Acetaminophen Induces an Antioxidative Response in Lettuce Plants
Abstract
1. Introduction
2. Results and Discussion
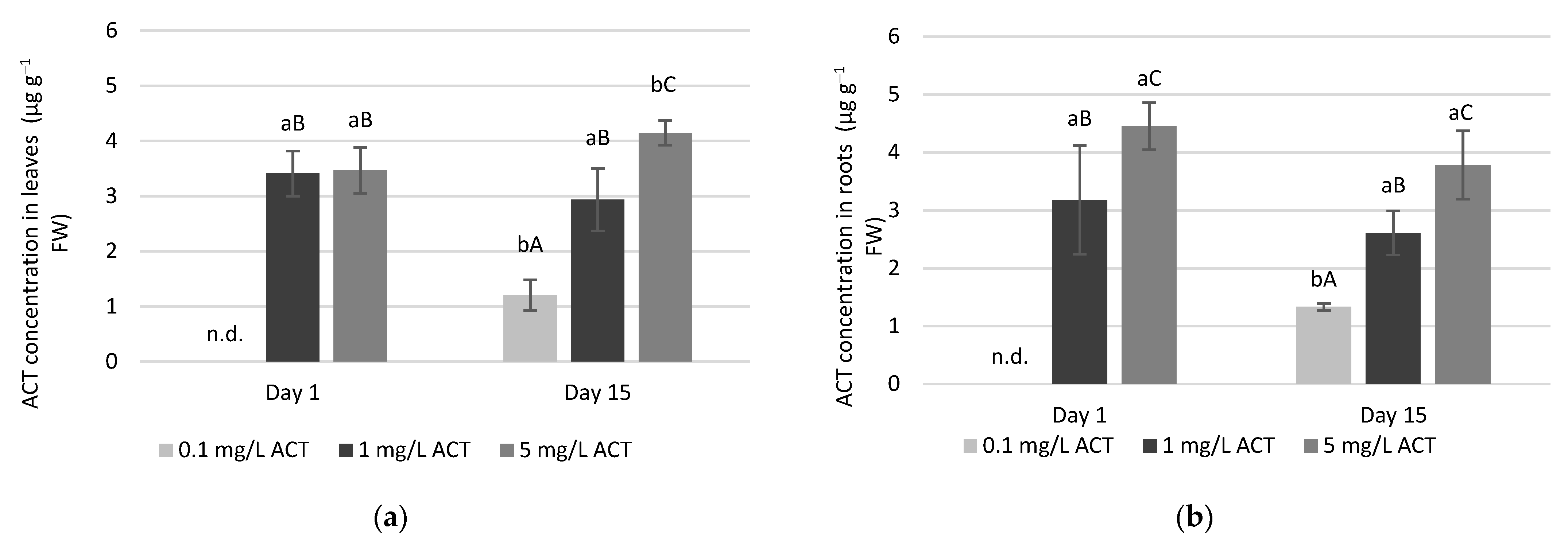
2.1. Acetaminophen Uptake in Leaves and Roots
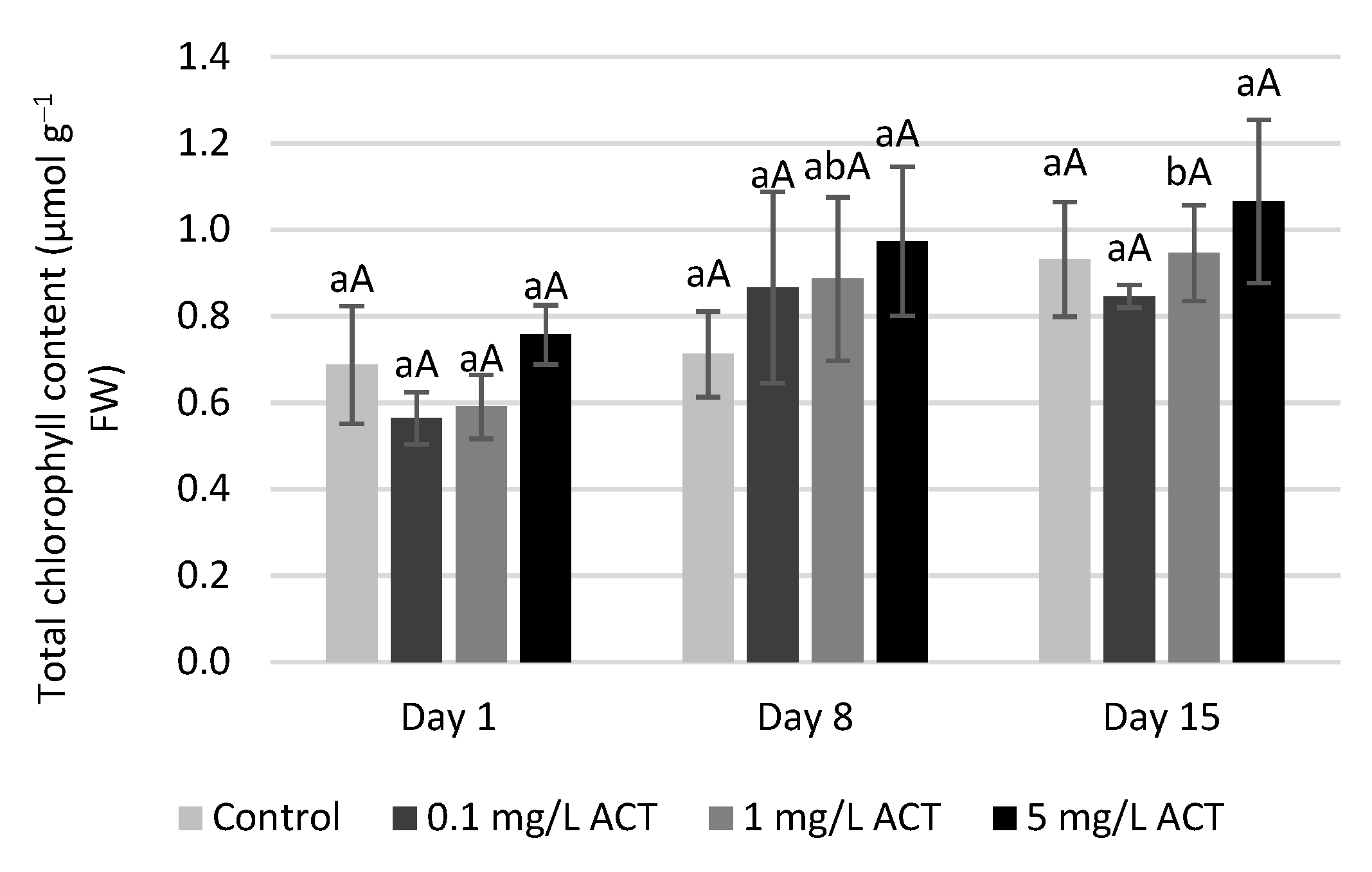
2.2. Biomass and Chlorophyll Parameters
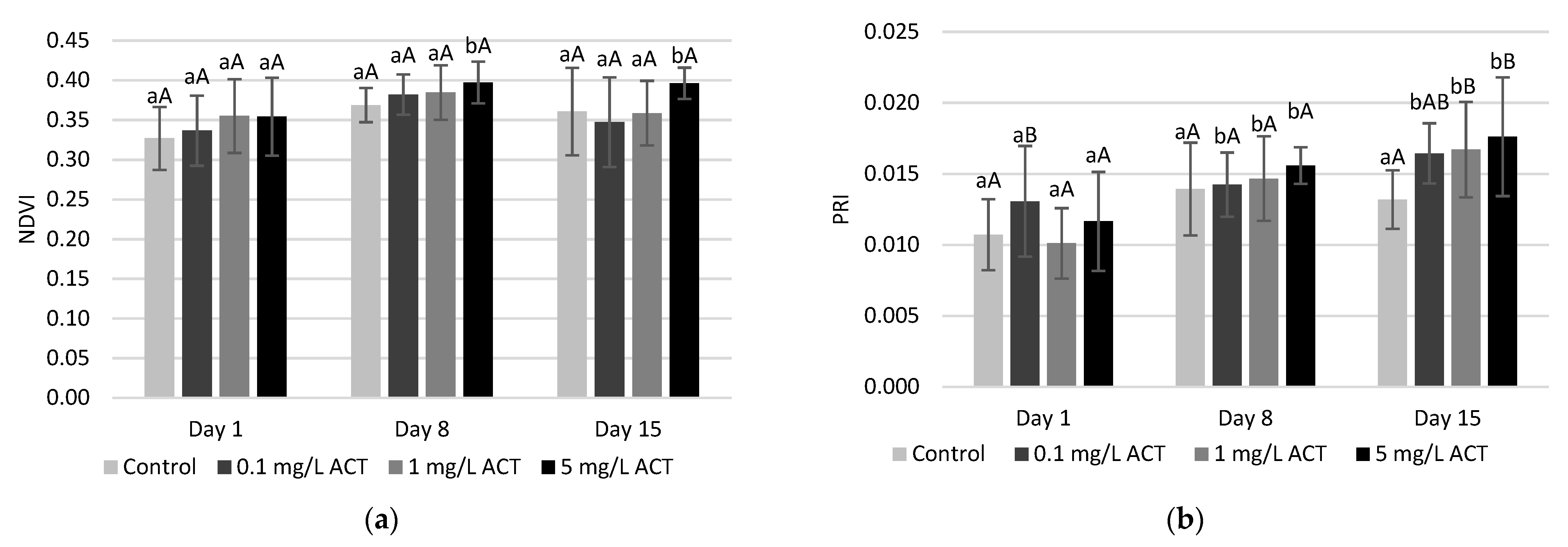
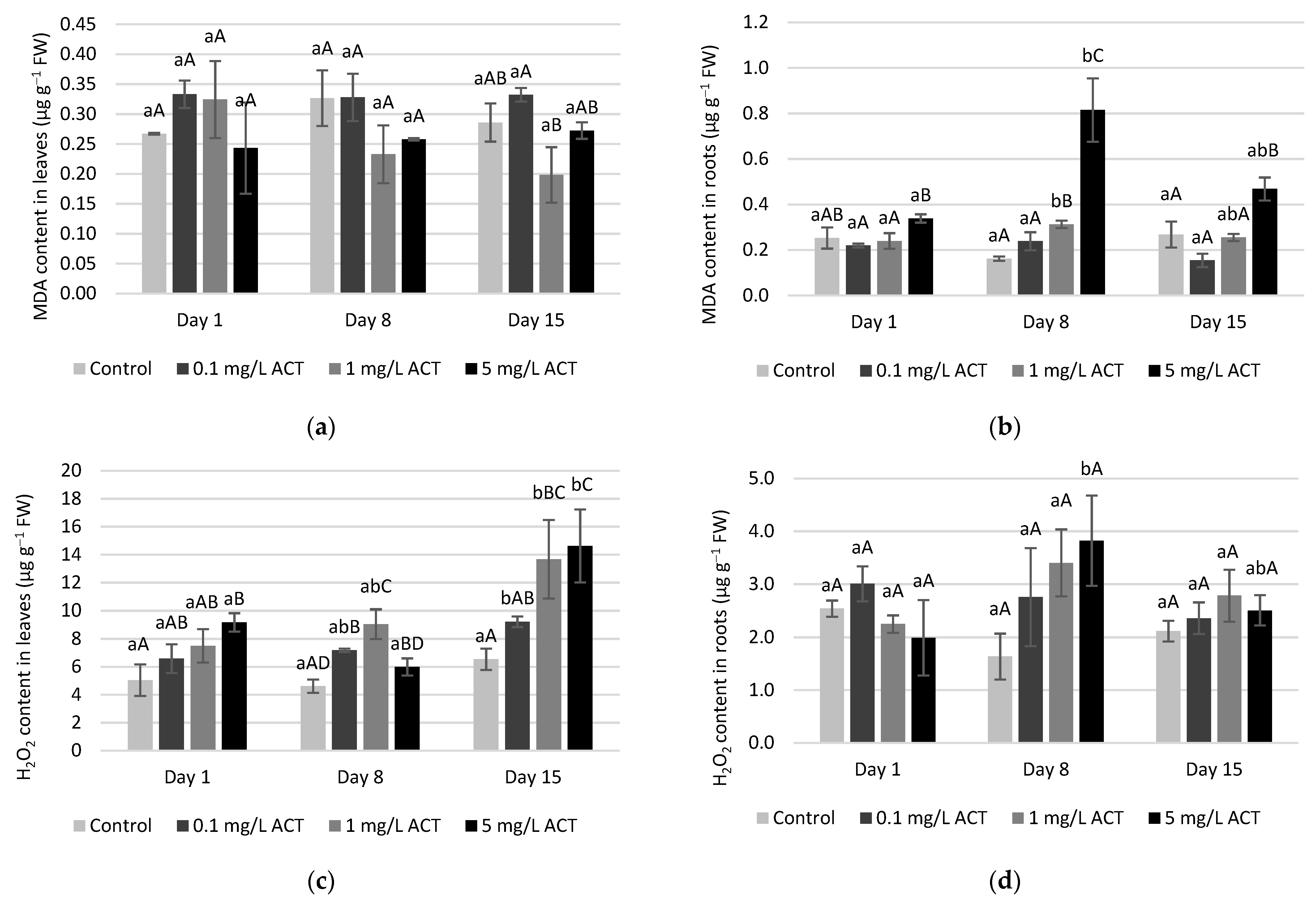
2.3. Stress Indicators Analysis
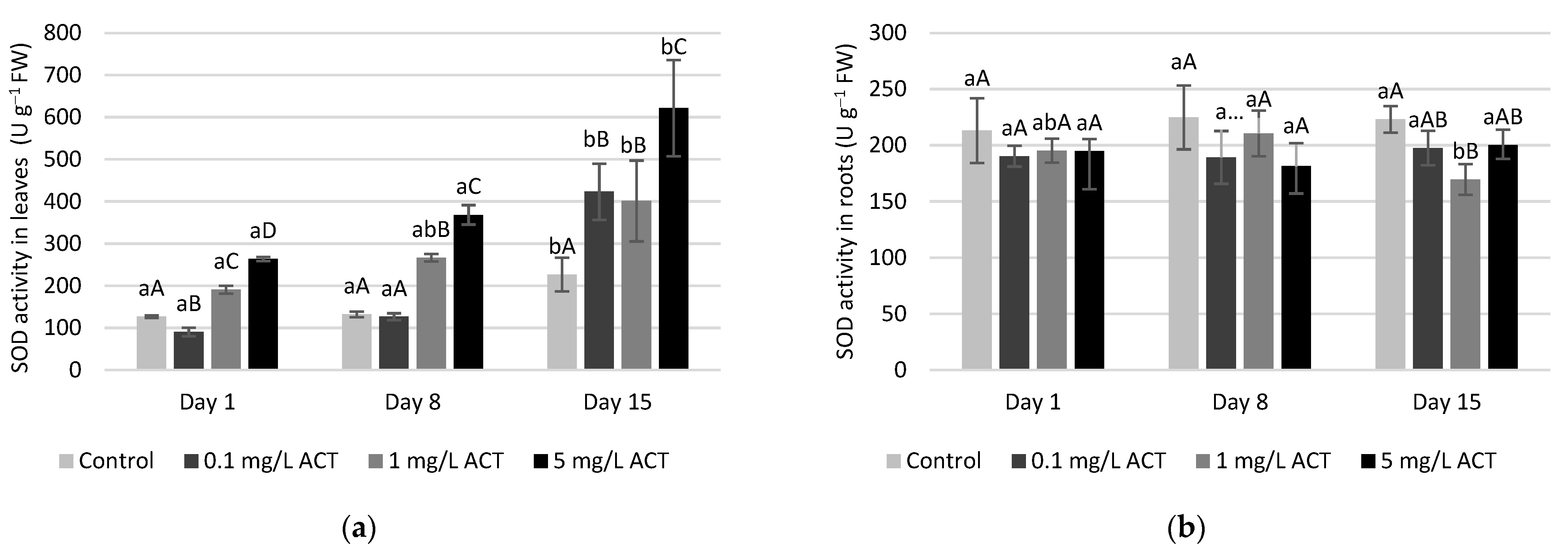
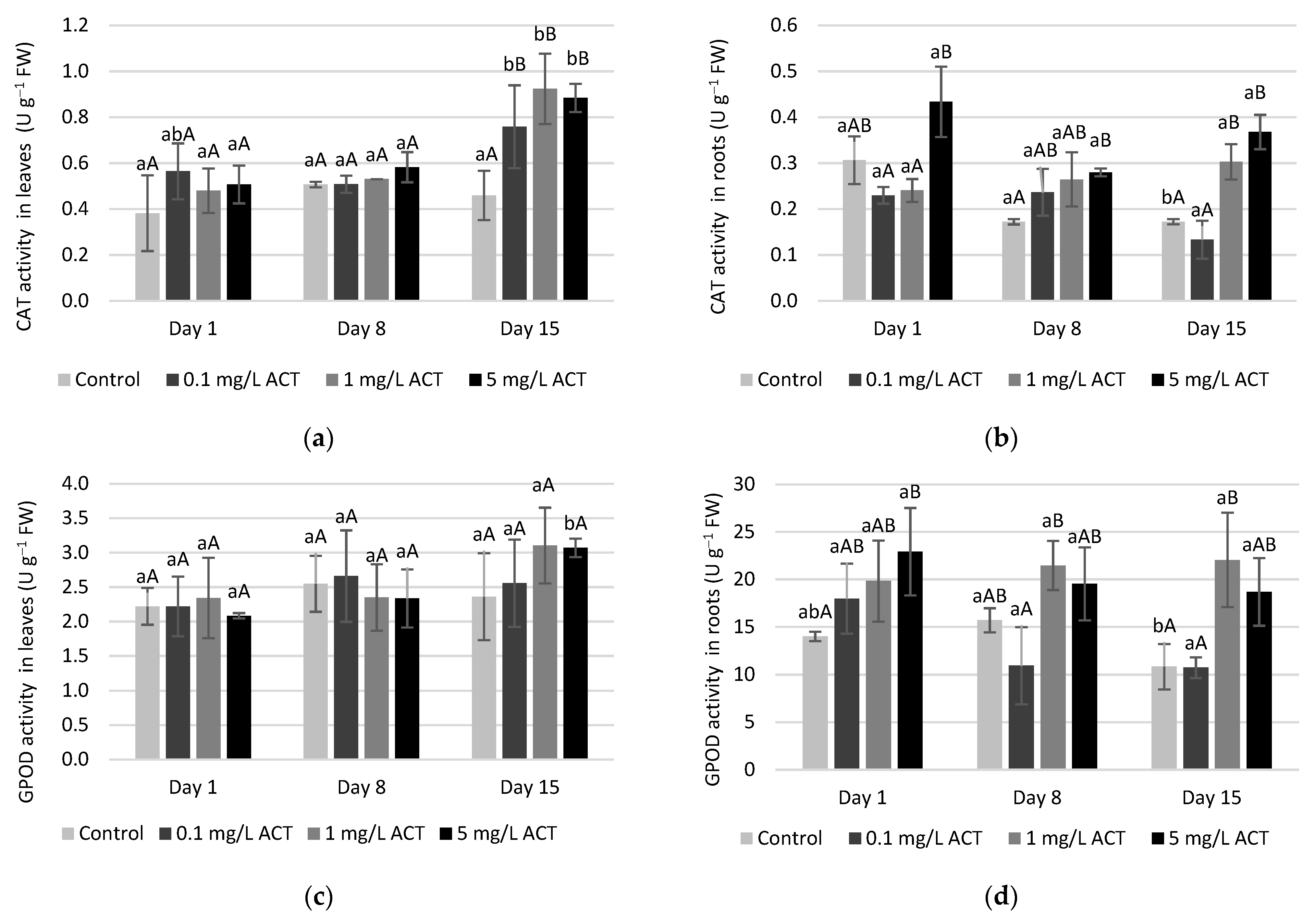
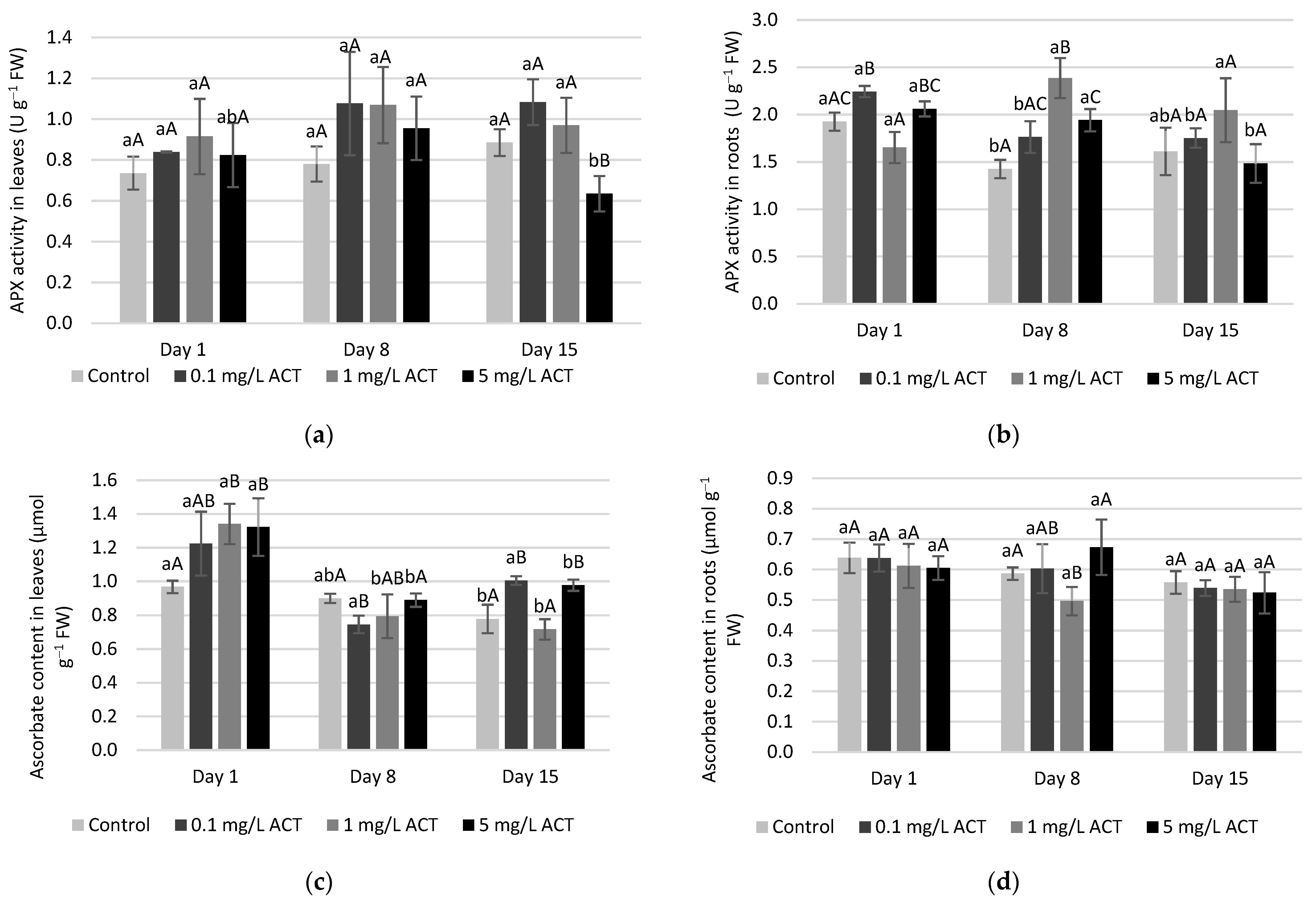
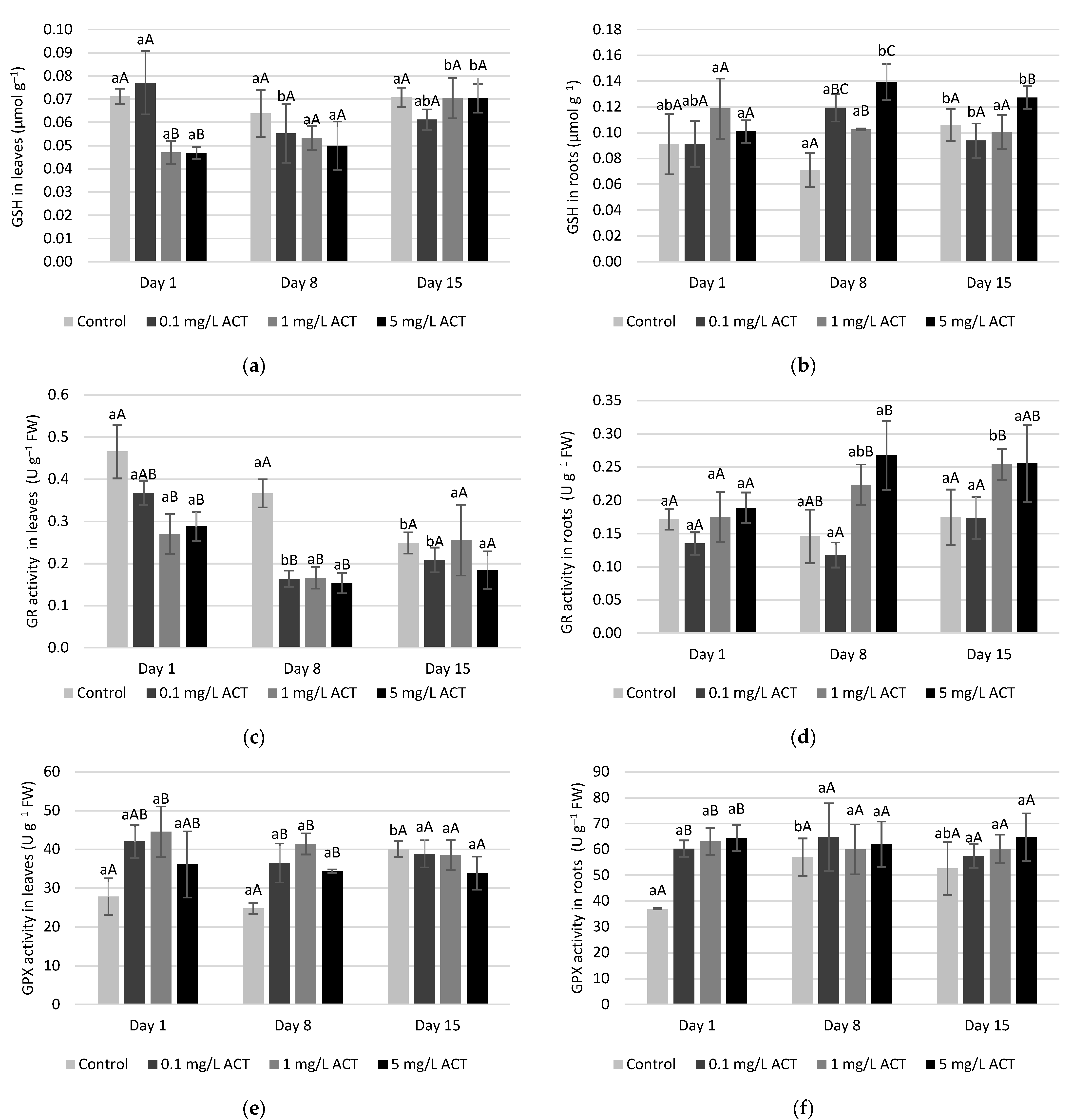
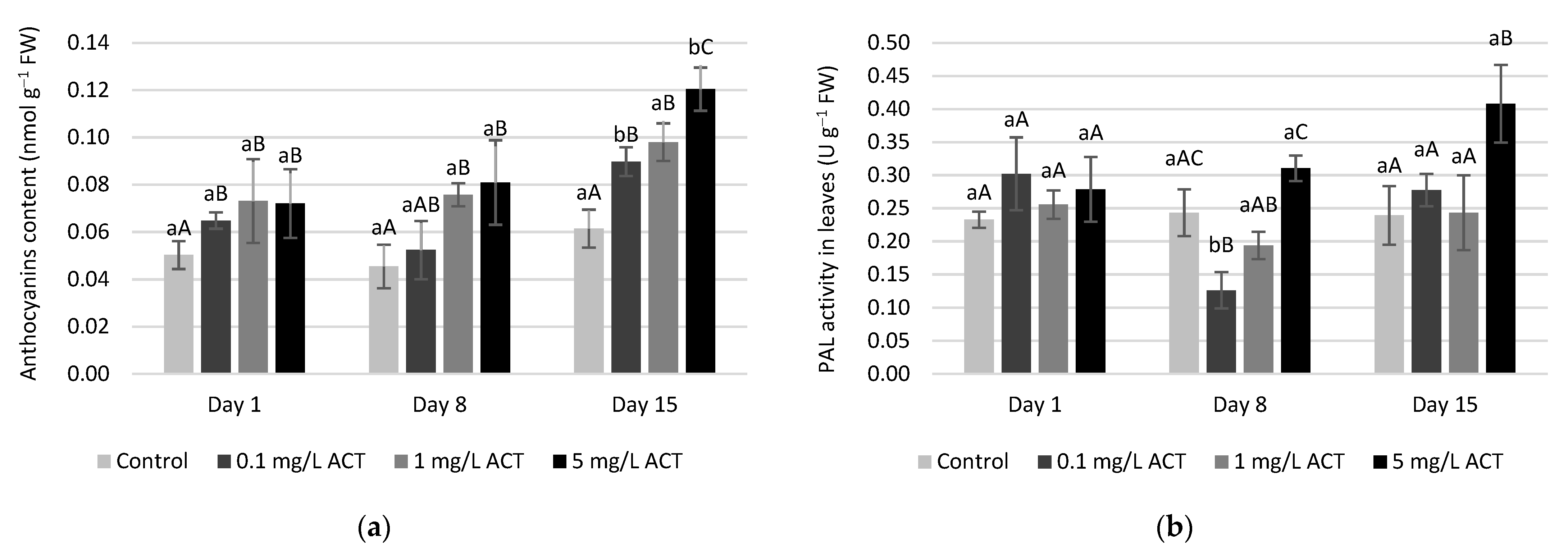
