The Orchid Velamen: A Model System for Studying Patterned Secondary Cell Wall Development?
Abstract
1. Introduction
2. Results
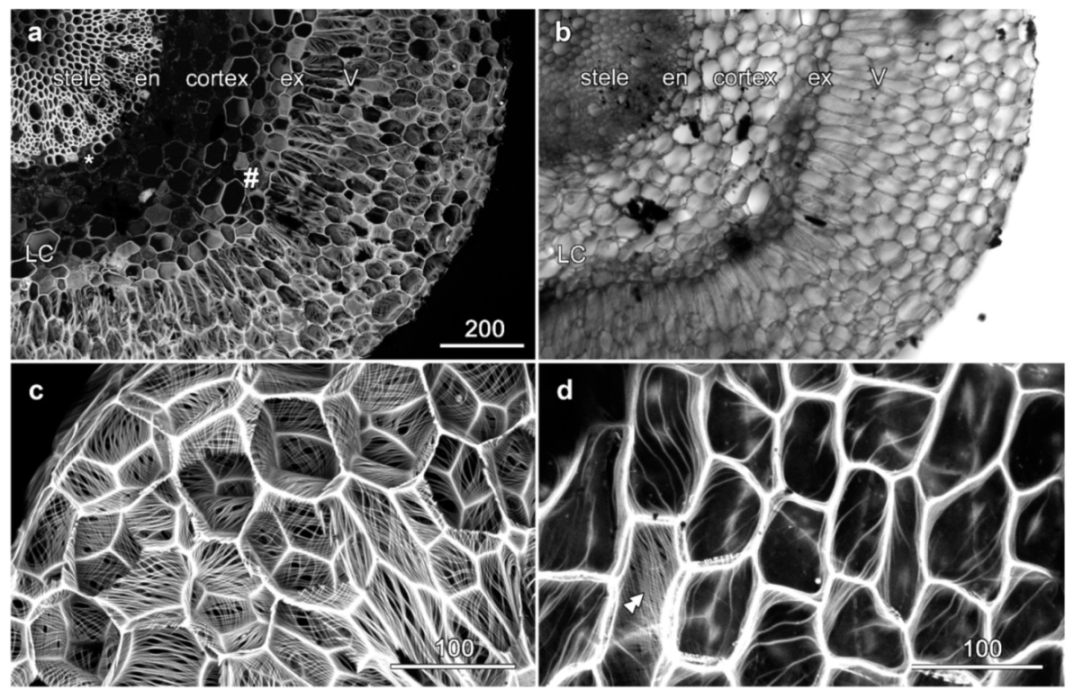
2.1. Velamen Structure in Miltoniopsis sp.
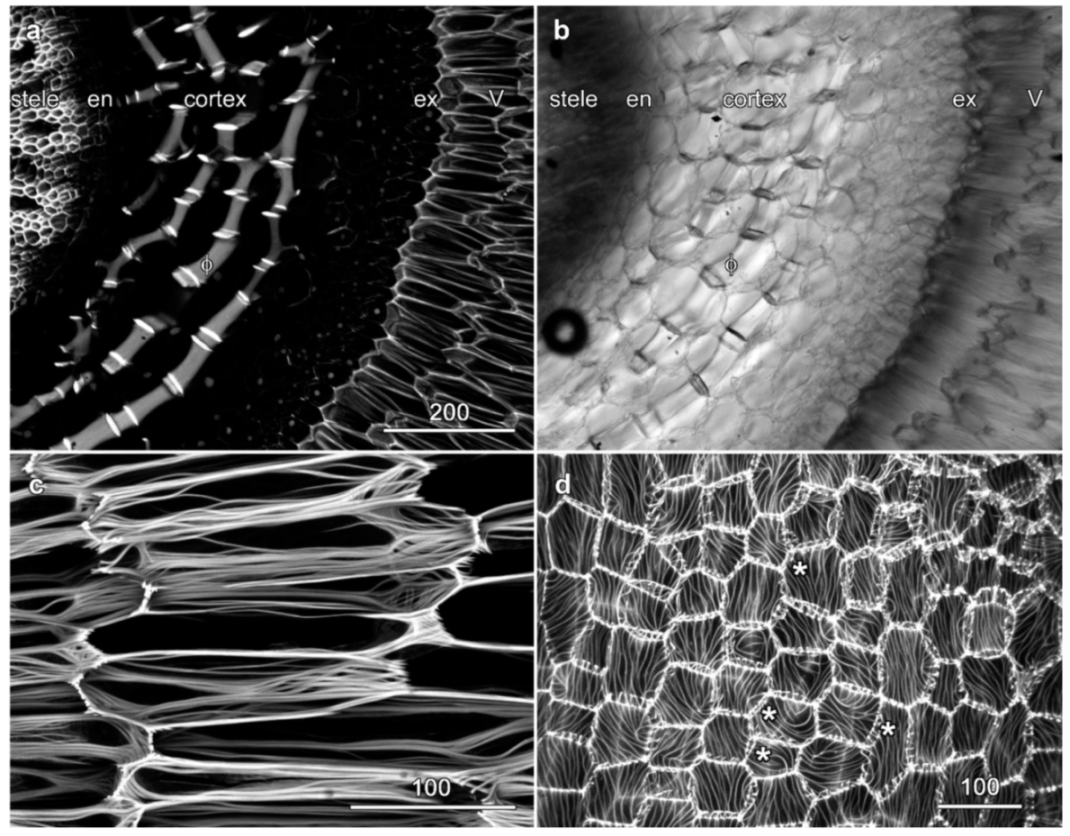
2.2. Velamen Structure in Laelia anceps
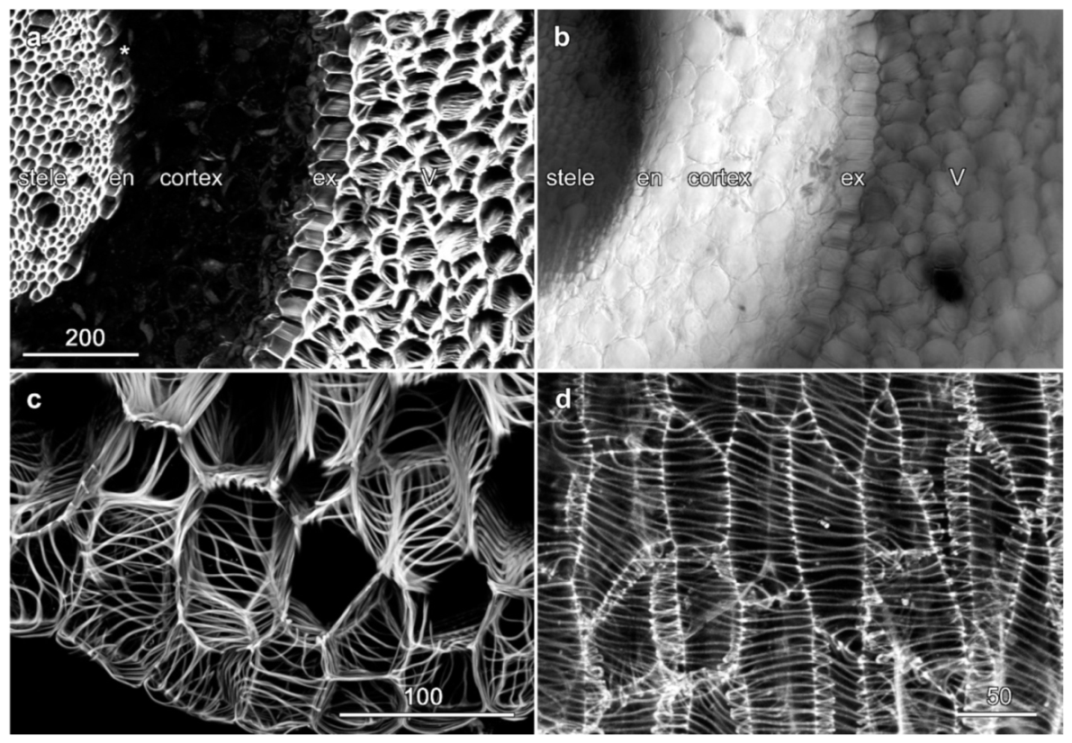
2.3. Velamen Structure in Dendrobium spp.
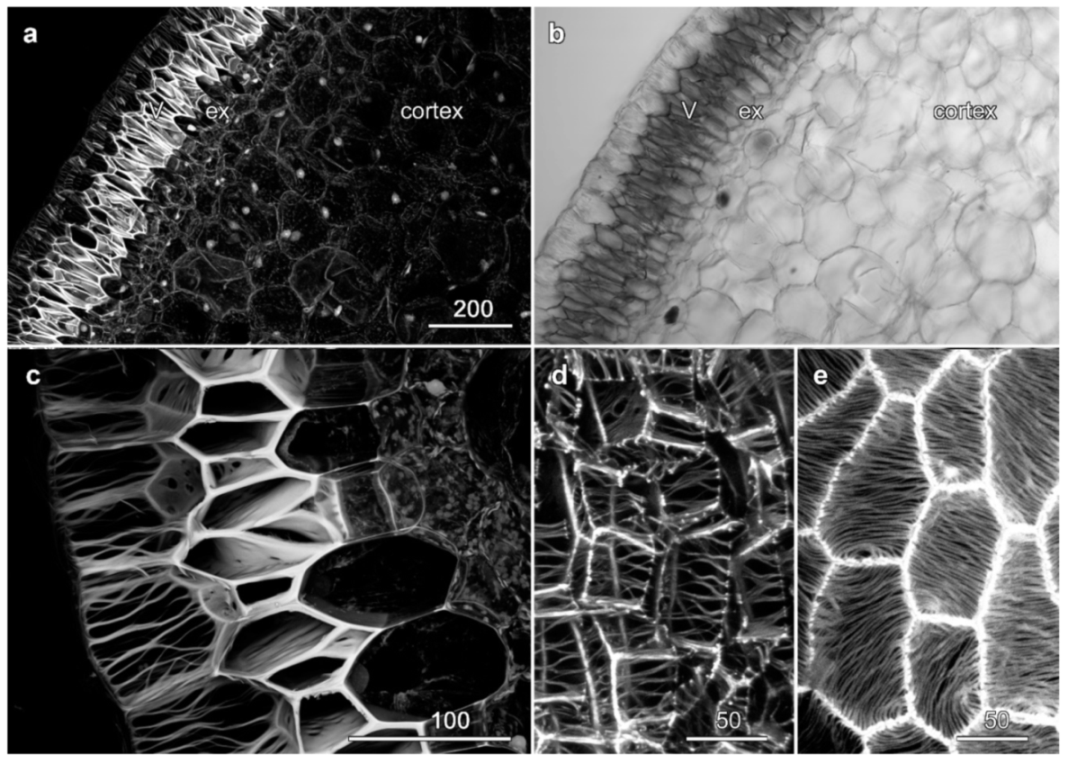
2.4. Velamen Structure in Phalaenopsis sp.
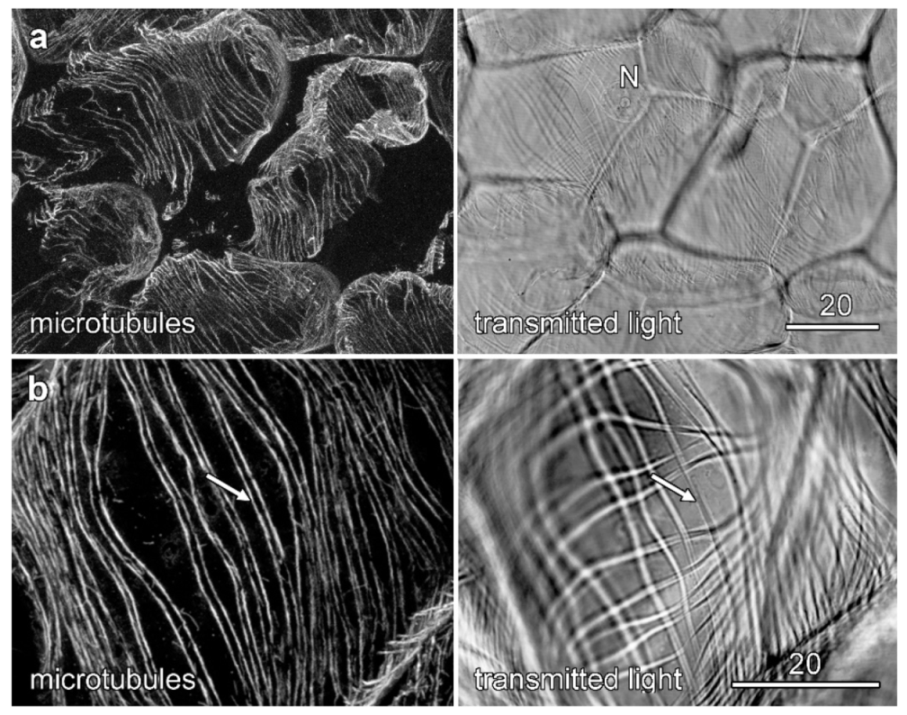
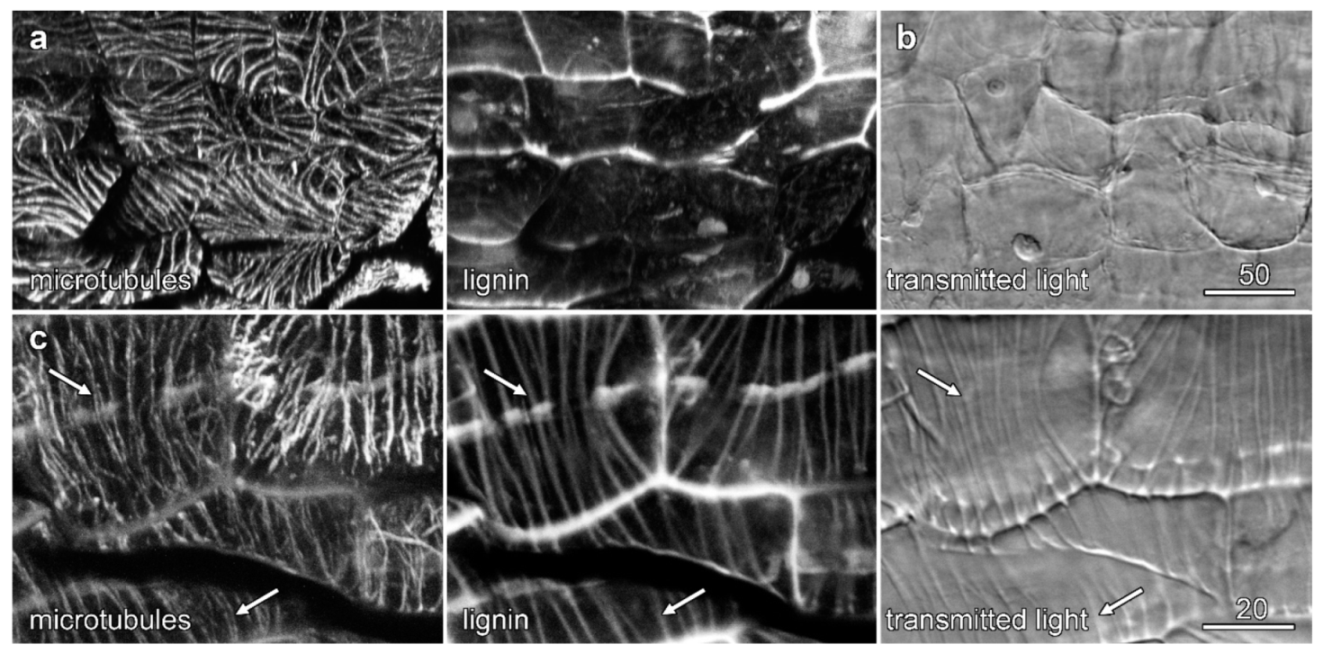
2.5. Cytoskeletal Organization during Velamen Development in Miltoniopsis
2.6. Microtubules and Velamen Development in Laelia
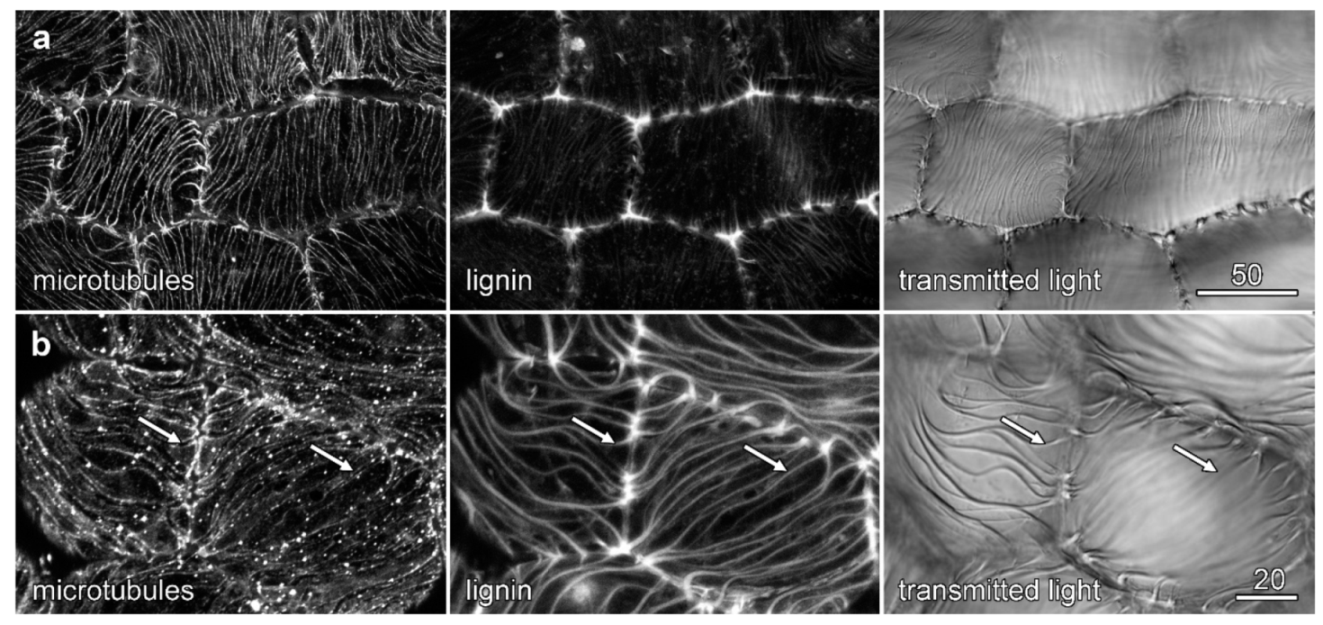
2.7. Microtubules and Velamen Development in Phalaenopsis
3. Discussion
3.1. Microtubules and Velamen Development
3.2. The Unusual Pattern of Microtubules That Flank the Cell Wall Ridges
3.3. Developing Orchid Roots as a Model System for Secondary Cell Wall Investigations?
- (i)
- secondary cell wall development occurring in a developmentally recognizable sequence at specific sites;
- (ii)
- secondary cell wall development showing similar characteristics to ‘important’ secondary cell walls, such as xylem development;
- (iii)
- secondary cell wall development in surface layers that are readily accessible for microscopy;
- (iv)
- a system readily adapted to tissue culture;
- (v)
- the ability to stably transform tissues so that they express fluorescent protein constructs;
- (vi)
- the availability of genomic information and access to mutants;
- (vii)
- and limited autofluorescence that would confound live cell imaging experiments.
4. Materials and Methods
4.1. Plant Material
4.2. Fixing Root Tissue
4.3. Cell Wall Labelling
4.4. Immunolabelling of the Cytoskeleton
