Evaluation of Growth, Yield, and Biochemical Attributes of Bitter Gourd (Momordica charantia L.) Cultivars under Karaj Conditions in Iran
Abstract
1. Introduction
2. Results
2.1. Variation of Vegetative Traits among the Cultivars
2.2. Variation of Reproductive Traits among the Cultivars
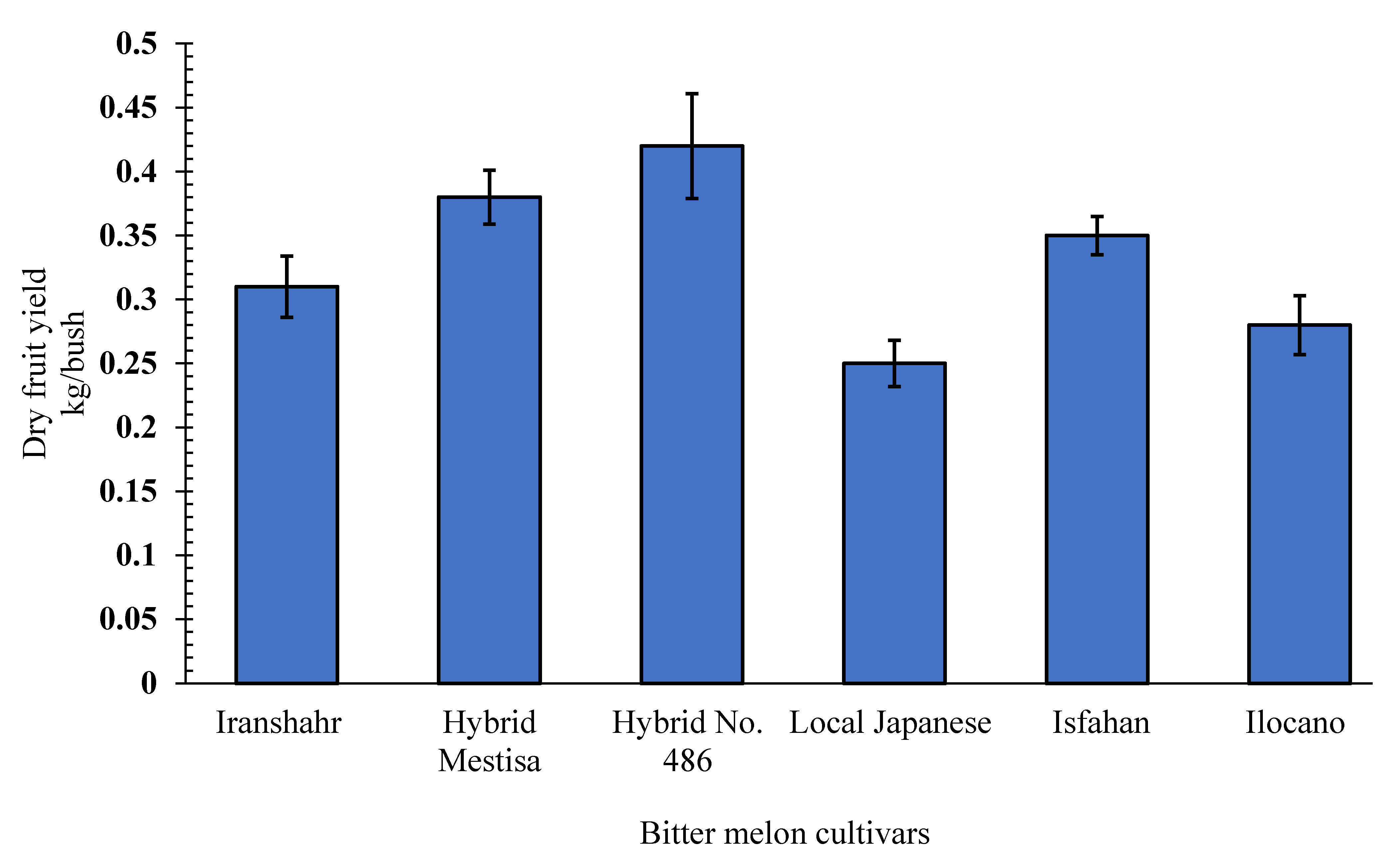
2.2.1. Dry Fruit Yield (g/Plant)
2.2.2. Fruit Number per Plant
2.2.3. Fruit Characteristics
2.2.4. Fruit Length
2.2.5. Fruit Diameter
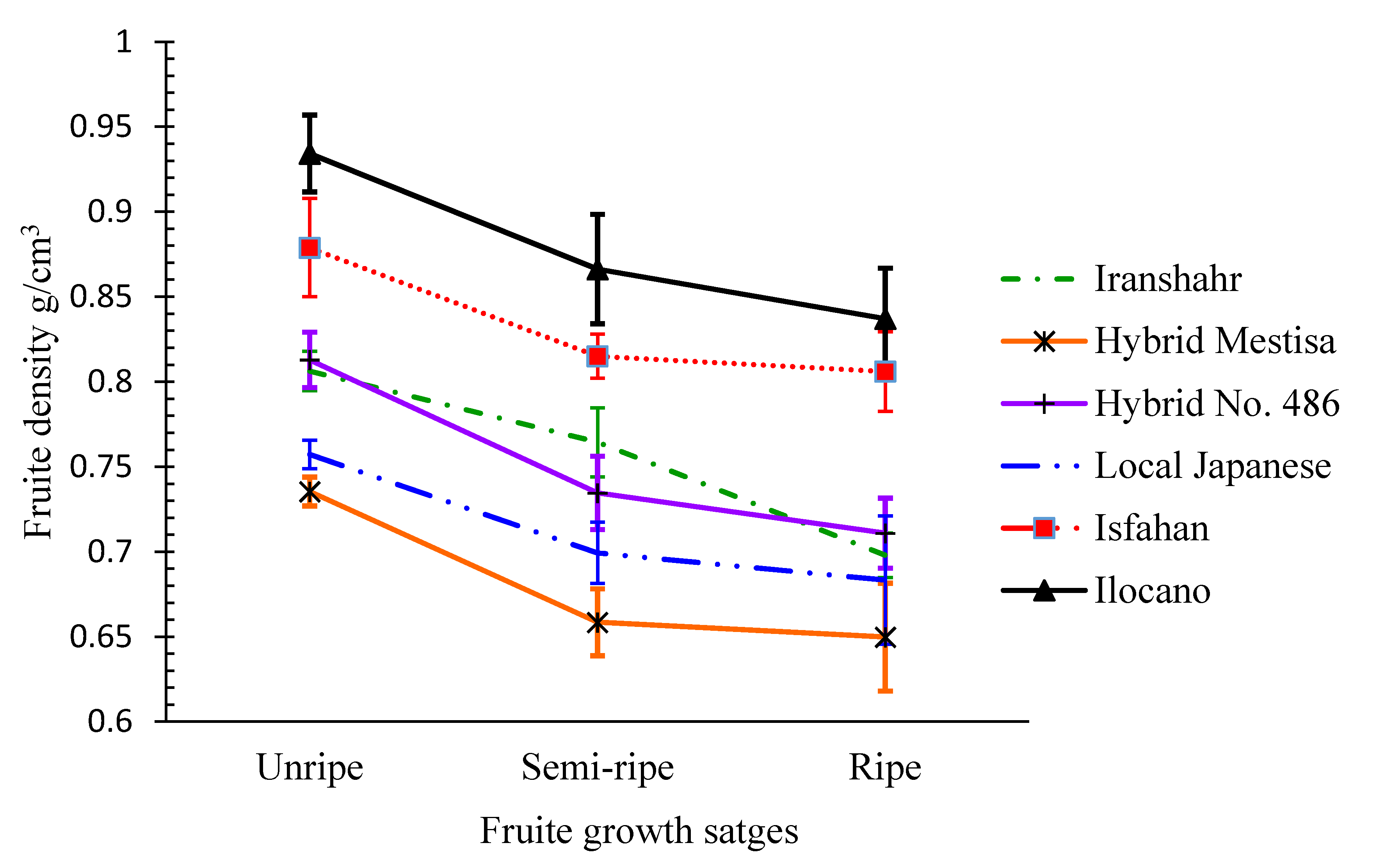
2.2.6. Fruit Density
2.2.7. Number of Seeds per Fruit
2.2.8. Thousand Seed Weight
2.2.9. Fruit Color
2.3. Phytochemical Characteristics
2.3.1. Total Phenol Content
2.3.2. Total Flavonoid Content
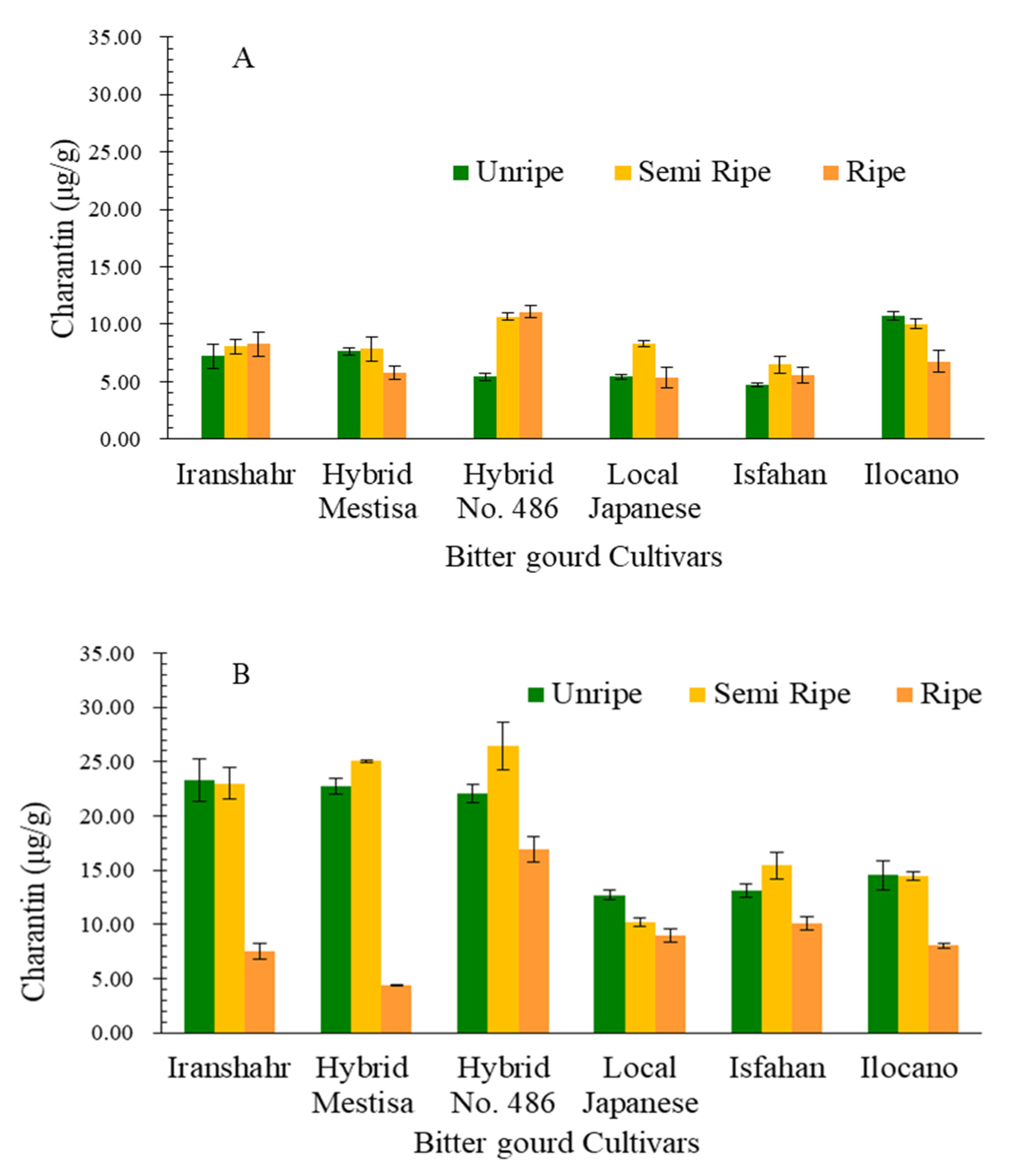
2.3.3. Fruit Charantin Content
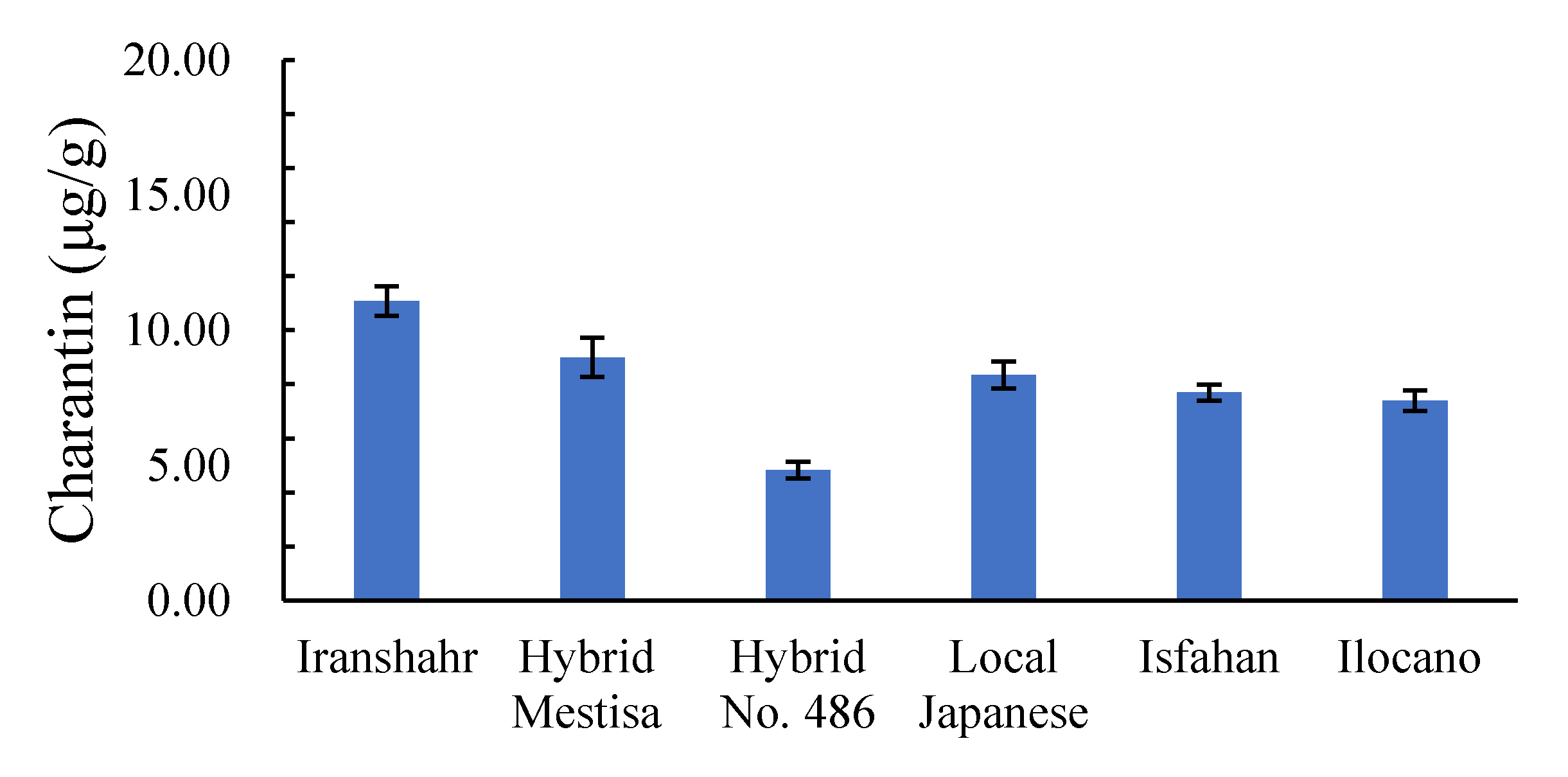
2.3.4. Leaf Charantin Content
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Plant Establishment in the Field
4.3. Experimental Design and Recorded Parameters
4.4. Phytochemical Analysis
4.5. Sample Preparation
4.6. Determination of Total Phenol Content
4.7. Determination of Total Flavonoid Content
4.8. Determination of Charantin
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.; Makaroff, L. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2007, 128, 40–50. [Google Scholar] [CrossRef]
- Mirzaei, M.; Rahmaninan, M.; Mirzaei, M.; Nadjarzadeh, A. Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes in Central Iran: Results from Yazd health study. BMC Public Health 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Roach, P.D. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food Rev. Int. 2016, 32, 181–202. [Google Scholar] [CrossRef]
- Raman, A.; Lau, C. Anti-diabetic properties and phytochemistry of Momordica charantia L.(Cucurbitaceae). Phytomedicine 1996, 2, 349–362. [Google Scholar] [CrossRef]
- Palada, M.; Chang, L.-C. Suggested cultural practices for Moringa. International Cooperators’ Guide AVRDC. AVRDC 2003, 3, 545. [Google Scholar]
- Bharathi, L.K.; John, K.J. Momordica Genus in Asia-An Overview; Springer: India, 2013. [Google Scholar]
- Semiz, A.; Sen, A. Antioxidant and chemoprotective properties of Momordica charantia L.(bitter melon) fruit extract. Afr. J. Biotechnol. 2007, 6, 6. [Google Scholar]
- Shukia, R.; Sharma, S.; Puri, D.; Prabhu, K.; Murthy, P. Medicinal plants for treatment of diabetes mellitus. Indian J. Clin. Biochem. 2000, 15, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Park, W.T.; Uddin, M.R.; Kim, Y.B.; Bae, H.; Kim, H.H.; Park, K.W.; Park, S.U. Variation of charantin content in different bitter melon cultivars. Asian J. Chem. 2014, 26, 309. [Google Scholar] [CrossRef]
