Breeding Buckwheat for Nutritional Quality in the Czech Republic
Abstract
:1. Introduction
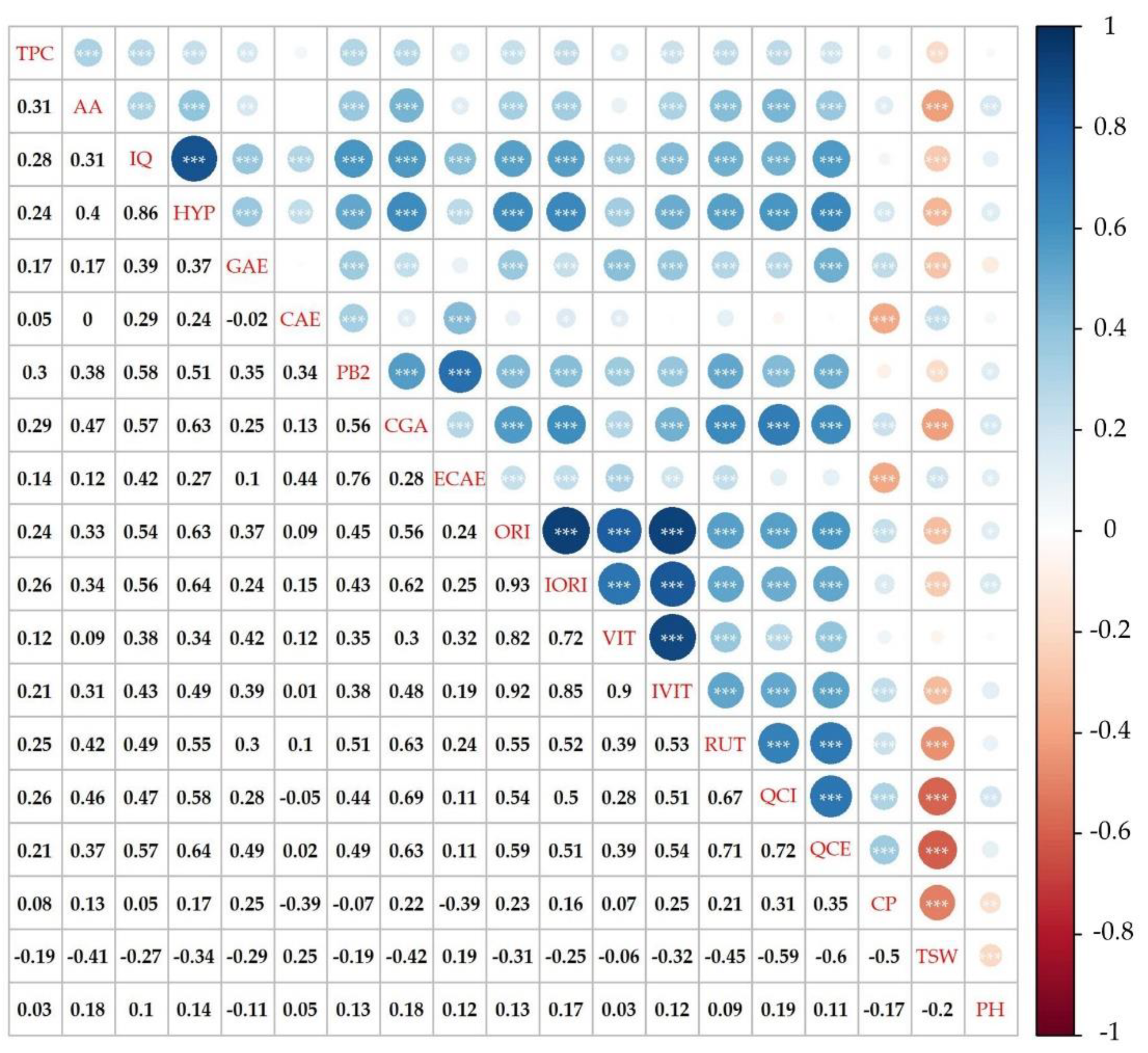
2. Results and Discussion
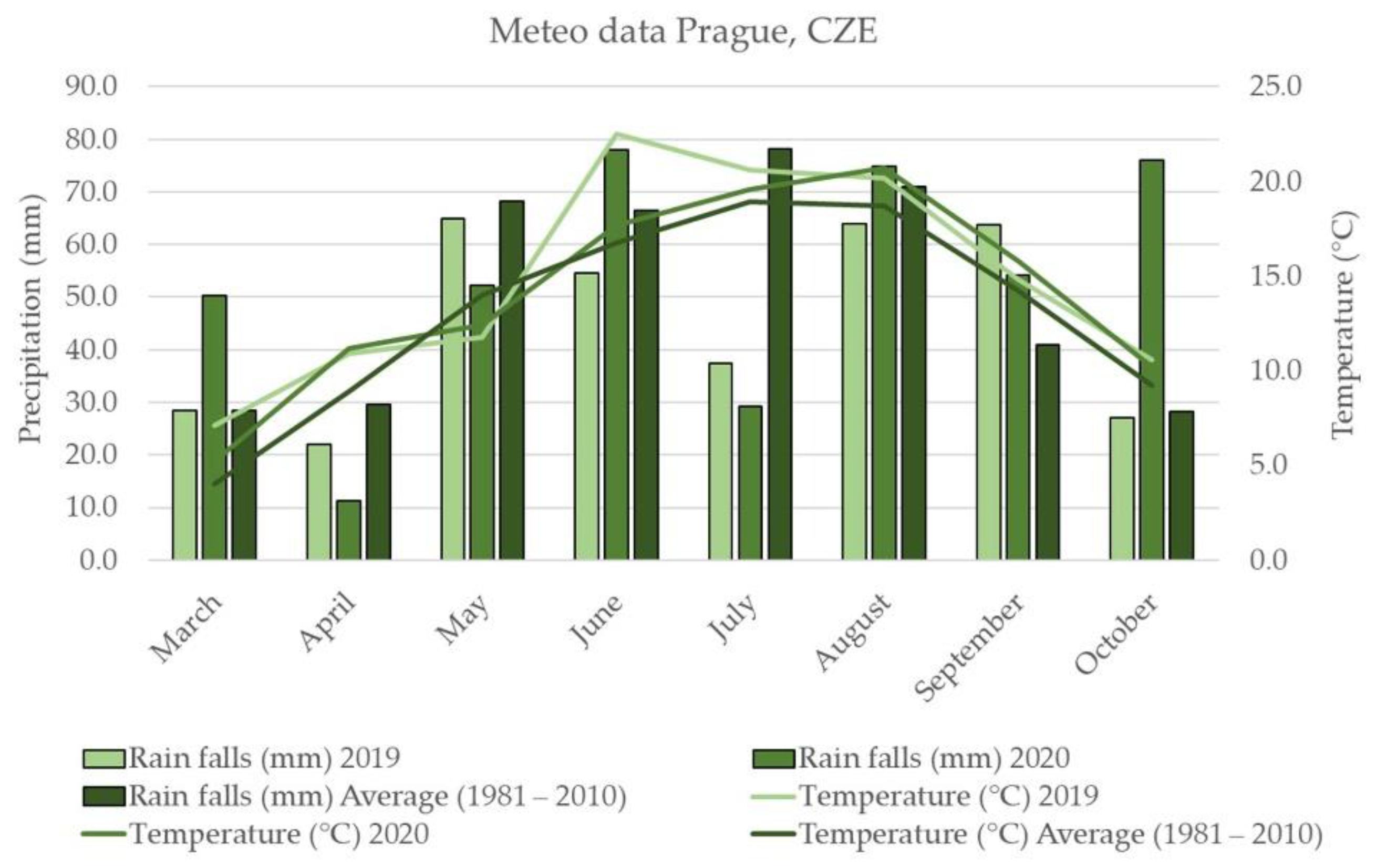
2.1. Weather Conditions
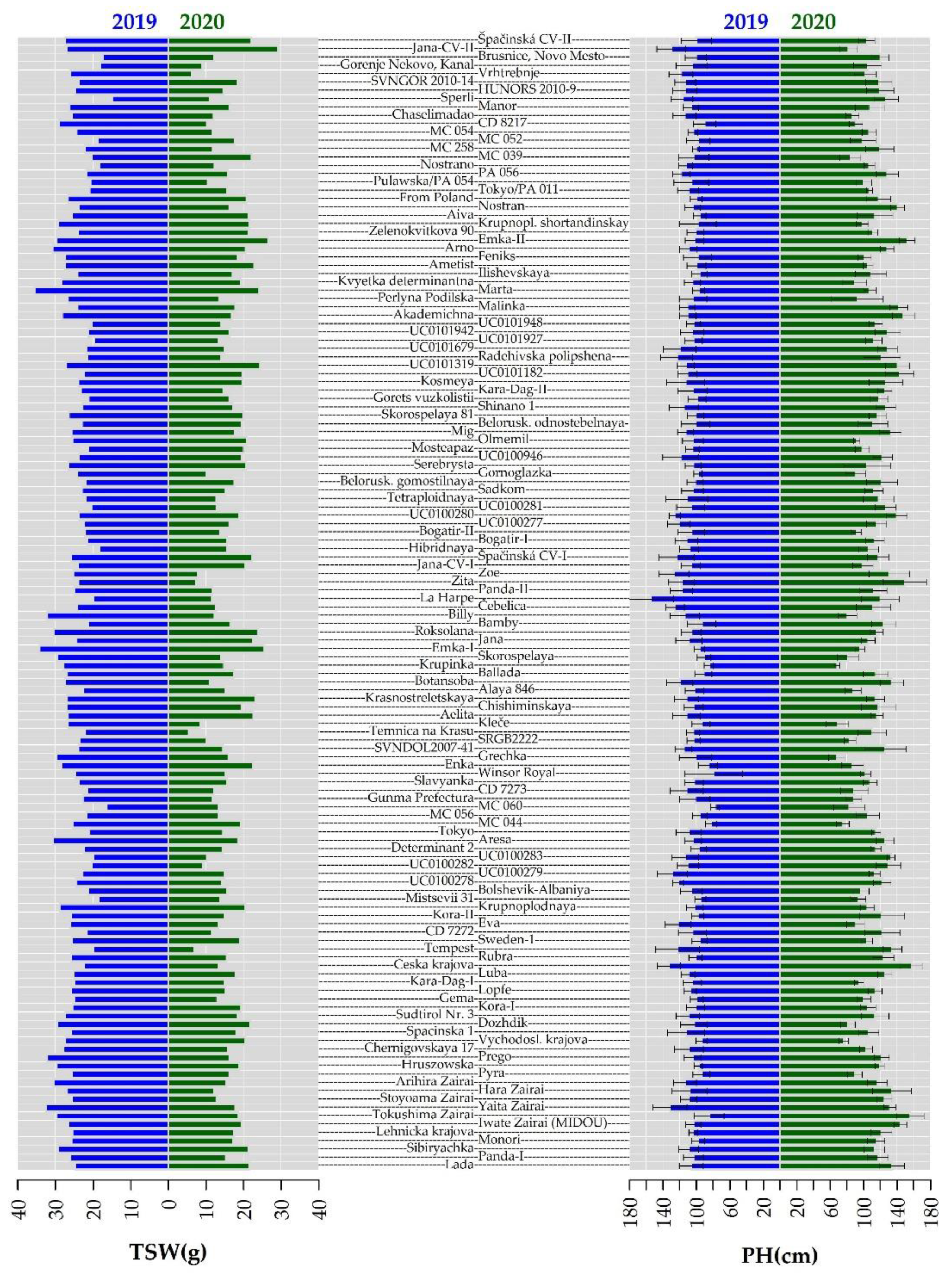
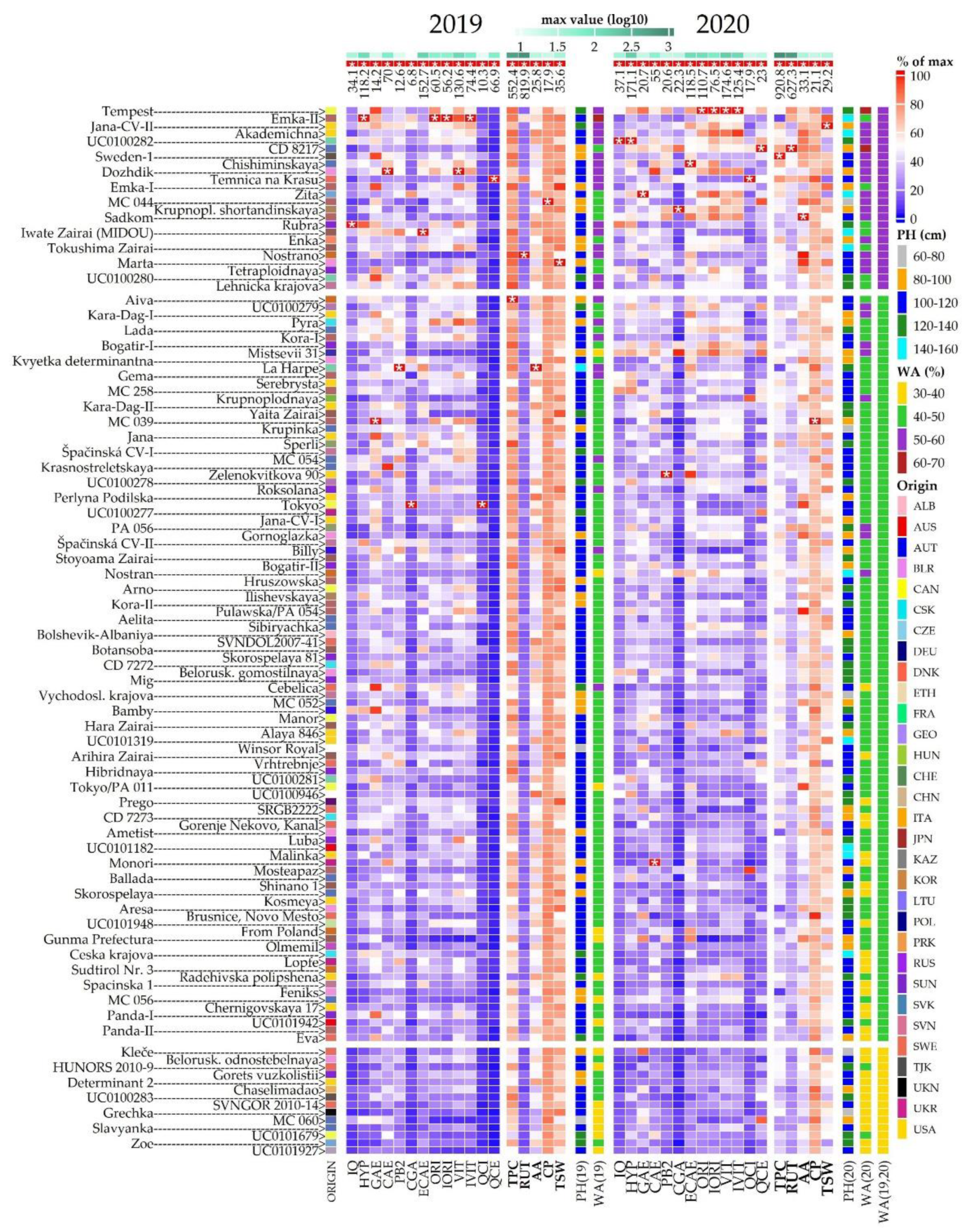
2.2. Morphological Evaluation
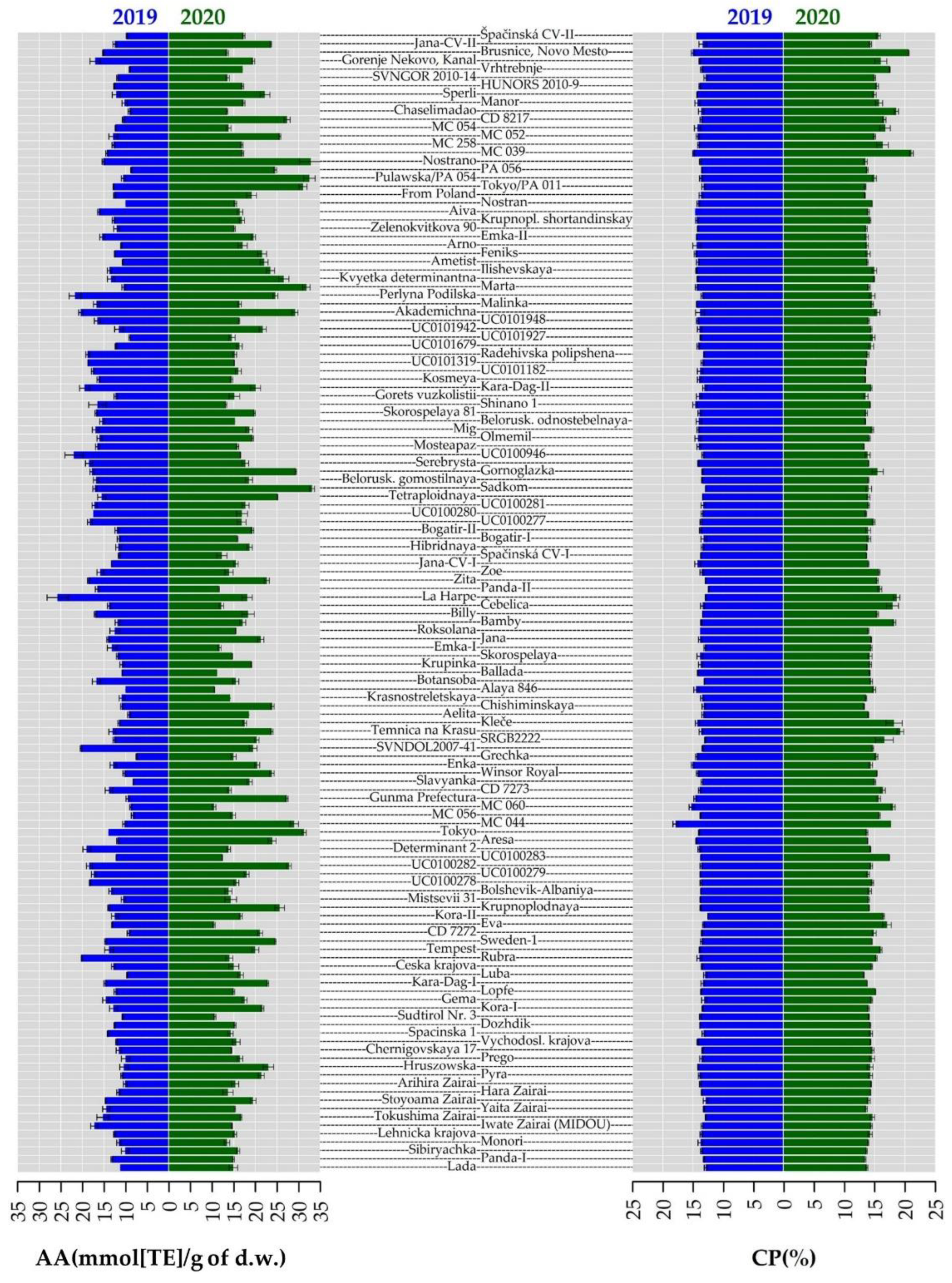
2.3. Crude Protein Content
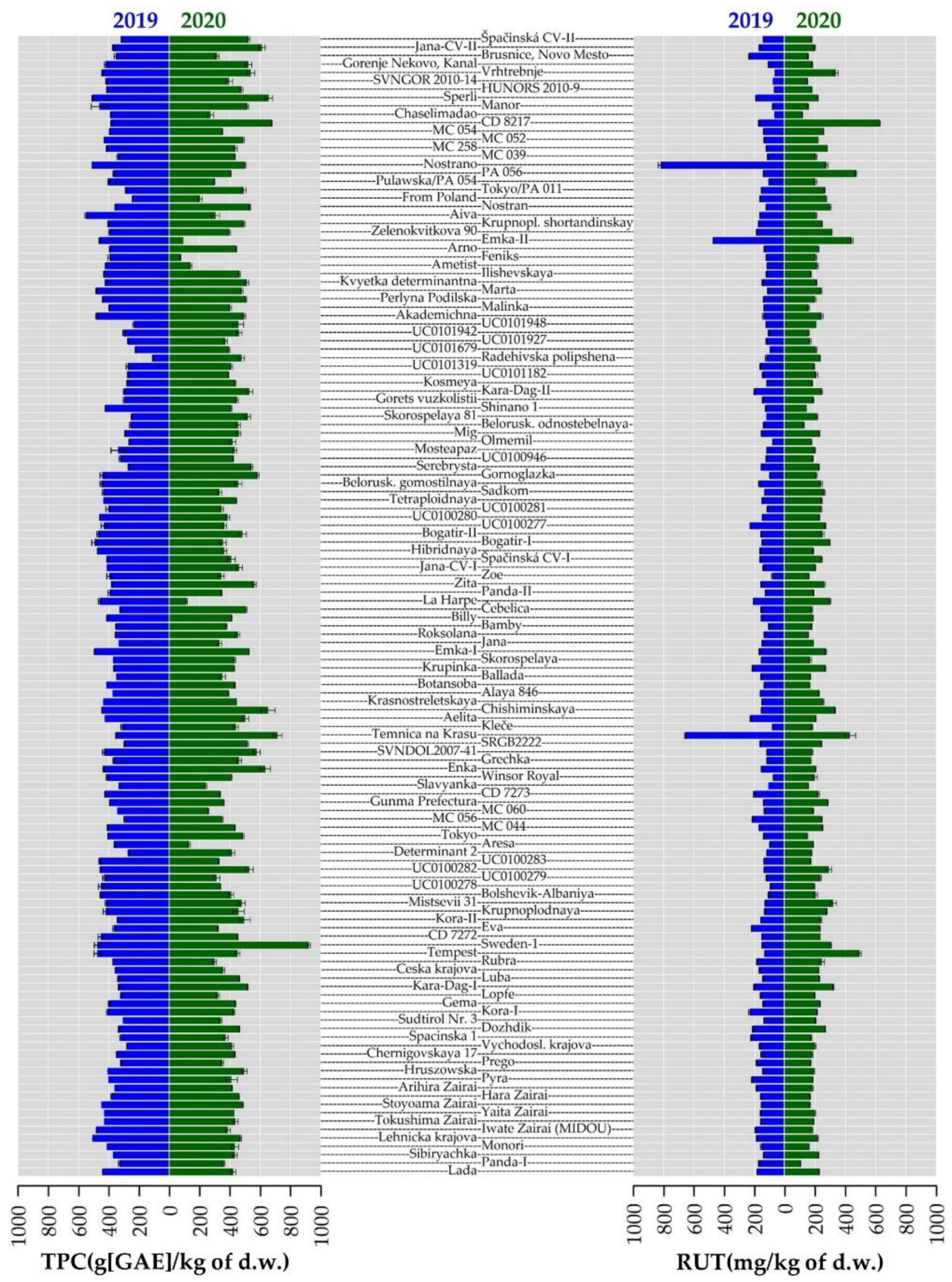
2.4. Total Phenolic Content and Antioxidant Activity
2.5. Phenolic Compounds Content
3. Materials and Methods
3.1. Plant Material
3.2. Weather Conditions
3.3. Chemicals
3.4. Standards Preparation and Sample Isolation
3.5. UHPLC-ESI-MS/MS Instrumentation
3.6. UHPLC-ESI-MS/MS Analysis
3.7. Determination of Phenolic Compound Concentration in Buckwheat Samples and Statistical Analysis
3.8. Chemical Analyses
3.9. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rodríguez, J.P.; Rahman, H.; Thushar, S.; Singh, R.K. Healthy and resilient cereals and pseudo-cereals for marginal agriculture: Molecular advances for improving nutrient bioavailability. Front. Genet. 