4.5. Confocal Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, R.H.; Hill, S.J.; Harris, P.J. Wide-angle X-ray scattering and solid-state nuclear magnetic resonance data combined to test models for cellulose microfibrils in mung bean cell walls. Plant Physiol. 2013, 163, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 2014, 22, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Baskin, T.I. On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 2001, 215, 150–171. [Google Scholar] [CrossRef]
- Kumar, M.; Campbell, L.; Turner, S. Secondary cell walls: Biosynthesis and manipulation. J. Exp. Bot. 2016, 67, 515–531. [Google Scholar] [CrossRef]
- Meents, M.J.; Watanabe, Y.; Samuels, A.L. The cell biology of secondary cell wall biosynthesis. Ann. Bot. 2018, 121, 1107–1125. [Google Scholar] [CrossRef] [PubMed]
- Smole, M.S.; Hribernik, S.; Kleinschek, S.; Kreže, T. Plant fibres for textile and technical applications. In Advances in Agrophysical Research; Grundas, S., Stepniewski, A., Eds.; IntechOpen: London, UK, 2013; pp. 369–397. [Google Scholar]
- Barnett, J.R.; Bonham, V.A. Cellulose microfibril angle in the cell wall of wood fibres. Biol. Rev. 2004, 79, 461–472. [Google Scholar] [CrossRef]
- Tabet, T.A.; Aziz, F.A. Cellulose microfibril angle in wood and its dynamic mechanical significance. In Cellulose: Fundamental Aspects; Van de Ven, T.G.N., Ed.; IntechOpen: London, UK, 2013; p. 142. [Google Scholar]
- Paredez, A.R.; Somerville, C.R.; Ehrhardt, D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006, 312, 1491–1495. [Google Scholar] [CrossRef]