- Gupta, N.; Bhardwaj, M.; Singh, S.; Sood, S. Correlation and path analysis of yield and yield components in some genetic stocks of bitter gourd (Momordica charantia L.). Sabrao J. Breed. Genet. 2015, 47, 475–481. [Google Scholar]
- Azizi, M. Change in content and chemical composition of Hypericum perforatum L. oil at three harvest time. J Herbs Spices Med. Plants 2008, 13, 79–85. [Google Scholar] [CrossRef]
- Franz, C. Nutrient and water management for medicinal and aromatic plants. Acta Hortic. 1983, 132, 203–216. [Google Scholar] [CrossRef]
- Palevitch, D. Recent advances in the cultivation of medicinal plants. Acta Hortic. 1987, 29–36. [Google Scholar] [CrossRef]
- Saranyadevi, G.; Lakshmanan, V.; Rohini, N. Performance evaluation and correlation analysis in mithipagal genotypes (Momordica charantia var. muricata). Electron. J. Plant Breed. 2017, 8, 652–659. [Google Scholar]
- Patil, D.; Dhanwate, K. Studies on growth of improved varieties of bitter guard (Momordica charantia L.). Asian J. Hortic. 2012, 7, 617–618. [Google Scholar]
- Heidari, S.; Azizi, M.; Soltani, F.; Hadian, J. Foliar application of Ca (NO3)2 and KNO3 affects growth, essential oil content, and oil composition of French tarragon. Ind. Crop. Prod. 2014, 62, 526–532. [Google Scholar] [CrossRef]
- Aminifard, M.; Aroiee, H.; Nemati, H.; Azizi, M.; Khayyat, M. Effect of nitrogen fertilizer on vegetative and reproductive growth of pepper plants under field conditions. J. Plant Nutr. 2012, 35, 235–242. [Google Scholar] [CrossRef]
- Rahmati, M.; Azizi, M.; Khayyat, M.H.; Nemati, H.; Asili, J. Yield and oil constituents of chamomile (Matricaria chamomilla L.) flowers depending on nitrogen application, plant density and climate conditions. J. Essent. Oil Bear. Plants 2011, 14, 731–741. [Google Scholar] [CrossRef]
- Rahmati, M.; Mirás-Avalos, J.M.; Valsesia, P.; Lescourret, F.; Génard, M.; Davarynejad, G.H.; Bannayan, M.; Azizi, M.; Vercambre, G. Disentangling the effects of water stress on carbon acquisition, vegetative growth, and fruit quality of peach trees by means of the QualiTree model. Front. Plant Sci. 2018, 9, 3. [Google Scholar] [CrossRef]
- Vibha, M.; Singh, D. Genetic evaluation of bitter gourd for yield parameter under tarai region of Uttarakhand. Int. J. Basic Appl. Agric. Res. 2017, 15, 126–128. [Google Scholar]
- Islam, M.; Mia, B.; Das, M.; Hossain, T.; Ahmed, J.; Hossain, M. Sex phenology of bitter gourd (Momordica charantia L.) landraces and its relation to yield potential and fruit quality. Pak. J. Agric. Sci. 2014, 51, 651–658. [Google Scholar]
