Fractionation and Distribution of Rare Earth Elements in Marine Sediment and Bioavailability in Avicennia marina in Central Red Sea Mangrove Ecosystems
Abstract
1. Introduction
2. Results
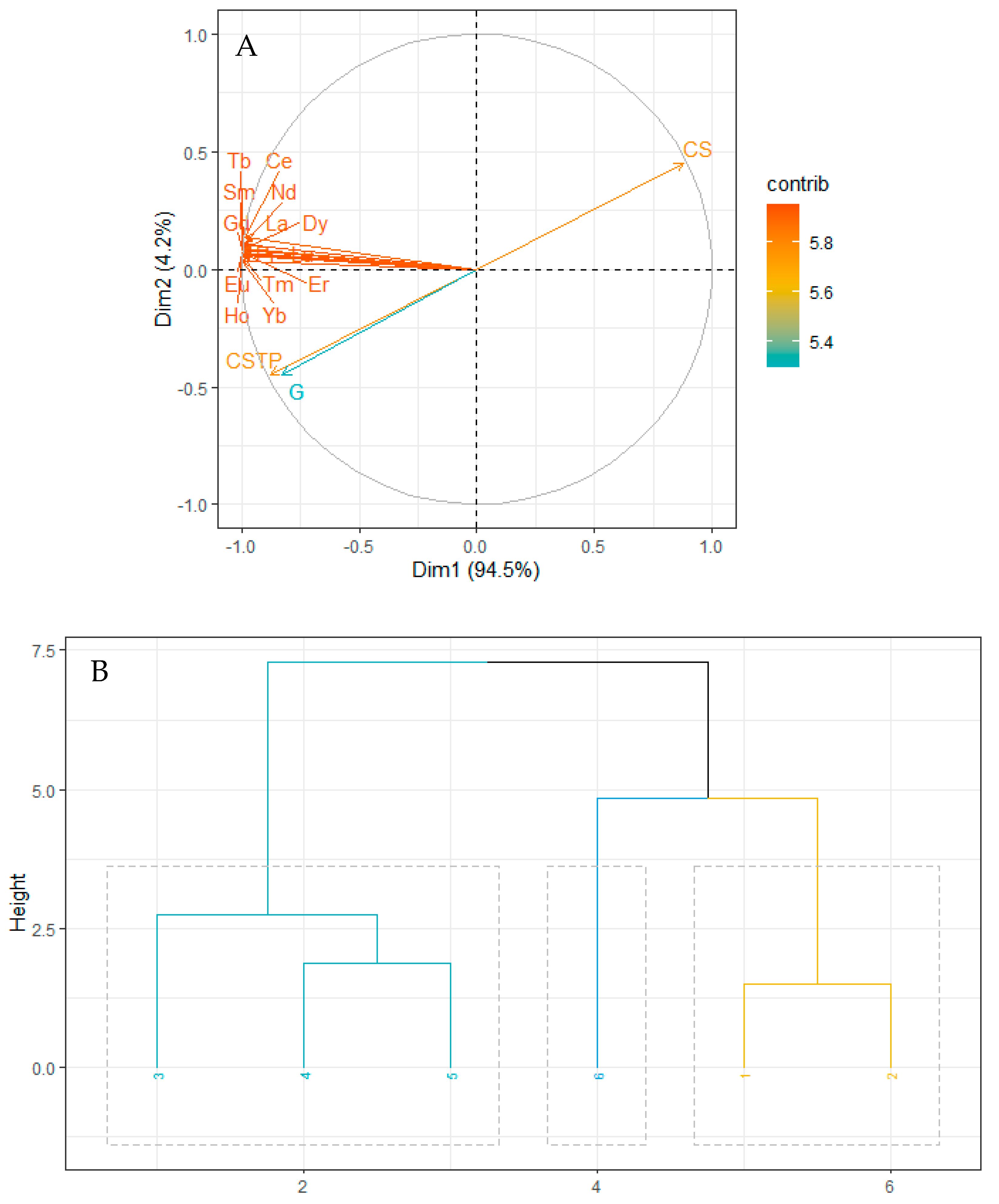
2.1. REE Composition in Sediment and Grain Sizes
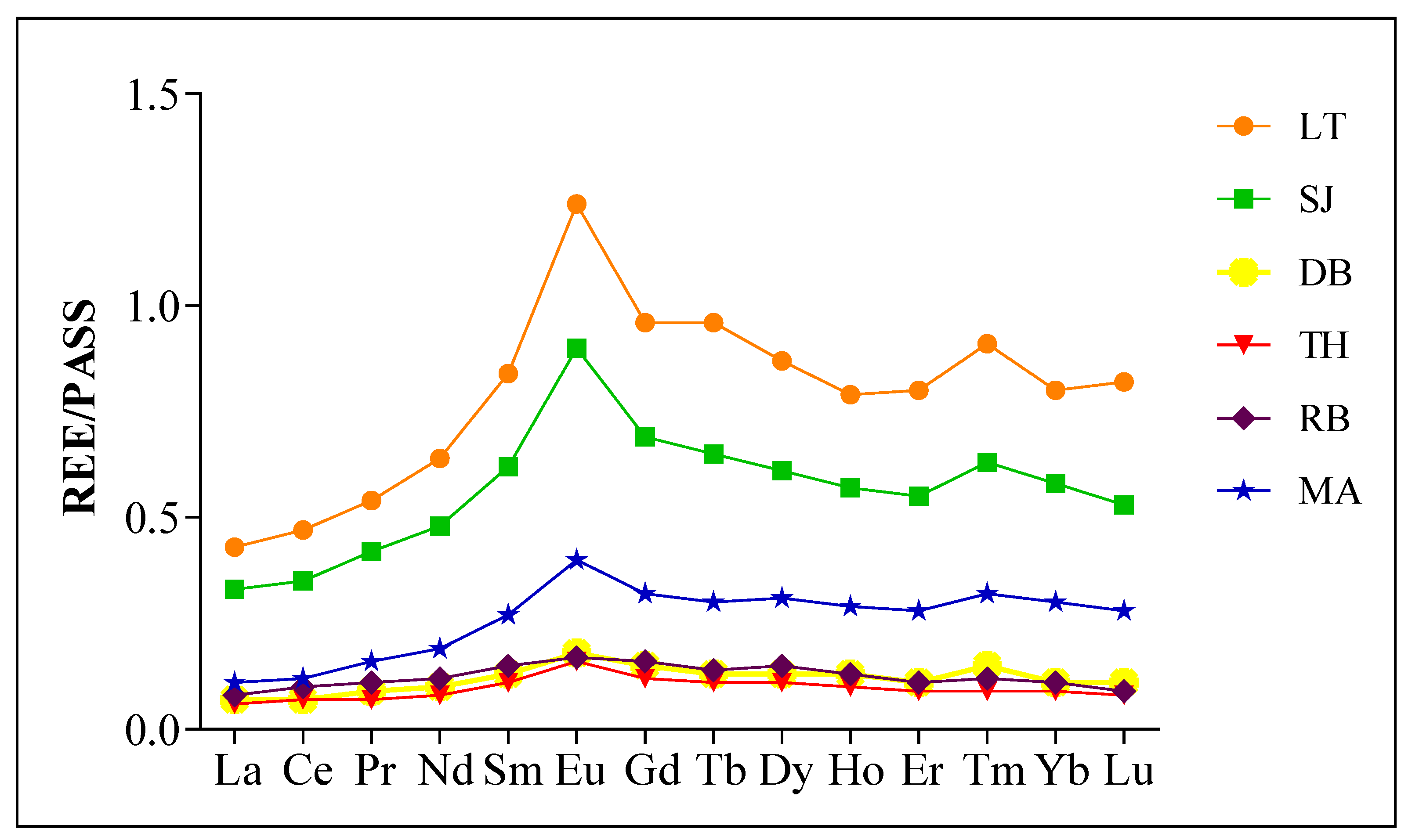
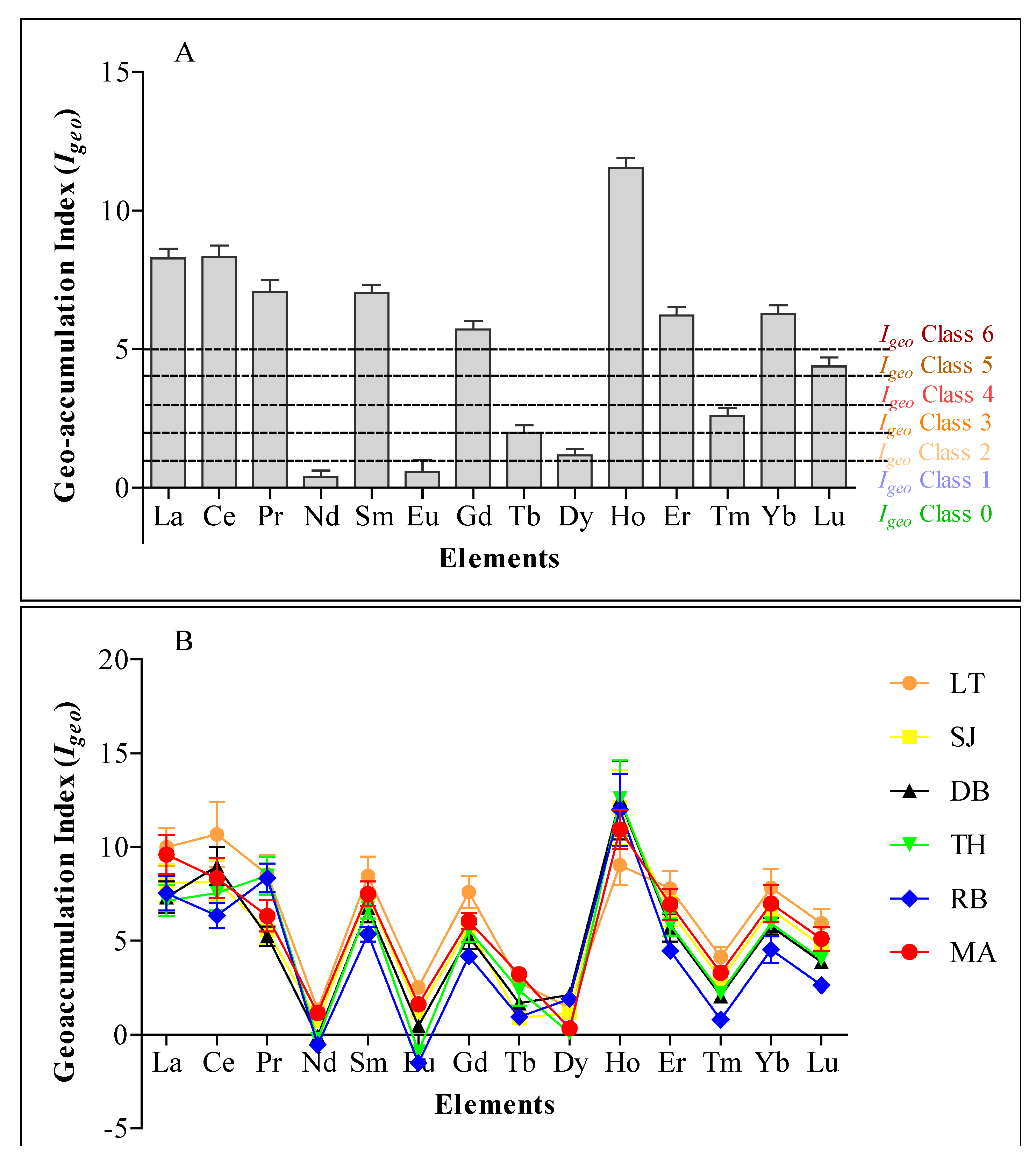
2.2. REE Fractionation and Geo-Accumulation Index (Igeo)
2.3. REE in Mangrove Avicennia marina and Bio-Concentration Factor (BCF)
3. Discussion
3.1. Influence of Sediment Grain Size on REE Concentrations and Fractionation
3.2. Fractionation of REE and Geo-Accumulation Index (Igeo)
3.3. REE in Mangrove Avicennia marina and Bio-Concentration Factor (BCF)
4. Materials and Methods
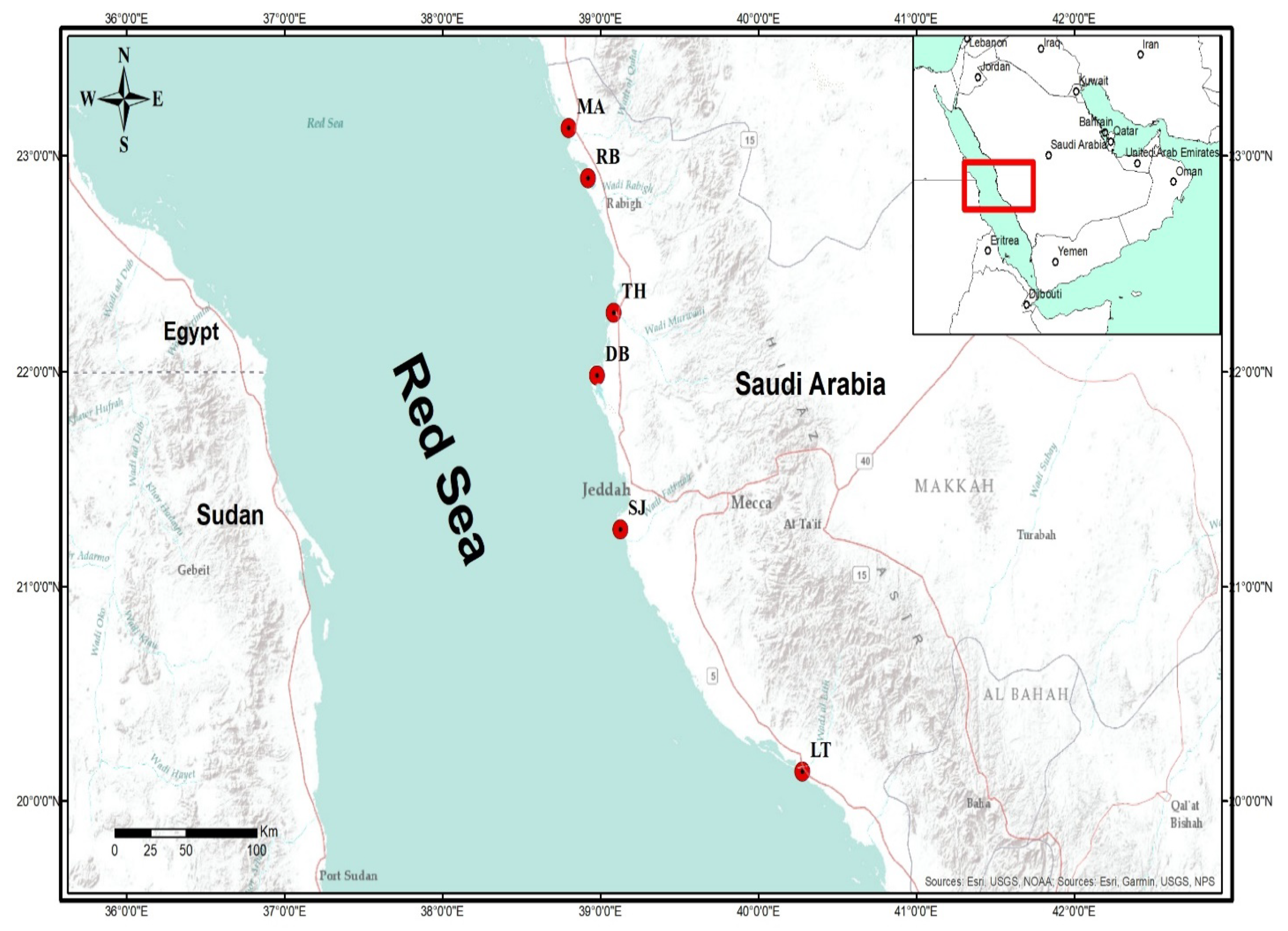
4.1. Study Area
4.2. Sampling and REE Analysis
4.3. Grain Size Analysis in Sediment
4.4. Geo-Accumulation (Igeo) and Bio-Concentration Factor (BCF)
4.5. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Chen, Z.; Chen, Z.; Ma, Q.; Zhang, Q. Rare earth elements in paddy fields from eroded granite hilly land in a southern China watershed. PLoS ONE 2019, 14, e0222330. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, T.O.P.; Pedreira, R.M.A.; Hatje, V. Distribution and fractionation of rare earth elements in sediments and mangrove soil profiles across an estuarine gradient. Chemosphere 2021, 264, 128431. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, K.H.; Cortés, A.; Leal, J.A.R.; Ramírez, A.G.; Durazo, J. Geochemistry of rare earth elements in groundwaters from a rhyolite aquifer, central México. In Rare Earth Elements in Groundwater Flow Systems; Springer: Dordrecht, The Netherlands, 2005; pp. 187–222. [Google Scholar]
- Sukumaran, P.V. Elements that rule the world: Impending REE metal crisis. J. Geol. Soc. India 2012, 80, 295. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, R.; Chen, Z.; Chen, M.; Yan, W.; Zhang, L.; Chen, H. Sources, transport and deposition of surface sediments from the South China Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2013, 71, 92–102. [Google Scholar] [CrossRef]
- Mandal, S.K.; Ray, R.; González, A.G.; Mavromatis, V.; Pokrovsky, O.S.; Jana, T.K. State of rare earth elements in the sediment and their bioaccumulation by mangroves: A case study in pristine islands of Indian Sundarban. Environ. Sci. Pollut. Res. 2019, 26, 9146–9160. [Google Scholar] [CrossRef]