- Gutierrez, R.; Lindeboom, J.J.; Paredez, A.R.; Emons, A.M.C.; Ehrhardt, D.W. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol. 2009, 11, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, J.C.; Taylor, N.G.; Turner, S.R. Control of cellulose synthase complex localization in developing xylem. Plant Cell 2003, 15, 1740–1748. [Google Scholar] [CrossRef]
- Wightman, R.; Turner, S.R. The roles of the cytoskeleton during cellulose deposition at the secondary cell wall. Plant J. 2008, 54, 794–805. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuda, H.; Shibaoka, H. Interrelation between the spatial disposition of actin filaments and microtubules during the differentiation of tracheary elements in cultured Zinnia cells. Protoplasma 1988, 143, 29–37. [Google Scholar] [CrossRef]
- Oda, Y.; Mimura, T.; Hasezawa, S. Regulation of secondary cell wall development by cortical microtubules during tracheary element differentiation in Arabidopsis cell suspensions. Plant Physiol. 2005, 137, 1027–1036. [Google Scholar] [CrossRef]
- Sampathkumar, A. Patterning and lifetime of plsma-membrane loalizd cellulose synthase is dependent on actin orgnization in Arabidopsis interphase cells. Plant. Physiol. 2013, 162, 675–688. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Goué, N.; Igarashi, H.; Ohtani, M.; Nakano, Y.; Mortimer, J.C.; Nishikubo, N.; Kubo, M.; Katayama, Y.; Kakegawa, K.; et al. VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol. 2010, 153, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Meents, M.J.; McDonnell, L.M.; Barkwill, S.; Sampathkumar, A.; Cartwright, H.N.; Demura, T.; Ehrhardt, D.W.; Samuels, A.L.; Mansfield, S.D. Visualization of cellulose synthases in Arabidopsis secondary cell walls. Science 2015, 350, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Scheneider, R.; Tang, L.; Lampugnani, E.R.; Barkwill, S.; Lathe, R.; Zhang, Y.; McFarlane, H.E.; Pesquet, E.; Niittyla, T.; Mansfield, S.D.; et al. Two complementary mechanisms underpin cell wall patterning during xylem vessel development. Plant Cell 2017, 29, 2433–2449. [Google Scholar] [CrossRef]