- Gupta, A.; Mishra, R.; Singh, A.; Srivastava, A.; Lal, R. Genetic variability and correlations of essential oil yield with agro-economic traits in Mentha species and identification of promising cultivars. Ind. Crop. Prod. 2017, 95, 726–732. [Google Scholar] [CrossRef]
- Robinson, R.; Decker-Walters, D. Cucurbits in Crop Production Science in Horticultures Series; CABI: USA, 1997. [Google Scholar]
- Rathod, V.; Narasegowda, N.C.; Papanna, N.; Simon, L. Evaluation of genetic diversity and genome fingerprinting of bitter gourd genotypes (Momordica charantia L.) by morphological and RAPD markers. Int. J. Plant Breed. 2008, 2, 79–84. [Google Scholar]
- Dhanwate, K.; Patil, D.; Patil, S.; Gaikwad, S. Studies on flowering days of improved varieties of bitter gourd (Momordica charantia L.). Asian J. Hortic. 2011, 6, 534–535. [Google Scholar]
- Resmi, J.; Sreelathakumary, I. Studies on genetic divergence in bitter gourd (Momordica charantia L.). J. Hortic. Sci. 2012, 7, 152–155. [Google Scholar]
- Singh, V.; Shah, K.; Rana, D. Performance of different bitter gourd (Momordica charantia L.) strain for growth, yield and quality traits under Garhwal hills. Plant Arch. 2016, 16, 815–820. [Google Scholar]
- Deyto, R.C.; Cervancia, C.R. Floral biology and pollination of Ampalaya (Momordica charantia L.). Philipp. Agric. Sci. 2009, 92, 8–18. [Google Scholar]
- Crisan, S.; Campeanu, G.; Halmagean, L. Study of Momordica charantia L. species grown on the specific conditions of Romania’s western part. J. Veg. Grow. 2008, 32, 425–428. [Google Scholar]
- Dalamu, B.T.; Gaikwad, A.; Saxena, S.; Bharadwaj, C.; Munshi, A. Morphological and molecular analyses define the genetic diversity of Asian bitter gourd (Momordica charantia L.). Aust. J. Crop. Sci. 2012, 6, 261–267. [Google Scholar]
- Singh, W.J.; Kandasamy, R. Genetic Diversity in Bitter Gourd (Momordica Charantia L.) Under Coastal Ecosystems. Plant Arch. 2020, 20, 1063–1066. [Google Scholar]
- Dey, S.; Singh, A.; Chandel, D.; Behera, T. Genetic diversity of bitter gourd (Momordica charantia L.) genotypes revealed by RAPD markers and agronomic traits. Sci. Hortic. 2006, 109, 21–28. [Google Scholar] [CrossRef]
- Morgan, W.; Midmore, D. Bitter Melon in Australia-A Report for the Rural Industries; RIRDC: Kingston, Australia, 2002. [Google Scholar]
- Liao, C.T.; Lin, C.H. Effect of flooding stress on photosynthetic activities of Momordica charantia. Plant Physiol. Biochem. (Paris) 1994, 32, 479–485. [Google Scholar]
- Chy, F.; Sarkar, S.; Islam, M.; Nurul, A. Nutrient and phytochemical analysis of four varieties of bitter gourd (Momordica charantia) grown in Chittagong hill tracts, Bangladesh. Asian J. Agric. Res. 2011, 5, 186–193. [Google Scholar]