2020, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Ohsako, T.; Li, C. Classification and systematics of the Fagopyrum species. Breed. Sci. 2020, 70, 93–100. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Deng, X.; Ruan, C.; Kreft, I.; Tang, Y.; Wu, T. Overview of buckwheat resources in the world. In Buckwheat Germplasm in the World; Zhou, M., Kreft, I., Suvorova, G., Tang, Y., Woo, S.H., Eds.; Elsevier Inc.: London, UK, 2018; p. 355. [Google Scholar]
- Ohnishi, O. Discovery of the wild ancestor of common buckwheat. Fagopyrum 1990, 11, 5–10. [Google Scholar]
- Ohnishi, O. Foreword. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M.K.I., Woo, S.H., Chrungoo, N., Wieslander, G., Eds.; Elsevier Inc.: London, UK, 2016. [Google Scholar]
- Kreft, I.; Chang, K.J.; Choi, Y.S.; Park, H.C. Ethnobotany of Buckwheat; Junsol Publishing Co.: Soul, Korea, 2003. [Google Scholar]
- Sytar, O. Phenolic acids in the inflorescences of different varieties of buckwheat and their antioxidant activity. J. King Saud Univ. Sci. 2015, 27, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Malhotra, N.; Sharma, K. Buckwheat (Fagopyrum sp.) genetic resources: What can they contribute towards nutritional security of changing world? Genet. Resour. Crop. Evol. 2020, 67, 1639–1658. [Google Scholar] [CrossRef]