- Gonzalez, V.; Vignati, D.A.L.; Leyval, C.; Giamberini, L. Environmental fate and ecotoxicity of lanthanides: Are they a uniform group beyond chemistry? Environ. Int. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Bosco-Santos, A.; Luiz-Silva, W.; da Silva-Filho, E.V.; de Souza, M.D.C.; Dantas, E.L.; Navarro, M.S. Fractionation of rare earth and other trace elements in crabs, Ucides cordatus, from a subtropical mangrove affected by fertilizer industry. J. Environ. Sci. 2017, 54, 69–76. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Hissler, C.; Stille, P.; Guignard, C.; Iffly, J.F.; Pfister, L. Rare Earth Elements as hydrological tracers of anthropogenic and critical zone contributions: A case study at the Alzette River basin scale. Procedia Earth Planet. Sci. 2014, 10, 349–352. [Google Scholar] [CrossRef]
- Duvert, C.; Cendón, D.I.; Raiber, M.; Seidel, J.-L.; Cox, M.E. Seasonal and spatial variations in rare earth elements to identify inter-aquifer linkages and recharge processes in an Australian catchment. Chem. Geol. 2015, 396, 83–97. [Google Scholar] [CrossRef]
- Pedreira, R.M.A.; Pahnke, K.; Böning, P.; Hatje, V. Tracking hospital effluent-derived gadolinium in Atlantic coastal waters off Brazil. Water Res. 2018, 145, 62–72. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Ecological risk assessment of heavy metal contamination in mangrove habitats, using biochemical markers and pollution indices: A case study of Avicennia marina L. in the Rabigh lagoon, Red Sea. Saudi J. Biol. Sci. 2020, 27, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Aljahdali, M.O.; Alhassan, A.B. Heavy metal accumulation and anti-oxidative feedback as a biomarker in seagrass Cymodocea serrulata. Sustainability 2020, 12, 2841. [Google Scholar] [CrossRef]
- Tyler, G.; Olsson, T. Rare earth elements in forest-floor herbs as related to soil conditions and mineral nutrition. Biol. Trace Elem. Res. 2005, 106, 177–191. [Google Scholar] [CrossRef]
- De Franca, E.J.; De Nadai Fernandes, E.A.; Turra, C.; Bacchi, M.A.; Elias, C.; Tagliaferro, F.; Moreira, C.; Ribeiro, A.; Figueira, R. Survey of lanthanoids in plants from a tropical region. Int. J. Environ. Health 2011, 5, 32–48. [Google Scholar] [CrossRef]