- Yaldız, G.; Sekeroglu, N.; Kulak, M.; Demirkol, G. Antimicrobial activity and agricultural properties of bitter melon (Momordica charantia L.) grown in northern parts of Turkey: A case study for adaptation. Nat. Prod. Res. 2015, 29, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Dudhat, M.; Patel, K. Evaluation of integrated nutrient management on the performance of quality and yield attributes of hybrid bitter gourd VNR 22 (Momordica charantia L.). J. Pharmacogn. Phytochem. 2020, 9, 1643–1645. [Google Scholar]
- Siemonsma, J.; Piluek, K. Plant Resources of South-East Asia, Vegetables, No. 8; Pudoc Scientific Publishers: Wageningen, The Netherlands, 1993; Volume 54, p. 50. [Google Scholar]
- Oronje, M.L.; Hagen, M.; Gikungu, M.; Kasina, M.; Kraemer, M. Pollinator diversity, behaviour and limitation on yield of karela (Momordica charantia L. Cucurbitaceae) in Western Kenya. Afr. J. Agric. Res. 2012, 7, 1629–1638. [Google Scholar]
- Desai, U.; Musmade, A. Pumpkins, squashes, and gourds. In Handbook of Vegetable Science and Technology; CRC Press: Boca Raton, FL, USA, 1998; pp. 291–354. [Google Scholar]
- Horax, R.; Hettiarachchy, N.; Islam, S. Total phenolic contents and phenolic acid constituents in 4 varieties of bitter melons (Momordica charantia) and antioxidant activities of their extracts. J. Food Sci. 2005, 70, C275–C280. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.; Chen, P. Extraction, quantification, and antioxidant activities of phenolics from pericarp and seeds of bitter melons (Momordica charantia) harvested at three maturity stages (immature, mature, and ripe). J. Agric. food Chem. 2010, 58, 4428–4433. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Tan, S.P.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Greenhouse-grown bitter melon: Production and quality characteristics. J. Sci. Food Agric. 2014, 94, 1896–1903. [Google Scholar] [CrossRef]
- Aminifard, M.; Aroiee, H.; Azizi, M.; Nemati, H.; Jaafar, H.Z. Effect of humic acid on antioxidant activities and fruit quality of hot pepper (Capsicum annuum L.). J. Herbs Spices Med. Plants 2012, 18, 360–369. [Google Scholar] [CrossRef]
- Nozad, M.; Khojastehpour, M.; Tabasizadeh, M.; Azizi, M.; Ashtiani, S.-H.M.; Salarikia, A. Characterization of hot-air drying and infrared drying of spearmint (Mentha spicata L.) leaves. J. Food Meas. Charact. 2016, 10, 466–473. [Google Scholar] [CrossRef]
