 2018, 56, 832–840. [Google Scholar] [CrossRef]
- Rastogi, A.; Kovar, M.; He, X.; Zivchak, M.; Kataria, S.; Kalaji, H.M.; Skalicky, M.; Ibrahimova, U.; Hussain, S.; Mbarki, S.; et al. JIP-test as a tool to identify salinity tolerance in sweet sorghum genotypes. Photosynthetica 2020, 58, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Albertsson, P.A. The structure and function of the chloroplast photosynthetic membrane—A model for the domain organization. Photosynth. Res. 1995, 46, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.J.; Zhang, Z.B. Specific roles of cyclic electron flow around photosystem I in photosynthetic regulation in immature and mature leaves. J. Plant Physiol. 2017, 209, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Szopkó, D.; Darkó, É.; Molnár, I.; Kruppa, K.; Háló, B.; Vojtkó, A.; Molnár-Láng, M.; Dulai, S. Photosynthetic responses of a wheat (Asakaze)–barley (Manas) 7H addition line to salt stress. Photosynthetica 2016, 54, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Milward, S.E.; Fan, D.Y.; Chow, W.S.; Badger, M.R. How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol. 2009, 149, 1560–1567. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids-pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Yotsova, E.K.; Dobrikova, A.G.; Stefanov, M.A.; Kouzmanova, M.; Apostolova, E.L. Improvement of the rice photosynthetic apparatus defence under cadmium stress modulated by salicylic acid supply to roots. Theor. Exp. Plant Physiol. 2018, 30, 57–70. [Google Scholar] [CrossRef]
- Gently, B.; Briantais, J.-M.; Baker, N. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Rohaček, K. Clorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationship. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Lichtenthaler, K.; Langsdorf, G.; Lenk, S.; Buschmann, C. Chlorophyll fluorescence imaging of photosynthetic activity with the flash-lamp fluorescence imaging system. Photosynthetica 2005, 43, 355–369. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Zivcak, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.I.; Lotfi, R.; Stypinski, P.; Samborska, L.A.; et al. Prompt chlorophyll fluorescence as a tool for phenotyping: An example of barley landcraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Tsimilli-Michael, M.; Strasser, R.J. The energy flux theory 35 years later: Formulations and applications. Photosynth. Res. 2013, 117, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Dankov, K.; Busheva, M.; Stefanov, D.; Apostolova, E. Relationship between the degree of carotenoid depletion and function of photosynthetic apparatus, J. Photochem. Photobiol. B 2009, 96, 49–56. [Google Scholar] [CrossRef] [PubMed]

| Variant | Chl a + b (µg/g DW) | Car (µg/g DW) | Chl a/b | Car/Chl |
|---|---|---|---|---|
| Shamal | ||||
| Control 50 mM NaCl 150 mM NaCl 200 mM NaCl 250 mM NaCl | 26017 ± 640 A 25124 ± 602 A 18739 ± 924 B 13694 ± 285 C 10814 ± 261 D | 4979 ± 111 A 4860 ± 55 A 4091 ± 273 B 2978 ± 125 C 2488 ± 72 D | 4.560 ± 0.035 D 4.602 ± 0.023 D 4.740 ± 0.010 C 4.926 ± 0.020 B 5.293 ± 0.055 A | 0.191 ± 0.002 B 0.193 ±0.003 B 0.223 ± 0.003 A 0.217 ± 0.007 A 0.230 ± 0.009 A |
| Kerala | ||||