- Krkošková, B.; Mrázová, Z. Prophylactic components of buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Podolska, G.; Gujska, E.; Klepacka, J.; Aleksandrowicz, E. Bioactive compounds in different buckwheat species. Plants 2021, 10, 961. [Google Scholar] [CrossRef]
- Skrabanja, V.; Kreft, I.; Golob, T.; Modic, M.; Ikeda, S.; Ikeda, K.; Kreft, S.; Bonafaccia, G.; Knapp, M.; Kosmelj, K. Nutrient content in buckwheat milling fractions. Cereal Chem. J. 2004, 81, 172–176. [Google Scholar] [CrossRef]
- Eggum, B.O.; Kreft, I.; Javornik, B. Chemical composition and protein quality of buckwheat (Fagopyrum esculen-tum MOENCH). Plant Foods Hum. Nutr. 1980, 30, 175–179. [Google Scholar] [CrossRef]
- Kasar, C.; Thanushree, M.P.; Gupta, S.; Inamdar, A.A. Milled fractions of common buckwheat (Fagopyrum escu-lentum) from the Himalayan regions: Grain characteristics, functional properties and nutrient composition. J. Food Sci. Tech. Mys. 2020, 11. [Google Scholar] [CrossRef]
- Domingos, I.F.; Bilsborrow, P.E. The effect of variety and sowing date on the growth, development, yield and quality of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Agron. 2021, 126, 126264. [Google Scholar] [CrossRef]
- Kalinova, J.P.; Vrchotova, N.; Triska, J. Phenolics levels in different parts of common buckwheat (Fagopyrwn esculentum) achenes. J. Cereal Sci. 2019, 85, 243–248. [Google Scholar] [CrossRef]
- Rauf, M.; Yoon, H.; Lee, S.; Hyun, D.Y.; Lee, M.C.; Oh, S.; Choi, Y.M. Evaluation of Fagopyrum esculentum Moench germplasm based on agro-morphological traits and the rutin and quercetin content of seeds under spring culti-vation. Genet. Resour. Crop Evol. 2020, 67, 1385–1403. [Google Scholar] [CrossRef] [Green Version]
- Ghiselli, L.; Tallarico, R.; Mariotti, M.; Romagnoli, S.; Baglio, A.P.; Donnarumma, P.; Benedettelli, S. Agronomic and nutritional characteristics of three buckwheat cultivars under organic farming in three environments of the Gar-fagnana mountain district. Ital. J. Agron. 2016, 11, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Ding, M.O.; Tang, Y.X.; Wu, Y.M.; Shao, J.R.; Zhou, M.L. Germplasm resources of buckwheat in China. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.H., Chrungoo, N., Wieslander, G., Eds.; Elsevier Inc.: London, UK, 2016; pp. 13–20. [Google Scholar]