- Brito, P.; Malvar, M.; Galinha, C.; Caçador, I.; Canário, J.; Araújo, M.F.; Raimundo, J. Yttrium and rare earth elements fractionation in salt marsh halophyte plants. Sci. Total Environ. 2018, 643, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; 312p. [Google Scholar]
- Liu, S.; Shi, X.; Yang, G.; Khokiattiwong, S.; Kornkanitnan, N. Distribution of major and trace elements in surface sediments of the western gulf of Thailand: Implications to modern sedimentation. Cont. Shelf Res. 2016, 117, 81–91. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Zhu, A.; Wang, K.; Chen, M.-T.; Khokiattiwong, S.; Kornkanitnan, N.; Shi, X. Distribution of rare earth elements in surface sediments of the western Gulf of Thailand: Constraints from sedimentology and mineralogy. Quat. Int. 2019, 527, 52–63. [Google Scholar] [CrossRef]
- Prasad, M.B.K.; Ramanathan, A.L. Distribution of rare earth elements in the Pichavaram mangrove sediments of the southeast coast of India. J. Coast. Res. 2008, 1, 126–134. [Google Scholar] [CrossRef]
- Shi, X.; Liu, S.; Fang, X.; Qiao, S.; Khokiattiwong, S.; Kornkanitnan, N. Distribution of clay minerals in surface sediments of the western Gulf of Thailand: Sources and transport patterns. J. Asian Earth Sci. 2015, 105, 390–398. [Google Scholar] [CrossRef]
- Agah, H.; Bastami, K.D.; Fumani, N.S. Ecological risk, source and preliminary assessment of metals in the surface sediments of Chabahar Bay, Oman Sea. Mar. Pollut. Bull. 2016, 107, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Ghosh, S.; Chakraborty, D.; Hazra, S.; Chaudhuri, P. Assessment of potentially toxic metal (PTM) pollution in mangrove habitats using biochemical markers: A case study on Avicennia officinalis L. in and around Sundarban, India. Mar. Pollut. Bull. 2018, 133, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P.; Nath, B.; Birch, G. Accumulation of trace metals in grey mangrove Avicennia marina fine nutritive roots: The role of rhizosphere processes. Mar. Pollut. Bull. 2014, 79, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, V.C.; Cabana, K.; LaValle, P.D. Relationship between grain size and heavy metals in sediments from beaches along the coast of Guyana. J. Coast. Res. 2003, 19, 600–608. [Google Scholar]
- Liu, S.; Liu, Y.; Yang, G.; Qiao, S.; Li, C.; Zhu, Z.; Shi, X. Distribution of major and trace elements in surface sediments of Hangzhou Bay in China. Acta Oceanol. Sin. 2012, 31, 89–100. [Google Scholar] [CrossRef]
