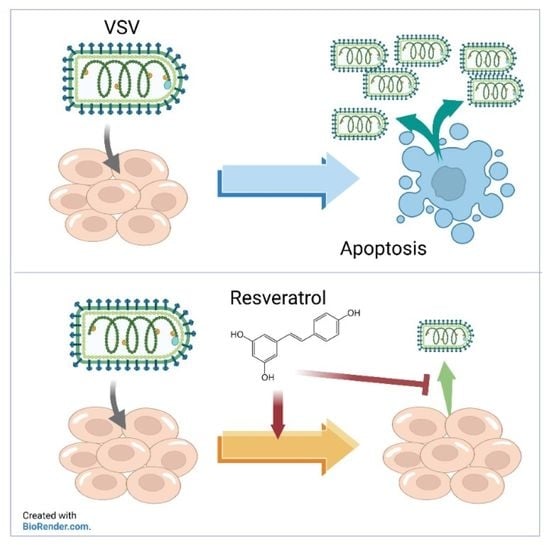
A Natural Botanical Product, Resveratrol, Effectively Suppresses Vesicular Stomatitis Virus Infection In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines and Cell Culture
2.3. Virus Infection
2.4. Cell Viability Assay
2.5. Fluorescence Analysis
2.6. Plaque Assay
2.7. Docking Prediction
2.8. Caspase-Glo® 3/7 Assay
2.9. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumberger, U. Growth of Vesicular Stomatitis Virus in Human Myeloblasts. Pathol. Microbiol. 1970, 36, 161–172. [Google Scholar] [CrossRef]
- Letchworth, G.; Rodriguez, L.; Del Cbarrera, J. Vesicular Stomatitis. Vet. J. 1999, 157, 239–260. [Google Scholar] [CrossRef]
- Rozo-Lopez, P.; Drolet, B.S.; Londoño-Renteria, B. Vesicular Stomatitis Virus Transmission: A Comparison of Incriminated Vectors. Insects 2018, 9, 190–205. [Google Scholar] [CrossRef]
- Whelan, S.P.J. Vesicular Stomatitis Virus. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 291–299. [Google Scholar]
- Stallknecht, D.E.; Perzak, D.E.; Bauer, L.D.; Murphy, M.D.; Howerth, E.W. Contact transmission of vesicular stomatitis virus New Jersey in pigs. Am. J. Vet. Res. 2001, 62, 516–520. [Google Scholar] [CrossRef]
- Quiroz, E.; Moreno, N.; Tesh, R.B.; Peralta, P.H. A Human Case of Encephalitis Associated with Vesicular Stomatitis Virus (Indiana Serotype) Infection. Am. J. Trop. Med. Hyg. 1988, 39, 312–314. [Google Scholar] [CrossRef]
- Overly, C.; Rieff, H.; Hollenbeck, P. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J. Cell Sci. 1996, 109 Pt 5, 971–980. [Google Scholar] [CrossRef]
- Kobinger, G.P.; Croyle, M.; Feldmann, H. Chapter 20—Ebola and Marburg. In Vaccines for Biodefense and Emerging and Neglected Diseases; Barrett, A.D.T., Stanberry, L.R., Eds.; Academic Press: London, UK, 2009; pp. 325–337. [Google Scholar]
- Jones, S.M.; Feldmann, H.; Ströher, U.; Geisbert, J.B.; Fernando, L.; Grolla, A.; Klenk, H.-D.; Sullivan, N.J.; Volchkov, V.E.; Fritz, E.A.; et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 2005, 11, 786–790. [Google Scholar] [CrossRef]
- Yahalom-Ronen, Y.; Tamir, H.; Melamed, S.; Politi, B.; Shifman, O.; Achdout, H.; Vitner, E.B.; Israeli, O.; Milrot, E.; Stein, D.; et al. A single dose of recombinant VSV-∆G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 6402. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.H.; Naczki, C.; Koumenis, C.; Lyles, D.S. Replication and Cytopathic Effect of Oncolytic Vesicular Stomatitis Virus in Hypoxic Tumor Cells In Vitro and In Vivo. J. Virol. 2004, 78, 8960–8970. [Google Scholar] [CrossRef] [PubMed]
- Cobleigh, M.A.; Bradfield, C.; Liu, Y.; Mehta, A.; Robek, M.D. The Immune Response to a Vesicular Stomatitis Virus Vaccine Vector Is Independent of Particulate Antigen Secretion and Protein Turnover Rate. J. Virol. 2012, 86, 4253–4261. [Google Scholar] [CrossRef] [PubMed]
- Forger, J.M., 3rd; Bronson, R.T.; Huang, A.S.; Reiss, C.S. Murine infection by vesicular stomatitis virus: Initial characterization of the H-2d system. J. Virol. 1991, 65, 4950–4958. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, A.J.; Ballington, J.R. Resveratrol, Pterostilbene, and Piceatannol in Vaccinium Berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Jin, Y.Y.; Chung, H.-J.; Kim, H.-J.; Baek, S.-H.; Hong, S.-T. Effect of the Resveratrol Rice DJ526 on Longevity. Nutrients 2019, 11, 1804–1817. [Google Scholar] [CrossRef]