- Bai, C.; Feng, M.; Hao, X.; Zhong, Q.; Tong, L.; Wang, Z. Rutin, quercetin, and free amino acid analysis in buckwheat (Fagopyrum) seeds from different locations. Genet. Mol. Res. 2015, 14, 19040–19048. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Yang, Q.H.; Gong, X.W.; Ma, H.C.; Dang, K.; Chen, G.H.; Gao, X.L.; Feng, B.L. Analysis of fla-vonoid metabolites in buckwheat leaves using UPLC-ESI-MS/MS. Molecules 2019, 24, 13. [Google Scholar]
- Kiprovski, B.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R.; Stampar, F.; Malencic, D.; Latkovic, D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 2015, 185, 41–47. [Google Scholar] [CrossRef]
- Huda, N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Penas, E.; Hernandez-Ledesma, B. Pseudocereal grains: Nutritional value, health ben-efits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 26. [Google Scholar] [CrossRef]
- Raguindin, P.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding buckwheat for nutritional quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Linington, S.; Hawkes, J.G.; Maxted, N.; Ford-Lloyd, B.V. The ex situ conservation of plant genetic resources. Kew Bull. 2002, 57, 506. [Google Scholar] [CrossRef]
- Gotor, E.; Alercia, A.; Rao, V.R.; Watts, J.; Caracciolo, F. The scientific information activity of Bioversity International: The descriptor lists. Genet. Resour. Crop Evol. 2008, 55, 757–772. [Google Scholar] [CrossRef]
- Rana, J.C.; Singh, M.; Chauban, R.S.; Chahota, R.K.; Sharma, T.R.; Yadav, R.; Achak, S. Genetic resources of buckwheat in India. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.H., Chrungoo, N., Wieslander, G., Eds.; Elsevier Inc.: London, UK, 2016; pp. 109–135. [Google Scholar]
- Siracusa, L.; Gresta, F.; Sperlinga, E.; Ruberto, G. Effect of sowing time and soil water content on grain yield and phenolic profile of four buckwheat (Fagopyrum esculentum Moench.) varieties in a Mediterranean environment. J. Food Compos. Anal. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- IPGRI. Descriptors for Buckwheat (Fagopyrum spp.). Available online: https://www.bioversityinternational.org/e-library/publications/detail/descriptors-for-buckwheat-fagopyrum-spp/ (accessed on 21 January 2021).