- Idris, N.A.; Collings, D.A. The life of phi: The development of phi thickenings in roots of the orchids of the genus Miltoniopsis. Planta 2015, 241, 489–506. [Google Scholar] [CrossRef]
- Aleamotuʻa, M.; McCurdy, D.W.; Collings, D.A. Phi thickenings in roots: Novel secondary wall structures responsive to biotic and abiotic stresses. J. Exp. Bot. 2019, 70, 4631–4641. [Google Scholar] [CrossRef] [PubMed]
- Idris, N.A.; Collings, D.A. Cell wall development in the velamen layer of the orchid Miltoniopsis investigated by confocal microscopy. Malays. J. Microsc. 2014, 10, 20–26. [Google Scholar]
- Olatunji, O.A.; Nengim, R.O. Occurrence and distribution of tracheoidal elements in the Orchidaceae. Bot. J. Linn. Soc. 1980, 80, 357–370. [Google Scholar] [CrossRef]
- Benzing, D.H.; Ott, D.W.; Friedman, W.E. Roots of Sobralia macrantha (Orchidaceae): Structure and function of the velamen-exodermis complex. Am. J. Bot. 1982, 69, 608–614. [Google Scholar] [CrossRef]
- Porembski, S.; Barthlott, W. Velamen radicum micromorphology and classification of Orchidaceae. Nord. J. Bot. 1988, 8, 117–137. [Google Scholar] [CrossRef]
- Zotz, G.; Winkler, U. Aerial roots of epiphytic orchids: The velamen radicum and its role in water and nutrient uptake. Oecologia 2013, 171, 733–741. [Google Scholar] [CrossRef]
- Hauber, F.; Konrad, W.; Nebelsick, A.R. Aerial roots of orchids: The velamen radicum as a porous material for efficient imbibition of water. Appl. Phys. A 2020, 126, 885. [Google Scholar] [CrossRef]