- Pallauf, K.; Rimbach, G.; Rupp, P.M.; Chin, D.; Wolf, I.M. Resveratrol and Lifespan in Model Organisms. Curr. Med. Chem. 2016, 23, 4639–4680. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Saqib, U.; Kelley, T.T.; Panguluri, S.K.; Liu, D.; Savai, R.; Baig, M.S.; Schürer, S.C. Polypharmacology or Promiscuity? Structural Interactions of Resveratrol With Its Bandwagon of Targets. Front. Pharmacol. 2018, 9, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613–107621. [Google Scholar] [CrossRef]
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.; Kehn-Hall, K.; Aggarwal, M.; Bracci, N.; Pan, H.-C.; Panny, L.; Lamb, R.; Lin, S.-C. Resveratrol Inhibits Venezuelan Equine Encephalitis Virus Infection by Interfering with the AKT/GSK Pathway. Plants 2021, 10, 346–362. [Google Scholar] [CrossRef]
- Lundberg, L.; Brahms, A.; Hooper, I.; Carey, B.; Lin, S.-C.; Dahal, B.; Narayanan, A.; Kehn-Hall, K. Repurposed FDA-Approved drug sorafenib reduces replication of Venezuelan equine encephalitis virus and other alphaviruses. Antivir. Res. 2018, 157, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Baer, A.; Kehn-Hall, K. Viral Concentration Determination Through Plaque Assays: Using Traditional and Novel Overlay Systems. J. Vis. Exp. 2014, e52065. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 3, W270–W277. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.; Thornton, J. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Payne, A.M.; Zorman, J.; Horton, M.; Dubey, S.; Ter Meulen, J.; Vora, K.A. Caspase Activation as a Versatile Assay Platform for Detection of Cytotoxic Bacterial Toxins. J. Clin. Microbiol. 2013, 51, 2970–2976. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kawai, A. Studies on the Antiviral Mechanisms of Protein Kinase Inhibitors K-252a and KT5926 against the Replication of Vesicular Stomatitis Virus. Biol. Pharm. Bull. 1998, 21, 498–505. [Google Scholar] [CrossRef]
- Chan, P.-M.; Tan, Y.-S.; Chua, K.-H.; Sabaratnam, V.; Kuppusamy, U.R. Attenuation of Inflammatory Mediators (TNF-α and Nitric Oxide) and Up-Regulation of IL-10 by Wild and Domesticated Basidiocarps of Amauroderma rugosum (Blume & T. Nees) Torrend in LPS-Stimulated RAW264.7 Cells. PLoS ONE 2015, 10, e0139593. [Google Scholar] [CrossRef]
- Emeny, J.M.; Morgan, M.J. Regulation of the Interferon System: Evidence that Vero Cells have a Genetic Defect in Interferon Production. J. Gen. Virol. 1979, 43, 247–252. [Google Scholar] [CrossRef]
- Hobbs, J.A.; Hommel-Berrey, G.; Brahmi, Z. Requirement of caspase-3 for efficient apoptosis induction and caspase-7 activation but not viral replication or cell rounding in cells infected with vesicular stomatitis virus. Hum. Immunol. 2003, 64, 82–92. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Amarante-Mendes, G.P.; Finucane, D.; Brunner, T.; Bossy-Wetzel, E.; Green, D.R. Propidium Iodide (PI) Uptake Assay to Detect Apoptosis. Cold Spring Harb. Protoc. 2006, 2006. [Google Scholar] [CrossRef] [PubMed]
- Agniswamy, J.; Fang, B.; Weber, I.T. Conformational similarity in the activation of caspase-3 and -7 revealed by the unliganded and inhibited structures of caspase-7. Apoptosis 2009, 14, 1135–1144. [Google Scholar] [CrossRef]
- Ni, C.-Z.; Li, C.; Wu, J.C.; Spada, A.P.; Ely, K.R. Conformational restrictions in the active site of unliganded human caspase-3. J. Mol. Recognit. 2003, 16, 121–124. [Google Scholar] [CrossRef]
- Wollmann, G.; Paglino, J.C.; Maloney, P.; Ahmadi, S.A.; Pol, A.N.V.D. Attenuation of vesicular stomatitis virus infection of brain using antiviral drugs and an adeno-associated virus-interferon vector. Virology 2015, 475, 1–14. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; El-Hack, M.E.A. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef]