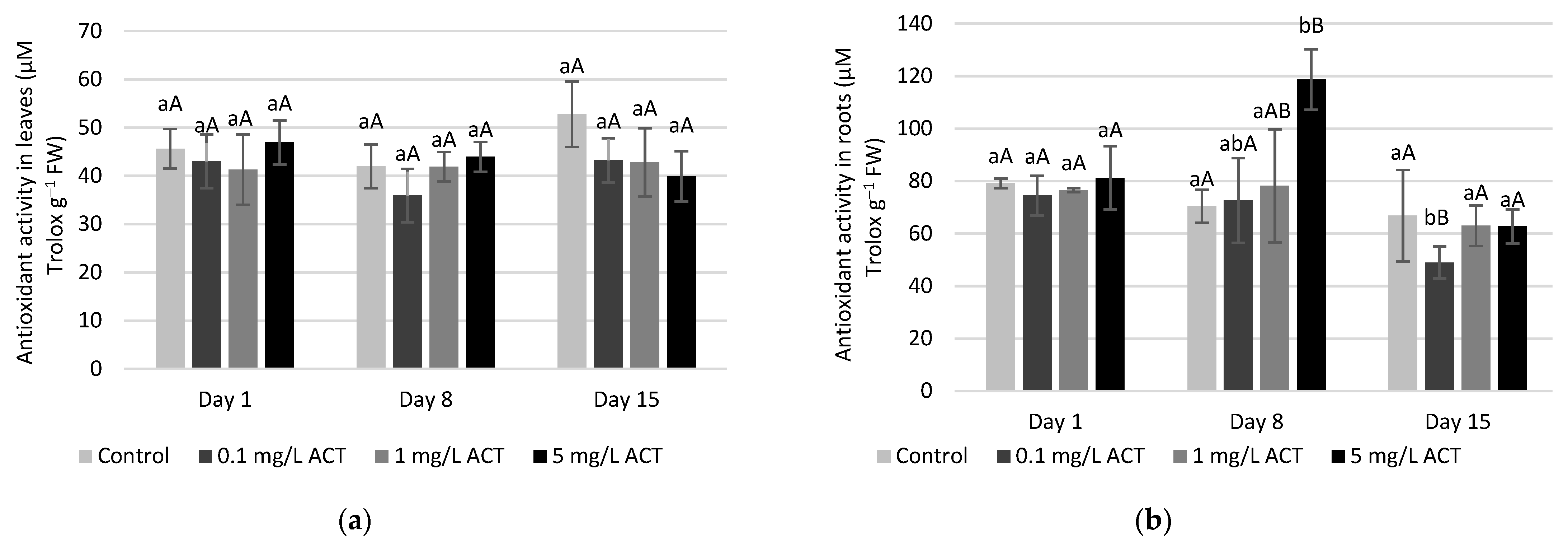
2.4. Antioxidative Enzymes and Metabolites Related to Stress Response
3. Materials and Methods
3.1. Experimental Setup
3.2. Activity of Antioxidant Enzymes
3.3. Photosynthetic Parameters, Total Chlorophyll and Anthocyanin Content
3.4. Hydrogen Peroxide Content and Lipid Peroxidation
3.5. Glutathione and Ascorbate Content
3.6. Antioxidant Activity
3.7. Acetaminophen Identification and Quantification
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Chen, J.; Wang, J.; Ma, Z.; Han, P.; Luan, Y.; Lu, A. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Sci. Total Environ. 2015, 521–522, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, E.; Cren-Olivé, C. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ. Pollut. 2011, 159, 2929–2934. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Palacios-Díaz, M.d.P.; Estévez, E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Cabrera, M.d.C. Occurrence of Pharmaceutical Compounds in Groundwater from the Gran Canaria Island (Spain). Water 2021, 13, 262. [Google Scholar] [CrossRef]
- Fram, M.S.; Belitz, K. Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci. Total Environ. 2011, 409, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dodgen, L.K.; Conkle, J.L.; Gan, J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: A review. Sci. Total Environ. 2015, 536, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Wychodnik, K.; Gałęzowska, G.; Rogowska, J.; Potrykus, M.; Plenis, A.; Wolska, L. Poultry Farms as a Potential Source of Environmental Pollution by Pharmaceuticals. Molecules 2020, 25, 1031. [Google Scholar] [CrossRef]
- Herklotz, P.A.; Gurung, P.; Vanden Heuvel, B.; Kinney, C.A. Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere 2010, 78, 1416–1421. [Google Scholar] [CrossRef]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity and degradation of antibiotic ofloxacin in duckweed (Spirodela polyrhiza) system. Ecotoxicol. Environ. Saf. 2019, 179, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bartrons, M.; Peñuelas, J. Pharmaceuticals and Personal-Care Products in Plants. Trends Plant Sci. 2017, 22, 194–203. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Mourato, M.; Reis, R.; Martins, L. Characterization of plant antioxidative system in response to abiotic stresses: A focus on heavy metal toxicity. In