- Caetano, M.; Prego, R.; Vale, C.; de Pablo, H.; Marmolejo-Rodríguez, J. Record of diagenesis of rare earth elements and other metals in a transitional sedimentary environment. Mar. Chem. 2009, 116, 36–46. [Google Scholar] [CrossRef]
- El-Taher, A.; Badawy, W.M.; Khater, A.E.M.; Madkour, H.A. Distribution patterns of natural radionuclides and rare earth elements in marine sediments from the Red Sea, Egypt. Appl. Radiat. Isot. 2019, 151, 171–181. [Google Scholar] [CrossRef]
- Sholkovitz, E.R. The geochemistry of rare earth elements in the Amazon River estuary. Geochim. Cosmochim. Acta 1993, 57, 2181–2190. [Google Scholar] [CrossRef]
- Kuss, J.; Garbe-Schönberg, C.-D.; Kremling, K. Rare earth elements in suspended particulate material of North Atlantic surface waters. Geochim. Cosmochim. Acta 2001, 65, 187–199. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A.; Dołęgowska, S. Rare earth and trace element signatures for assessing an impact of rock mining and processing on the environment: Wiśniówka case study, south-central Poland. Environ. Sci. Pollut. Res. 2016, 23, 24943–24959. [Google Scholar] [CrossRef]
- Sappal, S.M.; Ramanathan, A.; Ranjan, R.K.; Singh, G.; Kumar, A. Rare earth elements as biogeochemical indicators in mangrove ecosystems (Pichavaram, Tamilnadu, India). J. Sediment. Res. 2014, 84, 781–791. [Google Scholar] [CrossRef]
- Sow, M.A.; Payre-Suc, V.; Julien, F.; Camara, M.; Baque, D.; Probst, A.; Sidibe, K.; Probst, J.L. Geochemical composition of fluvial sediments in the Milo River basin (Guinea): Is there any impact of artisanal mining and of a big African city, Kankan? J. Afr. Earth Sci. 2018, 145, 102–114. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Spatial Variation of Metallic Contamination and Its Ecological Risk in Sediment and Freshwater Mollusk: Melanoides tuberculata (Müller, 1774)(Gastropoda: Thiaridae). Water 2020, 12, 206. [Google Scholar] [CrossRef]
- Ghosh, S.; Ram, S.S.; Bakshi, M.; Chakraborty, A.; Sudarshan, M.; Chaudhuri, P. Vertical and horizontal variation of elemental contamination in sediments of Hooghly Estuary, India. Mar. Pollut. Bull. 2016, 109, 539–549. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Metallic pollution and the use of antioxidant enzymes as biomarkers in Bellamya unicolor (Olivier, 1804)(Gastropoda: Bellamyinae). Water 2020, 12, 202. [Google Scholar] [CrossRef]