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of genetic diversity assessment in crop plants and its recent advances: An overview of its analytical perspectives. Genet. Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Michalová, A. In Study of relationships between yield and quality characters of common buckwheat (Fagopyrum esculen-tum Moench) Advance in Buckwheat Research. In Proceedings of the VII International Symposium on Buckwheat, Winnipeg, BA, Canada, 12–14 August 1998; pp. 188–196. [Google Scholar]
- Hlásná Čepková, P.; Janovská, D.; Stehno, Z. Assessment of genetic diversity of selected tartary and common buckwheat accessions. Span. J. Agric. Res. 2009, 7, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Morishita, T.; Hara, T.; Hara, T. Important agronomic characteristics of yielding ability in common buckwheat; ecotype and ecological differentiation, preharvest sprouting resistance, shattering resistance, and lodging resistance. Breed. Sci. 2020, 70, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Li, Y.; Nie, J.; Wang, C.; Huang, K.; Zhang, Y.; Zhang, Y.; She, H.; Liu, X.; Ruan, R.; et al. Effects of nitrogen fertilizer and planting density on the leaf photosynthetic characteristics, agronomic traits and grain yield in common buckwheat (Fagopyrum esculentum M.). Field Crop. Res. 2018, 219, 160–168. [Google Scholar] [CrossRef]
- Morishita, T.; Tetsuka, T. Year-to-year variation and variental difference of agronomic characters of common buckwheat in the kyushu area. Jpn. J. Crop. Sci. 2001, 70, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Saturni, L.F.G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as func-tional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Janovská, D.; Hlásná Čepková, P. Nutritional aspects of buckwheat in the Czech Republic. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.H., Chrungoo, N., Wieslander, G., Eds.; Elsevier Inc.: London, UK, 2016; pp. 177–192. [Google Scholar]
- Bonafaccia, G.; Fabjan, N. In Nutritional comparison of tartary buckwheat with common buckwheat and minor cereals. Zb. Bioteh. Fak. Univ. Ljubl. Kmet. 2003, 81, 349–355. [Google Scholar]
- Ikeda, K. Buckwheat composition, chemistry, and processing. Adv. Food Nutr. Res. 2002, 44, 395–434. [Google Scholar] [PubMed]
- Gorinstein, S.; Drzewiecki, J.; Delgado-Licon, E.; Pawelzik, E.; Ayala, A.L.M.; Medina, O.J.; Haruenkit, R.; Trakhtenberg, S. Relationship between dicotyledone-amaranth, quinoa, fagopyrum, soybean and monocots-sorghum and rice based on protein analyses and their use as substitution of each other. Eur. Food Res. Technol. 2005, 221, 69–77. [Google Scholar] [CrossRef]
- Luthar, Z.; Germ, M.; Likar, M.; Golob, A.; Vogel-Mikuš, K.; Pongrac, P.; Kušar, A.; Pravst, I.; Kreft, I. Breeding buckwheat for increased levels of rutin, quercetin and other bioactive compounds with potential antiviral effects. Plants 2020, 9, 1638. [Google Scholar] [CrossRef]
- Suzuki, T.; Noda, T.; Morishita, T.; Ishiguro, K.; Otsuka, S.; Brunori, A. Present status and future perspectives of breeding for buckwheat quality. Breed. Sci. 2020, 70, 48–66. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Peng, L.-X.; Wang, S.; Hu, Y.-B.; Zou, L. HPLC Fingerprint—Antioxidant properties study of buckwheat. J. Integr. Agric. 2012, 11, 1111–1118. [Google Scholar] [CrossRef]
- Lee, L.-S.; Choi, E.-J.; Kim, C.-H.; Sung, J.-M.; Kim, Y.-B.; Seo, D.-H.; Choi, H.-W.; Choi, Y.-S.; Kum, J.-S.; Park, J.-D. Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. J. Cereal Sci. 2016, 68, 181–186. [Google Scholar] [CrossRef]
- Klykov, A.G.; Moiseenko, L.M.; Barsukova, Y.N. Biological resources and selection value of species of Fagopy-rum Mill. Genus in the Far East of Russia. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.H., Chrungoo, N., Wieslander, G., Eds.; Elsevier Inc.: London, UK, 2016; pp. 51–60. [Google Scholar]
- Verardo, V.; Arraez-Roman, D.; Segura-Carretero, A.; Marconi, E.; Fernandez-Gutierrez, A.; Caboni, M.F. Identi-fication of buckwheat phenolic compounds by reverse phase high performance liquid chromatography-electrospray ionization-time of flight-mass spectrometry (RP-HPLC-ESI-TOF-MS). J. Cereal Sci. 2010, 52, 170–176. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Yoon, H.; Lee, S.; Hyun, D.Y.; Lee, M.-C.; Oh, S.; Rauf, M. Characterization of agro-morphological traits of tartary buckwheat germplasm under spring cultivation and analysis of health-related primary bioactive components in seeds by HPLC Method. J. Plant Biol. 2021, 64, 87–98. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinova, J.; Vrchotova, N. The influence of organic and conventional crop management, variety and year on the yield and flavonoid level in common buckwheat groats. Food Chem. 2011, 127, 602–608. [Google Scholar] [CrossRef]