- Zhang, M.; Hettiarachchy, N.S.; Horax, R.; Chen, P.; Over, K.F. Effect of maturity stages and drying methods on the retention of selected nutrients and phytochemicals in bitter melon (Momordica charantia) leaf. J. Food Sci. 2009, 74, C441–C448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Lee, H.-S.; Na, Y.-W.; Kang, M.-J.; Jeon, Y.-A.; Sung, J.-S.; Ma, K.-H.; Lee, S.-Y. Investigation of Physicochemical Properties According to Different Parts and Maturity of Momordica charantia L. Korean J. Plant Resour. 2015, 28, 382–390. [Google Scholar] [CrossRef][Green Version]
- Goo, K.S.; Ashari, S.; Basuki, N.; Sugiharto, A.N. The bitter gourd Momordica charantia L.: Morphological aspects, charantin and vitamin C contents, J. Agri. Vet. Sci. 2016, 9, 76–81. [Google Scholar]
- Habicht, S.D.; Kind, V.; Rudloff, S.; Borsch, C.; Mueller, A.S.; Pallauf, J.; Yang, R.-Y.; Krawinkel, M.B. Quantification of antidiabetic extracts and compounds in bitter gourd varieties. Food Chem. 2011, 126, 172–176. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.; Kannan, A.; Chen, P. Proximate composition and amino acid and mineral contents of Mormordica charantia L. pericarp and seeds at different maturity stages. Food Chem. 2010, 122, 1111–1115. [Google Scholar] [CrossRef]


| Cultivar | Leaf No Per Plant | Leaf Area (cm2/10 Leaf) | Plant Dry Weight (g) | Plant Height (cm) | Number of Branches/Plant |
|---|---|---|---|---|---|
| Iranshahr | 1735.33 ± 15 c | 64.7 ± 2.5 a | 541.00 ± 15 ab | 337.00 ± 12 ab | 128.00 ± 3.5 b |
| Hybrid Mestisa | 2312.66 ± 21 ab | 46.4 ± 1.6 b | 433.00 ± 17 abc | 311.00 ± 9 ab | 170.00 ± 6.7 ab |
| Hybrid No. 486 | 2756.33 ± 25 a | 60.8 ± 3.2 a | 666.00 ± 23 a | 355.00 ± 11 a | 168.00 ± 3.5 ab |
| Local Japanese | 2162.66 ± 14 bc | 58.5 ± 2.1 a | 489.00 ± 19 abc | 242.00 ± 18 bc | 146.00 ± 4.4 b |
| Isfahan | 1646.66 ± 9 c | 60.8 ± 2.4 a | 381.00 ± 21 bc | 332.00 ± 21 ab | 152.00 ± 4.6 ab |
| Ilocano | 2184.00 ± 11 ab | 15.5 ± 1.6 c | 222.00 ± 18 c | 175.00 ± 13 c | 229.00 ± 5.4 a |
| Traits | ||||||
|---|---|---|---|---|---|---|
| Cultivar | Days to First Male Flower | Days to First Female Flower | Node Number at 1st Female Flower Appear | Days Difference for Male and Female Flower Appearance | Male: Female Flower Ratio | Number of Fruits/Plant |
| Iranshahr | 21.00 b | 29.66 ab | 16.00 a | 8.66 ab | 15.46 bc | 33.18 b |
| Hybrid Mestisa | 19.00 b | 25.33 c | 9.00 b | 6.33 b | 17.11 ab | 33.07 b |
| Hybrid No. 486 | 19.33 b | 25.00 c | 12.6 ab | 5.66 b | 13.21 c | 34.35 b |
| Local Japanese | 25.33 a | 31.33 ab | 17.00 a | 6.00 b | 20.06 a | 24.51 c |
| Isfahan | 21.00 b | 33.00 a | 14.33 a | 12.00 a | 12.20 cd | 32.22 b |
| Ilocano | 19.33 b | 28.33 bc | 13.66 a | 9.00 ab | 9.03 d | 133.33 a |