- Balaram, V. Recent advances in the determination of elemental impurities in pharmaceuticals–Status, challenges and moving frontiers. Trac Trends Anal. Chem. 2016, 80, 83–95. [Google Scholar] [CrossRef]
- Zhou, H.; Chun, X.; Lü, C.; He, J.; Du, D. Geochemical characteristics of rare earth elements in windowsill dust in Baotou, China: Influence of the smelting industry on levels and composition. Environ. Sci. Process. Impacts 2020, 22, 2398–2405. [Google Scholar] [CrossRef]
- Cluis, C. Junk-greedy greens: Phytoremediation as a new option for soil decontamination. BioTeach J. 2004, 2, l-67. [Google Scholar]
- Cao, X.; Chen, Y.; Wang, X.; Deng, X. Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 2001, 44, 655–661. [Google Scholar] [CrossRef]
- Carpenter, D.; Boutin, C.; Allison, J.E.; Parsons, J.L.; Ellis, D.M. Uptake and effects of six rare earth elements (REEs) on selected native and crop species growing in contaminated soils. PLoS ONE 2015, 10, e0129936. [Google Scholar] [CrossRef]
- Analuddin, K.; Sharma, S.; Septiana, A.; Sahidin, I.; Rianse, U.; Nadaoka, K. Heavy metal bioaccumulation in mangrove ecosystem at the coral triangle ecoregion, Southeast Sulawesi, Indonesia. Mar. Pollut. Bull. 2017, 125, 472–480. [Google Scholar] [CrossRef]
- Mleczek, P.; Borowiak, K.; Budka, A.; Niedzielski, P. Relationship between concentration of rare earth elements in soil and their distribution in plants growing near a frequented road. Environ. Sci. Pollut. Res. 2018, 25, 23695–23711. [Google Scholar] [CrossRef] [PubMed]
- Rasul, N.M.A.; Stewart, I.C.F. Oceanographic and Biological Aspects of the Red Sea; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 3319994174. [Google Scholar]
- Almahasheer, H.; Duarte, C.M.; Irigoien, X. Nutrient limitation in central Red Sea mangroves. Front. Mar. Sci. 2016, 3, 271. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Munawar, S.; Khan, W.R. Monitoring Mangrove Forest Degradation and Regeneration: Landsat Time Series Analysis of Moisture and Vegetation Indices at Rabigh Lagoon, Red Sea. Forests 2021, 12, 52. [Google Scholar] [CrossRef]
- US E.P.A. Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. SW-846 Test Methods Eval. Available online: https://www.epa.gov/esam/us-epa-method-3051a-microwave-assisted-acid-digestion-sediments-sludges-and-oil (accessed on 21 January 2019).