- Vollmannová, A.; Musilová, J.; Lidiková, J.; Árvay, J.; Šnirc, M.; Tóth, T.; Bojňanská, T.; Čičová, I.; Kreft, I.; Germ, M. Concentrations of phenolic acids are differently genetically determined in leaves, flowers, and grain of common buckwheat (Fagopyrum esculentum Moench). Plants 2021, 10, 1142. [Google Scholar] [CrossRef]
- Borovaya, S.A.; Klykov, A.G. Some aspects of flavonoid biosynthesis and accumulation in buckwheat plants. Plant Biotechnol. Rep. 2020, 14, 213–225. [Google Scholar] [CrossRef]
- Hung, P.V.; Trinh, L.D.D.; Thuy, N.T.X.; Morita, N. Changes in nutritional composition, enzyme activities and bioactive compounds of germinated buckwheat (Fagopyrum esculantum Moench) under unchanged air and humidity conditions. Int. J. Food Sci. Technol. 2020, 9. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.-C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Morishita, T.; Shimizu, A.; Yamaguchi, H.; Degi, K. Development of common buckwheat cultivars with high antioxidative activity—‘Gamma no irodori’, ‘Cobalt no chikara’ and ‘Ruchiking’. Breed. Sci. 2019, 69, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Liu, S.; Yao, L.; Wang, L.; Li, C. Free and bound phenolics of buckwheat varieties: HPLC characterization, antioxidant activity, and inhibitory potency towards α-glucosidase with molecular docking analysis. Antioxidants 2019, 8, 606. [Google Scholar] [CrossRef] [Green Version]
- Janovska, D.; Štočková, L.; Stehno, Z. Evaluation of buckwheat sprouts as microgreens. Acta Agric. Slov. 2010, 95, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Guven, H.; Arici, A.; Simsek, O. Flavonoids in our foods: A short review. J. Basic Clin. Health Sci. 2019, 3, 96–106. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Bystrowska, B.; Francik, R. Identification of polyphenolic compounds and determination of antioxidant activity in extracts and infusions of buckwheat leaves. Eur. Food Res. Technol. 2017, 244, 333–343. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.S.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional composition and flavonoids content of flour from different buckwheat cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Zhang, S.D.; Zhang, H.Y.; Yang, X.J.; Cui, X.L.; Su, Z.H.; Hu, P. A Comparative Study on Polyphe-nolic Composition of Berries from the Tibetan Plateau by UPLC-Q-Orbitrap MS System. Chem. Biodivers. 2020, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Czech State Norm (CSN). EN ISO 712 (461014). Obiloviny a Výrobky z Obilovin, Stanovení Vlhkosti—Referenční Metoda; Úřad pro Technickou Normalizaci, Metrologii a Státní Zkušebnictví, vytiskl NORMSERVIS, s.r.o.: Praha, Czech Republic, 2010; Available online: http://www.technicke-normy-csn.cz/461014-csn-en-iso-712_4_85543.html (accessed on 21 May 2020). (In Czech)
- Czech State Norm (CSN). 5983-1 (467035), C.S.N.C.—Krmiva—Stanovení Obsahu Dusíku a Výpočet Obsahu Hrubého Proteinu—Část 1: Kjeldahlova Metoda; Český normalizační Institute, vytiskl XEROX, s.r.o.: Praha, Czech Republic, 2005; Available online: https://www.iso.org/standard/52374.html (accessed on 21 May 2020). (In Czech)
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Şensoy, Í.; Rosen, R.T.; Ho, C.-T.; Karwe, M.V. Effect of processing on buckwheat phenolics and antioxidant activity. Food Chem. 2006, 99, 388–393. [Google Scholar] [CrossRef]
| Type | Secondary Descriptors | Primary Descriptors | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Descriptor | IQ | HYP | GAE | CAE | PB2 | CGA | ECAE | ORI | IORI | VIT | IVIT | QCI | QCE | TPC | RUT | AA | CP | TSW |
| Coefficient | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 4 | 3 | 4 | 4 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janovská, D.; Jágr, M.; Svoboda, P.; Dvořáček, V.; Meglič, V.; Hlásná Čepková, P. Breeding Buckwheat for Nutritional Quality in the Czech Republic. Plants 2021, 10, 1262. https://doi.org/10.3390/plants10071262
Janovská D, Jágr M, Svoboda P, Dvořáček V, Meglič V, Hlásná Čepková P. Breeding Buckwheat for Nutritional Quality in the Czech Republic. Plants. 2021; 10(7):1262. https://doi.org/10.3390/plants10071262
Chicago/Turabian StyleJanovská, Dagmar, Michal Jágr, Pavel Svoboda, Václav Dvořáček, Vladimir Meglič, and Petra Hlásná Čepková. 2021. "Breeding Buckwheat for Nutritional Quality in the Czech Republic" Plants 10, no. 7: 1262. https://doi.org/10.3390/plants10071262
APA StyleJanovská, D., Jágr, M., Svoboda, P., Dvořáček, V., Meglič, V., & Hlásná Čepková, P. (2021). Breeding Buckwheat for Nutritional Quality in the Czech Republic. Plants, 10(7), 1262. https://doi.org/10.3390/plants10071262