Advances in Selected Plant Physiology Aspects; Montanaro, G., Dichio, B., Eds.; Intech: Rijeka, Croatia, 2012; pp. 23–44. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.I.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2011, 35, 454–484. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.V.; Mehta, V. Paracetamol: Mechanisms and updates. Contin. Educ. Anaesth. Crit. Care Pain 2013, 14, 153–158. [Google Scholar] [CrossRef]
- Huber, C.; Bartha, B.; Harpaintner, R.; Schroder, P. Metabolism of acetaminophen (paracetamol) in plants--two independent pathways result in the formation of a glutathione and a glucose conjugate. Environ. Sci. Pollut. Res. Int. 2009, 16, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kotyza, J.; Soudek, P.; Kafka, Z.; Vaněk, T. Phytoremediation of Pharmaceuticals—Preliminary Study. Int J. Phytorem 2010, 12, 306–316. [Google Scholar] [CrossRef] [PubMed]
- INFARMED, Medicamentos não Sujeitos a Receita Médica (MNSRM)—Monitorização das Vendas Fora das Farmácias. 2020. Available online: http://www.infarmed.pt/documents/15786/2210525/janeiro+a+dezembro/53e03bcd-4530-42ed-9114-d00190d0c4dd?version=1.0 (accessed on 12 April 2021).