- Aljahdali, M.O.; Alhassan, A.B.; Zhang, Z. Environmental Factors Causing Stress in Avicennia marina Mangrove in Rabigh Lagoon along the Red Sea: Based on a Multi-Approach Study. Front. Mar. Sci. 2021, 8, 328. [Google Scholar] [CrossRef]
- Stoffers, P.; Glasby, G.P.; Wilson, C.J.; Davis, K.R.; Walter, P. Heavy metal pollution in Wellington Harbour. N. Z. J. Mar. Freshw. Res. 1986, 20, 495–512. [Google Scholar] [CrossRef]
- Noack, C.W.; Dzombak, D.A.; Karamalidis, A.K. Rare earth element distributions and trends in natural waters with a focus on groundwater. Environ. Sci. Technol. 2014, 48, 4317–4326. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Greig, A.; Collerson, K.D.; Kamber, B.S. Rare earth element and yttrium variability in South East Queensland waterways. Aquat. Geochem. 2006, 12, 39–72. [Google Scholar] [CrossRef]


| Grain Size | Al Lith | South Jeddah | Dahaban | Thuwal | Rabigh | Mastorah |
|---|---|---|---|---|---|---|
| CS (0.063–2 mm) | 83.53 ± 12.54 | 88.83 ± 17.48 | 65.37 ± 13.95 | 52.53 ± 9.06 | 45.50 ± 7.52 | 72.48 ± 11.66 |
| CSTP (<0.063 mm) | 15.97 ± 2.55 | 10.53 ± 1.32 | 34.42 ± 2.17 | 47.15 ± 6.82 | 54.29 ± 9.31 | 27.06 ± 5.16 |
| G (>2 mm) | 0.49 ± 0.05 | 0.63 ± 0.06 | 0.20 ± 0.08 | 0.31 ± 0.06 | 0.20 ± 0.01 | 0.45 ± 0.03 |
| Texture | Sand | Sand | Sand | Sand | Loamy sand | Sand |
| Al Lith | South Jeddah | Dahaban | Thuwal | Rabigh | Mastorah | Average | p-Value | |
|---|---|---|---|---|---|---|---|---|
| La | 16.54 ± 0.32 | 12.59 ± 0.1 | 2.59 ± 0.03 | 2.26 ± 0.01 | 3.01 ± 0.1 | 4.36 ± 0.10 | 6.89 ± 1.50 | 0.001 |
| Ce | 37.36 ± 0.45 | 27.74 ± 0.19 | 5.84 ± 0.15 | 5.45 ± 0.15 | 7.88 ± 0.20 | 9.85 ± 0.12 | 15.69 ± 3.51 | 0.000 |
| Pr | 4.73 ± 0.02 | 3.73 ± 0.10 | 0.77 ± 0.03 | 0.66 ± 0.03 | 0.98 ± 0.03 | 1.43 ± 0.01 | 2.05 ± 0.07 | 0.000 |
| Nd | 21.60 ± 0.20 | 16.12 ± 0.19 | 3.36 ± 0.06 | 2.84 ± 0.02 | 4.20 ± 0.02 | 6.35 ± 0.11 | 9.08 ± 1.21 | 0.003 |
| Sm | 4.65 ± 0.10 | 3.43 ± 0.07 | 0.74 ± 0.02 | 0.61 ± 0.03 | 0.84 ± 0.01 | 1.48 ± 0.01 | 1.96 ± 0.06 | 0.000 |
| Eu | 1.34 ± 0.06 | 0.97 ± 0.03 | 0.19 ± 0.01 | 0.17 ± 0.02 | 0.19 ± 0.01 | 0.43 ± 0.01 | 0.55 ± 0.02 | 0.000 |
| Gd | 4.45 ± 0.21 | 3.20 ± 0.14 | 0.70 ± 0.02 | 0.58 ± 0.03 | 0.77 ± 0.03 | 1.48 ± 0.01 | 1.86 ± 0.06 | 0.000 |
| Tb | 0.74 ± 0.03 | 0.50 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.23 ± 0.01 | 0.30 ± 0.01 | 0.002 |