| Length of Fruit (cm) | Diameter of Fruit (cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unripe | Semi-Ripe | Aril Color Change | Ripe | Unripe | Semi-Ripe | Aril Color Change | Fruit Density (g/cm3) | |
| Iranshahr | 16.96 c | 19.00 c | 22.90 c | 25.16 b | 5.19 a | 6.00 a | 6.00 b | 0.76 bc |
| Hybrid Mestisa | 23 a | 28.93 a | 30.00 a | 32.16 a | 4.75 a | 5.25 a | 6.09 b | 0.65 e |
| Hybrid No. 486 | 21 ab | 25.00 b | 30.00 a | 32.50 a | 5.14 a | 5.77 a | 6.76 a | 0.73 cd |
| Local Japanese | 18.00 bc | 20.00 c | 24.43 bc | 27.16 b | 4.81 a | 5.72 a | 6.33 ab | 0.69 de |
| Isfahan | 20.30 ab | 23.93 b | 27.26 ab | 28.50 b | 5.06 a | 5.77 a | 6.43 ab | 0.81 ab |
| Ilocano | 6.70 d | 8.46 d | 9.63 d | 11.00 c | 3.61 b | 4.14 b | 4.43 c | 0.86 a |
| Cultivars | Traits | |||||
|---|---|---|---|---|---|---|
| Total Number of Seeds/Fruit | 1000-Seed Weight (g) | Color Index | ||||
| L* | a | b | ∆L | |||
| Iranshahr | 39.33 ± 3.1 a | 179.00 ± 3.5 c | 28.64 bc | −8.00 ab | 13.64 b | −65.66 bc |
| Hybrid Mestisa | 30.50 ± 2.6 abc | 190.50 ± 2.7 b | 41.36 a | −13.15 d | 21.53 a | −52.95 a |
| Hybrid No. 486 | 27.66 ± 1.7 c | 216.16 ± 1.9 a | 22.34 c | −7.37 a | 11.80 b | −71.96 c |
| Local Japanese | 29.00 ± 2.4 bc | 214.66 ± 4.1 a | 27.21 bc | −10.85 cd | 16.22 ab | −67.09 bc |
| Isfahan | 38.00 ± 3.1 ab | 157.33 ± 5.7 e | 29.36 b | −8.06 ab | 13.59 b | −64.95 b |
| Ilocano | 27.00 ± 4.2 c | 168.00 ± 6.2 d | 32.67 b | −10.07 bc | 15.63 b | −61.63 b |
| Fruit Phenol Content (mg GA eq.) | ||||||
|---|---|---|---|---|---|---|
| Unripe | Semi-Ripe | Ripe | ||||
| Cultivars | Shade Drying | Freeze Drying | Shade Drying | Freeze Drying | Shade Drying | Freeze Drying |
| Iranshahr | 9.56 ± 1.02 f–k | 22.51 ± 1.3 a–c | 5.09 ± 0.91 j–k | 10.49 ± 1.05 d–k | 7.96 ± 1.04 g–k | 12.01 ± 1.11 c–k |
| Hybrid Mestisa | 14.92 ± 2.01 a–j | 21.30 ± 0.95 a–d | 6.76 ± 0.82 i–k | 7.62 ± 1.63 h–k | 18.85 ± 2.13 a–g | 17.34 ± 1.65 a–i |
| Hybrid No. 486 | 10.78 ± 1.7 d–k | 25.17 ± 1.65 a | 9.43 ± 0.56 f–k | 16.56 ± 1.87 a–i | 20.70 ± 2.16 a–e | 24.12 ± 2.11 ab |
| Local Japanese | 8.34 ± 0.85 g–k | 17.87 ± 2.01 a–h | 8.76 ± 0.47 g–k | 15.47 ± 1.54 a–j | 15.72 ± 1.84 a–j | 13.78 ± 1.63 b–k |
| Isfahan | 12.26 ± 1.13 c–k | 20.15 ± 1.94 a–f | 3.57 ± 0.21 k | 13.99 ± 1.33 b–k | 18.42 ± 1.38 a–h | 12.22 ± 0.65 c–k |
| Ilocano | 9.64 ± 1.1 e–k | 11.54 ± 1.63 c–k | 7.62 ± 0.64 h–k | 6.65 ± 0.87 i–k | 11.84 ± 1.12 c–k | 15.21 ± 1.55 a–j |
| Drying Method | Development Stage | ||||
|---|---|---|---|---|---|
| Cultivars | Shade Drying | Lyophilized | Unripe | Semi-Ripe | Ripe |
| Iranshahr | 7.54 ± 1.60 d | 15.00 ± 0.50 bc | 16.04 ± 1.27 abc | 7.79 ± 1.32 ef | 9.98 ± 1.15 cdef |