- Favaro, S.P.; Alba, Y.C.; de Souza, A.D.V.; Vianna, A.C.A.; Roel, A.R. Characterization of lettuce (Lactuca sativa L.) grown with biopesticides and deltamethrin. Sci. Hortic. 2011, 130, 498–502. [Google Scholar] [CrossRef]
- Alkimin, G.D.; Daniel, D.; Frankenbach, S.; Serôdio, J.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of pharmaceutical toxic effects of non-standard endpoints on the macrophyte species Lemna minor and Lemna gibba. Sci. Total Environ. 2019, 657, 926–937. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wang, Q.; Jiao, L.; Hua, W.; Zhou, Q.; Huang, X. Photosynthesis, chlorophyll fluorescence characteristics, and chlorophyll content of soybean seedlings under combined stress of bisphenol A and cadmium. Environ. Toxicol. Chem. 2014, 33, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Field, C.B.; Goulden, M.L.; Griffin, K.L.; Hartley, A.E.; Joel, G.; Peñuelas, J.; Valentini, R. Relationships Between NDVI, Canopy Structure, and Photosynthesis in Three Californian Vegetation Types. Ecol. Appl. 1995, 5, 28–41. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Rem. Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Dias, M.C.; Figueiredo, P.; Duarte, I.F.; Gil, A.M.; Santos, C. Different responses of young and expanded lettuce leaves to fungicide Mancozeb: Chlorophyll fluorescence, lipid peroxidation, pigments and proline content. Photosynthetica 2014, 52, 148–151. [Google Scholar] [CrossRef]
- Dordio, A.V.; Belo, M.; Martins Teixeira, D.; Palace Carvalho, A.J.; Dias, C.M.B.; Picó, Y.; Pinto, A.P. Evaluation of carbamazepine uptake and metabolization by Typha spp., a plant with potential use in phytotreatment. Bioresour. Technol. 2011, 102, 7827–7834. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Ketsa, S. Cross reactivity between ascorbate peroxidase and phenol (guaiacol) peroxidase. Postharvest Biol. Technol. 2014, 95, 64–69. [Google Scholar] [CrossRef]
- Di Cori, P.; Lucioli, S.; Frattarelli, A.; Nota, P.; Tel-Or, E.; Benyamini, E.; Gottlieb, H.; Caboni, E.; Forni, C. Characterization of the response of in vitro cultured Myrtus communis L. plants to high concentrations of NaCl. Plant Physiol. Biochem. 2013, 73, 420–426. [Google Scholar] [CrossRef]
- Bartha, B.; Huber, C.; Harpaintner, R.; Schröder, P. Effects of acetaminophen in Brassica juncea L. Czern.: Investigation of uptake, translocation, detoxification, and the induced defense pathways. Environ. Sci. Pollut. Res. Int. 2010, 17, 1553–1562. [Google Scholar] [CrossRef]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolomics of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef]
- Vanderauwera, S.; Suzuki, N.; Miller, G.; van de Cotte, B.; Morsa, S.; Ravanat, J.-L.; Hegie, A.; Triantaphylidès, C.; Shulaev, V.; Van Montagu, M.C.E.; et al. Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. USA 2011, 108, 1711. [Google Scholar] [CrossRef]
- Gusman, G.S.; Oliveira, J.A.; Farnese, F.S.; Cambraia, J. Mineral nutrition and enzymatic adaptation induced by arsenate and arsenite exposure in lettuce plants. Plant Physiol. Biochem. 2013, 71, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Do, H.; Kim, I.-S.; Jeon, B.W.; Lee, C.W.; Park, A.K.; Wi, A.R.; Shin, S.C.; Park, H.; Kim, Y.-S.; Yoon, H.-S.; et al. Structural understanding of the recycling of oxidized ascorbate by dehydroascorbate reductase (OsDHAR) from Oryza sativa L. japonica. Sci. Rep. 2016, 6, 19498. [Google Scholar] [CrossRef]
- Passaia, G.; Margis-Pinheiro, M. Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 2015, 234, 22–26. [Google Scholar] [CrossRef]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzalka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Martins, L.L.; Mourato, M.P.; Cardoso, A.I.; Pinto, A.P.; Mota, A.M.; Goncalves, M.d.L.S.; de Varennes, A. Oxidative stress induced by cadmium in Nicotiana tabacum L.: Effects on growth parameters, oxidative damage and antioxidant responses in different plant parts. Acta Physiol. Plant 2011, 33, 1375–1383. [Google Scholar] [CrossRef]
- Pinto, F.R.; Mourato, M.P.; Sales, J.R.; Moreira, I.N.; Martins, L.L. Oxidative stress response in spinach plants induced by cadmium. J. Plant Nutr. 2017, 40, 268–276. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Gonçalves, E.F.; Lopes, C.; Amâncio, S. Physiological and agronomical responses to environmental fluctuations of two Portuguese grapevine varieties during three field seasons. Ciência Téc. Vitiv. 2018, 33, 1–14. [Google Scholar] [CrossRef]
- Murray, J.R.; Hackett, W.P. Dihydroflavonol Reductase Activity in Relation to Differential Anthocyanin Accumulation in Juvenile and Mature Phase Hedera helix L. Plant Physiol. 1991, 97, 343–351. [Google Scholar] [CrossRef]
- Fernández, R.; Bertrand, A.; Reis, R.; Mourato, M.P.; Martins, L.L.; González, A. Growth and physiological responses to cadmium stress of two populations of Dittrichia viscosa (L.) Greuter. J. Hazard. Mater. 2013, 244–245, 555–562. [Google Scholar] [CrossRef]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, I.; Martins, L.L.; Carvalho, L.; Oliveira, M.C.; Marques, M.M.; Mourato, M.P. Acetaminophen Induces an Antioxidative Response in Lettuce Plants. Plants 2021, 10, 1152. https://doi.org/10.3390/plants10061152
Leitão I, Martins LL, Carvalho L, Oliveira MC, Marques MM, Mourato MP. Acetaminophen Induces an Antioxidative Response in Lettuce Plants. Plants. 2021; 10(6):1152. https://doi.org/10.3390/plants10061152
Chicago/Turabian StyleLeitão, Inês, Luisa L. Martins, Luisa Carvalho, M. Conceição Oliveira, M. Matilde Marques, and Miguel P. Mourato. 2021. "Acetaminophen Induces an Antioxidative Response in Lettuce Plants" Plants 10, no. 6: 1152. https://doi.org/10.3390/plants10061152
APA StyleLeitão, I., Martins, L. L., Carvalho, L., Oliveira, M. C., Marques, M. M., & Mourato, M. P. (2021). Acetaminophen Induces an Antioxidative Response in Lettuce Plants. Plants, 10(6), 1152. https://doi.org/10.3390/plants10061152