| Dy | 4.09 ± 0.06 | 2.84 ± 0.01 | 0.63 ± 0.01 | 0.52 ± 0.03 | 0.68 ± 0.02 | 1.46 ± 0.01 | 1.70 ± 0.05 | 0.000 |
| Ho | 0.79 ± 0.02 | 0.57 ± 0.01 | 0.13 ± 0.01 | 0.10 ± 0.01 | 0.13 ± 0.01 | 0.29 ± 0.01 | 0.33 ± 0.02 | 0.000 |
| Er | 2.28 ± 0.02 | 1.58 ± 0.01 | 0.32 ± 0.01 | 0.25 ± 0.03 | 0.32 ± 0.01 | 0.81 ± 0.02 | 0.93 ± 0.04 | 0.000 |
| Tm | 0.37 ± 0.02 | 0.25 ± 0.02 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.004 |
| Yb | 2.25 ± 0.02 | 1.63 ± 0.03 | 0.31 ± 0.01 | 0.26 ± 0.01 | 0.31 ± 0.01 | 0.84 ± 0.02 | 0.94 ± 0.03 | 0.000 |
| Lu | 0.36 ± 0.03 | 0.23 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.12 ± 0.02 | 0.14 ± 0.01 | 0.000 |
| ∑REE | 101.53 ± 7.45 | 75.38 ± 5.55 | 15.79 ± 2.01 | 13.85 ± 1.65 | 19.50 ± 3.04 | 29.27 ± 3.92 | 42.56 ± 6.06 | 0.001 |
| (La/Yb)n | 0.54 ± 0.02 | 0.57 ± 0.03 | 0.61 ± 0.02 | 0.63 ± 0.04 | 0.72 ± 0.06 | 0.38 ± 0.01 | 0.57 ± 0.04 | 0.007 |
| (Sm/La)n | 1.94 ± 0.06 | 1.88 ± 0.04 | 1.96 ± 0.06 | 1.85 ± 0.05 | 1.91 ± 0.05 | 2.34 ± 0.14 | 1.98 ± 0.04 | 0.005 |
| (Yb/Sm)n | 0.95 ± 0.03 | 0.94 ± 0.08 | 0.84 ± 0.04 | 0.86 ± 0.03 | 0.73 ± 0.06 | 1.12 ± 0.09 | 0.91 ± 0.05 | 0.006 |
| R(M/L) | 0.24 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.03 | 0.21 ± 0.02 | 0.16 ± 0.04 | 0.30 ± 0.04 | 0.22 ± 0.02 | 0.032 |
| R(H/M) | −0.04 ± 0.02 | −0.05 ± 0.01 | −0.05 ± 0.01 | −0.13 ± 0.02 | −0.15 ± 0.01 | −0.01 ± 0.001 | −0.07 ± 0.002 | 0.004 |
| δCe | 0.97 ± 0.04 | 0.93 ± 0.03 | 0.95 ± 0.05 | 1.03 ± 0.33 | 1.05 ± 0.41 | 0.90 ± 0.06 | 0.97 ± 0.04 | 0.014 |
| δEu | 1.38 ± 0.43 | 1.37 ± 0.40 | 1.26 ± 0.24 | 1.36 ± 0.34 | 1.09 ± 0.20 | 1.37 ± 0.21 | 1.31 ± 0.25 | 0.021 |
| Al Lith | South Jeddah | Dahaban | Thuwal | Rabigh | Mastorah | Average | p-Value | |
|---|---|---|---|---|---|---|---|---|
| La | 0.68 ± 0.11 | 1.11 ± 0.03 | 0.50 ± 0.01 | 0.43 ± 0.04 | 0.57 ± 0.06 | 0.71 ± 0.05 | 0.67 ± 0.09 | 0.000 |
| Ce | 2.02 ± 0.15 | 1.46 ± 0.13 | 0.95 ± 0.18 | 0.82 ± 0.11 | 1.15 ± 0.08 | 1.42 ± 0.19 | 1.30 ± 0.02 | 0.000 |
| Pr | 0.24 ± 0.04 | 0.17 ± 0.03 | 0.12 ± 0.03 | 0.10 ± 0.02 | 0.13 ± 0.01 | 0.16 ± 0.02 | 0.15 ± 0.01 | 0.012 |
| Nd | 0.95 ± 0.09 | 0.67 ± 0.07 | 0.45 ± 0.04 | 0.39 ± 0.02 | 0.55 ± 0.08 | 0.61 ± 0.10 | 0.60 ± 0.03 | 0.000 |
| Sm | 0.22 ± 0.04 | 0.16 ± 0.02 | 0.10 ± 0.03 | 0.09 ± 0.01 | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.02 | 0.005 |