| Hybrid Mestisa | 13.51 ± 1.35 bc | 15.42 ± 1.10 b | 18.11 ± 1.15 ab | 7.19 ± 1.28 f | 18.09 ± 0.95 ab |
| Hybrid No. 486 | 13.64 ± 0.95 bc | 21.95 ± 1.25 a | 17.98 ± 1.50 ab | 13.00 ± 0.80 bcdef | 22.41 ± 1.34 a |
| Local Japanese | 10.94 ± 1.30 bcd | 15.71 ± 1.30 b | 13.10 ± 1.85 bcdef | 12.11 ± 0.125 bcdef | 14.75 ± 1.27 bcde |
| Isfahan | 11.42 ± 2.10 bcd | 15.45 ± 0.95 b | 16.21 ± 1.12 abc | 8.78 ± 1.55 def | 15.32 ± 1.15 bcd |
| Ilocano | 9.70 ± 1.12 cd | 11.14 ± 1.12 bcd | 10.59 ± 0.85 cdef | 7.13 ± 1.35 f | 13.53 ± 1.40 bcdef |
| Mean | 11.12 | 15.78 | 15.34 | 9.33 | 15.68 |
| Fruit Flavonoid Content (mg Quercetin eq.) | ||||||
|---|---|---|---|---|---|---|
| Unripe | Semi-Ripe | Ripe | ||||
| Cultivars | Shade-Drying | Freeze-Drying | Shade-Drying | Freeze-Drying | Shade-Drying | Freeze-Drying |
| Iranshahr | 15.63 ± 3.06 a–g | 18.44 ± 1.85 ab | 8.70 ± 0.88 d–k | 5.77 ± 0.55 i–k | 4.37 ± 0.35 k | 4.08 ± 0.34 k |
| Hybrid Mestisa | 18.08 ± 1.65 abc | 11.05 ± 1.66 a–k | 9.49 ± 0.65 d–k | 3.60 ± 0.61 k | 7.40 ± 0.62 g–k | 3.58 ± 0.61 k |
| Hybrid No. 486 | 19.42 ± 1.71 a | 16.07 ± 1.23 a–f | 11.94 ± 1.04 a–k | 14.07 ± 0.85 a–i | 9.61 ± 0.88 c–k | 9.63 ± 0.55 c–k |
| Local Japanese | 16.93 ± 1.35 a–d | 13.45 ± 1.45 a–j | 7.80 ± 1.32 f–k | 6.17 ± 0.55 h–k | 4.28 ± 0.55 k | 8.13 ± 0.81 e–k |
| Isfahan | 16.47 ± 1.45 a–e | 14.50 ± 1.66 a–h | 9.11 ± 1.12 d–k | 7.76 ± 0.77 f–k | 5.03 ± 0.41 jk | 6.46 ± 0.68 h–k |
| Ilocano | 15.21 ± 1.66 a–g | 8.51 ± 0.95 d–k | 7.94 ± 0.88 f–k | 11.08 ± 0.86 a–k | 10.37 ± 0.87 b–k | 5.86 ± 0.88 i–k |
| Drying Method | Development Stage | ||||
|---|---|---|---|---|---|
| Cultivars | Shade Drying | Lyophilized | Unripe | Semi-Ripped | Ripe |
| Iranshahr | 9.57 ± 0.85 abcd | 9.43 ± 0.45 bcd | 17.04 ± 1.60 ab | 7.24 ± 1.12 ef | 4.23 ± 0.50 f |
| Hybrid Mestisa | 11.66 ± 0.50 abc | 6.08 ± 1.20 d | 14.57 ± 1.40 abc | 6.55 ± 0.55 ef | 5.49 ± 0.25 f |
| Hybrid No. 486 | 13.66 ± 1.10 a | 13.26 ± 0.75 ab | 17.75 ± 1.35 a | 13.00 ± 1.50 abcd | 9.62 ± 0.30 cdef |
| Local Japanese | 9.67 ± 1.25 abcd | 9.25 ± 1.10 bcd | 15.19 ± 1.12 ab | 6.99 ± 0.55 ef | 6.21 ± 0.10 f |
| Isfahan | 10.20 ± 1.40 abcd | 9.57 ± 0.80 abcd | 15.49 ± 1.50 ab | 8.43 ± 0.65 def | 5.75 ± 0.70 f |
| Ilocano | 11.18 ± 1.35 abc | 8.48 ± 1.15 cd | 11.86 ± 0.50 bcde | 9.51 ± 0.80 cdef | 8.12 ± 0.35 def |
| Mean | 10.99 | 9.35 | 15.31 | 8.62 | 6.57 |
| Cultivars | Drying Method | Development Stage | ||||
|---|---|---|---|---|---|---|
| Shade-Drying | Lyophilized | Unripe | Semi-Ripe | Ripe | Mean | |
| Iranshahr | 7.87 ± 0.91 def | 17.93 ± 1.37 ab | 15.25 ± 1.51 bcd | 15.55 ± 1.02 bc | 7.91 ± 0.90 hij | 12.9 ± 1.14 |