- Pridgeon, A.M. The velamen and exodermis of orchid roots. In Orchid Biology Reviews and Perspectives; Comstock Publishing Associates: Ithaca, NY, USA, 1987. [Google Scholar]
- Figueroa, C.; Salazar, G.A.; Zavaleta, H.A.; Engelman, E.M. Root character evolution and systematics in Cranichidinae, Prescottiinae and Spiranthinae (Orchidaceae, Cranichideae). Ann. Bot. 2008, 101, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Aybeke, M. Comparative anatomy of selected rhizomatous and tuberous taxa of subfamilies Orchidoideae and Epidendroideae (Orchidaceae) as an aid to identification. Plant Syst. Evol. 2012, 298, 1643–1658. [Google Scholar] [CrossRef]
- Leroux, O.; Bagniewska-Zadworna, A.; Rambe, S.K.; Knox, J.P.; Marcus, S.E.; Bellefroid, E.; Stubbe, D.; Chabbert, B.; Habrant, A.; Claeys, M.; et al. Non-lignified helical cell wall thickenings in root cortical cells of Aspleniaceae (Polypodiales): Histology and taxonomical significance. Ann. Bot. 2011, 107, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.A.; Terán, L.; Mata, M.; Martinez, O.G.; Prado, J. Helical cell wall thickenings in root cortical cells of Polypodiaceae species from northwestern Argentina. Am. Fern J. 2013, 103, 225–240. [Google Scholar] [CrossRef]
- Zotz, G.; Schickenberg, N.; Albach, D. The velamen radicum is common among terrestrial monocotyledons. Ann. Bot. 2017, 120, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.R.A. Aspects of cell wall structure and the development of the velamen in Ansellia gigantea Reichb. f. Ann. Bot. 1974, 38, 495–505. [Google Scholar] [CrossRef]
- Falconer, M.M.; Seagull, R.W. Immunofluorescent and calcofluor white staining of developing tracheary elements in Zinnia elegans L. suspension cultures. Protoplasma 1985, 125, 190–198. [Google Scholar] [CrossRef]