| Eu | 0.05 ± 0.002 | 0.05 ± 0.002 | 0.04 ± 0.01 | 0.03 ± 0.001 | 0.04 ± 0.002 | 0.04 ± 0.001 | 0.04 ± 0.003 | 0.411 |
| Gd | 0.21 ± 0.03 | 0.14 ± 0.03 | 0.11 ± 0.02 | 0.08 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.03 | 0.13 ± 0.01 | 0.011 |
| Tb | 0.04 ± 0.003 | 0.04 ± 0.001 | 0.03 ± 0.001 | 0.02 ± 0.001 | 0.03 ± 0.002 | 0.03 ± 0.001 | 0.03 ± 0.003 | 0.390 |
| Dy | 0.18 ± 0.02 | 0.12 ± 0.01 | 0.09 ± 0.003 | 0.09 ± 0.001 | 0.10 ± 0.02 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.012 |
| Ho | 0.05 ± 0.003 | 0.04 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.002 | 0.03 ± 0.001 | 0.03 ± 0.002 | 0.04 ± 0.003 | 0.254 |
| Er | 0.10 ± 0.02 | 0.08 ± 0.002 | 0.06 ± 0.003 | 0.05 ± 0.001 | 0.05 ± 0.001 | 0.07 ± 0.002 | 0.07 ± 0.004 | 0.064 |
| Tm | 0.03 ± 0.007 | 0.03 ± 0.005 | 0.02 ± 0.004 | 0.02 ± 0.005 | 0.02 ± 0.006 | 0.02 ± 0.005 | 0.02 ± 0.001 | 0.843 |
| Yb | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.007 | 0.05 ± 0.007 | 0.06 ± 0.009 | 0.06 ± 0.008 | 0.06 ± 0.002 | 0.178 |
| Lu | 0.03 ± 0.007 | 0.03 ± 0.004 | 0.02 ± 0.006 | 0.02 ± 0.005 | 0.02 ± 0.004 | 0.02 ± 0.007 | 0.02 ± 0.001 | 0.898 |
| ∑REE | 3.56 ± 0.22 | 3.02 ± 0.06 | 3.69 ± 0.23 | 2.57 ± 0.17 | 2.26 ± 0.05 | 5.29 ± 0.92 | 3.40 ± 0.44 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhassan, A.B.; Aljahdali, M.O. Fractionation and Distribution of Rare Earth Elements in Marine Sediment and Bioavailability in Avicennia marina in Central Red Sea Mangrove Ecosystems. Plants 2021, 10, 1233. https://doi.org/10.3390/plants10061233
Alhassan AB, Aljahdali MO. Fractionation and Distribution of Rare Earth Elements in Marine Sediment and Bioavailability in Avicennia marina in Central Red Sea Mangrove Ecosystems. Plants. 2021; 10(6):1233. https://doi.org/10.3390/plants10061233
Chicago/Turabian StyleAlhassan, Abdullahi Bala, and Mohammed Othman Aljahdali. 2021. "Fractionation and Distribution of Rare Earth Elements in Marine Sediment and Bioavailability in Avicennia marina in Central Red Sea Mangrove Ecosystems" Plants 10, no. 6: 1233. https://doi.org/10.3390/plants10061233
APA StyleAlhassan, A. B., & Aljahdali, M. O. (2021). Fractionation and Distribution of Rare Earth Elements in Marine Sediment and Bioavailability in Avicennia marina in Central Red Sea Mangrove Ecosystems. Plants, 10(6), 1233. https://doi.org/10.3390/plants10061233