| Hybrid Mestisa | 7.09 ± 0.63 ef | 17.39 ± 0.31 abc | 15.21 ± 0.54 bcd | 16.44 ± 0.58 b | 5.06 ± 0.30 k | 12.2 ± 0.47 |
| Hybrid No. 486 | 9.06 ± 1.15 def | 21.79 ± 1.41 a | 13.72 ± 0.58 de | 18.54 ± 1.36 a | 14.01 ± 0.90 cde | 15.4 ± 0.94 |
| Local Japanese | 6.37 ± 0.41 f | 10.65 ± 0.3 def | 9.07 ± 0.33 hi | 9.28 ± 0.35 gh | 7.18 ± 0.75 j | 8.5 ± 0.47 |
| Isfahan | 5.60 ± 0.51 f | 12.89 ± 0.83 bcd | 8.93 ± 0.42 hij | 10.97 ± 0.96 fg | 7.84 ± 0.65 hij | 9.2 ± 0.67 |
| Ilocano | 9.15 ± 0.56 def | 12.36 ± 0.66 cde | 12.61 ± 0.85 ef | 12.26 ± 0.40 ef | 7.38 ± 0.60 ij | 10.8 ± 0.61 |
| Mean | 7.52 | 15.50 | 12.46 | 13.84 | 8.23 | 11.5 ± 0.71 |
| Clay | Silt | Sand | Texture | OC% | EC (Ds/m) | pH | N (total) | P (ava) | K (ava) | Fe | Zn | Cu | Mn | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg | ||||||||||||||
| 27 | 40 | 33 | Clay-loam | 0.63 ± 0.02 | 1.82 ± 0.21 | 7.56 ± 0.23 | 600 ± 15 | 27.6 ± 2.6 | 295 ± 6.3 | 7.97 ± 2.1 | 0.90 ± 0.03 | 2.22 ± 0.14 | 16.5 ± 0.3 | 0.44 ± 0.06 |
| Factor | Level | |
|---|---|---|
| 1 | Loading temperature | Room Temperature |
| 2 | Freezing rate | 0.5 °C/min |
| 3 | Freezing temperature | −40 °C |
| 4 | Complete freezing duration | 2 h |
| 5 | Temperature ramp rate during the primary drying | 8 °C/h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valyaie, A.; Azizi, M.; Kashi, A.; Sathasivam, R.; Park, S.U.; Sugiyama, A.; Motobayashi, T.; Fujii, Y. Evaluation of Growth, Yield, and Biochemical Attributes of Bitter Gourd (Momordica charantia L.) Cultivars under Karaj Conditions in Iran. Plants 2021, 10, 1370. https://doi.org/10.3390/plants10071370
Valyaie A, Azizi M, Kashi A, Sathasivam R, Park SU, Sugiyama A, Motobayashi T, Fujii Y. Evaluation of Growth, Yield, and Biochemical Attributes of Bitter Gourd (Momordica charantia L.) Cultivars under Karaj Conditions in Iran. Plants. 2021; 10(7):1370. https://doi.org/10.3390/plants10071370
Chicago/Turabian StyleValyaie, Akram, Majid Azizi, Abdolkarim Kashi, Ramaraj Sathasivam, Sang Un Park, Akifumi Sugiyama, Takashi Motobayashi, and Yoshiharu Fujii. 2021. "Evaluation of Growth, Yield, and Biochemical Attributes of Bitter Gourd (Momordica charantia L.) Cultivars under Karaj Conditions in Iran" Plants 10, no. 7: 1370. https://doi.org/10.3390/plants10071370
APA StyleValyaie, A., Azizi, M., Kashi, A., Sathasivam, R., Park, S. U., Sugiyama, A., Motobayashi, T., & Fujii, Y. (2021). Evaluation of Growth, Yield, and Biochemical Attributes of Bitter Gourd (Momordica charantia L.) Cultivars under Karaj Conditions in Iran. Plants, 10(7), 1370. https://doi.org/10.3390/plants10071370