- Zhao, X.; Tong, W.; Song, X.; Jia, R.; Li, L.; Zou, Y.; He, C.; Liang, X.; Lv, C.; Jing, B.; et al. Antiviral Effect of Resveratrol in Piglets Infected with Virulent Pseudorabies Virus. Viruses 2018, 10, 457–467. [Google Scholar] [CrossRef]
- Cui, Q.; Fu, Q.; Zhao, X.; Song, X.; Yu, J.; Yang, Y.; Sun, K.; Bai, L.; Tian, Y.; Chen, S.; et al. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PLoS ONE 2018, 13, e0192692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, L.; Zhao, X.; Chen, X.; Wang, L.; Geng, Z. Effect of dietary resveratrol supplementation on meat quality, muscle antioxidative capacity and mitochondrial biogenesis of broilers. J. Sci. Food Agric. 2018, 98, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Henson, D.A.; Sanderson, M.; Nieman, D.C.; Gillitt, N.D.; Lila, M.A. The Protective Effects of a Polyphenol-Enriched Protein Powder on Exercise-Induced Susceptibility to Virus Infection. Phytotherapy Res. 2014, 28, 1829–1836. [Google Scholar] [CrossRef]
- Johnson, J.E.; Nasar, F.; Coleman, J.W.; Price, R.E.; Javadian, A.; Draper, K.; Lee, M.; Reilly, P.A.; Clarke, D.K.; Hendry, R.M.; et al. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 2007, 360, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Agrawal, M.; Doré, S. Neuroprotective Properties and Mechanisms of Resveratrol in in Vitro and in Vivo Experimental Cerebral Stroke Models. ACS Chem. Neurosci. 2013, 4, 1151–1162. [Google Scholar] [CrossRef]
- Liu, J.; HuangFu, W.-C.; Kumar, K.S.; Qian, J.; Casey, J.P.; Hamanaka, R.B.; Grigoriadou, C.; Aldabe, R.; Diehl, J.A.; Fuchs, S.Y. Virus-Induced Unfolded Protein Response Attenuates Antiviral Defenses via Phosphorylation-Dependent Degradation of the Type I Interferon Receptor. Cell Host Microbe 2009, 5, 72–83. [Google Scholar] [CrossRef]
- Takashina, M.; Inoue, S.; Tomihara, K.; Tomita, K.; Hattori, K.; Zhao, Q.-L.; Suzuki, T.; Noguchi, M.; Ohashi, W.; Hattori, Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int. J. Oncol. 2017, 50, 787–797. [Google Scholar] [CrossRef]
- Duan, J.; Yue, W.; E, J.; Malhotra, J.; Lu, S.-E.; Gu, J.; Xu, F.; Tan, X.-L. In vitro comparative studies of resveratrol and triacetylresveratrol on cell proliferation, apoptosis, and STAT3 and NFκB signaling in pancreatic cancer cells. Sci. Rep. 2016, 6, 31672–31681. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-C.; Zhang, X.; Lehman, C.W.; Pan, H.-C.; Wen, Y.; Chen, S.-Y. A Natural Botanical Product, Resveratrol, Effectively Suppresses Vesicular Stomatitis Virus Infection In Vitro. Plants 2021, 10, 1231. https://doi.org/10.3390/plants10061231
Lin S-C, Zhang X, Lehman CW, Pan H-C, Wen Y, Chen S-Y. A Natural Botanical Product, Resveratrol, Effectively Suppresses Vesicular Stomatitis Virus Infection In Vitro. Plants. 2021; 10(6):1231. https://doi.org/10.3390/plants10061231
Chicago/Turabian StyleLin, Shih-Chao, Xiang Zhang, Caitlin W. Lehman, Han-Chi Pan, Ya Wen, and Shiow-Yi Chen. 2021. "A Natural Botanical Product, Resveratrol, Effectively Suppresses Vesicular Stomatitis Virus Infection In Vitro" Plants 10, no. 6: 1231. https://doi.org/10.3390/plants10061231
APA StyleLin, S.-C., Zhang, X., Lehman, C. W., Pan, H.-C., Wen, Y., & Chen, S.-Y. (2021). A Natural Botanical Product, Resveratrol, Effectively Suppresses Vesicular Stomatitis Virus Infection In Vitro. Plants, 10(6), 1231. https://doi.org/10.3390/plants10061231