- Oda, Y.; Hasezawa, S. Cytoskeletal organization during xylem cell differentiation. J. Plant Res. 2006, 119, 167–177. [Google Scholar] [CrossRef]
- Pesquet, E.; Korolev, A.V.; Calder, G.; Lloyd, C.W. The microtubule-associated protein AtMAP70-5 regulates secondary wall patterning in Arabidopsis wood cells. Curr. Biol. 2010, 20, 744–749. [Google Scholar] [CrossRef]
- Talbot, M.J.; Wasteneys, G.; McCurdy, D.W.; Offler, C.E. Deposition patterns of cellulose microfibrils in flange wall ingrowths of transfer cells indicate clear parallels with those of secondary wall thickenings. Funct. Plant Biol. 2007, 34, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Zhang, S.-B.; Xi, H.-P.; Bradshaw, C.J.A.; Zhang, J.-L. Processes controlling programmed cell death of root velamen radicum in an epiphytic orchid root. Ann. Bot. 2020, 126, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L. Autofluorescence in plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Matlashov, M.; Shcherbakova, D.M.; Alvelid, J.; Baloban, M.; Pennacchietti, F.; Shemetov, A.A.; Testa, I.; Verkhusha, V.V. A set of monomeric near-infrared fluorescent proteins for multicolor imaging across scales. Nat. Commun. 2020, 11, 239. [Google Scholar] [CrossRef]
- Yang, J.; Lee, H.-J.; Shin, D.; Oh, S.; Seon, J.; Paek, K.; Han, K.-H. Genetic transformation of Cymbidium orchid by particle bombardment. Plant Cell Rep. 1999, 18, 978–984. [Google Scholar] [CrossRef]
- Tee, C.S.; Maziah, M. Optimization of biolistic bombardment parameters for Dendrobium Sonia 17 calluses using GFP and GUS as the reporter system. Plant Cell Tissue Organ Cult. 2005, 80, 77–89. [Google Scholar] [CrossRef]