| Control 50 mM NaCl 150 mM NaCl 200 mM NaCl 250 mM NaCl | 45498 ± 356 a 39912 ± 340 b 28860 ± 540 c 19048 ± 350 d 13152 ± 141 e | 8907 ± 96 a 7904 ± 27 b 6174 ± 63 c 4987 ± 16 d 4176 ± 28 e | 4.449 ± 0.002 e 4.511 ± 0.007 d 4.624 ± 0.006 c 4.741 ± 0.003 b 4.908 ± 0.004 a | 0.197 ±0.002 d 0.200 ± 0.002 d 0.214 ± 0.003 c 0.262 ± 0.001 b 0.320 ± 0.003 a |
| Variant | ABS/RC | ETo/RC | REo/RC | φPo | φRo |
|---|---|---|---|---|---|
| Shamal | |||||
| Control 50 mM NaCl 150 mM NaCl 200 mM NaCl 250 mM NaCl | 2.821 ± 0.050 B 2.538 ± 0.047 C 2.452 ± 0.048 C 2.419 ± 0.029 C 4.309 ± 0.165 A | 1.297 ± 0.015 A 1.216 ± 0.013 B 1.249 ± 0.016 A,B 1.098 ± 0.020 C 0.850 ± 0.043 D | 0.657 ± 0.052 A 0.572 ± 0.013 A 0.513 ± 0.014 B 0.413 ± 0.014 C 0.356 ± 0.023 C | 0.791 ± 0.001 A 0.804 ± 0.002 A 0.794 ± 0.003 A 0.802 ± 0.003 A 0.418 ± 0.048 B | 0.233 ± 0.022 A 0.225 ± 0.001 A 0.209 ± 0.004 A 0.171 ± 0.004 B 0.066 ± 0.010 C |
| Kerala | |||||
| Control 50 mM NaCl 150 mM NaCl 200 mM NaCl 250 mM NaCl | 2.569 ± 0.050 b 2.304 ± 0.039 c 2.226 ± 0.073 c 2.507 ± 0.055 b 2.996 ± 0.067 a | 1.204 ± 0. 022 a 1.113 ± 0.018 b 1.051 ± 0.043 b 1.001 ± 0.068 b,c 0.835 ± 0.025 c | 0.507 ± 0.020 a 0.384 ± 0.018 b 0.339 ± 0.037 b,c 0.336 ± 0.060 b,c 0.245 ± 0.043 c | 0.796 ± 0.003 b 0.817 ± 0.004 a 0.809 ± 0.018 a,b 0.752 ± 0.008 c 0.760 ± 0.003 c | 0.197 ± 0.013 a 0.169 ± 0.009 a,b 0.145 ± 0.011 b,c 0.120 ± 0.013 c,d 0.091 ± 0.015 d |
| Variant | k1 | k2 | ΔA/A |
|---|---|---|---|
| Shamal | |||
| Control 50 mM NaCl 150 mM NaCl 200 mM NaCl 250 mM NaCl | 0.357 ± 0.033 B 0.376 ± 0.041 B 0.572 ± 0.065 A 0.502 ± 0.027 A 0.512 ± 0.038 A | 0.046 ± 0.005 A,B 0.058 ± 0.004 A 0.040 ± 0.003 B 0.045 ± 0.004 A,B 0.022 ± 0.001 C | 12.3 ± 0.6 A 12.7 ± 0.8 A 11.8 ± 0.2 A 11.5 ± 0.4 A 7.8 ± 0.8 B |
| Kerala | |||
| Control 50 mM NaCl 150 mM NaCl 200 mM NaCl 250 mM NaCl | 0.349 ± 0.036 a,b 0.395 ± 0.032 a 0.312 ± 0.041 a,b 0.261 ± 0.027 b 0.235 ± 0.026 b | 0.039 ± 0.004 a,b 0.045 ± 0.005 a 0.032 ± 0.002 b,c 0.024 ± 0.004 c 0.028 ± 0.002 b,c | 12.8 ± 0.5 a 11.5 ± 0.4 a 11.6 ± 0.5 a 9.3 ± 0.5 b 7.1 ± 0.6 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanov, M.A.; Rashkov, G.D.; Yotsova, E.K.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. Different Sensitivity Levels of the Photosynthetic Apparatus in Zea mays L. and Sorghum bicolor L. under Salt Stress. Plants 2021, 10, 1469. https://doi.org/10.3390/plants10071469
Stefanov MA, Rashkov GD, Yotsova EK, Borisova PB, Dobrikova AG, Apostolova EL. Different Sensitivity Levels of the Photosynthetic Apparatus in Zea mays L. and Sorghum bicolor L. under Salt Stress. Plants. 2021; 10(7):1469. https://doi.org/10.3390/plants10071469
Chicago/Turabian StyleStefanov, Martin A., Georgi D. Rashkov, Ekaterina K. Yotsova, Preslava B. Borisova, Anelia G. Dobrikova, and Emilia L. Apostolova. 2021. "Different Sensitivity Levels of the Photosynthetic Apparatus in Zea mays L. and Sorghum bicolor L. under Salt Stress" Plants 10, no. 7: 1469. https://doi.org/10.3390/plants10071469
APA StyleStefanov, M. A., Rashkov, G. D., Yotsova, E. K., Borisova, P. B., Dobrikova, A. G., & Apostolova, E. L. (2021). Different Sensitivity Levels of the Photosynthetic Apparatus in Zea mays L. and Sorghum bicolor L. under Salt Stress. Plants, 10(7), 1469. https://doi.org/10.3390/plants10071469