- Liau, C.-H.; You, S.-J.; Prasad, V.; Hsiao, H.-H.; Lu, J.-C.; Yang, N.-S.; Chan, M.-T. Agrobacterium tumefaciens-mediated transformation of an Oncidium orchid. Plant Cell Rep. 2003, 21, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Men, S.; Ming, X.; Wang, Y.; Liu, R.; Wei, C.; Li, Y. Genetic transformation of two species of orchid by biolistic bombardment. Plant Cell Rep. 2003, 21, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.-C.; Liu, K.-W.; Chen, L.-J.; He, Y.; Xu, Q.; Bianet, C.; et al. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2019, 47, 65–76. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Xu, Q.; Bian, C.; Tsai, W.-C.; Yeh, C.-M.; Liu, K.-W.; Yoshida, K.; Zhang, L.-S.; Chang, S.-B.; Chenet, F.; et al. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2019, 6, 19029. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Dievart, A.; Hsu, C.C.; Chious, S.Y.; Hunag, H.; Chen, H.H. Post genomics era for orchid research. Bot. Stud. 2017, 58, 61. [Google Scholar] [CrossRef]
- Collings, D.A.; Wasteneys, G.O. Actin microfilament and microtubule distribution patterns in the expanding root of Arabidopsis thaliana. Can. J. Bot. 2005, 83, 579–590. [Google Scholar] [CrossRef]
- Lux, A.; Morita, S.; Abe, J.; Ito, K. An improved method for clearing and staining free-hand sections and whole-mount samples. Ann. Bot. 2005, 96, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Kapp, N.; Barnes, W.J.; Richar, T.L.; Anderson, C.T. Imaging with the fluorogenic dye basic fuchsin reveals subcellular patterning and ecotype variation of lignification in Brachypodium distachyon. J. Exp. Bot. 2015, 66, 4295–4304. [Google Scholar] [CrossRef]
- Anderson, C.T.; Carroll, A.; Akhmetova, L.; Somerville, C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol. 2010, 152, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Ingerfeld, M.; Nair, H.; Chauhan, S.S.; Collings, D.A. Pontamine fast scarlet 4B: A new fluorescent dye for visualising cell wall organisation in radiata pine tracheids. Wood Sci. Technol. 2013, 47, 59–75. [Google Scholar] [CrossRef]
- Thomas, J.; Idris, N.A.; Collings, D.A. Pontamine fast scarlet 4B biflourescence, and measurements of cellulose microfibril angles. J. Microsc. 2017, 268, 13–27. [Google Scholar] [CrossRef]
- Collings, D.A.; Harper, J.D.I. Peroxisome aggregation during cytokinesis in different angiosperm taxa. Int. J. Plant Sci. 2008, 169, 241–252. [Google Scholar] [CrossRef][Green Version]


| Species | Velamen Thickening Class (as Defined by Porembski and Barthlott 1988) [24] |
|---|---|
| Dendrobium sp. | dendrobium |
| Laelia anceps | epidendrum |
| Miltoniopsis sp. | cymbidium |
| Phalaenopsis sp. | vanda |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, N.A.; Aleamotuʻa, M.; McCurdy, D.W.; Collings, D.A. The Orchid Velamen: A Model System for Studying Patterned Secondary Cell Wall Development? Plants 2021, 10, 1358. https://doi.org/10.3390/plants10071358
Idris NA, Aleamotuʻa M, McCurdy DW, Collings DA. The Orchid Velamen: A Model System for Studying Patterned Secondary Cell Wall Development? Plants. 2021; 10(7):1358. https://doi.org/10.3390/plants10071358
Chicago/Turabian StyleIdris, Nurul A., Maketelana Aleamotuʻa, David W. McCurdy, and David A. Collings. 2021. "The Orchid Velamen: A Model System for Studying Patterned Secondary Cell Wall Development?" Plants 10, no. 7: 1358. https://doi.org/10.3390/plants10071358
APA StyleIdris, N. A., Aleamotuʻa, M., McCurdy, D. W., & Collings, D. A. (2021). The Orchid Velamen: A Model System for Studying Patterned Secondary Cell Wall Development? Plants, 10(7), 1358. https://doi.org/10.3390/plants10071358








